Abstract
The molecular pathways leading to Alzheimer-type dementia are not well understood, but the amyloid β-protein is believed to be centrally involved. The quantity of amyloid β-protein containing plaques does not correlate well with clinical status, suggesting that if amyloid β-protein is pathogenic it involves soluble non-plaque material. Using 43 brains from the Newcastle cohort of the population-representative Medical Research Council Cognitive Function and Ageing Study, we examined the relationship between biochemically distinct forms of amyloid β-protein and the presence of Alzheimer-type dementia. Cortical samples were serially extracted with Tris-buffered saline, Tris-buffered saline containing 1% TX-100 and with 88% formic acid and extracts analysed for amyloid β-protein by immunoprecipitation/western blotting. The cohort was divisible into those with dementia at death with (n = 14) or without (n = 10) significant Alzheimer-type pathology, and those who were not demented (n = 19). Amyloid β-protein monomer in extracts produced using Tris-buffered saline and Tris-buffered saline containing 1% TX-100 were strongly associated with Alzheimer type dementia (P < 0.001) and sodium dodecyl sulphate-stable amyloid β-protein dimer was detected specifically and sensitively in Tris-buffered saline, Tris-buffered saline containing 1% TX-100 and formic acid extracts of Alzheimer brain. Amyloid β-protein monomer in the formic acid fraction closely correlated with diffuse and neuritic plaque burden, but was not specific for dementia. These findings support the hypothesis that soluble amyloid β-protein is a major correlate of dementia associated with Alzheimer-type pathology and is likely to be intimately involved in the pathogenesis of cognitive failure.
Keywords: Alzheimer’s disease pathology, Alzheimer’s disease, amyloid β-protein, biochemistry, cognitive impairment
Introduction
By current criteria a positive diagnosis of Alzheimer’s disease requires both the clinical confirmation of dementia and post-mortem detection of amyloid plaques and neurofibrillary tangles in the neocortex of the brain (Mirra et al., 1991; Ball et al., 1997). Extensive evidence supports a pathogenic role for the principal proteinaeous component of plaques, the amyloid β-protein (Aβ), and it is widely believed that an imbalance in Aβ metabolism initiates a cascade of events that ultimately results in Alzheimer’s disease (Hardy and Allsop, 1991; Selkoe, 1991; Hardy and Selkoe, 2002). Yet this hypothesis remains controversial because amyloid burden does not correlate well with disease (Katzman et al., 1988; Dickson et al., 1991; Terry et al., 1991).
If plaques containing fibrillar, insoluble forms of Aβ do not correlate with severity of dementia, and if Aβ is pathogenic, this suggests that non-fibrillar soluble forms of Aβ may mediate disease (Klein et al., 2001; Selkoe, 2002; Walsh et al., 2003). Indeed, biochemical studies of post-mortem brain have revealed strong correlations between the levels of water-soluble Aβ and severity of cognitive impairment (Lue et al., 1999; Wang et al., 1999). In those studies the term ‘soluble Aβ’ was an operational definition, embracing all forms of Aβ that remained in aqueous solution following high-speed centrifugation of brain extracts (Kuo et al., 1996; Lue et al., 1999; McLean et al., 1999; Wang et al., 1999). Most studies of ‘soluble Aβ’ have employed enzyme-linked immunosorbent assay (ELISA) methods that do not disclose the aggregation state of the species detected and appear to preferentially detect Aβ monomer (Morishima-Kawashima and Ihara, 1998; Enya et al., 1999; Stenh, 2005). Although the detection methods used provide little information about the assembly state of Aβ, the fact that they are not sedimented by ultracentrifugation indicates that they are not fibrillar in nature. In a study in which the buffer-soluble fraction of human cerebral cortex was examined by sensitive western blotting, monomeric ∼4 kDa Aß, and low-n sodium dodecyl sulphate (SDS)-stable Aβ oligomers (∼8 and ∼12 kDa) were detected (McLean et al., 1999), indicating that the soluble phase of Alzheimer’s disease brain contains at least two physically distinct non-fibrillar Aβ species.
Nonetheless, questions still remain regarding the distribution of Aβ in the ageing brain, its relationship with plaques and tangles and the way in which these pathologies interact with other pathologies and the clinical manifestations of dementia. In this study, we addressed these questions using a donor cohort from the prospective population-based Medical Research Council Cognitive Function and Ageing Study. The use of this population-representative sample builds on previous results from case-control studies by allowing the distribution of Aβ in the population to be assessed and minimizing selection biases when testing the relationship with other pathologies and dementia. A population-based cohort provides results that are applicable to the population including individuals with mixed pathology, discordant clinical and pathological findings and others who represent groups of the population less likely to be recruited into clinical studies. Using serial extraction of cortical tissue and immunoprecipitation/western blotting for detection we examined the relationship between Aβ species, the burden of Alzheimer-type pathology and dementia at death. This novel approach quantifies Aβ species that are not detected by ELISA and facilitates the simultaneous assessment of both Aβ monomer and SDS-stable Aβ assemblies. The predominant Aβ species migrated on SDS-polyacrylamide gel electrophoresis as monomers and dimers, with trimers and higher molecular weight species only occasionally detected. Water- and triton-soluble Aβ, but not formic acid-extracted Aβ, was specifically elevated in brains from demented people with Alzheimer’s disease pathology. Water-soluble and triton-soluble Aβ monomer was readily detected in the majority of Alzheimer’s disease donors, and only rarely detected in non-Alzheimer’s disease donors. A similar pattern was also seen with water-soluble and triton-soluble SDS-stable Aβ dimer. Detection of dimer was less sensitive than monomer but was strongly associated with the presence of Alzheimer’s disease. These results suggest that both water-soluble and triton-X100 extracted SDS-stable Aβ dimers are intimately linked to pathogenesis.
Materials and methods
Reagents and antibodies
Unless otherwise stated, all chemicals were from Sigma (Arklow, Co. Wicklow, Republic of Ireland). Synthetic Aβ1-42 was purchased from the Keck laboratory (Yale University, CT). Monoclonal antibodies 21F12 and 2G3, which specifically recognize Aβ terminating at residue 42 and 40, respectively and 3D6 a monoclonal antibody to the extreme N-terminus of Aβ (Johnson-Wood et al., 1997) were gifts from Drs Peter Seubert and Dale Schenk (Elan Pharmaceuticals, San Francisco, CA, USA). 6E10, a monoclonal antibody to residues 1–16 of Aβ, was from Signet (Dedham, MA, USA). AW8, a novel anti-Aβ polyclonal antibody was raised to aggregated synthetic Aβ1-42 and recognizes synthetic Aβ by dot blot, western blot and ELISA. AW8 is capable of immunoprecipitating Aβ from culture medium, CSF and human brain extracts, and when used for immunohistochemistry AW8 recognizes both diffuse and neuritic plaques (Supplementary Fig. S1). Fluorochrome-coupled anti-mouse IR800 antibody was from Rockland (Gilbertsville, PA, USA).
Cognitive function and ageing study cohort
Forty-three brains were used in the present study representing all but two donations (from which tissue was available) made prior to August 2006 to the Newcastle Medical Research Council Cognitive Function and Ageing Study centre. Medical Research Council Cognitive Function and Ageing Study is a population-representative longitudinal study of ageing and cognition with a programme of brain donation and has been described in full previously (Brayne, 2006). The Medical Research Council Cognitive Function and Ageing Study neuropathology cohort has been shown to be highly representative of the older population (Matthews, 2009). All procedures for brain donation and for use of the tissue in this research were approved by a multicentre Research Ethics committee.
