Abstract
Aging‐related neurodegenerative diseases (NDs) are the culmination of many different genetic and environmental influences. Prior studies have shown that RNAs are pathologically altered during the inexorable course of some NDs. Recent evidence suggests that microRNAs (miRNAs) may be a contributing factor in neurodegeneration. miRNAs are brain‐enriched, small (∼22 nucleotides) non‐coding RNAs that participate in mRNA translational regulation. Although discovered in the framework of worm development, miRNAs are now appreciated to play a dynamic role in many mammalian brain‐related biochemical pathways, including neuroplasticity and stress responses. Research about miRNAs in the context of neurodegeneration is accumulating rapidly, and the goal of this review is to provide perspective for these new data that may be helpful to specialists in either field. An overview is provided about the normal functions for miRNAs, including some of the newer concepts related to the human brain. Recently published studies pertaining to the roles of miRNAs in NDs––including Alzheimer's disease, Parkinson's disease and triplet repeat disorders—are described. Finally, a discussion is included with theoretical syntheses and possible future directions in exploring the nexus between miRNA and ND research.
Keywords: Alzheimer's disase, miRNAs, microRNAs, microarray, Parkinson's disease, review
INTRODUCTION: PERTINENT GENERAL ASPECTS OF NEURODEGENERATIVE DISEASES
“Neurodegeneration” is a term that has been used to refer to differing topics. Abundant nerve cell death occurs in the course of normal brain development, and many pediatric neurological diseases are characterized by pathological degeneration of neurons and/or muscles. Phenomena involving nerve cell death also occur in animal models and cells in culture. This review is focused upon the human‐specific neurodegenerative diseases (NDs) that afflict mainly the elderly, particularly trinucleotide repeat diseases, Alzheimer's disease (AD) and the synucleinopathies, such as Parkinson's disease (PD).
In the course of NDs, neurons lose their connections and die prematurely. Although highly relevant to “physiological” cell death, the role of apoptosis in most NDs has not been conclusively proven, and will not be discussed here in great detail. Each nosological ND entity has its own set of pathological processes, some of which are presumably unique. However, there are six general ideas that are relevant at least circumstantially to how microRNA (miRNA) biochemistry may interact with the pathogenesis of NDs.
-
(i)
Most NDs are not inherited in patterns that reflect simple Mendelian genetics
-
2
Most prevalent subtypes of NDs, such as AD and PD, are inherited in a manner termed “sporadic” (as opposed to “familial”), influenced by alleles with limited genetic penetrance, or are caused by genetic and/or environmental influences as yet uncharacterized (28, 102). These facts may shift the focus of ND genetic studies away from “traditional” genes which constitute only ∼1%–2% of human DNA, and towards the other ∼50% of transcribed DNA about which we are mostly ignorant (48, 96).
-
(ii)
Brains of human ND patients show evidence of chronic stress
-
4
ND brains show evidence of considerable cellular stress (77, 105). Although this may be expected in disease processes that last for long periods and involve abundant cell death, this response may prove contributory because the stress response itself could play a role in the “cascade” effect (see below) that result ultimately in neurological deficits.
-
(iii)
NDs may be exacerbated in the clinicobiological sense by the aberrant stimuli of developmental pathways
-
6
Some biochemical pathways that are up‐regulated during normal brain development, but down‐regulated in normal adulthood, are then aberrantly up‐regulated again in the course of NDs. These include pathways involved in cell–cell signaling, cell division, neuroplasticity and apoptosis (see reviews 3, 17, 91). In the case of the microtubule‐associated protein tau (MAPT), there is an mRNA splicing variant that is more highly represented both in early development and in some neurodegenerative diseases (57, 88). Furthermore, the developmentally up‐regulated phosphorylation of MAPT is also up‐regulated in AD neurofibrillary pathology (39, 119). These are only a few examples of how the up‐regulation of developmental pathways in the adult milieu may contribute to the pathological progression of some NDs.
-
(iv)
RNA is pathologically altered in NDs
-
8
RNA biochemists are well aware that RNA is labile in even tightly controlled circumstances. In the course of some NDs, brain RNAs becomes pathologically altered. These changes have been reviewed elsewhere (81, 88, 118), but include aberrant RNA oxidation, RNA degradation, altered RNA splicing and ribosomal changes which cause mRNA translational frame‐shifting abnormalities.
-
(v)
Human NDs are chronic conditions that last for decades rather than just for several years
-
10
Recent studies have established the surprising chronicity of the processes underlying NDs. Using AD as an example, the time from patients' clinical diagnosis to death is typically ∼8 years (126). However, the underlying pathological processes of AD occur over many decades. Persons at risk for developing AD in their seventh or eighth decade already show brain metabolic abnormalities by PET scan even in their third decade of life (103, 112). These are important data because they show that ND pathology involves a long‐evolving shift in cellular equilibrium, which could be “tipped” even by subtle genetic and/or environmental influences.
-
(vi)
Pathogenetic mechanisms of ND involve multiple distinct steps
-
12
Most NDs are probably not just a “one‐hit” phenomenon, but rather they probably involve a sequential progression of pathological processes that result collectively in neuronal death and/or compromised connectivity. In the case of AD, the most prevalent hypothesis is the “amyloid cascade hypothesis” (43, 44), and both neuritic amyloid plaques and neurofibrillary tangles are apparently required to produce the advanced clinical stages of the disease (as described in 129).
These six features of NDs dovetail on some of the known characteristics of miRNA biochemistry in the brain. As described below, miRNAs derive from “non‐traditional genes”; are related to pathways of cellular stress and neurodevelopment; may be altered by the changes seen in ND brains such as oxidation; and may ultimately contribute to step(s) that culminate in chronic brain diseases.
