Abstract
Cellular signaling can inhibit the membrane Na+-K+ pump via protein kinase C (PKC)-dependent activation of NADPH oxidase and a downstream oxidative modification, glutathionylation, of the β1 subunit of the pump α/β heterodimer. It is firmly established that cAMP-dependent signaling also regulates the pump, and we have now examined the hypothesis that such regulation can be mediated by glutathionylation. Exposure of rabbit cardiac myocytes to the adenylyl cyclase activator forskolin increased the co-immunoprecipitation of NADPH oxidase subunits p47phox and p22phox, required for its activation, and increased superoxide-sensitive fluorescence. Forskolin also increased glutathionylation of the Na+-K+ pump β1 subunit and decreased its co-immunoprecipitation with the α1 subunit, findings similar to those already established for PKC-dependent signaling. The decrease in co-immunoprecipitation indicates a decrease in the α1/β1 subunit interaction known to be critical for pump function. In agreement with this, forskolin decreased ouabain-sensitive electrogenic Na+-K+ pump current (arising from the 3:2 Na+:K+ exchange ratio) of voltage-clamped, internally perfused myocytes. The decrease was abolished by the inclusion of superoxide dismutase, the inhibitory peptide for the ϵ-isoform of PKC or inhibitory peptide for NADPH oxidase in patch pipette solutions that perfuse the intracellular compartment. Pump inhibition was also abolished by inhibitors of protein kinase A and phospholipase C. We conclude that cAMP- and PKC-dependent inhibition of the cardiac Na+-K+ pump occurs via a shared downstream oxidative signaling pathway involving NADPH oxidase activation and glutathionylation of the pump β1 subunit.
Keywords: Cyclic AMP (cAMP); Na,K-ATPase; Protein Chemical Modification; Protein Kinase A (PKA); Reactive Oxygen Species (ROS); Adrenergic; Glutathionylation; Oxidative Signaling
Introduction
The membrane Na+-K+ pump transports three Na+ ions out of and two K+ ions into cells against their electrochemical gradient using energy derived from hydrolysis of ATP. The Na+ and K+ ion gradients generated by the pump serve in secondary co- and counter-transport processes that maintain gradients for H+, Ca2+, Cl−, and various organic molecules. Changes in the intracellular Na+ concentration can have wide-ranging effects on the intracellular milieu and, hence, cell function, including excitability, energy metabolism, and excitation-contraction coupling. Regulation of the Na+-K+ pump is, therefore, important for cell homeostasis.
The Na+-K+ pump is a heterodimer that consists of a large α subunit and a much smaller β subunit. Each exists in several isoforms with α1 and β1 the most abundantly expressed (1). Hydrolysis of ATP and transport of Na+ and K+ are mediated by the α subunit. The β subunit has a chaperone role in mediating membrane integration of newly synthesized α subunits. In addition, it influences transport properties of the assembled α/β heterodimer. A conformational rearrangement of the heterodimer during the catalytic cycle may couple the β subunit to function (2).
Oxidative stimuli induce glutathionylation, a reversible oxidative modification, of Cys-46 of the β1 subunit. Glutathionylation and an associated pump inhibition are abolished by mutation of Cys-46 indicating a causal relationship between the oxidative modification and pump function. This is supported by the absence of an effect of oxidative stimuli when expressed pump heterodimers contain wild-type β2 or β3 subunits that do not have a Cys-46 (3). The physiological relevance of glutathionylation is suggested by its coupling to hormone receptors and kinase-dependent signaling pathways. Exposure of cardiac myocytes to angiotensin II (Ang II)3 increases glutathionylation and inhibits pump activity via protein kinase C (PKC)-dependent activation of NADPH oxidase (3, 4). Conversely, exposure of myocytes to β3 adrenergic receptor agonists decreases glutathionylation from base line and stimulates pump activity via activation of nitric oxide (NO) synthase and NO-dependent downstream pathways (5).4 Thus, pump stimulation and inhibition are mediated by decreasing or increasing the degree of Cys-46 glutathionylation from base line.
Cyclic AMP-dependent messenger pathways have also been reported to regulate the Na+-K+ pump. Such regulation occurs in many different tissues (6), including the heart (7–16), and is reported to cause either pump stimulation or inhibition. In this study we show that the adenylyl cyclase activator forskolin can induce glutathionylation of the β1 Na+-K+ pump subunit in cardiac myocytes. As expected from the glutathionylation of the subunit (3), exposure of voltage-clamped myocytes to forskolin decreased electrogenic Na+-K+ pump current (Ip). Parallel studies on intact myocytes and functional studies on voltage-clamped, internally perfused myocytes implicated the same oxidative signaling pathway in the forskolin-induced glutathionylation of the β1 subunit and decrease in Ip.