Assessment of dementia at death
The Medical Research Council Cognitive Function and Ageing Study diagnosis of dementia at death has been described in detail previously (Savva, 2009). In brief, algorithmic assessment of dementia status at each interview was made using the Geriatric Mental State examination to which the Automated Geriatric Examination for Computer Assisted Taxonomy (Copeland, 1990) was applied. The Geriatric Mental State examination was augmented with questions from the Cambridge examination for mental disorders in the elderly (CAMDEX) (Huppert, 1995). A diagnosis of dementia was made at each assessment based on the full Geriatric Mental State-Automated Geriatric Examination for Computer Assisted Taxonomy algorithm, which is equivalent to the Psychiatry Diagnostic and Statistical Manual of Mental Disorders (3rd Edition Revised). A diagnosis of dementia at death was based on combining the interviews conducted before death, information from death certification and a retrospective informant interview. Of 43 samples included in this study, 19 died without dementia and 24 died with dementia.
Assessment of Alzheimer pathology
Pathological evaluation of the Cognitive Function and Ageing Study cohort has been described in detail previously (Savva, 2009). Assessment of plaques and tangles associated with Alzheimer’s disease was conducted by neuropathologists, blind to clinical data using immunohistochemical or tinctorial methods. The severity of diffuse plaques and neuritic plaques was scored semi-quantitatively according to the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) protocol as either ‘none’, ‘low’, ‘intermediate’ or ‘high’ in the frontal (Brodmann area 8/9), temporal (Brodmann area 21/22), parietal and occipital neocortices (Mirra et al., 1991). Tangle pathology was also evaluated in neocortex and hippocampus using the CERAD method and Braak stage assigned to each case (Braak and Braak, 1991). Based on the neuritic plaque scores, a CERAD neuropathological diagnosis was made of ‘normal brain’ (absent plaques), ‘plaques and tangles insufficient for Alzheimer’s disease’ (low plaques) or ‘Alzheimer’s disease’ (intermediate or high plaques).
Human brain homogenate preparation
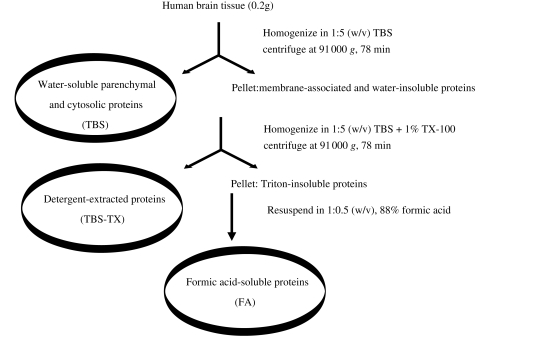
Frozen samples of frontal (Brodmann area 8/9) and temporal (Brodmann area 21/22) cortices weighing ∼0.5 g were dissected to produce 0.2 g aliquots and used to prepare homogenates (Fig. 1). Brain tissue (0.2 g) was homogenized with 25 strokes of a Dounce homogenizer (Fisher, Ottawa, Canada) in 1 ml of freshly prepared ice-cold Tris-buffered saline (TBS), containing 5 mM ethylenediaminetetraacetic acid (EDTA), 5 mM ethylene glycol tetraacetic acid (EGTA), 10 mg/ml leupeptin, 1 mg/ml aprotinin, 1 mg/ml pepstatin A, 1 mM Pefabloc, 2 mM 1,10-phenanthroline supplemented with the Sigma cocktail of phosphatase inhibitors. Homogenates were then centrifuged at 91 000 g and 4°C in a TLA-55 rotor (Beckman Coultour, Fullerton, CA, USA) for 78 min (Fig. 1). The supernatant referred to as the TBS extract was divided into 300 μl aliquots and stored at –80°C. The pellet was re-homogenized (1:5 w/v) in TBS containing 1% Triton-X 100 (TBS-TX) plus inhibitors, centrifuged as before, the supernatant removed, aliquoted and stored. The pellet was re-suspended in 88% formic acid (1:0.5 w/v) with gentle agitation overnight at 4°C. Next day, the formic acid extracts were aliquoted and transferred to –80°C pending analysis.
Figure 1.
Serial extraction of water-soluble, detergent-soluble and formic acid-soluble Aβ. Human brain tissue was homogenized in 5 vol Tris-buffered saline (TBS), centrifuged at 91 000g for 78 min and the supernatant designated as the TBS extract. The pellet was re-homogenized in 5 vol of Tris-buffered saline containing 1% TX-100 (TBS-TX), centrifuged and the supernatant removed (TBS-TX extract). The remaining pellet was then extracted in 88% formic acid (FA extract).
Quantitation of Aβ in brain extracts
All extraction and quantitation of Aβ was performed blind to clinical and pathological findings. Cortical Aβ levels were determined using a sensitive immunoprecipitation/western blotting protocol used to detect Aβ in culture medium, CSF and brain extracts (Walsh et al., 2000; Klyubin et al., 2008; Shankar et al., 2008). Three-hundred-microlitre samples of TBS or TBS-TX extracts were diluted to 1 ml with TBS or TBS-TX, respectively and used for immunoprecipitation. Immediately prior to analysis formic acid extracts were clarified and neutralized. Aliquots of extracts (100 µl) were centrifuged at 1800 g and 4°C for 10 min and the upper 75 µl removed and samples of supernatant (25 μl) neutralized by addition of 1 M unbuffered Tris containing 0.1% phenol red (650 μl) and the resultant solution (675 μl) used without further dilution. To reduce detection of proteins that non-specifically bind to Protein A Sepharose all samples were pre-incubated with Protein A Sepharose (25 µl) with gentle agitation for 1 h at 4°C, the Protein A Sepharose sedimented and the supernatant used for immunoprecipitation. AW8 antisera were added to samples at a ratio of 1 vol AW8 to 80 vol sample and incubated overnight with Protein A sepharose (25 μl) at 4°C. Antigen–antibody–Protein A sepharose complexes were collected and washed as described previously and the Aβ liberated by heating at 100°C for 6 min in 2× SDS sample buffer (Walsh et al., 2000). Samples were then electrophoresed on 16% polyacyrlamide Tris-Tricine gels (Schagger and von Jagow, 1987) and proteins transferred onto 0.2 μm nitrocellulose (Optitran, Schleicher and Schüll, Germany) at 400 mA for 2 h. To improve detection of Aβ, filters were microwaved (Ida et al., 1996) at 800 Hz for 1.5 min in phosphate-buffered saline and after 3.5 min turned and microwaved again. After a further 3.5 min, membranes were removed from phosphate-buffered saline and incubated in 5% (w/v) fat-free milk in TBS at 4°C overnight. Membranes were immunoblotted using a combination of the C-terminal monoclonal antibodies, 2G3 and 21F12 (each at 1 μg/ml) and detected using fluorochrome-coupled anti-mouse secondary antibody (1 : 2500), (Rockland, Gilbertsville, PA, USA). Images were collected by scanning at 800 nm at a resolution of 169 μm using a Li-COR Odyssey near infrared imaging system (Li-COR Biosciences, Lincoln, NE, USA). Aβ detected in immunoprecipitations of brain extracts was quantified by reference to known amounts of synthetic Aβ1-42 (5, 10, 20 ng per well) loaded on each gel. To facilitate direct comparison of Aβ concentrations measured on different blots the same synthetic standards were used for all analyses and only blots on which there was a linear correlation between Aβ concentration and band intensity with an r2 value ≥ 0.90 were used. The reliable detectable limit of the western blotting protocol was ∼2.5 ng; therefore, samples containing <2.5 ng of Aβ were rated as below the detection limit.