INTRODUCTION: GENERAL ASPECTS ABOUT TRANSCRIPTIONAL REGULATION AND miRNAs
In mammalian cells, mRNA levels and protein levels tend to correlate quite poorly (88). This may be because translation (RNA→Protein) is even more important than transcription (DNA→RNA) as a nidus for gene expression regulation. More than 95% of human cellular RNAs are “non‐coding” RNAs, that is, other than mRNA, and most of these molecules participate in translational regulation (48, 96). These and other data have led researchers to seek a better understanding of mRNA translation.
miRNAs are small non‐coding RNAs with potent biological activities involved in mRNA translational regulation (see Figure 1). Derived from genes that reside between or within protein‐coding genes, miRNAs may be transcribed by RNA polymerase II (usually) or by RNA polymerase III (16, 19, 66). The precursor RNAs are processed endonucleolytically and exported to the cytoplasm where they are further trimmed by dicer protein to their mature form (89). These “mature”, short (∼22 nucleotides in length), single‐stranded miRNAs are bound intimately to argonaute proteins, which apparently mediate miRNAs' ultimate functions (20, 42). Argonaute proteins and miRNA biochemical machinery are universal among eukaryotes, but great diversity is evident in the particular characteristics of miRNA functions in vivo. The canonical function of argonaute‐bound miRNAs is the suppression of target mRNAs. In other words, an mRNA that is “targeted” by miRNAs may be present by a cDNA microarray experiment, reverse transcriptase PCR, Northern blot or other “expression profiling” technique; however, there would be little or no polypeptide deriving from that mRNA.
Figure 1.
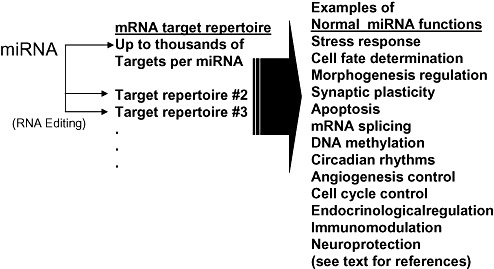
In the mammalian brain as elsewhere, miRNAs subserve multiple fundamental biological roles. Many miRNAs are thought to interact with thousands of different targets. Post‐transcriptional RNA editing may change mRNA target specifity. References for the different hypothesized functions of miRNAs are given in the text.
Biochemical mechanisms by which miRNAs work are probably numerous depending on the availability of local regulatory factors (see for example 53, 60, 67, 95, 97). miRNAs are a relatively recent discovery, having been first recognized in animals in the context of worm development. In the Caenorhabditis elegans heterochronic pathway, miRNAs participate in the sequential process of cell fate determination by decreasing the production of certain proteins despite high levels of their cognate mRNAs (36, 65, 104). This system provides one contextual paradigm for miRNA action, in which a “one miRNA, one/few mRNA target(s)” rule seems to apply in a set developmental staging schema. However, the characteristics of miRNA‐related biochemical pathways have provided many surprises, and more are no doubt yet to come.
One unexpected characteristic of some human miRNAs is their very high RNA copy number per cell. Particular miRNAs can be represented by 1000–30 000 copies per cell or more (1, 72). By comparison, the large majority of mRNAs typically number less than 100 copies per cell (21, 115).
If miRNAs participate in gene expression regulation and are represented in high numbers per cell, how extensive is the cellular impact of miRNAs? Numerous studies have now established that miRNAs can have a profound cellular impact by dint of sheer biochemical promiscuity: each miRNA can regulate hundreds, thousands or more mRNA targets. This was first indicated by predictive bioinformatics, which showed that evolutionarily conserved “miRNA recognition elements” are present in the 3′ untranslated region (3′UTR) of very many mRNAs per miRNA (7, 38, 56, 59, 69, 70, 83). Further studies have exploited the fact that in some circumstances, miRNAs actually cause down‐regulation of target mRNA, in addition to protein, so that cDNA microarrays could be compared between functionally miRNA‐transfected cells and controls (41, 73, 75, 124). These studies have shown convincingly that each miRNA can regulate thousands of different mRNA targets. Hence, these small non‐coding RNAs, which are present in very high gene copy numbers and which work through a powerful translational regulation paradigm, can modify an exceptionally large number of genes.
Recent studies have revealed intriguing novel aspects of miRNA function (see Figure 2):
Figure 2.
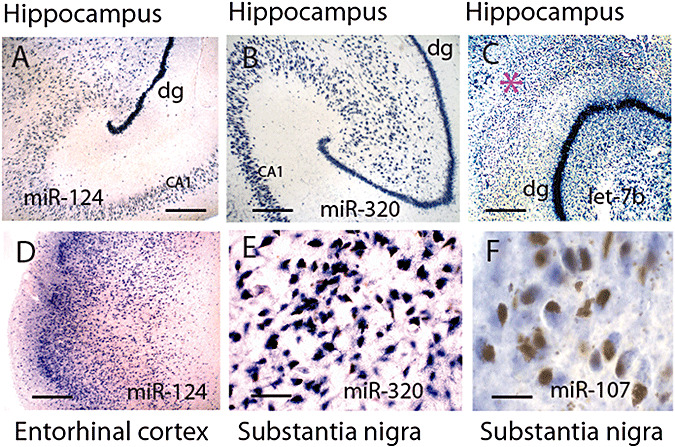
In situ hybridization (ISH) from normal human brains for miRNAs gives an idea of how miRNAs are expressed in neuroanatomical areas that are vulnerable to NDs. A–C show hippocampus. A,B shows miRNAs (miR‐124 and miR‐320) that are expressed predominantly in neurons. By contrast, let‐7b is expressed in both neurons and glia, so cells outside the neuronal layers (such as the molecular layer, pink star, of the dentate granules, dg) are stained. D shows that cells of the superficial layer of entorhinal cortex are also stained avidly using a miR‐124 probe. In the substantia nigra, miR‐320 labels the pigment‐containing cells (E), but miR‐107 only labels them barely if at all (F). Scale bars: A–D = 500 µM; E = 125 µM; F = 62.5 µM. ISH was performed according to the protocol previously described (89) except fixed brain was cut with a freezing microtome.
-
•
Some miRNAs are edited post‐transcriptionally, and thus their targets can be profoundly altered (15, 76, 93).
-
•
Some human miRNAs are targeted to the nucleus for as yet unknown reasons (49).
-
•
miRNAs are shown to alter mRNA splicing and/or DNA methylation in trans (14, 18, 34, 80).
-
•
miRNAs (like siRNAs) may recognize mRNAs outside of the 3′UTR, opening up new possibilities for complex gene expression regulation (84).
-
•
Viral‐derived miRNAs/siRNAs can play important roles in host cell biochemistry (23, 30, 111).
-
•
Under some circumstances, miRNAs can increase translation of targeted mRNAs (10, 11, 47). See note added in proof at end of article.
