Summary
This article is dedicated to our teacher, Prof. Erich Kuhn, Heidelberg, on the occasion of his 88th birthday on 23th November 2008. In contrast to muscular dystrophies, the muscle channelopathies, a group of diseases characterised by impaired muscle excitation or excitation-contraction coupling, can fairly well be treated with a whole series of pharmacological drugs. However, for a proper treatment proper diagnostics are essential. This article lists state-of-the-art diagnostics and therapies for the two types of myotonic dystrophies, for recessive and dominant myotonia congenita, for the sodium channel myotonias, for the primary dyskalemic periodic paralyses, for central core disease and for malignant hyperthermia susceptibility in detail. In addition, for each disorder a short summary of aetiology, symptomatology, and pathogenesis is provided.
Keywords: Chloride and sodium channel myotonias, periodic paralyses, malignant hyperthermia and central core disease
Introduction
Research into myotonias and periodic paralyses began in Ulm in the early 1980s (1). With the arrival of molecular genetics, the 1990s provided the first possibilities to apply human genetic diagnostics to this group of hereditary diseases. The desire to have “their” mutation identified brought many affected families to ask the specialists for diagnostic and therapeutic assistance. The growing interest in research into neuromuscular diseases, created with the detection of the “Duchenne gene”, induced the University of Ulm to formally establish a Muscle Centre at its Faculty of Medicine in 1993. National and international collaborations between physicians caring for affected families were soon established to collect the necessary numbers of patients for a statistically significant mapping of the mutated genes. This in turn made it imperative that the families entering the gene search for a certain disease really all had the same nosologic identity. Modern molecular genetics requires clear-cut diagnostic criteria. To achieve this, Alan Emery, in the 1990s Director of the European Neuromuscular Centre (ENMC), assigned various experts to compile diagnostic criteria for all neuromuscular disorders. The specialists of the Ulm Muscle Centre contributed the Chapter on myotonias and periodic paralyses to this pioneering booklet (2). Then, at the end of the 1990s, the German Neurological Society decided to have guidelines elaborated for all neurological diseases, and again the experts of the Ulm Muscle Centre were asked to contribute the part on what during an ENMC workshop held at Ulm in 1993 had meanwhile been dubbed “muscle channelopathies”. The resulting book has almost 500 pages (3). The following guidelines have grown out of this chapter as a revised, much expanded, and illustrated update. The discussed diseases are classified in Table I.
Table 1. Listing of the main muscle channelopathies of man, as treated in this article.
| Classification of the Muscle Channelopathies |
| I. Myotonic Dystrophies |
| Myotonic dystrophy type 1 (DM1, Curschmann-Steinert) |
| Myotonic dystrophy type 2 (DM2, PROMM) |
| II. Chloride Channel Myotonias |
| Recessive myotonia congenita (Becker) |
| Dominant myotonia congenita (Thomsen) |
| III. Sodium Channel Myotonias |
| Paramyotonia congenita (Eulenburg) |
| Potassium-aggravated myotonia (PAM) |
| Myotonia fluctuans |
| Myotonia permanens |
| IV. Dyskalemic Episodic Paralyses |
| Hyperkalaemic periodic paralysis (HyperPP) |
| Hypokalaemic periodic paralysis (HypoPP) |
| Normokalaemic periodic paralysis (NormoPP) |
| Andersen syndrome |
| V. Calcium release channelopathies |
| Malignant hyperthermia (MH) susceptibility |
| Central core disease (CCD) |
I. Myotonic dystrophies
In this first group of two diseases, DM1 and DM2, the molecular pathomechanism is not based on mutations in genes coding for ion channels, as is the case in the diseases listed under II-V of Table 1. The symptoms rather stem from mutations in genes with nucleotide repeats of not strictly defined length. The two diseases are caused by serious expansions of such repeats. Ribonucleic acid (RNA) transcripts of these mutant expansions accumulate in cell nuclei, where they interfere with the normal RNA metabolism of the cell. The mutant RNA transcripts sequester CUG-binding protein 1 (CUG-BP1) and muscleblind-like proteins (MBNL) which normally regulate alternative splicing (4). Several genes, including the gene CLCN1 which codes for the muscular chloride channel, are abnormally spliced in DM1 and DM2. The name-giving symptom of myotonia most likely stems from increased or alternative splicing leading to non-functional chloride channel gene products. Thus, although the myotonic dystrophies are not channelopathies in the usual sense, the myotonia is still generated by abnormally functioning muscular chloride channels.
1. Myotonic dystrophy type 1 (DM1, Curschmann-Steinert disease)
Myotonic dystrophy type 1 (DM1) is the most common muscle disease of adult age in Europe (prevalence 5.5/100,000). It is genetically transmitted as a dominant trait. The main symptoms of this multisystemic disorder are distal muscular weakness, myotonia and cataracts.
Aetiology/Pathophysiology
The disease is caused by the expansion of a CTG repeat on chromosome 19q13.3 at the non-translated 3’ end of the gene coding for dystrophia myotonica protein kinase (DMPK). The symptoms are usually the more severe, the longer the expansion is. Pre-mRNA transcripts of the expanded CTG repeat accumulate in inclusions of the cell nuclei and in the myoplasm where they may increase or decrease the function of RNA-binding proteins. The observed multisystemic defects are at least in part explained by the fact that altered RNA-binding protein may influence the splice apparatus of transcription factors so that altered splicing may lead to dysfunctioning splice variants for a variety of proteins (4).
Clinical symptoms
Patients present with both muscular and extramuscular symptoms. Of the muscular symptoms the most prominent ones are myotonia, in particular in the hands and legs, muscular weakness and atrophy. The muscular weakness sets in distally and in the flexors of the head. Involvement of the facial muscles results in a so-called facies myopathica. Later in the course of the disease, the proximal extremities are also affected.
Of the extramuscular symptoms, cataracts in the posterior part of the capsule are the most common ones. Polychromatic inclusions are only seen during a certain time period.
DM1 is often combined with diabetes mellitus (insulin resistance). Another common finding is primary hypogonadism, particularly in males. Males also frequently have frontal balding. A high fraction of the patients has restricted cognitive ability, with a tendency to dissimulate. Tiredness during the day may prevail with and without an apnea syndrome.
A severe congenital form exists that often shows symptoms even before birth. Post partum babies present with floppy infant syndrome. They have a high palate and at times keep their mouth open; they have difficulties with sucking. In general, their psychomotor development is retarded. This form is almost exclusively transmitted by a symptomatic mother, very rarely by a symptomatic father. The expansion of the CTG repeat is then always > 1,000.
Diagnostics
Obligatory
Tests of the creatine kinase (CK) and transaminases including gamma-GT, blood sugar, HBA1C and the parameters of the thyroid gland should be performed.
The electromyography (EMG) should be investigated for myotonic discharges and myopathic changes.
A search for myotonic cataracts should be performed by an experienced ophthalmologist.
Molecular genetic search for an expansion of the CTG repeat.
An electrocardiogram (ECG) investigation should search for defects in the cardiac impulse conduction.
Optional
Echocardiography in search of a rare (1-2%) cardiomyopathy.
In case of clinically manifest hypogonadism, the hormone status should be determined in consideration of a possible substitution therapy
Magnetic resonance investigation (MRI) of the muscles for a determination of the progression.
Brain MRI to estimate cerebral involvement.
Determination of serum immune globulines as an additional serologic parameter (in about 50% of patients IgG and/or IgM are decreased).
Therapy
Lifelong physiotherapeutic treatment of the muscular weakness is recommended in order to counteract contractures and a progression of the muscular weakness.
Of all drugs tested for treating the myotonia, mexiletine was found to be the most effective one (5), but because of a possible blocking of the cardiac impulse conducting system, treatment with this antiarrhythmic drug is recommended with limitations and only if the ECG is regularly controlled. This check should also comprise the drug serum concentration (6).
Creatine monophosphate has no evidently positive effect on the muscular weakness (7, 8).
A diabetic metabolic status and failures of the thyroid function should be treated under the usual caveats (6).
When failure of the cardiac impulse conduction is manifest, a pacemaker should be implanted (6, 9).