EXPERIMENTAL PROCEDURES
Cells
Ventricular myocytes were isolated from rabbit hearts as described previously (17). They were stored at room temperature until used for experimentation and were used on the day of isolation only. Experimental protocols were approved by our institutional animal ethics and care committee.
Protein Co-immunoprecipitation and Detection of Glutathionylated Protein
Cells were lysed in ice-cold buffer containing 150 mmol/liter NaCl, 50 mmol/liter Tris·HCl (pH 8.0), EDTA, and 1% Triton X-100. Protease inhibitors were added. The lysates were clarified by centrifugation at 16,000 × g for 20 min and incubated with monoclonal antibody to the protein of interest or control IgGs. Protein A/G plus-agarose beads (40 μl of a 50% slurry) were added to the supernatant for a further incubation of 1 h at 4 °C. Immunoprecipitated proteins were eluted by boiling for 5 min in Laemmli sample buffer. Immune complexes were analyzed by SDS/PAGE and Western blot by probing with antibodies to ϵPKC (BD Biosciences), the receptor for the activated kinase (ϵRACK, BD Biosciences), p22phox (Santa Cruz Biotechnology), p47phox (Santa Cruz Biotechnology), and the α1 or β1 subunit of Na+-K+-ATPase (Upstate Biotechnology). To detect S-glutathionylation of pump subunits, myocytes were loaded with biotinylated glutathione (GSH). After lysis, the biotin-tagged glutathionylated subfraction was precipitated using streptavidin-Sepharose beads (18) and immunoblotted for α1 and β1 Na+-K+ pump subunits. In separate experiments the β1 subunit immunoprecipitate was immunoblotted with an antibody against glutathionylated protein (anti-GSH antibody, Invitrogen). Western blots were quantified by densitometry using a Las-4000 image reader and Multi Gauge 3.1 software (Fuji Photo Film Co., Ltd.) and normalized against relevant control. Exposure times were adjusted to ensure that the variation in signal intensity was in the linear dynamic range.
Fluorescence Microscopy
The oxidative fluorescent dye dihydroethidium (DHE) was used to image intracellular O2˙̄ as described previously (19, 20). Myocytes were incubated in the dark in Krebs solution containing 2 μmol/liter DHE for 20 min at 37 °C. In some experiments they were preincubated in 170 IU/ml pegylated superoxide dismutase (pegylated SOD, covalently attached polyethylene glycol polymer chain to SOD, which enhances cell association of the enzyme), 10 μmol/liter apocynin, or 10 μmol/liter myristoylated ϵPKC inhibitory peptide for 20 min before loading with DHE. Myocytes were then exposed to control solutions or solutions containing 100 nmol/liter forskolin for 10 min before fixation in 2% paraformaldehyde on ice for 4 min. They were washed and mounted on poly-l-lysine-coated glass slides in Vectashield and examined under a laser scanning confocal microscope (Nikon C1) equipped with an argon-krypton laser. The excitation wavelength was 488 nm, and the emission wavelength was 585 nm. The fluorescence images were obtained using constant settings of scanning speed, pinhole diameter, and voltage gain. Myocytes representative of each experimental condition were selected randomly for quantification of fluorescence intensity (Photoshop, Adobe). Only myocytes with clear striations and a rod-like shape were included in the analysis. The average intensity for cells from each experiment was normalized against its control (100%).