-
•
Numerous cell‐ and organism‐level functions as diverse as immunomodulation (26, 27, 108), endocrine function (29, 101, 109), circadian rhythms (24, 92), metabolic pathways (125), limb morphogenesis (45) and angiogenesis (63, 100, 120, 128) have been hypothesized to be regulated by miRNAs.
miRNAs IN CELLULAR STRESS
As mentioned above, cellular stress is a conspicuous feature of ND brains. A detailed catalog of the many roles now thought to be played by miRNAs in stress is beyond the scope of this review. However, miRNAs' expressions have been shown to change dramatically in response to cold and heat stress, hypoxia, oxidative stressors, arsenic, irradiation, nutrient deprivation and other stresses (2, 25, 32, 51, 79, 82, 107, 110, 121). Typically, in stress conditions, a subset of miRNAs are increased, and a subset are decreased. The subset of miRNAs that are altered in one stress condition and in one cell type are not necessarily related to the miRNAs which change expression upon a different stressful stimulus. To illustrate the point, miR‐93 is up‐regulated in titanium‐induced stress in a human osteoblast cell line and in the liver of chronically tamoxifen‐fed rats, but down‐regulated in hypoxic human trophoblasts; however, miR‐93 showed no evident expression change in folate deficiency or arsenic exposure in a lymphoblastic cell line (31, 82, 94, 99).
The biochemical effects of miRNAs during cellular stress can change dramatically. For example, stress may alter a miRNA : mRNA interaction to produce translational up‐regulation of target mRNAs (10, 11, 47), which is the opposite of “canonical” miRNA activity. This has prompted speculation that miRNAs may constitute a “safeguard against turmoil”, by altering globally the parameters of translational machinery in the cells during stress (68). Plant miRNAs also participate in the stress response in part through a ubiquitin pathway (5, 25); this is intriguing partly because plant miRNA paradigms have been fundamentally important in understanding the anciently evolved miRNA pathways. In summary, during any conditions that are attended by cellular stress, such as NDs, it can be expected that miRNA expression patterns will change, which would be expected in turn to alter the translation of a large number of different cellular mRNAs.
miRNAs IN THE MAMMALIAN BRAIN
miRNAs are enriched in human and rodents brain: relative to other organs, more miRNAs are expressed in brain and at somewhat higher levels (4, 6, 9, 114, but see 37, 122). Very high‐throughput sequencing experiments have been interpreted to suggest that the number of miRNAs expressed in human brain is over 1000, although currently only ∼550 have been annotated in all humans (8). It has been speculated that miRNAs and other non‐coding RNAs may serve to act as a “complexity multiplier” to help translate the ∼25 000 human protein‐coding genes into the human cerebral cortex with its ∼1013 neurons, 1018 synapses and the staggeringly complex processes of neurodevelopment and neurophysiological network integration (35, 88).
The expression of brain miRNAs alters in the course of brain development. Accordingly, some miRNAs are relatively enriched during early development in the mammalian brain, and some are relatively enriched during later development (85, 90). These changes may relate to biochemical cues in cell fate determination, apoptosis and/or cell division programming (116). Some miRNAs are also relatively enriched in different neuronal nuclei, and/or different cell populations, including neurons or glia (117; see Figure 2). For example, it was recently shown that some rat miRNAs are relatively specific to the hippocampus relative to the rest of rat brain, and another miRNA may be enriched in the substantia nigra (46, 58). Furthermore, some miRNAs are relatively enriched in some neuronal cell compartments (somatodendritic vs. axonal) (64).
The function of brain miRNAs are evidently not related only to cell fate determination along developmental lineages. miRNAs may also play important roles in neuroplasticity and in many other neurobiological functions. These are beyond the scope of this review, and well discussed previously (61, 62). However, it bears repeating that probably most of the functions for miRNAs in the brain as elsewhere have yet to be discovered. The miRNA system is adaptable and researchers should be as open‐minded as possible in developing testable hypotheses about physiologically relevant miRNA actions.
miRNAs IN NEURODEGENERATION—AN EXPANDING RESEARCH CORPUS
The current data that explicitly pertain to miRNAs and neurodegeneration must be characterized as preliminary, reflecting that our understanding of miRNAs is still in its infancy. However, recent studies have begun providing important glimpses into the functions of miRNAs in neuroprotection and neurodegeneration. These studies have been performed across a variety of cells and organisms. A table of some studies that include animal models is provided (Table 1).
Table 1.
| Experimental system | Study relating miRNAs and ND | Notes | References |
|---|---|---|---|
| Fly eye | Dicer knock‐down potentiates neurodegeneration in response to SCA3 and MAPT toxicity | Implicates miRNAs in neuroprotection from ND‐related neurotoxicity | (12, 13) |
| Mouse cerebellum | Dicer knock‐down leads to cerebellar neurodegeneration and ataxia | Analagies are drawn to spinocerebellar ataxia | (113) |
| Mouse and human substantia nigra | miR‐133b is down‐regulated in Parkinson's patients and may play a role in dopaminergic neuronal development | An important connection between novel new roles for miRNA function as well as ND‐related dysfunction | (58) |
| Human brain and primary human neural cultures | A subset of miRNAs is increased in AD hippocampus and also in cell culture due to reactive oxygen species such as aluminum and iron sulfate | Draws a first direct link between oxidative stress, miRNAs, and NDs | (78, 79) |
Abbreviations: AD = Alzheimer's disease; miRNA = microRNA; ND = neurodegenerative diseases; SCA3 = spinocerebellar ataxia type 3.
With regard to the functions of miRNAs in neuroprotection and NDs, experiments in invertebrates have provided important data. Research from Nancy Bonini's laboratory have shown that miRNAs can play an important role in modulating the nerve cell toxicity of pathologically altered SCA3 (spinocerebellar ataxia type 3) protein (12, 13). Increased number of polyglutamine repeats in SCA3 is what mediates the human clinical phenotype (106), and also correlates with cellular toxicity in the fly eye (13). SCA3 polyglutamine toxicity is greatly potentiated when miRNA pathways are inhibited. A particular miRNA (ban) is a factor of neuroprotection both in SCA3‐ and MAPT‐related neurodegeneration in flies (12, 13). Also in flies, the miRNA miR‐7 has been shown to potentiate stable EGFR signaling in photoreceptor cells (71). Still another fly miRNA (miR‐14) also serves to help enable appropriate homeostasis in Drosophila eye cells, and particularly to play a role in adapting to stress (127). Taken together, these studies from different laboratories in flies demonstrate that animal miRNAs play a neuroprotective role in stress‐ and ND‐relevant neuroprotection.