Clinicopathological classification
For the primary analysis, our sample was divided into three groups based on their clinical and pathological profile, those without dementia and those with dementia and Alzheimer’s disease pathology and those with dementia but no significant Alzheimer’s disease pathology (DNAD). Of the 24 participants who died with dementia, 14 had plaques and tangles sufficient for a CERAD diagnosis of Alzheimer’s disease (mean age at death: 87.9 years ± 5.8) and 10 did not (DNAD: mean age: 90.4 years ± 7.9). Seventeen of the 19 participants who died without dementia did not have a neuropathological Alzheimer’s disease diagnosis (mean age: 81.2 years ± 9.0), while two (76 and 82 years) did meet the CERAD criteria for a pathological diagnosis of Alzheimer’s disease. Owing to the small numbers, all those with no dementia were included as a single group in our primary analysis. To elucidate further the relationship between TBS, TBS-TX and formic acid soluble Aβ with specific features of Alzheimer’s disease pathology, we conducted a secondary analysis of the relationship between these fractions and neuritic plaques, diffuse plaques and tangles after stratifying by dementia status at death.
Statistical analysis
The distribution of detectable Aβ was highly skewed and in many cases; no soluble Aβ was detected, and so non-parametric statistical methods were used. Fisher’s exact tests were used to compare the proportions of brains in which soluble Aβ was detected across the Alzheimer’s disease, DNAD and non-dementia groups.
Results
Characteristics of the cohort
The demographic and pathological details of the cohort are presented in Table 1 . There was considerable overlap in the age ranges of all three groups. Those who died without dementia were, on average, younger than those who died with dementia, while the DNAD group were older at death than the Alzheimer’s disease group. There was also a large degree of overlap in pathological findings between the groups. In addition to the two non-dementia cases with a pathological diagnosis of Alzheimer’s disease, 8 (42%) of the non-demented group and 5 (50%) of those with dementia but not Alzheimer’s disease had detectable plaque and tangle pathology and cerebrovascular disease was a common finding across all groups.
Table 1.
Demographic and pathological profile of samples used
| No dementia n = 19 | Dementia without Alzheimer's pathology n = 10 | Dementia with Alzheimer's pathology n = 14 | |
|---|---|---|---|
| Number of female (%) | 8 (42) | 6 (60) | 11 (79) |
| Age at death (median, IQR) | 81 (74–87) | 92 (85–95) | 87.5 (84–93) |
| Years since last cognitive assessment (median, IQR) | 0.9 (0.6–1.1) | 1.5 (0.6–2.9) | 1.0 (0.6–2.3) |
| MMSE at last assessment (median, IQR) | 26 (23–28) | 14.5 (0–18) | 5 (0–11) |
| CAMCOG at last assessment (median, IQR) | 88 (79–95) | 4 (0–64) | 2 (0–27) |
| Hours post-mortem delay (median, IQR) | 24 (12–55)a | 18 (12–30)a | 18 (14–24)a |
| Number of normal brains (%) | 7 (37) | 1 (10) | 0 (0) |
| Plaques and tangles insufficient for AD n (%) | 8 (42) | 5 (50)b | 0 (0) |
| Alzheimer's disease n (%) | 2 (11) | 0 (0) | 14 (100) |
| Vascular disease n (%) | 7 (37) | 5 (50)b | 6 (43) |
| Lewy body dementia or Parkinson’s disease n (%) | 1 (5) | 3 (30) | 1 (7) |
aData on post-mortem delay interval were available for only 35 of the 43 cases studied. b50% of cases in the DNAD group had no plaque or tangle pathology, and the other 50% of cases had some plaque and tangle pathology, but this was insufficient to diagnose Alzheimer’s disease. MMSE = Mini Mental State Examination; CAMCOG = Cambridge Cognitive Examination; AD = Alzheimer’s disease.
Immunoprecipitation/western blotting analysis of TBS extracts reveals the presence of Aβ in certain brain samples
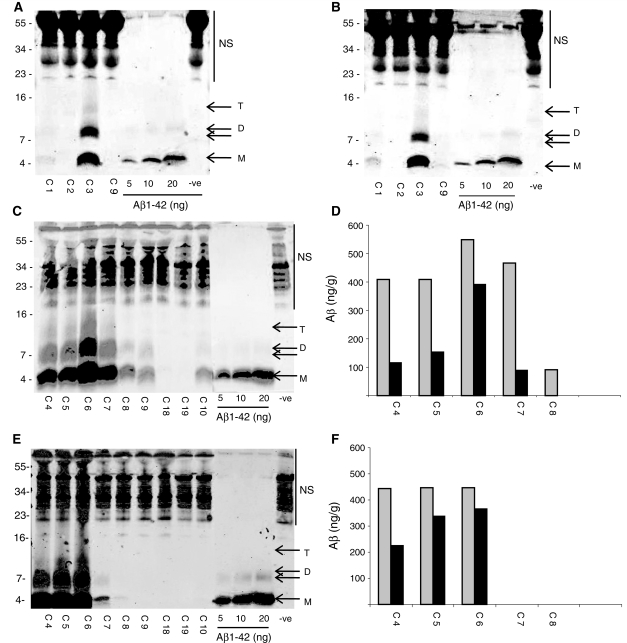
In a pilot study employing serial extraction and immunoprecipitation/Western blotting analysis of 10 brain samples we found abundant Aβ monomer and SDS-stable dimer in the TBS, TBS-TX and guanidine hydrochloride extracts of three Alzheimer’s disease and one Down’s syndrome brain, but little or no TBS-soluble or TBS-TX-soluble Aβ in six non-Alzheimer’s disease brains (Shankar et al., 2008). Here, we first sought to validate the qualitative and quantitative reproducibility of our extraction and detection protocols. When samples from the same homogenate were used for separate immunoprecipitation/western blotting analysis similar qualitative results were obtained. In the example shown the same homogenates from four different donor samples were analysed on two separate occasions (Fig. 2A and B). Overall, the pattern and relative abundance of Aβ species detected in all four samples was similar for both immunoprecipitation/western blotting analyses, but the absolute numeric values show variation in the order of 10–40%. Variation in the amount detected was not a result of non-linear detection of Aβ, since the Aβ concentrations detected in samples were within the linear range of detection for synthetic Aβ standards (Supplementary Fig. 2A, B). Moreover, immunoprecipitation/western blotting analysis of TBS brain extract indicated that this technique produced reliable quantitation of Aβ in samples that had been serially diluted with buffer (Supplementary Fig. 2C) and confirmed that the immunoprecipitation antibody was not limiting. Thus, the source of the variation in the numeric quantitation of Aβ detected by immunoprecipitation/western blotting of the same sample on 2 different days is unclear, but was consistent across 25 duplicate homogenates analysed (data not shown). Since the immunoprecipitation/western blotting protocol yielded consistent qualitative results, we went on to assess the reproducibility of detection using two homogenates produced from adjacent tissue samples from the same ∼0.5 g cube of cortex.
Figure 2.