The cataracts should be operated when indicated
At the later states, hypersomnia might develop. In such cases, modafinil (200-400 mg daily) in an open study was reported to exert a positive effect (10). This was not confirmed in a more recent study (11).
Genetic counselling of the family is a must!
Risks and complications with operative treatments
Myotonic reactions due to depolarising muscle relaxants.
Apnoea after general anaesthesia, particularly after barbiturates.
Cataract operations: eye infection.
Pacemaker implantation: local infections, bleedings, pacemaker dislocation.
2. Myotonic dystrophy type 2 (DM2/PROMM)
Like DM1, myotonic dystrophy type 2 (DM2) is a multisystemic disorder. Its main manifestations are myotonia, cataract and proximal muscle weakness. Because of the latter symptom, the disease was first called Proximal Myotonic Myopathy (PROMM) (12). Its prevalence in Germany seems to be similarly high as that of DM1, but is much lower in other countries.
Aetiology/Pathophysiology
Responsible is an expansion of a CCTG repeat on chromosome 3q2.13 in the first intron of the gene coding for zinc finger protein 9 (ZNF9) (13). The length of the extension does not correspond with the severity of the disease.
Clinical symptoms
The progression of DM2 is as a rule much milder than that of DM1. The muscular symptoms are usually not very pronounced, in particular not in the hands and legs. Weakness and atrophy prevail distinctly more proximally. First symptoms appear often at the pelvic flexors and extensors, later, occasionally also the muscles of the distal extremities are affected. A facies myopathica, as in DM1, is rare. Patients suffer from myalgias. As in DM1, the most prominent extramuscular symptoms are cataracts in the posterior parts of the lens capsule, often only in the form of strong opacities, but sometimes also as polychromatic cataracts. Other extra-ocular symptoms are primary hypogonadism (particularly in men), frontal balding (much rarer than in DM1) and diabetes mellitus (probably insulin resistance). Also rarer than in DM1 are cognitive restrictions. A congenital form is unknown.
Diagnostics/Therapy
Are the same as with DM1 (6), except that in DM2, there is less need to treat the myotonia or the hypersomnia. However, frequent occurrence of a distinct status of exhaustion is very typical for DM2. The myalgias are often resistant to therapy. In some patients, treatment with gabapentin up to 4 x 400 mg, diclofenac 2 x 50 mg, or creatine monohydrate 4 g/day had a positive effect on the pain symptoms. In a controlled study, pain tended to be relieved in some patients by creatine monohydrate even though muscular strength was not significantly improved (14).
II. Chloride channel myotonias
The name-giving symptom of these diseases is a disturbance of muscle relaxation (myotonia) experienced by the patients as muscle stiffness. Thomsen was the first to describe the symptom in a family where the mode of inheritance was dominant. Later, Becker discovered that in many families with similar symptoms the mode of inheritance was recessive. Since Becker myotonia is much more common (prevalence 1:25,000) than Thomsen myotonia (prevalence 1:400,000), and as a rule is a much more severe disease, we have decided to deal with it first in this article, in contrast to common usage. A characteristic sign for all chloride channel myotonias is the athletic habitus, the warm-up phenomenon, i.e. an improvement of muscle stiffness by repeated muscle contractions, and that males are usually more severely affected than females. The myotonia is pronounced during pregnancy and hypothyroidism. The condition may be already present at birth, but becomes usually manifest during childhood (15).
Aetiology/Pathophysiology
Disease-causing are missense or nonsense mutations or deletions in the CLCN1 gene on chromosome 7q encoding the major muscle chloride channel, CLC1. The mutations lead to faulty or missing chloride channels in the fibre membrane (Fig. 1). The activity of the chloride channels at the resting potential of the muscle fibres is decreased and this increases the excitability of the membrane (16–18). Series of involuntary action potentials following voluntary action potentials result in muscle stiffness.
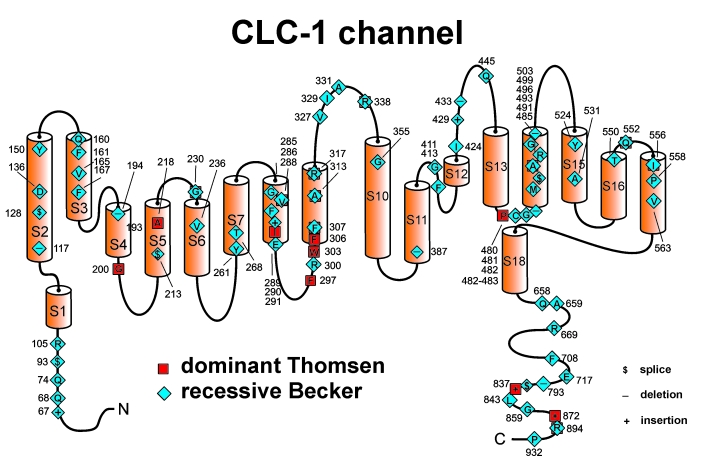
Figure 1.
Membrane topology model of the ClC1 monomer. The functional channel is an antiparallel-assembled homodimer. It possesses two independent ion-conducting pores each with a fast opening mechanism of its own, two selectivity filters, and two voltage sensors. The channel is functional without any other subunits. Symbols are used for mutation-caused amino acid substitutions leading to either recessive Becker or dominant Thomsen myotonia congenita. The amino acids at which substitutions occur are indicated by one-letter abbreviations. They are numbered according to the protein sequence.
1. Recessive myotonia congenita (Becker)
Clinical symptoms
The major symptom is the stiffness that occurs in all skeletal muscles upon frightening, during very sudden movements in a starting reaction, or simply during voluntary movements performed after a period of rest. The patients are also often much impeded by a transient weakness following the stiffness. Toddlers tend to common falls and clumsiness in grasping and walking. Continuous small involuntary muscle contractions may lead to an athletic build of the patients even though muscle strength may not be increased. Muscle shortening due to continuous involuntary contractions may limit bilateral dorsiflection of the wrist or foot and lead to toe-walking and development of a compensatory lordosis. The leg and gluteal muscles are often markedly hypertrophic. In some patients, especially the older ones, the neck, shoulder and arm muscles appear poorly developed resulting in a characteristic disproportionate figure. Very disabling is the mentioned transient weakness. It lasts only a few seconds following initial contractions and superimposes the myotonic stiffness. The symptoms can progress until adulthood. Patients with severe Becker myotonia are limited in their choice of occupation and are unsuited for military service. A few Becker patients show a permanent, late-onset weakness in some muscle groups, distal muscle atrophy, and unusually high serum CK levels, making the differentiation from myotonic dystrophies difficult. Life expectancy is normal.
Diagnostics
Obligatory
Search for myotonia by having the patient repeatedly open and close the fist and looking for the speed of finger opening (warm-up phenomenon).
A warm-up phenomenon of the eyelid muscles is further evidence for the diagnosis of a chloride channel myotonia whereas sodium channel myotonia mostly is associated with paradoxical eyelid myotonia.
A slight blow on the tongue or the muscles of the extremities to elicit percussion myotonia.
Search for lid-lag.
Determination of the CK and transaminases. The CK is, as a rule, not more increased than 2 times whereas in sodium channel myotonias it is often higher.
Several muscles should be examined with the EMG in search for myotonic discharges.
A molecular genetics test should be performed to verify the recessive trait (usually there are mutations on both alleles which leads to a premature stop codon) and to exclude sodium channel myotonia.
Optional
In unclear cases apply molecular diagnostics to exclude DM1 or DM2.
Therapy
Treatment of the muscle stiffness is only indicated if the myotonia compromises the every day life or when reduction of symptoms is required for professional or social activities.
Therapy of first choice is mexiletine (2-3 x 200 mg/day). A cardiologic test should precede administration, and the tolerance should be occasionally controlled. Stepwise reduction of the dose is recommended for the ending of a treating period. Note the low therapeutic ratio of the agent (particularly children should avoid dehydration). Cave: affection of cardiac impulse conduction!