In addition to the studies in invertebrates, recent mammalian studies have also suggested a role for miRNAs in NDs and neuroprotection. Workers in Paul Greengard's laboratory used transgenic mice to give support to the hypothesis that miRNA function is critical for mammalian neuronal survival (and conversely, compromising miRNAs causes neurodegeneration) (113). In this study, the cerebellar Purkinje cell‐specific Pcp2 promoter was used to drive a Cre recombinase causing the dicer gene (and the production of most mature miRNAs) to be knocked down beginning in the second week of life with Pcp2 gene activation. Loss of cerebellar expression of both dicer and most mature miRNAs is followed by Purkinje cell death and dendritic withering neuropathologically, and an ataxia phenotype develops in the mice. The authors hypothesize that as dicer down‐regulation causes neuronal cell death, then some human neurodegenerative diseases may be caused by loss of small regulatory RNAs. As dicer is knocked out in the cerebellum, the authors conclude that their work may be relevant to spinocerebellar ataxias.
In another important work concerning miRNAs in NDs, research from Asa Abeliovich's laboratory indicates that a particular miRNA may play a significant role in PD (58). The authors show experimentally that miR‐133b is relatively highly expressed at the tissue level in midbrain under normal conditions, but not during PD. Furhthermore, knocking out miRNAs generally in vivo, or miR‐133b by itself in culture, dramatically decreases tyrosine hydroxylase and dopamine transporter levels in dopaminergic neurons. The data also support the hypothesis that miR‐133b and the paired‐like homeodomain transcription factor PITX3 regulate each other's expression. This study is important for a number of reasons. First, it indicates a discrete role for a particular miRNA in dopaminergic function. Second, previously, no particular miRNA–mRNA pair had been strongly implicated in a prevalent neurodegenerative disease. In other words, these investigators have provided plausible molecular neurobiological breakthroughs for both miRNA function and dysfunction. This study demonstrates also how miRNAs can be an important component of a “downward spiral” during NDs.
Two other excellent recent papers (78, 79) came from Walter Lukiw's laboratory. DNA arrays were analysed to evaluate the expression of a subset of 12 miRNAs in the AD hippocampus in comparison with non‐demented controls and fetal brain. The results of the expression profiling was that miR‐9 is elevated in both fetal hippocampus and AD hippocampus, miR‐128a is elevated in AD but neither fetus nor non‐demented controls, and miR‐125b shows a trend to be increased in AD that is not statistically significant (78). In a second study from the same group (79), cultured human fetal brain‐derived primary neural (HN) cells, which included both astrocytes and neurons, were treated with metal salts, such as aluminum and iron sulfates that stimulate reactive oxygen species (ROS). The RNA from HN cells was then compared using the same DNA miRNA array that was used in the study of AD brains. Remarkably, the HN cells treated using conditions that activate ROS also had essentially the same changes (increased expression of miR‐9, miR‐128 and, to a lesser extent, miR‐125b) vs. controls, as were seen in the AD brain in comparison with non‐demented brains. The authors make several inferences from these data. First, these data provide evidence in support of the hypothesis that ROS influence AD brain through pathways specifically mediated by miRNAs. Second, miR‐125b, which trends higher in both AD brain and in ROS‐treated HN cells, is predicted to target the mRNA of synapsin I and synapsin II, and thus down‐regulation of synapsins in AD may be partially explained by miR‐125b up‐regulation (78, 79).
Some genes have been associated most strongly with NDs, and it would be interesting to see how strongly these genes are in turn regulated by miRNAs. Two examples are presented (Figure 3): beta‐amyloid precursor protein and alpha‐synuclein, which are important in the pathogenesis of AD and PD, respectively. An important characteristic of both of these genes is that studies have shown that the disease phenotypes in humans can be caused solely by increasing the expression of the genes (50, 87). As shown in Figure 3, each of these genes is also predicted to be the target of multiple miRNAs. These predictions are not validated; however, they derive from only a single miRNA prediction database and still more miRNAs could easily target each of these (as well as many other) mRNAs.
Figure 3.
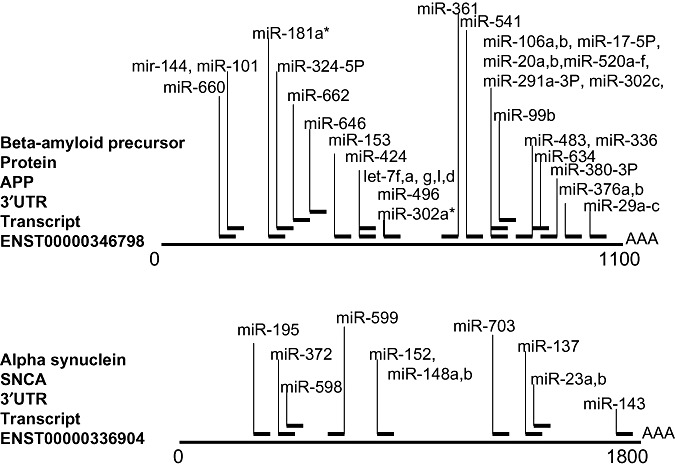
Not only does a given miRNA recognize many mRNA targets, but the 3′ untranslated region (3′UTR) of many mRNAs are also the potential targets for many different miRNAs. Here are two examples that are relevant to NDs, the beta‐amyloid precursor protein (APP) and alpha‐synuclein (SNCA). miRNA target predictions are from the miRBASE registry (http://microrna.sanger.ac.uk/targets/v4/), and have not been validated. APP is predicted to be recognized by the products of 41 different miRNA genes, and SNCA is predicted to be recognized by that of 12.