Repeated immunoprecipitation/western analysis of the same brain homogenate produced similar results, while the amount of Aβ detected varied in homogenates prepared from different pieces of tissue from the same cortical sample. The same Tris-buffered saline (TBS) homogenates from four different donor samples (frontal cortex) were analysed by immunoprecipitation/western blotting on two different occasions [(A) and (B)]. The donor codes C1, C2, C3 and C9 and lanes containing synthetic Aβ are indicated. Molecular weight markers are on the left. NS indicates non-specific immunoreactive bands detected when TBS buffer alone was immunoprecipitated (−ve). Immunoprecipitation/western analysis of TBS extracts from duplicate homogenates [(C) and (E)] and their corresponding quantitation (nanogram Aβ per gram of wet weight brain tissue) are shown in (D) and (F). Grey bars represent Aβ monomer and the black bars Aβ dimer. As above, molecular weight standards are on the left and NS indicates non-specific immunoreactive bands. M and D denote Aβ monomer and SDS-stable dimer and T indicates SDS-stable trimer. Aβ monomer and dimer were detected in the first set of homogenates for cases C4, C5, C6, C7, C8, C9 and C10, but in the second set of homogenates Aβ was not detected in case C9 or C10 (E).
All samples that produced Aβ monomer concentrations ≥400 ng/g in the first homogenate analysed (Fig. 2C and D) also yielded detectable amounts of Aβ monomer when a second homogenate was prepared and analysed (Fig. 2E and F). Importantly, in three out of four samples where Aβ was detected in both homogenates, the estimated concentration was similar. For instance, in the example shown, monomer and dimer concentrations never differed by more than ∼30% and 50%, respectively. However, in certain samples, considerable variation was observed (e.g. C7, Fig. 2C and E). Together, these data indicate that while our immunoprecipitation/western blotting protocol is suitable to discriminate between samples that have detectable Aβ from those that do not, the usefulness of the absolute values is limited. Moreover, there also appears to be an inherent variability in the detection of Aβ in duplicate tissue samples from the same donor sample (Fig. 2C–F); thus, in order to obtain meaningful results it would be necessary to analyse homogenates prepared from a large piece of tissue or from several pieces of tissue from the same region. Given the limited amount of tissue available, we chose the latter approach.
Aβ monomer and SDS-stable oligomers are detected in serial extracts of human cortex
Having established the limitations of our extraction and detection techniques, we then went on to apply these to the study of 43 brains obtained from the Newcastle-upon-Tyne cohort of the Cognitive Function and Ageing study. In each case, two homogenates were prepared from 0.2 g sections of adjacent tissue from both temporal (Brodmann area 21/22) and frontal (Brodmann area 8/9) cortices. Two homogenates from the temporal cortex and two homogenates from the frontal cortex were analysed by immunoprecipitation/western blotting. Average results from duplicate frontal and duplicate temporal homogenates were used for statistical analysis and the presence and concentration of Aβ in the TBS, TBS-TX and formic acid extracts analysed with respect to the dementia status of the brain donor.
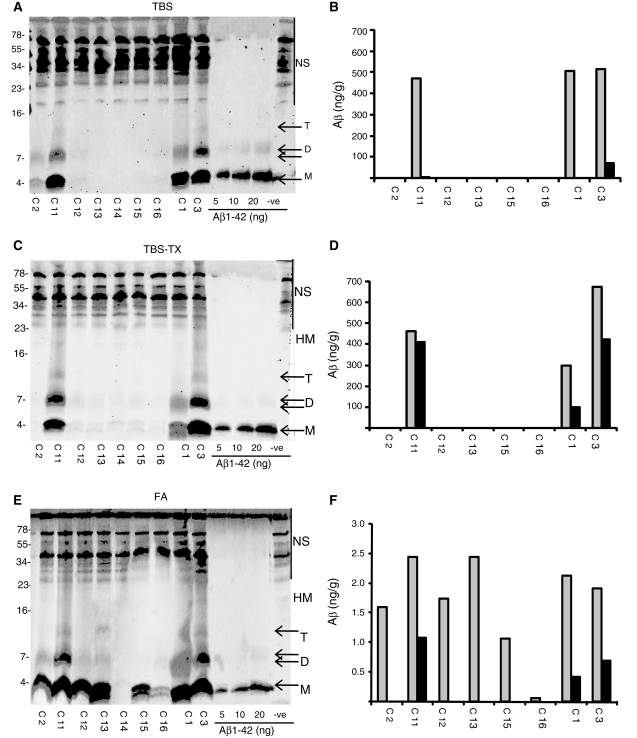
Certain samples of temporal cortex serially extracted with TBS, TBS-TX and formic acid and analysed by immunoprecipitation/western blotting were found to contain both Aβ monomer and higher molecular weight SDS-stable Aβ species (Fig. 3). Based on comparison with molecular weight standards, the three most common Aβ species migrated with estimated molecular weights of 4.1 kDa (monomer), 7.5 kDa (dimer) and 12.1 kDa (trimer). In a few cases, a specific band with an approximate molecular weight of 17.8 kDa (tetramer) was also discerned and in several cases a smear between ∼18 and 30 kDa was detected. The specificity of these bands as authentic full-length Aβ species was confirmed by their detection using the N-terminal specific antibodies 3D6 and 6E10 (Supplementary Fig. S3). Due to the presence of non-specific bands detected in the range of ∼30-75 kDa, it was not possible to discern the presence of specific bands with molecular weights ≥30 kDa (Fig. 3). Formic acid extracts of samples C12, C13, C15 and C16 all exhibited appreciable levels of Aβ, whereas TBS, TBS-TX extracts of the same samples contained no Aβ. Moreover, analysis with 40- and 42-specific antibodies revealed a preponderance of Aβ40 species in the TBS and TBS-TX fractions, whereas Aβ42 was the dominant species in formic acid extracts (Supplementary Fig. 4). The different distribution of Aβ isoforms present in the TBS and formic acid extracts indicates that the Aβ detected in aqueous extracts is likely to represent authentic soluble Aβ and not merely protein released by partial disruption of Aβ42-rich senile plaques. In addition, certain samples extracted with formic acid produced a smear of Aβ immunoreactive material between 7 and 105 kDa (Fig. 3E, Supplementary Fig. 3E). These results are in keeping with the notion that Aβ42 is the major Aβ species deposited in human brain (Roher et al., 1993) and that formic acid extraction solubilizes highly aggregated Aβ42, some of which is not completely depolymerized to monomer.
Figure 3.
Aβ monomer and SDS-stable dimer are detected in cortical samples serially extracted with Tris-buffered saline (TBS), Tris-buffered saline containing 1% TX-100 (TBS-TX) and formic acid. Aliquots of nine brain samples (0.2 g, temporal cortex) were serially extracted and analysed. Western blots of TBS (A), TBS-TX (C) and formic acid (E) extracts are shown. NS indicates non-specific immunoreactive bands detected in buffer alone (−ve) and molecular weight markers are indicated on the left. The concentration of Aβ present in each extract was determined by comparison with the synthetic Aβ standards shown and estimated using Li-COR software (B–F). M = monomer; D = dimer; T = trimer; HM = high molecular weight Aβ species larger than trimer; FA = formic acid.