Therapies of second choice are carbamazepine (up to 3 x 200 mg) or phenytoin (3 x 100 mg/day). Cave: low sodium serum levels and affection of cardiac impulse conduction with phenytoin!
Depolarising agents such as succinylcholine can cause a severe myotonic reaction of all muscles. They may impair intubation and ventilation and should therefore be avoided.
If tocolysis is required, feneterol is contraindicated.
Genetic counseling of the family is recommended.
2. Dominant myotonia congenita (Thomsen)
Is caused by a missense mutation on one allele that exerts a dominant effect on the homodimeric channel complex. Usually, the open probability of the mutant channel complex is reduced at the resting membrane potential and only increases at large membrane depolarization.
Except the few Thomsen patients who harbor a severe dominant mutation, myotonic stiffness, muscle hypertrophy and transient weakness are less pronounced than in Becker myotonia and muscle shortening is absent.
Diagnostics
As in Becker myotonia.
Therapy
In contrast to Becker patients, the majority of Thomsen patients needs no medication. Continual slight exercise maintains the “warmed-up” state.
III. Sodium channel myotonias
This group of diseases comprises the long known paramyotonia congenita (Eulenburg) and the fairly recently detected potassium-aggravated myotonia, a set of disorders with varied severity of the myotonia.
Aetiology/Pathophysiology
The diseases are caused by point mutations in the gene SCN4A on chromosome 17q23 that codes for the sodium channel Nav 1.4, of skeletal muscle (Fig. 2). The mutations lead to sodium channels in the sarcolemma that show impaired inactivation (19). This process may be impaired in two different ways, i.e., (i) by imperfect inactivation with incomplete closure of the affected channels at the end of the depolarisation phase of the action potential, or (ii) by a slowing of the inactivation process. In either case the result is an increased inflow of Na+ into the muscle fibres and a transient intracellular Na+ accumulation (20). If the Na+ inflow is only slightly increased, repetitive action potentials are generated that lead to the symptom of myotonia (potassium-sensitive myotonia). If the Na+ inflow is considerably increased, the cell membranes tend to depolarise for a longer time and to a degree where intact Na+ channels are inactivated. This leads to inexcitability of the sarcolemma. Hyperactivity and hypoactivity may be overlapping so that muscles may become both stiff and weak at the same time.
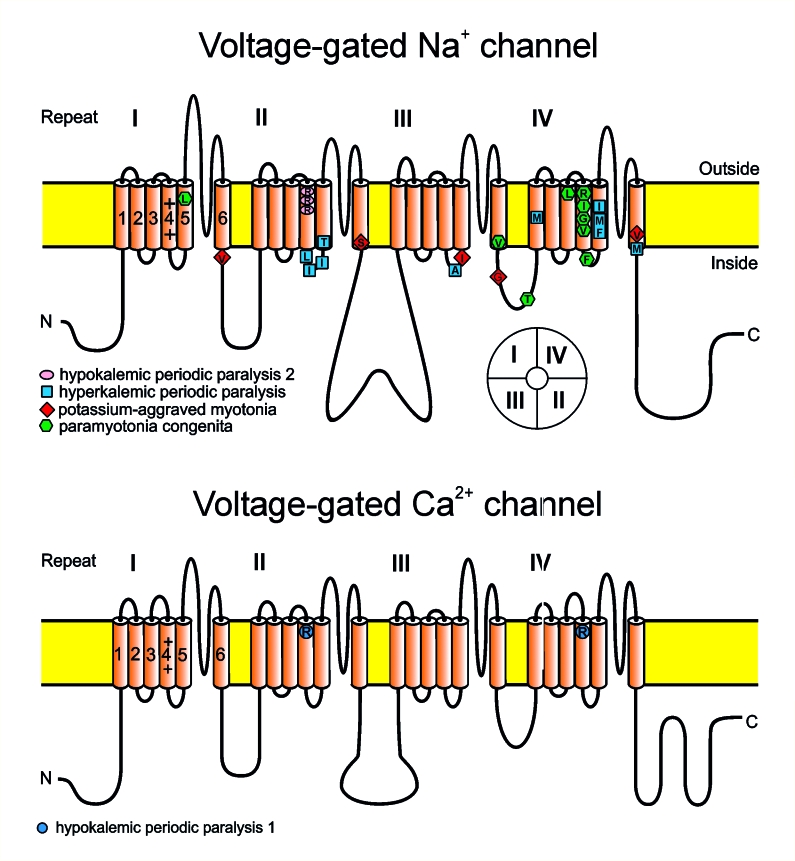
Figure 2.
The voltage-gated Na+ and Ca2+ channels unfolded. The α subunit consists of four highly homologous domains I-IV with six transmembrane segments each (S1-S6). The S5-S6 loops and the transmembrane segments S6 form the ion selective pore; the S4 segments contain positively charged residues conferring voltage dependence to the protein. The S4 segments are thought to move outward upon depolarisation thereby inducing channel opening. The repeats are connected by intracellular loops. In the Na+ channel (upper panel), the III-IV linker, contains the supposed inactivation particle whereas the slowly activating and inactivating L-type Ca2+ channel (lower panel) does not possess a fast inactivation gate. When inserted into the membrane, the four repeats of the protein fold to generate a central pore as schematically indicated on the right bottom of the figure (see insert). Mutation-caused amino acid substitutions associated to the various diseases are indicated.
1. Paramyotonia congenita (Eulenburg)
This disease impresses both patients and investigators by the clear-cut dependency of its symptoms on the ambient temperature. The prevalence is 1:250,000. Before the era of molecular genetics there was a long-lasting argument whether paramyotonia and hyperkalaemic periodic paralysis are a nosological entity. Anyway, this separation was useful because the treatment of the two symptoms is different.
Aetiology/Pathophysiology
The mutations are preferentially situated in the S4 voltage sensor of repeat IV which is involved in fast channel inactivation and in the loop connecting repeats III and IV which contains the inactivation particle (Fig. 2).
Clinical symptoms
In a warm environment the symptoms are usually light or absent. When the ambient temperature decreases and the muscles have to work in the cold, they become increasingly myotonic. With sustained labour they become very weak and this weakness may last for several hours even during rewarming. These symptoms can already be noted at birth. They persist throughout life. In some families, paramyotonia patients also show episodes of hyperkalaemic paralyses from adolescence onwards. The myotonia usually affects the eye-lids, the extra-ocular muscles, the face and neck, the distal parts of the upper extremities and the lower extremities.
A very characteristic finding is the paradoxical myotonia, so called because, in contrast to the warm-up phenomenon in myotonia congenita, the muscle stiffness increases with repetitive movements. In the cold, the face becomes stiff like a mask. The fingers assume a painless flexed position. If hypothermia has been induced, e.g., during swimming in cold water or during general anaesthesia, a generalized weakness may ensue, and only because the diaphragm is pretty well prevented from getting cold, restriction of the respiratory muscles is usually prevented.
Myotonia, if not obvious, can be detected with the following test: with multiple forced closure of the eyes, eye-opening becomes very difficult, in particular when a cold and wet cloth had before been laid on the eyes. Myotonia is less frequently observed after closure of the fist, in percussion tests, or in the “staircase test” (introduced to show myotonia of the legs during climbing stairs). In certain forms of the disease (e.g., with the R1448C/P mutations) distal atrophies may be seen.
Diagnostics
Obligatory
Investigation of the EMG in the muscles of an extremity during cooling.
Determination of the CK and transaminases. The CK is often increased by more than a factor of 2.
The mutation should be defined using molecular genetics because the indicated therapy depends on it.
Optional
Muscle biopsy in unclear cases.
Therapy
Treatment is only indicated when the symptoms impair the everyday life. If medication is desired, regular cardiac controls are highly recommended.
The medication of choice depends on the mutation. Mexiletine (3 x 100 mg up to 3 x 200 mg) is best for R1448H/C/P patients (21), propafenon (2 x 150 mg to 2 x 300 mg) or flecainide (2 x 50-100 mg) is best for T1313M patients (22). These recommendations pertain to prophylactic needs, and the drugs should then be taken 2 days before an expected situation
For continuous medication 2-3 x 200 mg mexiletine are recommended, as it staffs off the cold-induced myotonia and paralysis. Before drug administration a cardiologic test is recommended (5, 23).