Aside from the associations between miRNA changes and “classic” NDs, it is also notable that miRNAs may play a role in other degenerative brain diseases, including the fragile X mental retardation syndrome. Fragile X‐associated tremor/ataxia syndrome (FXTAS) affects generally male carriers of expanded triplet CGG repeats on the fragile X mental retardation 1 (FMR1) gene. The polypeptide product of FMR1 is the protein FMRP, which is effectively silenced during the disease (36, 123). Neuropathologically, FXTAS syndrome is characterized by intranuclear inclusions, white matter changes and atypical appearing astrocytes profiles (40). Numerous lines of evidence suggest that FMRP interacts with the miRNA machinery, including miRNAs themselves (22, 33, 52, 55, 98). One hypothesis for the etiology of FMR is that a miRNA derived from the triplet repeat expansion in FMR1's 3′UTR mediates a methylation event that inhibits profoundly the transcription of FMR1 (54, 98). This hypothesis has been supported experimentally (74).
miRNAs IN NEURODEGENERATION— THEORETICAL ASPECTS AND QUESTIONS FOR THE FUTURE
Most of the details of how miRNAs participate in neurodegeneration have yet to be worked out. Hence, at this point, one may consider that the current research corpus is mostly a platform for future gains. Figure 4 shows some of the possibilities related to the interactions between miRNAs and NDs. Here are questions that may merit some reflection:
Figure 4.
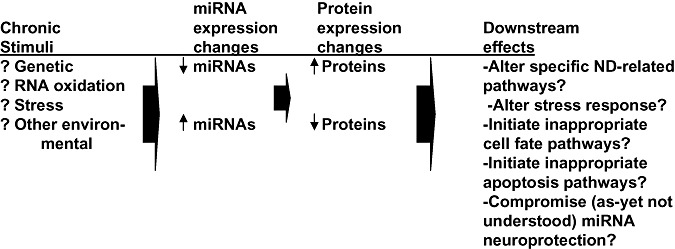
A schematic diagram about some roles miRNAs may play in NDs. The brains of persons at risk for NDs are subjected to chronic stimuli that can perturb specific and/or global miRNA expression. Either the aberrant stimulation or inhibition of miRNA expression may be associated with pathways involved in NDs or neuroprotection.
-
(i)
Neurons are distinguished geometrically by their exceptionally asymmetric shape, with complicated functions requiring protein translation long distances—in some places, many centimeters—from the cell nucleus. Do miRNAs play a role in localized control of mRNA translation, and if so, does perturbation of that localized translational control contribute to NDs?
-
(ii)
If RNAs are altered in NDs, are miRNAs altered also, and does ND‐related miRNA alterations (eg, oxidation) alter biochemical function?
-
(iii)
miRNAs are implicated in cell fate determination, apoptosis, cell division and stress, all of which are pathways thought to be aberrantly up‐regulated in ND brains. Could the miRNA‐related pathways relevant to earlier development be pathogenetic in the adult brain milieu?
-
(iv)
Do risk factors for NDs (eg, the Apolipoprotein epsilon 4 allele) affect miRNA brain expression?
-
(v)
To what extent are genes that are allelic for NDs, and for increased or decreased risk for NDs, target for miRNA regulation?
-
(vi)
Is RNA editing of miRNAs changed in NDs?
-
(vii)
What are the prospects for using miRNA‐related biochemistry for ND therapeutics?
CONCLUSIONS
The regulation of human genomic expression is tremendously complex—it has to be in order to provide an information template for the human brain. We have only begun to understand the function of non‐coding RNAs, such as miRNAs, in providing a complexity multiplier for the genome in the human CNS. It is hoped that research into the normal biochemical brain processes will dovetail with a better understanding of the pathological processes that these same molecules and pathways play. Tantalizing preliminary evidence suggests that miRNAs may play an important role in NDs.
REFERENCES
- 1. Allawi HT, Dahlberg JE, Olson S, Lund E, Olson M, Ma WP et al (2004) Quantitation of microRNAs using a modified Invader assay. RNA 10:1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ambros V (2003) MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113:673–676. [DOI] [PubMed] [Google Scholar]
- 3. Arendt T (2005) Alzheimer's disease as a disorder of dynamic brain self‐organization. Prog Brain Res 147:355–78. [DOI] [PubMed] [Google Scholar]
- 4. Babak T, Zhang W, Morris Q, Blencowe BJ, Hughes TR (2004) Probing microRNAs with microarrays: tissue specificity and functional inference. RNA 10:1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bari R, Datt Pant B, Stitt M, Scheible WR (2006) PHO2, microRNA399, and PHR1 define a phosphate‐signaling pathway in plants. Plant Physiol 141:988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baskerville S, Bartel DP (2005) Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bentwich I (2005) Prediction and validation of microRNAs and their targets. FEBS Lett 579:5904–5910. [DOI] [PubMed] [Google Scholar]
- 8. Berezikov E, Thuemmler F, Van Laake LW, Kondova I, Bontrop R, Cuppen E, Plasterk RH (2006) Diversity of microRNAs in human and chimpanzee brain. Nat Genet 38:1375–1377. [DOI] [PubMed] [Google Scholar]
- 9. Beuvink I, Kolb FA, Budach W, Garnier A, Lange J, Natt F et al (2007) A novel microarray approach reveals new tissue‐specific signatures of known and predicted mammalian microRNAs. Nucleic Acids Res 35:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W (2006) Relief of microRNA‐mediated translational repression in human cells subjected to stress. Cell 125:1111–1124. [DOI] [PubMed] [Google Scholar]
- 11. Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W (2006) Stress‐induced reversal of microRNA repression and mRNA P‐body localization in human cells. Cold Spring Harb Symp Quant Biol 71:513–521. [DOI] [PubMed] [Google Scholar]
- 12. Bilen J, Liu N, Bonini NM (2006) A new role for microRNA pathways: modulation of degeneration induced by pathogenic human disease proteins. Cell Cycle 5:2835–2838. [DOI] [PubMed] [Google Scholar]
- 13. Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM (2006) MicroRNA pathways modulate polyglutamine‐induced neurodegeneration. Mol Cell 24:157–163. [DOI] [PubMed] [Google Scholar]
- 14. Bland CS, Cooper TA (2007) Micromanaging alternative splicing during muscle differentiation. Dev Cell 12:171–172. [DOI] [PubMed] [Google Scholar]
- 15. Blow MJ, Grocock RJ, Van Dongen S, Enright AJ, Dicks E, Futreal PA et al (2006) RNA editing of human microRNAs. Genome Biol 7:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borchert GM, Lanier W, Davidson BL (2006) RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol 13:1097–1101. [DOI] [PubMed] [Google Scholar]
- 17. Bothwell M, Giniger E (2000) Alzheimer's disease: neurodevelopment converges with neurodegeneration. Cell 102:271–273. [DOI] [PubMed] [Google Scholar]