Since for TBS and TBS-TX extracts the abundance of Aβ species >10 kDa was generally low, further analysis was limited to consideration of Aβ monomer and SDS-stable Aβ dimer. In TBS extracts of temporal cortices, 37% of samples had appreciable Aβ monomer and 23% had discernable monomer and dimer (Figs 4 and 5). The pattern in the TBS-TX extracts was similar, but not identical, 16% of samples had discernable quantities of Aβ monomer and 19% of samples had Aβ dimer (Figs 4B and 5B). The slight difference between the prevalence of monomer and dimer arose because one sample had dimer, but not monomer. In contrast, large quantities of Aβ monomer were evident in 60% of samples extracted with formic acid, whereas only 14% of samples extracted with formic acid contained appreciable dimer (Figs 4C and 5C).
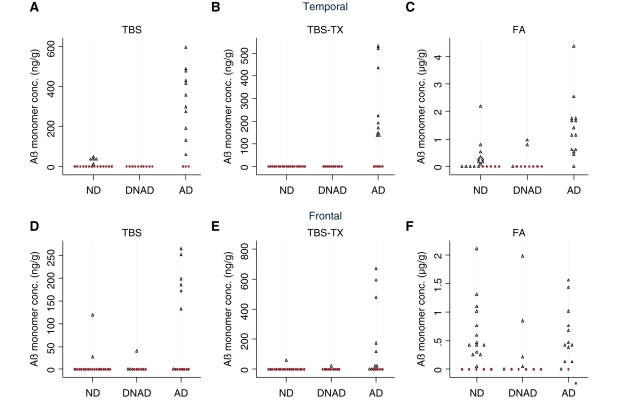
Figure 4.
Aβ monomer is specifically detected in Tris-buffered saline (TBS) and Tris-buffered saline containing 1% TX-100 (TBS-TX) extracts of Alzheimer brain. Average quantifiable monomeric (∼4 kDa) Aβ detected in duplicate homogenates of temporal (A–C) and frontal (D–F) cortices from 43 human brains. The presence of dementia was based on the most recent participant interview, information from death certification, and a retrospective informant interview. Alzheimer’s disease was defined as a donor with dementia plus a pathological CERAD rating of probable/definite Alzheimer’s disease. All samples with detectable levels of Aβ are indicated by black open triangles and those lacking detectable Aβ are shown as red filled circles. ND = not demented, n = 19; DNAD = demented not Alzheimer type, n = 10; AD = Alzheimer Disease, n = 14; FA = formic acid.
Figure 5.
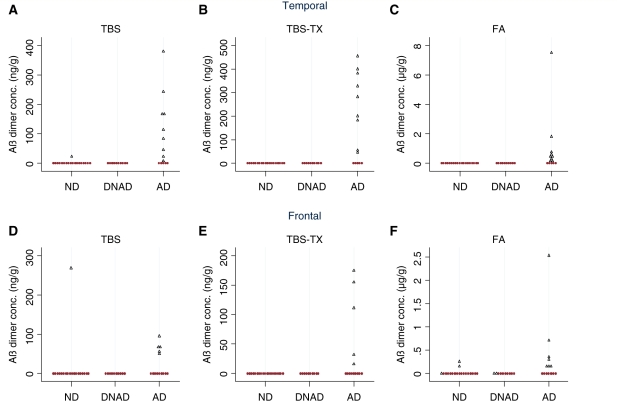
Aβ dimer is present in Tris-buffered saline (TBS), Tris-buffered saline containing 1% TX-100 (TBS-TX) and formic acid extracts of Alzheimer’s disease brain. Average quantifiable dimeric (∼7.5 kDa) Aβ in homogenates of temporal and frontal cortices from 43 human brains is shown. Disease status was assigned as described in the ‘Materials and methods’ section and Aβ concentrations are the average values obtained from duplicate homogenates. Samples with detectable levels of Aβ dimer are indicated by blue open triangles and those lacking detectable dimer are shown as red filled circles. ND = not demented, n = 19; DNAD = demented not Alzheimer’s disease; n = 10; AD = Alzheimer’s disease, n = 14; FA = formic acid.
The detection of Aβ monomer and dimer in the serial extracts of the frontal cortex showed a similar trend (Figs 4D–F and 5D–F). In TBS extracts, 26% of samples had discernable quantities of Aβ monomer and 14% had monomer and dimer. Similarly, 20% of samples had discernable quantities of Aβ monomer in the TBS-TX extract but only 12% of samples had monomer and dimer. As in the temporal cortex, large quantities of Aβ monomer were evident in the majority (67%) of frontal samples extracted with formic acid whereas only 20% of samples had appreciable Aβ dimer. Overall, the number of donor samples that had detectable levels of dimer, relative to samples that had monomer alone was higher in TBS and TBS-TX extracts than in formic acid extracts, but dimer was seldom seen in the absence of monomer. Moreover, in TBS extracts of both temporal and frontal cortices with the exception of one sample (C2), the level of dimer was always lower than the level of monomer. A similar pattern was evident in the TBS-TX, with the exception of one frontal sample that had equal levels of monomer and dimer, and three temporal donor samples (C7, C6 and C4), which had larger values for dimer than monomer and C17 that had dimer but no monomer.
Detection of Aβ monomer in TBS and TBS-TX extracts of temporal and frontal cortices is associated with Alzheimer-type dementia
The presence of detectable Aβ monomer levels in the water-soluble fraction of temporal cortex discriminated the Alzheimer’s disease, DNAD and non-dementia groups, with 11/14 of Alzheimer’s disease samples having detectable monomer, whereas no DNAD samples and only 5/19 of non-dementia samples had appreciable monomer (Fig. 4A, P < 0.001). An even more striking pattern was observed in the TBS-TX extract with monomer detected in 9/14 of Alzheimer’s disease samples but not in non-dementia or DNAD, (P < 0.001, Fig. 4B). In contrast, despite being detected in all 14 Alzheimer’s disease samples, detection of Aβ in formic acid extracts was less specific for Alzheimer’s disease with 14/19 of non-dementia and 3/10 of DNAD samples also containing monomer, (P = 0.001, Fig. 4C).
The pattern observed in the frontal cortex was similar, albeit not identical to that seen in the temporal cortex. Monomer was not detected as frequently in the TBS extract (7/14 of Alzheimer’s disease samples, 2/19 of non-dementia and 3/10 of DNAD samples; P = 0.032), although it still discriminated the groups. As with temporal cortex, TBS-TX extracts from frontal cortex continued to show the highest association with co-existing Alzheimer’s disease pathology and dementia group, with only 1/10 DNAD and 1/19 non-dementia samples having appreciable monomer compared to 9/14 Alzheimer’s disease samples (P < 0.001) (Fig. 4E). Juxtaposed to this, monomer levels in the formic acid extracts from the frontal cortex could not distinguish the groups, with 12/14 Alzheimer’s disease, 5/10 of DNAD and 14/19 of non-dementia samples containing quantifiable monomer (P = 0.15).
SDS-stable Aβ dimer is detected specifically and sensitively in TBS, TBS-TX and formic acid extracts of Alzheimer brain
In addition to Aβ monomer, SDS-stable dimers were also detected in certain brain samples and appear to be even more strongly associated with Alzheimer’s disease. In TBS extracts of temporal cortex only 1/19 non-dementia and 0/10 DNAD samples had dimer, whereas, 9/14 Alzheimer’s disease samples had dimer, P < 0.001 (Fig. 5A). The sensitivity for detecting Alzheimer’s disease based on the presence of dimer in TBS-TX extract was identical to that given by the presence of dimer in TBS extract, but the detection of TBS-TX dimer was more strongly associated with Alzheimer’s disease since dimer was not detected in any non-dementia or DNAD samples despite being seen in 9/14 Alzheimer’s disease samples, P < 0.001 (Fig. 5B). In the formic acid extract, dimer also appeared to be associated with Alzheimer’s disease being detected in 9/14 Alzheimer’s disease samples but not in any of the non-Alzheimer’s disease samples (Fig. 5C).