Measurement of Ip in Voltage-clamped Myocytes
Solutions and voltage clamp protocol were designed to minimize non-pump membrane currents in voltage-clamped myocytes. We used wide-tipped patch pipettes (4–5 μm) filled with solutions containing 5 mmol/liter HEPES, 2 mmol/liter MgATP, 5 mmol/liter EGTA, 70 mmol/liter potassium glutamate, 10 mmol/liter sodium glutamate, and 80 mmol/liter tetramethylammonium chloride. The solution also contained 0.01 mmol/liter l-arginine when indicated. Myocytes were initially superfused with solution containing 140 mmol/liter NaCl, 5.6 mmol/liter KCl, 2.16 mmol/liter CaCl2, 1 mmol/liter MgCl2, 10 mmol/liter glucose, 0.44 mmol/liter NaH2PO4, 10 mmol/liter HEPES, and subsequently with a solution that was nominally Ca2+-free and contained 2 mmol/liter BaCl2 and 0.2 mmol/liter CdCl2. Na+-containing compounds in this solution were replaced with N-methyl-d-glutamine (21) to avoid transmembrane Na+ influx that might cause an increase in the intracellular Na+ concentration and secondary pump stimulation. This solution also included 100 nmol/liter forskolin when indicated. Because forskolin is expected to activate a large Cl− current, we voltage-clamped myocytes at the equilibrium potential for Cl−, calculated from the composition of the superfusate and pipette solution (−14 mV). Ip was identified as the difference between stable holding current before and after Na+-K+ pump blockade with100 μmol/liter ouabain. Na+-K+ pump currents are small relative to other membrane currents, and it is important for their accurate measurement that holding currents are stable before and after superfusion of ouabain. We used predetermined criteria for stability of holding currents, and the shift in the currents was induced by ouabain (22). Ip was normalized for membrane capacitance and, hence, cell size. We switched to the ouabain-containing superfusate 8–10 min after the whole-cell configuration had been established in all experiments. As the effect of ouabain is not reversible within the time frame that stable holding currents can be reliably measured (22, 23), the suspension of myocytes was removed at the conclusion of the experimental protocol, and a new aliquot of myocytes was added to the tissue bath in ouabain-free solution. As a consequence, only one myocyte was studied per bath.
Statistical Analysis
Results are expressed as the mean ± S.E. One-way analysis of variance was used for analysis of co-immunoprecipitation data, and a Student's t test was used for paired data, with single tail distribution for analysis of differences in DHE fluorescence intensity levels between experiments and controls. Student's t test for unpaired data was used for analysis of patch clamp data. p < 0.05 is regarded as significant in all comparisons.
RESULTS
Forskolin Induces Glutathionylation of the β1 Pump Subunit and Decreases Its Co-immunoprecipitation with the α1 Subunit
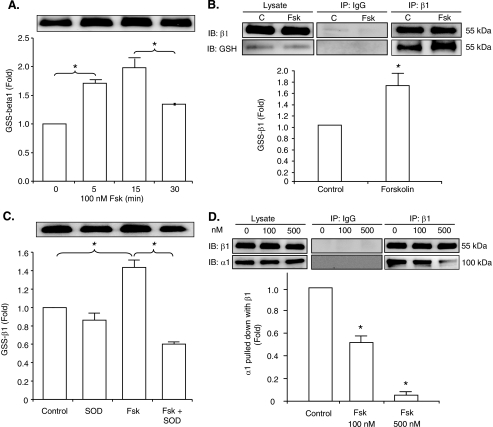
To examine the effect of cAMP-dependent signaling on glutathionylation of the pump β1 subunit (3), myocytes were loaded with biotin-tagged GSH. They were then exposed to control solution or solution containing 100 nmol/liter forskolin for 5, 15, or 30 min. The cells were lysed, and the glutathionylated protein subfraction was pulled down with streptavidin beads and immunoblotted with β1 subunit antibody. Fig. 1A shows that there was an increase in glutathionylation of the β1 subunit after 5 min of exposure to forskolin. The increase was sustained with exposure for 15 min, but there was a subsequent decrease by 30 min.5 Fig. 1B shows that forskolin also increased glutathionylation of the β1 subunit as detected by the independent GSH antibody technique (3). As shown in Fig. 1C, preincubation of myocytes in solutions containing pegylated SOD abolished the forskolin-induced glutathionylation.
FIGURE 1.
Effect of forskolin on glutathionylation of the Na+-K+ pump β1 subunit and α1/β1 subunit interaction. A, the immunoblot shows biotinylated GSS-β1 subunit after exposure of myocytes loaded with biotin-GSH to forskolin for 5, 15, or 30 min or vehicle control. B, shown is the effect of forskolin on glutathionylation of the β1 subunit as shown by immunoblotting (IB) the β1 subunit immunoprecipitate (IP) with GSH antibody. The immunoblot of the cell lysate and non-immune IgG control are also shown. C, control. C, shown is the effect of SOD on forskolin-induced glutathionylation as detected by the biotin-GSH technique. D, shown is the effect of forskolin on Na+-K+ pump α1/β1 subunit co-immunoprecipitation. Representative α1 and β1 subunit immunoblots after immunoprecipitation with β1 subunit antibody are shown. The histograms show mean densitometry of blots from three-four experiments, each normalized against control (%). IP indicates the antibody used for immunoprecipitation. IB indicates the antibody used for immunoblot. The asterisk indicates significant difference versus control.