- 18. Boutz PL, Chawla G, Stoilov P, Black DL (2007) MicroRNAs regulate the expression of the alternative splicing factor nPTB during muscle development. Genes Dev 21:71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai X, Hagedorn CH, Cullen BR (2004) Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. Rna 10:1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carmell MA, Xuan Z, Zhang MQ, Hannon GJ (2002) The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev 16:2733–2742. [DOI] [PubMed] [Google Scholar]
- 21. Carter MG, Sharov AA, VanBuren V, Dudekula DB, Carmack CE, Nelson C, Ko MS (2005) Transcript copy number estimation using a mouse whole‐genome oligonucleotide microarray. Genome Biol 6:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caudy AA, Myers M, Hannon GJ, Hammond SM (2002) Fragile X‐related protein and VIG associate with the RNA interference machinery. Genes Dev 16:2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen J, Li WX, Xie D, Peng JR, Ding SW (2004) Viral virulence protein suppresses RNA silencing‐mediated defense but upregulates the role of microrna in host gene expression. Plant Cell 16:1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP et al (2007) Obrietan, microRNA modulation of circadian‐clock period and entrainment. Neuron 54:813–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiou TJ (2007) The role of microRNAs in sensing nutrient stress. Plant Cell Environ 30:323–332. [DOI] [PubMed] [Google Scholar]
- 26. Chowdhury D, Novina CD (2005) RNAi and RNA‐based regulation of immune system function. Adv Immunol 88:267–292. [DOI] [PubMed] [Google Scholar]
- 27. Cobb BS, Hertweck A, Smith J, O'Connor E, Graf D, Cook T et al (2006) A role for dicer in immune regulation. J Exp Med 203:2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coppede F, Mancuso M, Siciliano G, Migliore L, Murri L (2006) Genes and the environment in neurodegeneration. Biosci Rep 26:341–367. [DOI] [PubMed] [Google Scholar]
- 29. Cuellar TL, McManus MT (2005) MicroRNAs and endocrine biology. J Endocrinol 187:327–332. [DOI] [PubMed] [Google Scholar]
- 30. Cullen BR (2006) Viruses and microRNAs. Nat Genet 38(Suppl.):S25–S30. [DOI] [PubMed] [Google Scholar]
- 31. Donker RB, Mouillet JF, Nelson DM, Sadovsky Y (2007) The expression of Argonaute2 and related microRNA biogenesis proteins in normal and hypoxic trophoblasts. Mol Hum Reprod 13: 273–279. [DOI] [PubMed] [Google Scholar]
- 32. Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP (2005) Cold stress‐induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci USA 102:1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duan R, Jin P (2006) Identification of messenger RNAs and microRNAs associated with fragile X mental retardation protein. Methods Mol Biol 342:267–276. [DOI] [PubMed] [Google Scholar]
- 34. Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E et al (2007) MicroRNA‐29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA 104:15805–15810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fields RD, Nelson PG (1992) Activity‐dependent development of the vertebrate nervous system. Int Rev Neurobiol 34:133–214. [DOI] [PubMed] [Google Scholar]
- 36. Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S et al (1991) Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67:1047–1058. [DOI] [PubMed] [Google Scholar]
- 37. Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C et al (2007) Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 67:2456–2468. [DOI] [PubMed] [Google Scholar]
- 38. Ghosh Z, Chakrabarti J, Mallick B (2007) miRNomics—the bioinformatics of microRNA genes. Biochem Biophys Res Commun 363:6–11. [DOI] [PubMed] [Google Scholar]
- 39. Goedert M (2004) Tau protein and neurodegeneration. Semin Cell Dev Biol 15:45–49. [DOI] [PubMed] [Google Scholar]
- 40. Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A et al (2006) Neuropathology of fragile X‐associated tremor/ataxia syndrome (FXTAS). Brain 129(Pt 1):243–255. [DOI] [PubMed] [Google Scholar]
- 41. Grimson A, Farh KK, Johnston WK, Garrett‐Engele P, Lim LP, Bartel DP (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hammond SM (2005) Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett 579:5822–5829. [DOI] [PubMed] [Google Scholar]
- 43. Hardy J (2006) Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis 9(3 Suppl.):151–153. [DOI] [PubMed] [Google Scholar]
- 44. Hardy JA, Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256:184–185. [DOI] [PubMed] [Google Scholar]
- 45. Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ (2005) The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA 102:10898–10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He X, Zhang Q, Liu Y, Pan X (2007) Cloning and identification of novel MicroRNAs from rat hippocampus. Acta Biochim Biophys Sin (Shanghai) 39:708–714. [DOI] [PubMed] [Google Scholar]
- 47. Huang J, Liang Z, Yang B, Tian H, Ma J, Zhang H (2007) Derepression of micro‐RNA‐mediated protein translation inhibition by apolipoprotein B mRNA‐editing enzyme catalytic polypeptide‐like 3G (APOBEC3G) and its family members. J Biol Chem 282:33632–33640. [DOI] [PubMed] [Google Scholar]
- 48. Huttenhofer A, Schattner P, Polacek N (2005) Non‐coding RNAs: hope or hype? Trends Genet 21:289–297. [DOI] [PubMed] [Google Scholar]
- 49. Hwang HW, Wentzel EA, Mendell JT (2007) A hexanucleotide element directs microRNA nuclear import. Science 315:97–100. [DOI] [PubMed] [Google Scholar]
- 50. Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P et al (2004) Causal relation between alpha‐synuclein gene duplication and familial Parkinson's disease. Lancet 364:1169–1171. [DOI] [PubMed] [Google Scholar]
- 51. Ishii H, Saito T (2006) Radiation‐induced response of micro RNA expression in murine embryonic stem cells. Med Chem 2: 555–563. [DOI] [PubMed] [Google Scholar]
- 52. Ishizuka A, Siomi MC, Siomi H (2002) Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev 16:2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jackson RJ, Standart N (2007) How do microRNAs regulate gene expression? Sci STKE 2007:Jan 2;2007(367):re1. Review. [DOI] [PubMed] [Google Scholar]
- 54. Jin P, Alisch RS, Warren ST (2004) RNA and microRNAs in fragile X mental retardation. Nat Cell Biol 6:1048–1053. [DOI] [PubMed] [Google Scholar]
- 55. Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA et al (2004) Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci 7:113–117. [DOI] [PubMed] [Google Scholar]