There was also a positive relationship between the presence of SDS-stable dimer in extracts of frontal cortex and dementia with Alzheimer-type pathology. In the TBS extracts, only 1 out of 19 non-dementia samples had detectable dimer (300 ng/g; Fig. 5D) and dimer was not detected in DNAD donor tissue. As with temporal cortex, the presence of dimer in the TBS-TX fraction of frontal cortex was strongly associated with Alzheimer’s disease but not particularly sensitive, compared to TBS-TX extracts of temporal cortex (5/14 samples Fig. 5E versus 9/14 samples Fig. 5B). The presence of dimer in formic acid extracts of frontal cortex (7/14) more sensitively identified Alzheimer’s disease samples than did dimer in frontal TBS or TBS-TX extracts, but detection of dimer in formic acid extracts was less specific for Alzheimer’s disease (3/19 non-dementia and 2/10 DNAD samples contained detectable dimer; P = 0.12) (Fig. 4F).
TBS and TBS-TX Aβ is only detected in cortical tissue that contains diffuse plaques
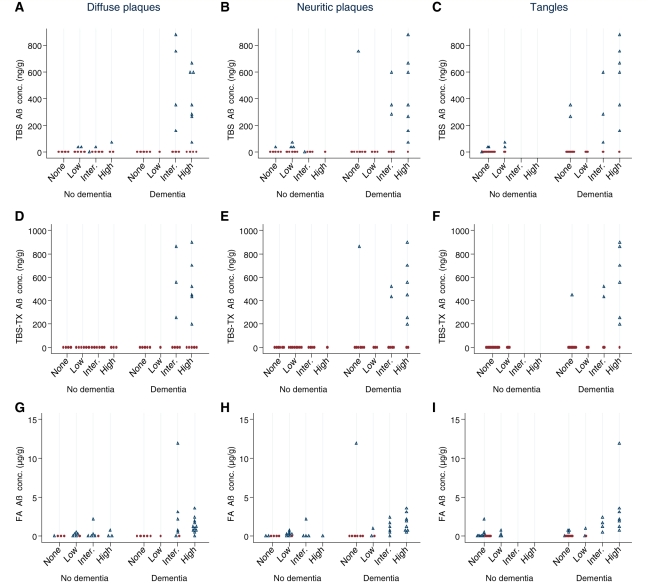
Having established a clear link between the presence of water-soluble and triton-X100-soluble Aβ and Alzheimer’s disease, we turned our attention to investigate the relationship between Aβ and amyloid plaques. Under the CERAD protocol, plaques are divided into diffuse and neuritic depending on the morphology and texture of amyloid deposition combined with the presence or absence of abnormal neurites. Stratification of brains based on severity of diffuse plaque pathology revealed that those lacking discernible diffuse plaques did not contain either Aβ monomer or dimer in TBS or TBS-TX extracts of tissue adjacent to that used for pathological analysis (Fig. 6A and D and Supplementary Fig. 5A and D). In brains that contained only infrequent diffuse plaques, little or no Aβ was detected in the TBS or TBS-TX extracts, whereas Aβ was detected in a significant number of brains with intermediate or high diffuse plaque pathology. Importantly, the levels of soluble Aβ in brains from demented individuals with intermediate or high diffuse plaques tended to be higher than in samples from non-demented individuals with similar levels of diffuse pathology. As with diffuse plaques, the number of brains with detectable Aβ in TBS and TBS-TX extracts tended to increase with the extent of neuritic plaque pathology but the pattern was less clear cut (Fig. 6B and E and Supplementary Fig. 5B and E). Soluble Aβ was detected in a significant number of brains with no or small numbers of neuritic plaques but in each instance these samples contained diffuse plaques. Consideration of Aβ found in formic acid extracts revealed a strong correlation with neuritic plaques. For instance, in temporal cortex only 3/13 samples that lacked discernible neuritic plaques contained formic acid-extractable Aβ, whereas 31/32 samples that had pathologically detectable Aβ deposits contained Aβ in the corresponding formic acid extract (Fig. 6H) with a similar pattern evident in frontal cortex (Supplementary Fig. 5H). A comparable, but slightly less robust, correlation was observed between the presence of formic acid extractable Aβ and diffuse plaques (Fig. 6G and Supplementary Fig. 5G). These data strongly support the notion that the formic acid fraction used in this study is largely derived from both neuritic and diffuse plaques. Specifically, Aβ was detected in the formic acid fraction from samples with intermediate diffuse plaque pathology in the absence of neuritic plaques, but was not detected in samples with only small numbers of diffuse plaques (data not shown). This, together with our finding that a small number of samples lacking any plaque type contained formic acid extractable Aβ, suggests heterogeneity in the distribution of diffuse plaques in tissue samples used for pathology and biochemistry and is in line with our biochemical analysis, which revealed variation in the detection of Aβ between homogenates prepared from adjacent tissue samples (Fig. 2).
Figure 6.
Comparison of Aβ levels and severity of pathological lesions in temporal cortex. The severity of diffuse plaque pathology was compared with Aβ levels in Tris-buffered saline (TBS; A), Tris-buffered saline containing 1% TX-100 (TBS-TX) (D) and formic acid (FA; G) extracts and for neuritic plaque pathology (B, E and H). Similarly, the severity of tangle pathology was compared with Aβ levels in TBS (C), TBS-TX (F) and formic acid (I) extracts. Samples of frontal cortex from 43 cases were analysed. Aβ values are the composite of Aβ monomer and dimer concentration and are the mean of duplicate homogenates. All samples with detectable levels of Aβ monomer and/or dimer are indicated by black open triangles and those lacking detectable Aβ are shown as red filled circles.
TBS and TBS-TX Aβ is detected in most brains that have tangles
Next, we investigated the relationship between Aβ and neurofibrillary tangles. Most of the brains examined lacked neurofibrillary tangles in these cortical regions so that only ∼40% of temporal samples and 29% of frontal samples contained tangles. Of those samples that did contain tangles, 60% also contained TBS and TBS-TX extractable Aβ (Fig. 6C and F and Supplementary Fig. 5C and F) with <20% of tangle-free samples containing TBS or TBS-TX soluble Aβ. In contrast, the presence and abundance of tangles and formic acid extracted Aβ differed substantially from that seen in the TBS and TBS-TX fractions. For instance, in the temporal cortex ∼60% of samples that lacked tangles contained detectable Aβ, whereas 17/18 samples that contained neurofibrillary tangles contained formic acid soluble Aβ (Fig. 6C and F).
Discussion
Burgeoning data from cell and animal models support the notion that non-fibrillar Aβ is synaptotoxic and memory impairing (Klein et al., 2001; Walsh and Selkoe, 2007). Yet, only a few studies have investigated the relationship between non-fibrillar Aβ and the presence/severity of Alzheimer’s disease (Lue et al., 1999; McLean et al., 1999; Wang et al., 1999). In a study using 23 Alzheimer’s disease, 10 pathological ageing (amyloid pathology, but not demented) and 10 normal control samples, Wang and colleagues reported that both water-soluble and formic acid-soluble Aβ levels were consistently higher in cortical extracts from Alzheimer’s disease brain than extracts from either pathological ageing or normal controls (Wang et al., 1999). In contrast, an examination of eight Alzheimer’s disease, eight ‘high Alzheimer’s disease pathology’ controls (i.e. cognitively normal individuals with sufficient plaques and tangles to meet criteria for Alzheimer’s disease) and eight normal controls, concluded that only water-soluble Aβ levels could distinguish Alzheimer’s disease, high pathological controls and normal controls, and that insoluble Aβ levels did not discriminate between Alzheimer’s disease and ‘high Alzheimer’s disease pathology’ controls (Lue et al., 1999).