Under the administration of one of the above-mentioned antiarrhythmic drugs, patients with cardiac insufficiency or arrhythmias should have no more than 20% prolongation of their QRS time and the QT time should not exceed 500 ms. The absolute QTc time should remain stable.
The medication of second choice is carbamazepine (up to 3 x 200 mg retard).
A warm environment is always a good prophylaxis. If, however, cold-induced weakness has set in, restitution of force is not speeded up in a warm environment.
Genetic counselling of the family is recommended.
2. Potassium-aggravated myotonias (PAM)
This class comprises at least two forms, a light one (myotonia fluctuans) and a severe one (myotonia permanens). Both forms were only detected as nosological entities after molecular genetics had become available. Peter Emil Becker, who had separated the recessive form of chloride channel myotonia (that now carries his name) from dominant Thomsen myotonia, listed many families with dominant mode of inheritance as “strange” Thomsen cases. These had PAM as it turned out later. This separation lead to the experience that Thomsen mutations are rare, whereas PAM families are more frequent.
In contrast to paramyotonia congenita or hyperkalaemic periodic paralysis, PAM patients show no muscle weakness and hardly any cold sensitivity. However, they show an aggravation of their myotonia after ingestion of potassium, which distinguishes them from patients with chloride channelopathies.
Aetiology/Pathophysiology
Most Nav 1.4 mutations are located at inner parts of the transmembrane segments or in intracellular loops and affect structures that form the three-dimensional docking site for the fast inactivation particle (Fig. 2). Increase in extracellular potassium opens the mutant channels which do not adopt the fast inactivated state and initiate repetitive action potentials.
A. Myotonia fluctuans
In this form of the disease the myotonic symptoms are so mild that at times the patients hardly notice them.
Clinical symptoms
Myotonic stiffness occurs during or after protracted work in a warm environment (24). There are no signs of muscle weakness. The sensitivity to cold is very mild, if at all present.
Diagnostics
Obligatory
EMG recordings should be done while muscle stiffness is present, i.e. 20 min after a work-out (23).
The CK should be tested; it is often increased by more than a factor of 2.
A mutation search in SCN4A should be performed to confirm the diagnosis of a sodium channel myotonia.
Optional
In contrast to chloride channel myotonia, the sodium channel myotonia can be aggravated by an oral potassium load (60 mMol).
Therapy
Patients must be advised to refrain from potassium-rich food, such as dried fruits and vegetables, and from nuts and meat.
They should also avoid stressful labour like hiking tours.
A certain form of the disease (sometimes called “acetazolamide-sensitive” myotonia) reacts positively to acetazolamide (2-4 x 500 mg) (23).
B. Myotonia permanens
This is the most severe form of myotonic disease. In bad situations the patients may experience an insufficiency of breathing caused by cramping of their thoracic muscles.
Clinical symptoms
Persistent muscle stiffness that may fluctuate at a high level. Affection of the thorax is occasionally possible, e.g., when a patient is alarmed or frightened. The symptoms are rarely already present at birth.
Diagnostics
The same as with myotonia fluctuans except for the test with a potassium load.
Cave: This poatassium loading test must by no means be performed with a patient having myotonia permanens because of the potential development of a life-threatening muscle stiffness.
Therapy
The first choice in medication is mexiletine 2-3 x 200 mg or propafenon (2 x 150-300 mg) or flecainide (2 x 50-100 mg) long-term or for 2-3 days before a work load.
Drugs of second choice are: carbamazepine (up to 3 x 200 mg). If general anaesthesia is inevitable, hyperkalaemia, hypothermia and hypoglycaemia should be avoided.
Depolarising muscle relaxants, in particular succinylcholine are contraindicated (25).
IV. Dyskalaemic episodic paralyses
Under this headline, we will discuss a family of four diseases characterised by occasional incidences of pareses that might grow into full-fledged, though transient paralyses. In contrast to the historical names, these incidences occur as episodic events rather than in a periodic manner. In fact, the occurrence of the episodes can vary between once in a lifetime and almost daily. The “attacks”, as these events of muscle weakness are usually called, may be associated with changes in the serum potassium that are of diagnostic value.
The pareses or paralytic attacks are caused by transient phases of reduced excitability of the muscle fibres. Certain mutations may also lead to a permanent muscle weakness (15). The muscle fibres then develop morphologic changes, such as vacuoles and tubular aggregates. These changes are not specific for the various diseases.
1. Hyperkalaemic periodic paralysis (HyperPP)
Aetiology
See under the headline: Sodium channel myotonias. The prevalence is about 1:250,000.
Pathophysiology
Most Nav 1.4 mutations are situated at inner parts of the transmembrane segments or in intracellular protein loops and affect structures that form the three-dimensional docking site for the fast inactivation particle (Fig. 2), whereby the malformations reduce the affinity between the “latch bar and the catch”. The mutant channels do not adopt the fast or slow inactivated state and, in contrast to normal Na+ channels, re-open or flicker between the inactivated and the open state, yielding a gain-of-function defect. As a result, sodium influx is increased and causes a sustained membrane depolarisation that increases the electrical driving force for potassium. K+ released from muscle elevates the serum potassium level. The sodium influx into the muscle fibres is accompanied by a water influx, which causes increase of all serum concentrations including that of potassium. This is a vicious cycle that spreads out and affects the surrounding muscle fibres (26).
Clinical symptoms
Attacks of weakness may first appear during childhood, sometimes not before the patients are young adults. The attacks may be singular or frequent events; in some cases they recur daily. They may last for half an hour to 2 hours; rarely do they last for up to 2 hours. Finally they disappear spontaneously. During the attack-free interval fluctuations of the muscle strength are often noted. The attacks often begin in a phase of bodily rest after exhaustion. There is a propensity for attacks in the morning, in particular, when no carbohydrates have yet been ingested.
At the beginning of an attack, the serum potassium may be increased to 6 mM and in this phase, there are slight paresthesias in the extremities and fasciculation-like twitches as a sign of hyperactivity of the peripheral nerves.
During the attack the tidal volume of the lungs may be reduced to a life-threatening degree, but there are no problems with speaking or swallowing.
Only with one common mutation (T740M) the disease is often accompanied by a progressive myopathy and a permanent weakness (26).
Diagnostics
Obligatory
A key parameter is serum potassium. It should be monitored several times between attacks and, if possible, repeatedly during attacks.
An ECG should be taken at rest to exclude the long-QT syndrome and ventricular arrhythmias.
Recording of the EMG in particular at the beginning of an attack (myotonic activity excludes the possibility of hypokalaemic periodic paralysis).
Determination of the CK and transaminases. The CK is often more than 2 times increased.
Molecular genetic diagnostics in SCN4A and KCNJ2 to differentiate against HypoPP and Andersen syndrome (see below). Investigation of KCNE3 is not necessary (27).
Optional
Long-term ECG and ECG under load to exclude excessive ventricular arrhythmias.
Muscle biopsy if the molecular genetics result is unclear.
Therapy
During the attack
Shortening of an attack by slight labour or ingestion of carbohydrates (2 g glucose/kg body weight)
Inhalation of an α-mimetic drug (which activates the Na/K pump): 3 puffs = 1.3 mg metaproterenole, repeatable after 15 min, or 2 puffs = 0.18 mg albuterol, or 2 puffs = 0.1 mg salbutamol (26).
Acetazolamide (2 x 500 mg) or calcium gluconate 0.5-2g i.v. are not always effective.
Prophylactive therapy
First choice is hydrochlorothiazide 25 mg every other day to 75 mg daily. The serum potassium level should be daily controlled and should not fall below 3.0 mM. The sodium value should not be higher than 135 mM
Second choice is acetazolamide 2-4 x 250 mg daily, depending on the tolerance. The aim of the therapy is a permanently low or slightly subnormal potassium level.
General anaesthesia: Hyperthermia and hypoglykemia should be avoided. Therefore, depolarising muscle relaxants should not be administered (25, 28).