- 56. John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS (2004) Human MicroRNA targets. PLoS Biol2 2:e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kar A, Kuo D, He R, Zhou J, Wu JY (2005) Tau alternative splicing and frontotemporal dementia. Alzheimer Dis Assoc Disord 19(Suppl. 1):S29–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E et al (2007) RNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kiriakidou M, Nelson PT, Kouranov A, Fitziev P, Bouyioukos C, Mourelatos Z, Hatzigeorgiou A (2004) A combined computational‐experimental approach predicts human microRNA targets. Genes Dev 18:1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kiriakidou M, Tan GS, Lamprinaki S, De Planell‐Saguer M, Nelson PT, Mourelatos Z (2007) An mRNA m7G cap binding‐like motif within human Ago2 represses translation. Cell 129: 1141–1151. [DOI] [PubMed] [Google Scholar]
- 61. Kosik KS (2006) The neuronal microRNA system. Nat Rev Neurosci 7:911–920. [DOI] [PubMed] [Google Scholar]
- 62. Kosik KS, Krichevsky AM (2005) The elegance of the MicroRNAs: a neuronal perspective. Neuron 47:779–782. [DOI] [PubMed] [Google Scholar]
- 63. Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S (2007) Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 101:59–68. [DOI] [PubMed] [Google Scholar]
- 64. Kye MJ, Liu T, Levy SF, Xu NL, Groves BB, Bonneau R, Lao K, Kosik KS (2007) Somatodendritic microRNAs identified by laser capture and multiplex RT‐PCR. Rna 13:1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin‐4 encodes small RNAs with antisense complementarity to lin‐14. Cell 75:843–854. [DOI] [PubMed] [Google Scholar]
- 66. Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN (2004) MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23:4051–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leung AK, Sharp PA (2006) Function and localization of microRNAs in mammalian cells. Cold Spring Harb Symp Quant Biol 71:29–38. [DOI] [PubMed] [Google Scholar]
- 68. Leung AK, Sharp PA (2007) microRNAs: a safeguard against turmoil? Cell 130:581–585. [DOI] [PubMed] [Google Scholar]
- 69. Lewis BP, Shih IH, Jones‐Rhoades MW, Bartel DP, Burge CB (2003) Prediction of mammalian microRNA targets. Cell 115:787–798. [DOI] [PubMed] [Google Scholar]
- 70. Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20. [DOI] [PubMed] [Google Scholar]
- 71. Li X, Carthew RW (2005) A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell 123:1267–1277. [DOI] [PubMed] [Google Scholar]
- 72. Lim LP, Lau NC, Weinstein EG, Abdelhakim A, Yekta S, Rhoades MW et al (2003) The microRNAs of Caenorhabditis elegans. Genes Dev 17:991–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lim LP, Lau NC, Garrett‐Engele P, Grimson A, Schelter JM, Castle J et al (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433: 769–773. [DOI] [PubMed] [Google Scholar]
- 74. Lin SL, Chang SJ, Ying SY (2006) First in vivo evidence of microRNA‐induced fragile X mental retardation syndrome. Mol Psychiatry 11:616–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Linsley PS, Schelter J, Burchard J, Kibukawa M, Martin MM, Bartz SR et al (2007) Transcripts targeted by the microRNA‐16 family cooperatively regulate cell cycle progression. Mol Cell Biol 27:2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Luciano DJ, Mirsky H, Vendetti NJ, Maas S (2004) RNA editing of a miRNA precursor. Rna 10:1174–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lukiw WJ (2004) Gene expression profiling in fetal, aged, and Alzheimer hippocampus: a continuum of stress‐related signaling. Neurochem Res 29:1287–1297. [DOI] [PubMed] [Google Scholar]
- 78. Lukiw WJ, Micro (2007) RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport 18:297–300. [DOI] [PubMed] [Google Scholar]
- 79. Lukiw WJ, Pogue AI (2007) Induction of specific micro RNA (miRNA) species by ROS‐generating metal sulfates in primary human brain cells. J Inorg Biochem 101:1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Makeyev EV, Zhang J, Carrasco MA, Maniatis T (2007) The MicroRNA miR‐124 promotes neuronal differentiation by triggering brain‐specific alternative pre‐mRNA splicing. Mol Cell 27:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Markesbery WR, Lovell MA (2007) Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch Neurol 64:954–956. [DOI] [PubMed] [Google Scholar]
- 82. Marsit CJ, Eddy K, Kelsey KT (2006) MicroRNA responses to cellular stress. Cancer Res 66:10843–10848. [DOI] [PubMed] [Google Scholar]
- 83. Maziere P, Enright AJ (2007) Prediction of microRNA targets. Drug Discov Today 12:452–458. [DOI] [PubMed] [Google Scholar]
- 84. Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM et al (2006) A pattern‐based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 126:1203–1217. [DOI] [PubMed] [Google Scholar]
- 85. Miska EA, Alvarez‐Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P et al (2004) Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol 5:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moss EG, Lee RC, Ambros V (1997) The cold shock domain protein LIN‐28 controls developmental timing in C. elegans and is regulated by the lin‐4 RNA. Cell 88:637–646. [DOI] [PubMed] [Google Scholar]
- 87. Mrak RE, Griffin WS (2004) Trisomy 21 and the brain. J Neuropathol Exp Neurol 63:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nelson PT, Keller JN (2007) RNA in brain disease: no longer just “the messenger in the middle”. J Neuropathol Exp Neurol 66:461–468. [DOI] [PubMed] [Google Scholar]
- 89. Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z (2003) The microRNA world: small is mighty. Trends Biochem Sci 28:534–540. [DOI] [PubMed] [Google Scholar]
- 90. Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RH, Mourelatos Z (2006) RAKE and LNA‐ISH reveal microRNA expression and localization in archival human brain. Rna 12:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nguyen MD, Mushynski WE, Julien JP (2002) Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ 9:1294–1306. [DOI] [PubMed] [Google Scholar]
- 92. O'Neill JS, Hastings MH (2007) Circadian clocks: timely interference by MicroRNAs. Curr Biol 17:R760–R762. [DOI] [PubMed] [Google Scholar]
- 93. Ohman M (2007) A‐to‐I editing challenger or ally to the microRNA process. Biochimie 89:1171–1176. Epub 2007 Jun 8. [DOI] [PubMed] [Google Scholar]
- 94. Palmieri A, Brunelli G, Guerzoni L, Lo Muzio L, Scarano A, Rubini C et al (2007) Comparison between titanium and anatase miRNAs regulation. Nanomedicine 3:138–143. [DOI] [PubMed] [Google Scholar]