In this study, we sought to examine the relationship between Aβ monomer and SDS-stable Aβ dimers and the presence or absence of Alzheimer-type dementia in water-soluble, triton-X100-soluble and formic acid-soluble fractions of human brain. This builds on previous findings in that the tissue samples used were obtained from a well-characterized population-based study. Donors were segregated post hoc into comparison groups using both clinical and pathological data. Consequently, we were able to determine the relationships between soluble Aβ, dementia and other pathological findings with minimal selection bias, and our results are informative about the whole of the older population.
The cohort is representative of the complexity of the relationship between dementia and pathology that is seen in the population. Our study diagnosis of Alzheimer’s disease includes those individuals who die with dementia and a sufficient burden of plaques and tangles for a pathological diagnosis of Alzheimer’s disease to be made, irrespective of the presence of other pathology. It is unlikely that Alzheimer’s disease is the sole cause of cognitive impairment within our Alzheimer’s disease group, reflecting the large proportion of the older population who die with mixed neuropathology. Nevertheless, we have shown that soluble Aβ is strongly associated with both dementia at death, and the presence of plaques and tangles. Our secondary analysis was independently stratified on both pathological and clinical findings, and confirms the specificity of soluble Aβ to both clinical dementia and pathology associated with Alzheimer’s disease without recourse to a clinicopathological Alzheimer’s disease definition.
Aβ concentrations were examined with respect to the presence or absence of dementia at death, since this is the most reliable estimate of cognitive state close to death that was available. Our dementia diagnosis makes use of multiple sources of information including multiple cognitive and psychological assessments, death certification data and retrospective interviews with knowledgeable informants and is made independently of any pathological finding. More detailed continuous cognitive measures are only available at assessments undertaken some time before death and using these measures would provide a less reliable indication of the relationship between soluble Aβ and cognitive status at death. Nevertheless, the relationship of soluble Aβ concentrations to domains and severity of cognitive deficits remains an important question that may be addressed in a larger population-based study in the future.
Unlike most studies measuring cerebral Aβ, we did not employ a sandwich-based ELISA, but rather used a sensitive immunoprecipitation/western blotting method. This method offers four major advantages over ELISA: (i) a polyclonal antibody was used for immunoprecipitation, which facilitates detection of a variety of N-terminally truncated Aβ species; (ii) the immunoprecipitation conditions were optimized so that the immunoprecipitating antibody was always present in excess, enabling analysis of even the most concentrated samples; (iii) several commonly used ELISAs do not discriminate between detection of p3 and Aβ (Lue et al., 1999), whereas immunoprecipitation/western blotting does; and (iv) this immunoprecipitation/western blotting method allows for the detection of both Aβ monomer and SDS-stable Aβ aggregates, whereas ELISA does not detect SDS-stable aggregates (Enya et al., 1999; Stenh, 2005). Thus, in this manner, we were able to analyse the levels of Aβ monomer and SDS-stable Aβ oligomers in distinct biochemical fractions of brain.
The levels of Aβ detected in brain extracts varied between samples within the same group and substantially between samples from different groups. In agreement with prior studies, the vast majority of Aβ present in human brain was detected in the formic acid fraction (Tamaoka, 1994; Lue et al., 1999; Naslund et al., 2000) and the ratio of Aβ42/Aβ40 was higher in this fraction than in the TBS or TBS-TX fractions. Although the range of the Aβ levels reported here (based on the sum of Aβ monomer, SDS-stable dimer and trimer), 14.5–941 ng/g (TBS), 1.7–1493 ng/g (TBS-TX) and 0.03–118.6 µg/g (formic acid), are in good agreement with several published studies (Kuo et al., 1996; McLean et al., 1999; Steinerman et al., 2008), it is worth noting that there are huge variations in the levels of cerebral Aβ reported in the literature. For instance, for soluble Aβ, values of 1.9–103.8 pg/g (Lue et al., 1996) to 5.3–1460 ng/g (Tamaoka, 1994) have been detected. Variations in the concentration of insoluble Aβ are equally striking with differences between studies spanning three orders of magnitude from the pg/g to μg/g range (Kuo et al., 1996; Enya et al., 1999; McLean et al., 1999; Wang et al., 1999; Shankar et al., 2008; Steinerman et al., 2008). These differences are likely to result from several factors, including patient selection, sites sampled, inclusion of meninges, different centrifugation speeds/durations, different antibodies and/or assay systems. Variations in centrifugation conditions are of particular importance because soluble Aβ is defined by its ability to remain in solution following centrifugation and this in turn can lead to variations in the estimation of insoluble Aβ. The extraction and centrifugation conditions used in this study are comparable with those employed in two key prior studies (Lue et al., 1999; McLean et al., 1999) so that the biochemical fractions generated should be similar.
In keeping with earlier reports, we found that the levels of TBS-soluble Aβ monomer were specifically elevated in Alzheimer’s disease with little or no monomer detected in demented samples that lacked Alzheimer’s disease-type pathology. In addition, only a few non-demented samples had discernible Aβ monomer and those that did tended to have very low levels. A similar pattern was also seen in the TBS-TX fraction, but the presence of Aβ monomer allowed even greater discrimination between Alzheimer’s disease, DNAD and non-dementia. In contrast, the presence and abundance of Aβ monomer detected in formic acid extracts was not strongly associated with Alzheimer’s disease. The presence of SDS-stable Aβ dimer in the TBS and TBS-TX fractions was highly associated with Alzheimer’s disease and tended to discriminate between Alzheimer’s disease, non-dementia and DNAD better than Aβ monomer. Moreover, even dimer detected in formic acid extracts tended to be associated with Alzheimer’s disease.
These results, together with our recent finding that brain-derived Aβ dimers block long-term potentiation and impair memory consolidation (Shankar et al., 2008), strongly suggest a pathogenic role for water-soluble, diffusible, SDS-stable Aβ oligomers. Prior studies indicated that water-soluble Aβ was elevated in Alzheimer’s disease and the current study extends those findings by demonstrating that this elevation is not only limited to Aβ monomer, but also includes elevation of SDS-stable Aβ dimers. Moreover, the finding that both Aβ monomer and SDS-stable Aβ dimers are elevated in the TBS-TX fraction suggests that at least a portion of water-soluble Aβ may be associated with membranes and could represent the active synaptotoxic species binding to its cognate target. Further, the finding that Aβ dimer levels in temporal cortex better predict the presence of disease than dimer in the frontal lobe is in good agreement with positron emission tomography studies that consistently show significantly greater reductions of glucose metabolism in the temporal cortex than in the frontal cortex (Piert, 1996).