Genetic couseling of the family is recommended.
2. Familial hypokalaemic periodic paralysis (HypoPP)
Aetiology
Mutations in genes coding for two different types of muscular ion channels can cause hypokalaemic periodic paralysis with virtually identical clinical symptoms. More common are point mutations in CACNA1S on chromosome 1q31-32 for the muscular L-type calcium channel Cav1.1; less frequent are point mutations in SCN4A on chromosome 17q23 coding for the muscular sodium channel Nav 1.4 (29, 30, 33).
Pathophysiology
The hypokalaemia-induced weakness was explained by the in-vitro finding that in a 1mM K+ solution the muscle fibres were depolarized to -55mV (34, 35). The effects of the mutations on the channel pore currents suggested reduced function and were not able to satisfactorily explain the triggering by hypokalaemia (27). Mutation-induced cation leaks through an aberrant pore open at the resting potential were described (36, 37). Such a cation leak can counteract the hyperpolarising K+ currents and increase the tendency of the muscle fibre membrane to depolarise (33, 38).
Clinical symptoms
The first attacks of weakness usually become manifest in the adolescence, in severe cases or in connexion with infections they may occur earlier. The attacks may be singular events or may recur weekly. Between attacks there is often a fluctuating weakness. The attacks may last from 3 to 6 hours up to several days. They end spontaneously, however slowly. Often they appear in the second half of the night or in the morning after a day with a strong work load or an excessive intake of carbohydrates. In rare cases (R528H mutation) a transient phase of myotonia may be noticable at the beginning of the attack. The ventilatory tidal volume can be restricted to a life-threatening extent. With age, the majority of patients develops a progressive permanent weakness with myopathy with or without vacuoles (39).
Diagnostics
Obligatory
The key parameter is serum potassium. It should be monitored several times between attacks and, if possible, repeatedly during attacks.
An ECG should be taken at rest to exclude the long-QT syndrome and ventricular arrhythmias.
Recording of the EMG in particular at the beginning of an attack (myotonic activity is more likely in hyperkalaemic periodic paralysis).
Determination of the CK and transaminases. The CK is often more than 2 times increased.
Molecular genetic diagnostics in SCN4A and KCNJ2 to differentiate against HypoPP and Andersen syndrome (see below). Investigation of KCNE3 not necessary (40).
Optional
Long-term ECG and ECG under load to exclude excessive ventricular arrhythmias.
Muscle biopsy if the molecular genetic result is unclear.
Therapy
During the attack
Shortening of an attack by slight labour.
Administration of potassium, either per os in form of tablets or, in severe cases, as infusion.
Prophylactic therapy
Avoidance of longer periods of rest.
Sodium-poor diet.
Avoidance of carbohydrate-rich meals.
Avoidance of strong corporal activities.
Carboanhydrase inhibitors (CAI) like acetazolamide (dosage as low as possible: 125 mg/day every second day up to 2 x 250 mg/day) or dichlorphenamide (41). Rests of dichlorphenamide are only available in Italy (Antidrasi®).
Alternatively or in combination with CAIs, potassium sparing diuretics like spironolactone 100-200 mg/day or triamterene 150 mg/day or furosemide can be given (15); the newer aldosterone antagonist eplerenone (Inspra®) has much less hormonal effects than spironolactone and should be preferred.
General anaesthesia: avoidance of hypothermia und hyperglycaemia; avoidance of depolarising muscle relaxants like succinylcholine (25).
Genetic counselling of the family is recommended.
3. Familial normokalaemic periodic paralysis (NormoPP)
The term normokalaemic periodic paralysis was originally given to a variant described in the 1960s. The disorder resembled HyperPP in many aspects; the only real differences were that (i) even during serious attacks the concentration of serum potassium was not increased, and (ii) administration of glucose did not have a beneficial effect (15). The existence of NormoPP as a nosologic entity was questioned because of the potassium sensitivity of the patients and the identification of the most frequent HyperPP mutations T704M or M1592V in NormoPP families including the original family.
Recently, a potassium-sensitive type of PP with normokalaemia and episodes of weakness reminiscent of those in both hyperkalaemic (initiation of an attack by potassium) and hypokalaemic forms (duration of attacks) was reported (42). This phenotype, also named normokalaemic PP, is caused by SCN4A mutations at deeper locations of the voltage sensors S4 which are exposed to the extracellular space at larger depolarisation, e.g. amino acid 675. Future studies will show whether NormoPP is a separate clinical entity. The diagnostics are as described for the two more common forms of the disease. The prophylaxis consists of avoidance of hyperkalaemia and the administration of acetazolamide.
4. Andersen syndrome
Aetiology/Pathophysiology
The syndrome, sometimes also called Andersen-Tawil syndrome, is caused by mutation in KCNJ2, the gene coding for the inward rectifying potassium channel Kir2.1 (43) (Fig. 3). Mutant Kir2.1 channels conduct a reduced potassium current which results in an impairment of the of the correct resting membrane potential, i.e. the muscle cells may depolarize which renders them inexcitable. Muscle weakness or paralysis ensues, i.e. episodes of paralysis of the hyper-, normo-, or hypokalaemic form may occur. Since KCNJ2 is also expressed in other tissues, e.g. in heart, also arrhythmias may occur, both during paralytic attacks and in the attack free interval. Patients also may present with skeletomuscular dysmorphia (44–46).
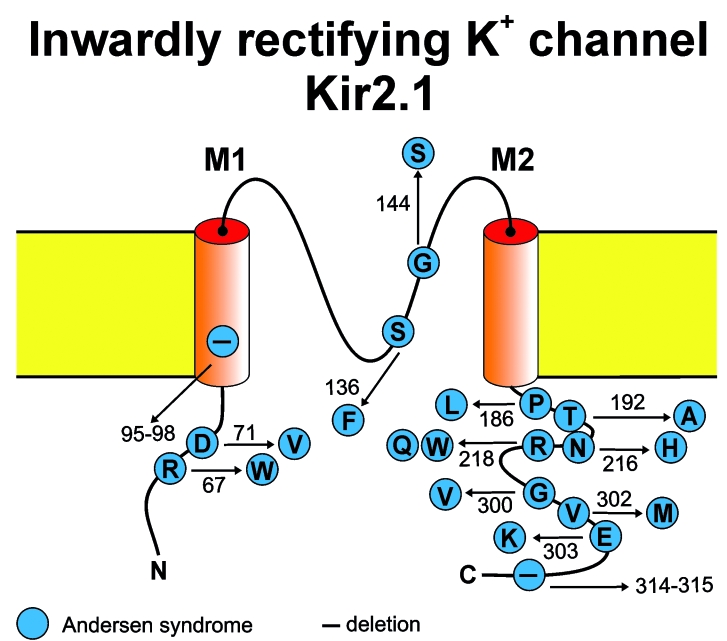
Figure 3.
Membrane topology model of the voltage-independent inward rectifying Kir2.1 potassium channel of skeletal muscle encoded by KCNJ2. Amino acid substitutions in this channel cause Andersen syndrome.
Clinical Symptoms
The full-fledged syndrome encompasses the trias of periodic paralyses, cardiac arrhythmias and dysmophia. But only part of the patients show them all. The congenital dysmorphies comprise those of the face (hypertelorism, low-set ears, hypoplasia of the mandible) as well as those of the hands and feet (e.g. syndaktyly). Episodes of paralysis and/or arrhythmias manifest themselves usually in early adolescence. The episodes of paralyses can be of the hypo-, normo- or hyperkalaemic form. The ECG often shows ventricular extrasystoles as well as pathologic T-U morphology with an increased and delayed U wave. In contrast, the Q-T time is only little increased. Therefore, in comparison with the long QT syndrome, syncopes and sudden death are rare.
Diagnostics
Obligatory
Investigation of the ECG (at rest, under load and long-term).
Echocardiography.
Determination of the serum potassium (if possible during a paralytic attack).
Determination of the CK,
Molecular genetic diagnostic in KCNJ2, CACNA1S and SCN4A for a differentiation from hypo- or hyperkalaemic periodic paralysis.