- 95. Parker R, Sheth U (2007) P bodies and the control of mRNA translation and degradation. Mol Cell 25:635–646. [DOI] [PubMed] [Google Scholar]
- 96. Pearson H (2006) Genetics: what is a gene? Nature 441:398–401. [DOI] [PubMed] [Google Scholar]
- 97. Pillai RS (2005) MicroRNA function: multiple mechanisms for a tiny RNA? Rna 11:1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Plante I, Davidovic L, Ouellet DL, Gobeil LA, Tremblay S, Khandjian EW, Provost P (2006) Dicer‐Derived MicroRNAs are utilized by the fragile X mental retardation protein for assembly on target RNAs. J Biomed Biotechnol 2006:64347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pogribny IP, Tryndyak VP, Boyko A, Rodriguez‐Juarez R, Beland FA, Kovalchuk O (2007) Induction of microRNAome deregulation in rat liver by long‐term tamoxifen exposure. Mutat Res 619:30–37. [DOI] [PubMed] [Google Scholar]
- 100. Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K et al (2006) MicroRNAs modulate the angiogenic properties of HUVECs. Blood 108:3068–3071. [DOI] [PubMed] [Google Scholar]
- 101. Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE et al (2004) A pancreatic islet‐specific microRNA regulates insulin secretion. Nature 432:226–230. [DOI] [PubMed] [Google Scholar]
- 102. Price DL, Sisodia SS, Borchelt DR (1998) Genetic neurodegenerative diseases: the human illness and transgenic models. Science 282:1079–1083. [DOI] [PubMed] [Google Scholar]
- 103. Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D et al (2004) Functional brain abnormalities in young adults at genetic risk for late‐onset Alzheimer's dementia. Proc Natl Acad Sci USA 101:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE et al (2000) The 21‐nucleotide let‐7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403:901–906. [DOI] [PubMed] [Google Scholar]
- 105. Reynolds A, Laurie C, Lee Mosley R, Gendelman HE (2007) Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol 82:297–325. [DOI] [PubMed] [Google Scholar]
- 106. Riess O, Rub U, Pastore A, Bauer P, Schols L (2007) SCA3: neurological features, pathogenesis and animal models. Cerebellum 30:1–13. [DOI] [PubMed] [Google Scholar]
- 107. Rocha S (2007) Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem Sci 32:389–397. [DOI] [PubMed] [Google Scholar]
- 108. Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR et al (2007) Requirement of bic/microRNA‐155 for normal immune function. Science 316:608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S et al (2006) MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol 24:4677–4684. [DOI] [PubMed] [Google Scholar]
- 110. Van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN (2006) A signature pattern of stress‐responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103:18255–18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Scaria V, Hariharan M, Maiti S, Pillai B, Brahmachari SK (2006) Host‐virus interaction: a new role for microRNAs. Retrovirology 68:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, Stern Y (2005) APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry 76:1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Schaefer A, O'Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P (2007) Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med 204:1553–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sempere LF, Freemantle S, Pitha‐Rowe I, Moss E, Dmitrovsky E, Ambros V (2004) Expression profiling of mammalian microRNAs uncovers a subset of brain‐expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 5:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sheng HZ, Lin PX, Nelson PG (1994) Analysis of multiple heterogeneous mRNAs in single cells. Anal Biochem 222:123–30. [DOI] [PubMed] [Google Scholar]
- 116. Singh SK (2007) miRNAs: from neurogeneration to neurodegeneration. Pharmacogenomics 8:971–978. [DOI] [PubMed] [Google Scholar]
- 117. Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG (2005) Regulation of miRNA expression during neural cell specification. Eur J Neurosci 21:1469–1477. [DOI] [PubMed] [Google Scholar]
- 118. Smith MA, Nunomura A, Zhu X, Takeda A, Perry G (2000) Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer disease. Antioxid Redox Signal 2:413–420. [DOI] [PubMed] [Google Scholar]
- 119. Stoothoff WH, Johnson GV (2005) Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta 1739:280–297. [DOI] [PubMed] [Google Scholar]
- 120. Suarez Y, Fernandez‐Hernando C, Pober JS, Sessa WC (2007) Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res 100:1164–1173. [DOI] [PubMed] [Google Scholar]
- 121. Sunkar R, Chinnusamy V, Zhu J, Zhu JK (2007) Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci 12:301–309. [DOI] [PubMed] [Google Scholar]
- 122. Tang X, Gal J, Zhuang X, Wang W, Zhu H, Tang G (2007) A simple array platform for microRNA analysis and its application in mouse tissues. Rna 13:1803–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A et al (1991) Identification of a gene (FMR–1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914. [DOI] [PubMed] [Google Scholar]
- 124. Wang X, Wang X (2006) Systematic identification of microRNA functions by combining target prediction and expression profiling. Nucleic Acids Res 34:1646–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wilfred BR, Wang WX, Nelson PT (2007) Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR‐103/107 regulates human metabolic pathways. Mol Genet Metab 91:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Williams MM, Xiong C, Morris JC, Galvin JE (2006) Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology 67:1935–1941. [DOI] [PubMed] [Google Scholar]
- 127. Xu P, Vernooy SY, Guo M, Hay BA (2003) The Drosophila microRNA Mir‐14 suppresses cell death and is required for normal fat metabolism. Curr Biol 13:790–795. [DOI] [PubMed] [Google Scholar]
- 128. Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G (2005) Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 280:9330–9335. [DOI] [PubMed] [Google Scholar]
Note added in proof
After submission of the manuscript, excellent additional work of miRNAs up‐regulated targeted mRNAs was published by the laboratory of Joan Steitz.
- 129. Nelson PT, Jicha GA, Schmitt FA, Liu HL, Davis DG et al. Clinicopathological Correlations in a Large Alzheimer's Disease Center Autopsy Cohort: Neuritic Plaques and Neurofibrillary Tangles “Do Count” When Staging Disease Severity. J Neuropathol Exp Neurol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