In addition to investigating the relationship between different forms of Aβ and clinical and pathologically confirmed Alzheimer’s disease, we also examined the relationship between Aβ and the pathological hallmarks of Alzheimer’s disease. In regard to tangle pathology, TBS- and TBS-TX-soluble Aβ were detected in a majority of donor samples with intermediate or high neurofibrillary tangles but, because this is a population representative cohort, the number of neurofibrillary tangle-positive samples was relatively small and precluded meaningful statistical analysis. Moreover, given that it has been previously documented that SDS-stable Aβ dimers appear in cortex during ageing and are frequently found in the hippocampus in the absence of neurofibrillary tangles, one might not anticipate a simple correlation between soluble Aβ and tangle pathology (Funato et al., 1998, 1999; Enya et al., 1999). In contrast, the prevalence of amyloid plaques in all three donor categories allowed a detailed examination of the relationship between soluble Aβ and plaques. In agreement with McLean and colleagues, we found a good correlation between the levels of soluble Aβ and neuritic plaques (McLean et al., 1999), a similar relationship between TBS Aβ and diffuse plaques, and a near identical pattern for TBS-TX Aβ and both types of plaques. However, it is unclear if the TBS-soluble Aβ species are free-floating ‘plaque-independent Aβ∋ or are released from plaques by homogenization. If the latter is true, our results suggest that only certain plaques are amenable to disruption and that the ability to release Aβ is ‘disease-specific’. Consequently, future studies designed to better understand the relationship between soluble Aβ, particularly SDS-stable dimer, and plaques could provide valuable insights as to how best to target synaptotoxic water-soluble Aβ.
Funding
The European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement No. 200611 (DMW); grants from the Welcome Trust (867660, DMW), National Institutes of Health (IRO1AGO27443, DMW & DJS) and Medical Research Council, UK (MRC CFAS, grant number G9901400). J.M.M is an Irish Research Council for Science, Engineering and Technology (IRCSET) postgraduate fellow.
Conflict of interest: D.M.W. is a consultant and member of the scientific advisory board of Senexis, plc. D.J.S. is a director of Elan Pharmaceuticals.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Monoclonal antibodies 3D6, 2G3 and 21F12 were kindly provided by Drs. P. Seubert and D. Schenk (Elan Pharmaceuticals, San Francisco, CA, USA). We thank Carlo Sala Frigerio for assistance with preparation of figures.
Glossary
Abbreviations
- Aβ
amyloid β-protein
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- DNAD
dementia but no significant Alzheimer’s disease pathology
- ELISA
enzyme-linked immunosorbent assay
- SDS
sodium dodecyl sulphate
- TBS
tris-buffered saline
- TBS-TX
Tris-buffered saline containing 1% TX-100
References
- Ball M, Braak H, Coleman P, Dickson D, Duyckaerts C, Gambetti P, et al. Consensus recommendations for the postmortem diagnosis of Alzheimers-disease. Neurobiol Aging. 1997;18:S12. [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brayne C, McCracken C, Matthews FE. Medical Research Council Cognitive Function and Ageing Study (CFAS). Cohort profile: the Medical Research Council Cognitive Function and Ageing Study (CFAS) Int J Epidemiol. 2006;35:1140–5. doi: 10.1093/ije/dyl199. [DOI] [PubMed] [Google Scholar]
- Copeland JRM, Dewey ME, Griffiths-Jones HM. Dementia and depression in elderly persons: AGECAT compared with DSM III and pervasive illness. Int J Geriatr Psychiatry. 1990;5:47–51. [Google Scholar]
- Dickson D, Crystal H, Mattiace L, Masur D, Blau A, Davis P, et al. Identification of normal and pathological aging in prospectively studied nondemeneted elderly individuals. Neurobiol Aging. 1991;13:179–89. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Enya M, Morishima-Kawashima M, Yoshimura M, Shinkai Y, Kusui K, Khan K, et al. Appearance of sodium dodecyl sulfate-stable amyloid beta-protein (Abeta) dimer in the cortex during aging. Am J Pathol. 1999;154:271–9. doi: 10.1016/s0002-9440(10)65273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato H, Enya M, Yoshimura M, Morishima-Kawashima M, Ihara Y. Presence of sodium dodecyl sulfate-stable amyloid β-protein in the hippocampus CA1 not exhibiting neurofibrillary tangle formation. Am J Pathol. 1999;155:23–8. doi: 10.1016/s0002-9440(10)65094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, et al. Quantitation of amyloid beta-protein (Aβ) in the cortex during aging and in Alzheimer’s-disease. Am J Pathol. 1998;152:1633–40. [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. 1991;12:383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Huppert FABC, Gill C, Paykel ES, Beardsall L. CAMCOG-a concise neuropsychological test to assist dementia diagnosis: sociodemographic determinants in an elderly population sample. Br J Clin Psychol. 1995;34:529–41. doi: 10.1111/j.2044-8260.1995.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Ida N, Hartmann T, Pantel J, Schroder J, Zerfass R, Forstl H, et al. Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996;271:22908–14. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, et al. Amyloid precursor protein processing and Aß42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci. 1997;94:1550–5. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davis P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia:a subgroup with preserved mentalk status and numerous neocortical plaques. Ann Neurol. 1988;23:138–44. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–24. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, et al. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–7. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y-M, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, et al. Water-soluble Aβ (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271:4077–81. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- Lue LF, Brachova L, Civin WH, Rogers J. Inflammation, Abeta deposition, and neurofibrillary tangle formation as correlates of Alzheimer's disease neurodegeneration. J Neuropathol Exp Neurol. 1996;55:1083–8. [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–62. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the medical research council cognitive function and ageing study. PloS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mirra S, Heyman A, McKeel D, Sumi S, Crain B, Brownless L, et al. The Consortium to Establish a Registry for Alzheimer's disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Ihara Y. The presence of amyloid ß-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry. 1998;37:15247–53. doi: 10.1021/bi981843u. [DOI] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. J Am Med Assoc. 2000;283:1571–7. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Piert M, Koeppe RA, Giordani B, Berent S, Kuhl DE. Diminished glucose transport and phosphorylation in Alzheimer's disease determined by dynamic FDG-PET. J Nucl Med. 1996;37:201–8. [PubMed] [Google Scholar]
- Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, et al. β-amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer's disease. Proc Natl Acad Sci. 1993;90:10836–40. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva G, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, Neuropathology, and Dementia. N Eng J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–98. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta dimers isolated directly from Alzheimer's disease brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinerman JR, Irizarry M, Scarmeas N, Raju S, Brandt J, Albert M, et al. Distinct pools of beta-amyloid in alzheimer disease-affected brain. Arch Neurol. 2008;65:906–12. doi: 10.1001/archneur.65.7.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenh C, Englund H, Lord A, Johansson AS, Almeida CG, Gellerfors P, et al. Amyloid-beta oligomers are inefficiently measured by enzyme-linked immunosorbent assay. Ann Neurol. 2005;58:147–50. doi: 10.1002/ana.20524. [DOI] [PubMed] [Google Scholar]
- Tamaoka A, Kondo T, Odaka A, Sahara N, Sawamura N, Ozawa K, et al. Biochemical evidence for the long-tail form (Abeta 1-42/43) of amyloid beta protein as a seed molecule in cerebral deposits of Alzheimer's disease. Biochem Biophys Res Commun. 1994;205:834–42. doi: 10.1006/bbrc.1994.2740. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Selkoe DJ. The many faces of Aß: Structures and activity. Curr Med Chem Immununol EndocrMetab Agent. 2003;3:277–91. [Google Scholar]
- Walsh DM, Selkoe DJ. Abeta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. Detection of intracellular oligomers of amyloid ß-protein in cells derived from human brain. Biochemistry. 2000;39:10831–9. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999;158:328–37. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]