Optional
Muscle biopsy when the molecular genetic diagnostics give unclear results.
Therapy
During the attack
Shortening of the attack by a slight labour.
Normalisation of the serum potassium.
Prophylactic therapy
Continual movement, avoidance of longer periods of rest.
Avoidance of strong exercise.
Carboanhdrase inhibitors (CAI) such as acetazolamide (dose as low as possible: 125 mg/day every second day up to 2 x 250 mg/day) for the prophylaxis of attacks of weakness.
Extrasystoles should not be treated at any rate; arrhythmias often disappear with tachycardia, e.g. during intermediate labour or fever.
Treatment with sodium or calcium channel blockers and also with beta-blockers is common, but often not effective.
According to earlier reports imipramin may be effective.
Amiodaron, actually contraindicated with the long-QT syndrome, should be given only in severe cases because of severe side effects (47).
When rhythmogenic syncopes are present, the implantation of a pacemaker or of a combined pacemaker/defribrillator may be indicated. All drugs that increase the QT time are contraindicated as a matter of principle.
General anaesthesia: dyskalaemic states and hypothermia should be avoided. By the same token, depolarising muscle relaxants like succinylcholine are forbidden.
V. Calcium release channelopathies
Under this headline we will discuss mutation-induced dysfunctions of the calcium release channel of skeletal muscle, also named ryanodine receptor type 1 (RyR1) that is under the control of the voltage-dependent dihydropyridine-sensitive Ca2+ channel Cav1.1. The mutations can cause a dominantly inherited predisposition of clinically inconspicuous individuals to life-threatening events during general anaesthesia or to a congenital myopathy such as central core disease.
1. Malignant hyperthermia (MH) susceptibility
Aetiology/Pathophysiology
More than 30 disease-causing point mutations in the RyR1 gene have been identified in man, most of them substituting amino acids in the cytoplasmic part, the so-called “foot” of the RyR1 protein (Fig. 4) (for review see ref. 48). Functionally, hypersensitivity of RyR1 to anaesthetic triggering agents has been shown to be pathogenetically causative in functional tests of muscle, myotubes, isolated RyR1 protein, and heterologously expressed full-length receptors (49, 50). The triggering substances lead to an uncontrolled calcium release from the sarcoplasmic reticulum via RyR1 (51). The myoplasmic Ca2+ elevation causes glycogenolysis that results in excess lactate production, and hyperactivation of the oxidative cycle with increased ATP depletion, high oxygen consumption and carbon dioxide production. If the myoplasmic Ca2+ concentration exceeds the mechanical threshold, muscle contractures occur.
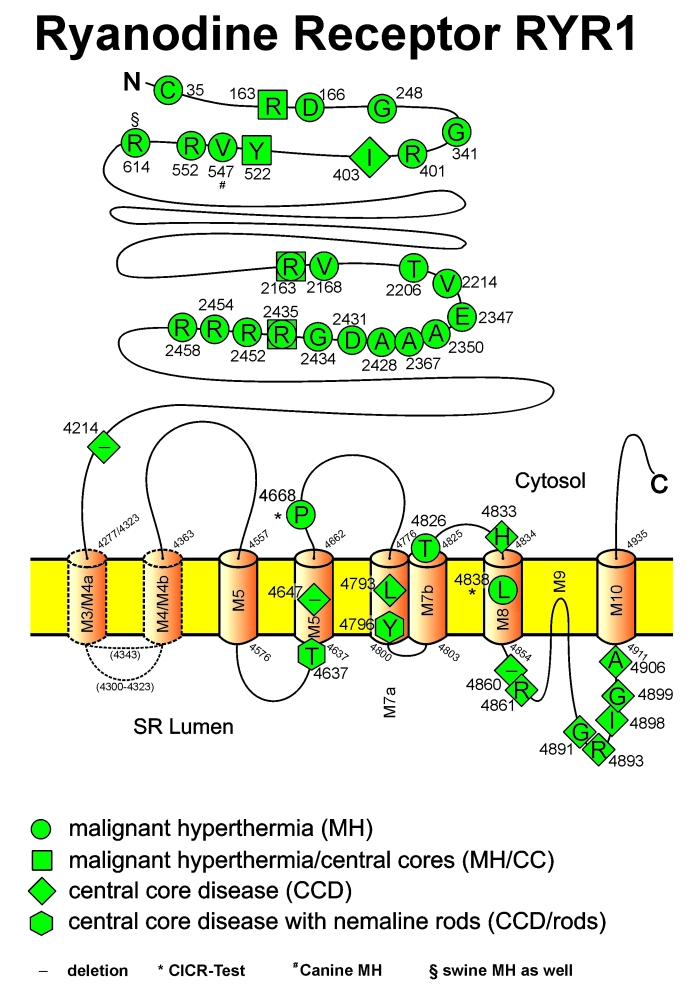
Figure 4.
Model of the homotetrameric ryanodine receptor, the calcium release channel situated in the membrane of the sarcoplasmic reticulum (SR). The cytosolic part of the protein complex, the so-called foot, bridges the gap between the transverse tubular system and the SR. Mutations have been described for the skeletal muscle ryanodine receptor (RyR1), which cause susceptibility to malignant hyperthermia (MHS) and central core disease (CCD). Conventional 1-letter abbreviations are used for the replaced amino acids.
Clinical symptoms
The combination of volatile anaesthetics or depolarising muscle relaxants often leads to a masseter spasm and heat production. Tachycardia may be observed as an early sign of the hypermetabolism which leads to hypercapnia, hypoxaemia, metabolic and respiratory acidosis. Hyperthermia and muscle contractures may be a late sign in some cases. Rhabdomyolysis can occur and lead to subsequent elevation of CK, to hyperkalaemia, which may potentially cause ventricular fibrillations, and to myoglobinuria with the possibility of renal failure. If an episode is survived, normalisation of oedematous muscle can occur within weeks but extended rhabdomyolysis may lead to incomplete regeneration and permanent weakness.
Outside general anaesthesia, most individuals do not present with symptoms, but some suffer from muscle cramping and a few from heat strokes under heavy exertion in hot environments (so-called awake episode).
Diagnostics
Slight familial CK elevations up to twice normal at rest, whereas higher values are indicative of recent rhabdomyolysis or a hereditary myopathy.
A functional test on biopsied skeletal muscle, the in vitro contracture test (IVCT) (52).
In addition, muscle histology, histochemistry and, where indicated by the former, electron microscopy should be performed. Individuals with MH susceptibility may have cores in the muscle, but the diagnosis of CCD should be limited to those with a clinical myopathy and abundance of central cores in type 1 fibres.
Molecular genetics of the RyR1 gene
Therapy
During the crisis
The most effective treatments are immediate stop of administration of triggering agents, change of the gassing tubes and rapid infusion of dantrolene, a specific RyR1 channel blocker
Further treatment aims at correction of hyperkalaemia and prevention of secondary complications
Prophylactic therapy
Total intravenous anaesthesia is the method of choice when regional techniques are inappropriate.
Safe drugs are propofol, opioids, nitrous oxide, barbiturates, benzodiazepines and all local anaesthetics.
Awake episodes can be prevented by competitive sports.
2. Central core disease (CCD)
Aetiology/pathophysiology
With a few exceptions all mutations are situated in the C terminus of the RyR1 protein thought to form the channel pore region (Fig. 4). Expression of these mutations in non-muscle cells led to the finding of a leaky Ca2+ release channel compatible with the view of a myoplasmic Ca2+ overload responsible for the mitochondrial and cellular damages (53). A selective disruption of the orthograde excitation-contraction coupling process has been found in a skeletal muscle expression system suggesting a dominant negative effect of the CCD mutations on the voltage-controlled Ca2+ release (54). This functional disruption may contribute to the muscle weakness and atrophy experienced by the patients.
The current understanding of CCD suggests a strong link between subcellular Ca2+ metabolism and the pathophysiologic mechanism of the disease (55, 56). This is corroborated by clinical evidence of MH susceptibility of CCD patients and pathological contractures in the IVCT. However, in some cases the IVCT gives negative results (57). The location of the mutation seems to be decisive, because CCD mutations in the C terminal region of the RyR1 protein are associated with excitation-contraction uncoupling or a partially depleted sarcoplasmic reticulum through a constant Ca2+ leak. Both mechanisms lead to a reduced Ca2+ release which explains the muscle weakness and the lower in-vitro sensitivity to Ca2+ releasing drugs (58, 59). Most other mutations behave like MH mutations and give strong contractures in the IVCT. The clinical situation for the IVCT negative mutations is not clear. Hence, every CCD patient should be regarded as MH susceptible and therefore receive non-triggering anaesthesia except for patients with a clearly negative IVCT result.
Clinical symptoms
CCD is a congenital myopathy with muscle weakness of variable degree. The myopathy is characterized by congenital muscle hypotonia (floppy infant syndrome), proximally pronounced weakness, delayed motor development, and slight CK elevation. The clinical expression of the disease is highly variable between and in families from asymptomatic up to permanent weakness which may cause severe disability in daily life (60, 61). Later in life, muscle strength usually improves except for rare cases showing progressive muscle weakness. It is one of the rare known myopathies for which strong physical exercise seems to be beneficial (62) although exercise-induced muscle cramps are often reported. In addition, skeletal anomalies such as congenital hip displacement and scoliosis are frequent.
Usually, the mode of inheritance is autosomal dominant. Recently recessive transmission has been described for variant forms of CCD (63). There is also an overlap of CCD with other myopathies (e.g., nemaline myopathy, multi-minicore disease) (64).
Diagnostics
Obligatory
Serum CK levels are normal or slightly elevated.
Pathognomonic is the abundance of central cores along type 1 muscle fibres. The cores are structured or unstructured and lack oxidative enzyme activity.
Molecular genetics of RyR1 exons.
Optional
A functional test on skeletal muscle biopsy, the IVCT to clarify the susceptibility to MH of the underlying mutation.
Therapy
Physical exercise seems to be beneficial.
Acknowledgments
We are grateful to Dr C. Schneider-Gold for helpful discussions. We thank the German Research Foundation (DFG; JU470/1-2) for financial support, the Periodic Paralysis Association of the USA (PPA) for referring patients to our molecular genetics lab, and our patients for their invaluable collaboration.
Laboratory for Human Genetic Diagnostics
Specialised in myotonic dystrophies, channelopathies and malignant hyperthermia.
Prof Dr. Frank Lehmann-Horn, Institute of Applied Physiology, Albert-Einstein-Allee 11, D-89069 Ulm/ Germany. Ph.: +49 731 500 23250. Fax: +49 731 500 23260.
References
- 1. Rüdel R, Lehmann-Horn F. Membrane changes in cells from myotonia patients. Physiol Rev 1985;65:310-56. [DOI] [PubMed] [Google Scholar]
- 2. Emery AEH. Diagnostic criteria for neuromuscular disorders. London: Royal Society of Medicine Press 1997. [Google Scholar]
- 3. Diener HC. Leitlinien für Diagnostik und Therapie in der Neurologie. 3. Auflage. Stuttgart: Thieme 2008. [Google Scholar]
- 4. Mankodi A, Takahashi MP, Jiang H, et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell 2002;10:35-44. [DOI] [PubMed] [Google Scholar]
- 5. Kwieciński H, Ryniewicz B, Ostrzycki A. Treatment of myotonia with antiarrhythmic drugs. Acta Neurol Scand 1992;86:371-5. [DOI] [PubMed] [Google Scholar]
- 6. Harper P, Monckton DG. Myotonic dystrophy. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd ed. New York: McGraw-Hill 2004. pp. 1039-76. [Google Scholar]
- 7. Walter MC, Reilich P, Lochmüller H, et al. Creatine monohydrate in myotonic dystrophy: a double-blind, placebo-controlled clinical study. J Neurol 2002;249:1717-22. [DOI] [PubMed] [Google Scholar]
- 8. Tarnopolsky M, Mahoney D, Thompson T, et al. Creatine monohydrate supplementation does not increase muscle strength, lean body mass or phosphocreatine in patients with myotonic dystrophy type 1. Muscle Nerve 2004;29:51-8. [DOI] [PubMed] [Google Scholar]
- 9. Lazarus A, Varin J, Babuty D, Anselme F, et al. Long-term follow-up of arrhythmias in patients with myotonic dystrophy treated by pacing. J. Am Coll Cardiol 2002;40:1645-52. [DOI] [PubMed] [Google Scholar]
- 10. Damian MS, Gerlach A, Schmidt F, et al. Modafinil for excessive daytime sleepiness in myotonic dystrophy. Neurology 2001;56:794-6. [DOI] [PubMed] [Google Scholar]
- 11. Wintzen AR, Lammers GJ, van Dijk JG. Does modafinil enhance activity of patients with myotonic dystrophy: a double blind placebo controlled cross-over study. J Neurol 2007;254:26-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ricker K, Koch MC, Lehmann-Horn F, et al. Proximal myotonic myopathy: a new dominant disorder with myotonia muscle weakness, and cataracts. Neurology 1994;44:1448–52. [DOI] [PubMed] [Google Scholar]
- 13. Liquori CL, Ricker K, Moseley ML, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 2001;293:864-7. [DOI] [PubMed] [Google Scholar]
- 14. Schneider-Gold C, Beck M, Wessig C, et al. Creatine monohydrate in DM2/PROMM. A double blind placebo-controlled clinical study. Neurology 2003;60:500-2. [DOI] [PubMed] [Google Scholar]
- 15. Lehmann-Horn F, Rüdel R, Jurkat-Rott K. Nondystrophic myotonias and periodic paralysis. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd ed. New York: McGraw-Hill; 2004. pp. 1257-300. [Google Scholar]
- 16. Rüdel R, Ricker K, Lehmann-Horn F. Transient weakness and altered membrane characteristic in recessive generalized myotonia (Becker). Muscle Nerve 1988;11:202-11. [DOI] [PubMed] [Google Scholar]
- 17. Koch MC, Steinmeyer K, Lorenz C, et al. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science 1992;257:797-800. [DOI] [PubMed] [Google Scholar]
- 18. Pusch M, Steinmeyer K, Koch MC, et al. Mutations in dominant human myotonia congenita drastically alter the voltage dependence of the CIC-1 chloride channel. Neuron 1995;15:1455-63. [DOI] [PubMed] [Google Scholar]
- 19. Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiological Reviews 1999;79:1317-71. [DOI] [PubMed] [Google Scholar]
- 20. Weber MA, Nielles-Vallespin S, Essig M, et al. Muscle Na+ channelopathies: MRI detects intracellular 23Na accumulation during episodic weakness. Neurology 2006;67:1151-8. [DOI] [PubMed] [Google Scholar]
- 21. Mohammadi B, Jurkat-Rott K, Alekov A, et al. Preferred mexiletine block of human sodium channels with IVS4 mutations and its pH-dependence. Pharmacogenet Genomics 2005;15:235-44. [DOI] [PubMed] [Google Scholar]
- 22. Alfonsi E, Merlo IM, Tonini M, et al. Efficacy of propafenone in paramyotonia congenita. Neurology 2007;68:1080-1. [DOI] [PubMed] [Google Scholar]
- 23. Heatwole CR, Moxley RT 3rd. The nondystrophic myotonias. Neurotherapeutics 2007;4:238-51. [DOI] [PubMed] [Google Scholar]
- 24. Ricker K, Moxley RT 3rd, Heine R, et al. Myotonia fluctuans, a third type of muscle sodium channel disease. Arch Neurol 1994;51:1095-102. [DOI] [PubMed] [Google Scholar]
- 25. Klingler W, Lehmann-Horn F, Jurkat-Rott K. Complications of anesthesia in neuromuscular disorders. Neuromuscular Disord 2005;15:195-206. [DOI] [PubMed] [Google Scholar]
- 26. Jurkat-Rott K, Lehmann-Horn F. Genotype-phenotype correlation and therapeutic rationale in hyperkalemic periodic paralysis. Neurotherapeutics 2007;4:216-24. [DOI] [PubMed] [Google Scholar]
- 27. Jurkat-Rott K, Lehmann-Horn F. Muscle channelopathies and critical points in functional and genetic studies. J Clin Invest 2005;115:2000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricker K. Muscle ion channel myotonia. In: Brandt L, Caplan R, Dichgans J, Diener HC, Kennard C, editors. Neurological disorders. Course and treatment. 2nd Edition. USA: Elsevier Science 2003. [Google Scholar]
- 29. Fontaine B, Vale-Santos J, Jurkat-Rott K, et al. Mapping of the hypokalaemic periodic paralysis (HypoPP) locus to chromosome 1q31-32 in three European families. Nature Genetics 1994;6:267-72. [DOI] [PubMed] [Google Scholar]
- 30. Jurkat-Rott K, Lehmann-Horn F, Elbaz A, et al. A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet 1994;3:1415-9. [DOI] [PubMed] [Google Scholar]
- 31. Jurkat-Rott K, Mitrovic N, Hang C, et al. Voltage sensor sodium channel mutations cause hypokalemic periodic paralysis type 2 by enhanced inactivation and reduced current. Proc Natl Acad Sci USA 2000;97:9549-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sternberg D, Maisonobe T, Jurkat-Rott K, et al. Hypokalemic periodic paralysis type 2 caused by mutations at codon 672 in the muscle sodium channel gene SCN4A. Brain 2001;124:1091-9. [DOI] [PubMed] [Google Scholar]
- 33. Carle T, Lhuillier L, Luce S, et al. Gating defects of a novel Na+ channel mutant causing hypokalemic periodic paralysis. Biochem Biophys Res Commun 2006;348:653-61. [DOI] [PubMed] [Google Scholar]
- 34. Rüdel R, Lehmann-Horn F, Ricker K, et al. Hypokalemic periodic paralysis: In vitro investigation of muscle fibre membrane parameters. Muscle Nerve 1984;7:110-20. [DOI] [PubMed] [Google Scholar]
- 35. Ruff RL. Insulin acts in hypokalaemic periodic paralysis by reducing inward rectifier K+ current. Neurology 1999;53:1556-63. [DOI] [PubMed] [Google Scholar]
- 36. Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature 2007;446:76-8. [DOI] [PubMed] [Google Scholar]
- 37. Struyk AF, Cannon SC. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J Gen Physiol 2007;130:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jurkat-Rott K, Lehmann-Horn F. Do hyperpolarisation-induced proton currents contribute to the pathogenesis of hypokalemic periodic paralysis, a voltage sensor channelopathy? J Gen Physiol 2007;130:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jurkat-Rott K, Weber MA, Fauler M, et al. Cellular sodium overload and K+-dependent depolarisation indicate a nonselective cation leak. Submitted to Proc Natl Acad Sci USA (Author: accepted? In press?) [Google Scholar]
- 40. Jurkat-Rott K, Lehmann-Horn F. Periodic paralysis mutation MiRP2-R83H in controls: interpretations and general recommendation. Neurology 2004; 62:1012-5. [DOI] [PubMed] [Google Scholar]
- 41. Tawil R, McDermott MP, Brown R Jr, et al. Randomized trials of dichlorophenamide in the periodic paralyses. Working Group on Periodic Paralysis. Ann Neurol 2000;47:46-53. [PubMed] [Google Scholar]
- 42. Vicart S, Sternberg D, Fournier E, et al. New mutations of SCN4A cause a potassium-sensitive normokalemic periodic paralysis. Neurology 2004;63:2120-7. [DOI] [PubMed] [Google Scholar]
- 43. Plaster NM, Tawil R, Tristani-Firouzi M, et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell 2001;105:511-9. [DOI] [PubMed] [Google Scholar]
- 44. Tawil R, Ptaček LJ, Pavlakis SG, et al. Andersen’s syndrome: potassium-sensitive periodic paralysis, ventricular ectopy, and dysmorphic features. Ann Neurol 1994;35:326-30. [DOI] [PubMed] [Google Scholar]
- 45. Andelfinger G, Tapper AR, Welch RC, et al. KCNJ2 mutation results in Andersen’s syndrome with sex-specific cardiac and skeletal muscle phenotypes. Am J Hum Genet 2002;71:663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Venance SL, Cannon SC, Fialho D, et al. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain 2006;129:8-17. [DOI] [PubMed] [Google Scholar]
- 47. Junker J, Haverkamp W, Schulze-Bahr E, et al. Amiodarone and acetazolamide for the treatment of genetically confirmed severe Andersen syndrome. Neurology 2002;59:466. [DOI] [PubMed] [Google Scholar]
- 48. Jurkat-Rott K, McCarthy T, Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve 2000;23:4-17. [DOI] [PubMed] [Google Scholar]
- 49. Censier K, Urwyler A, Zorzato F, et al. Intracellular calcium homeostasis in human primary muscle cells from malignant hyperthermia-susceptible and normal individuals. Effect of overexpression of recombinant wild-type and Arg163Cys mutated ryanodine receptors. J Clin Invest 1998;101:1233-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dietze B, Henke J, Eichinger HM, et al. Malignant hyperthermia mutation Arg615Cys in the porcine ryanodine receptor alters voltage dependence of Ca2+ release. J Physiol 2000;526:507-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iaizzo PA, Klein W, Lehmann-Horn F. Fura-2 detected myoplasmic calcium and its correlation with contracture force in skeletal muscle from normal and malignant hyperthermia susceptible pigs. Pflügers Archiv - European J Physiol 1988;411:648-53. [DOI] [PubMed] [Google Scholar]
- 52. Brandt A, Schleithoff L, Jurkat-Rott K, et al. Screening of the ryanodine receptor gene in 105 malignant hyperthermia families: novel mutations and concordance with the in-vitro contracture test. Hum Mol Gen 1999;8:2055-62. [DOI] [PubMed] [Google Scholar]
- 53. Lynch PJ, Tong J, Lehane M, et al. A mutation in the transmembrane/luminal domain of the ryanodine receptor is associated with abnormal Ca2+ release channel function and severe central core disease. Proc Natl Acad Sci USA 1999;96:4164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Avila G, O’Brien JJ, Dirksen RT. Excitation-contraction uncoupling by a human central core disease mutation in the ryanodine receptor. Proc Natl Acad Sci USA 2001;98:4215-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Avila G, Dirksen RT. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol 2001; 118:277-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Robinson R, Carpenter D, Shaw MA, et al. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat 2006;27:977-89. [DOI] [PubMed] [Google Scholar]
- 57. Halsall PJ, Bridges LR, Ellis FR, et al. Should patients with central core disease be screened for malignant hyperthermia? J Neurol Neurosurg Psychiatry 1996;61:119-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dirksen RT, Avila G. Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1. Biophys J 2004;87:3193-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Du GG, Khanna VK, Guo X, et al. Central core disease mutations R4892W, I4897T and G4898E in the ryanodine receptor isoform 1 reduce the Ca2+ sensitivity and amplitude of Ca2+-dependent Ca2+ release. Biochem J 2004;382:557-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Cauwer H, Heytens L, Martin JJ. Workshop report of the 89th ENMC International Workshop: Central Core Disease, 19th-20th January 2001, Hilversum, The Netherlands. Neuromuscul Disord 2002;12:588-95. [DOI] [PubMed] [Google Scholar]
- 61. North K. Congenital myopathies. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd edition. New York: McGraw-Hill 2004. pp. 1473-533. [Google Scholar]
- 62. Hagberg JM, Carroll JE, Brooke MH. Endurance exercise training in a patient with central core disease. Neurology 1980;30:1242-4. [DOI] [PubMed] [Google Scholar]
- 63. Zhou H, Jungbluth H, Sewry CA, et al. Molecular mechanisms and phenotypic variation in RYR1-related congenital myopathies. Brain 2007;130:2024-36. [DOI] [PubMed] [Google Scholar]
- 64. Wu S, Ibarra MC, Malicdan MC, et al. Central core disease is due to RYR1 mutations in more than 90% of patients. Brain 2006;129:1470-80. [DOI] [PubMed] [Google Scholar]