The interaction of the pump β subunit with the catalytic α subunit is important for function, and glutathionylation of the β1 subunit is associated with a reduction in its co-immunoprecipitation with the α1 subunit (3). We examined the effect of forskolin on α1/β1 subunit co-immunoprecipitation. Myocytes were exposed to 100 or 500 nmol/liter forskolin for 15 min before lysis. The lysate was immunoprecipitated with β1 subunit antibody and immunoblotted with α1 subunit antibody. Fig. 1D shows that forskolin reduced α1/β1 subunit co-immunoprecipitation.
Forskolin Induces ϵPKC-dependent Activation of NADPH Oxidase
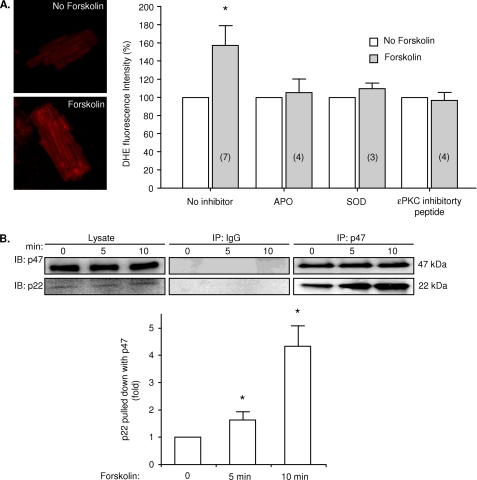
As the Na+-K+ pump subunits co-immunoprecipitate with the membrane-associated p22phox subunits of NADPH oxidase in cardiac myocytes (4) and SOD abolished the forskolin-induced glutathionylation, we examined if forskolin activates NADPH oxidase. Two techniques were used, O2˙̄-sensitive DHE fluorescence and co-immunoprecipitation of the cytosolic p47phox NADPH oxidase subunit with p22phox, reflecting the translocation that is necessary for activation. We loaded myocytes with DHE and exposed them to 100 nmol/liter forskolin for 10 min or to control solutions. Representative micrographs and a summary histogram are shown in Fig. 2A. Forskolin increased the intensity of DHE fluorescence. Preincubation of myocytes in solutions containing 170 IU/ml pegylated SOD or 10 μmol/liter NADPH oxidase inhibitor apocynin blocked the increase in fluorescence supporting the specificity of the fluorescence signal and implicating the source of O2˙̄. Phosphorylation of the p47phox by PKC, but not PKA, can activate NADPH oxidase (24), and we have previously found that activation of NADPH oxidase can be inhibited by an ϵPKC-inhibitory peptide (4). To examine if the forskolin-induced increase in DHE fluorescence might be dependent on ϵPKC, we incubated myocytes with 10 μmol/liter membrane-permeable, myristoylated ϵPKC inhibitory peptide. It abolished the forskolin-induced increase in fluorescence. To examine if forskolin increases co-immunoprecipitation of the p47phox NADPH oxidase subunit with the p22phox subunit, we exposed myocytes to 100 nmol/liter forskolin or to control solutions for 15 min. Cell lysate was immunoprecipitated with antibody to the p47phox subunit, and the precipitate was immunoblotted with antibody to p22phox. Fig. 2B shows that forskolin increased the co-immunoprecipitation.
FIGURE 2.
Effect of forskolin on myocyte O2˙̄-sensitive DHE fluorescence and NADPH oxidase activation. A, confocal fluorescent micrographs and mean DHE-fluorescence intensity of control myocytes and myocytes exposed to forskolin are shown. Forskolin increased the fluorescence intensity. This increase was abolished by pegylated-SOD, apocynin (APO), or myristoylated ϵPKC inhibitory peptide. The number of experiments is shown in parentheses. B, shown is co-immunoprecipitation of p47phox and p22phox subunits of NADPH oxidase. Representative immunoblots of p47phox and p22phox immunoprecipitated with antibody to the p47phox subunit after exposure of myocytes to forskolin are shown. IP indicates the antibody used for immunoprecipitation. IB indicates the antibody used for immunoblot. The histogram shows the mean densitometric measurements of immunoblots from three experiments standardized to the value of control samples. The asterisk represents significant difference compared with control.
Forskolin Inhibits Ip in Cardiac Myocytes
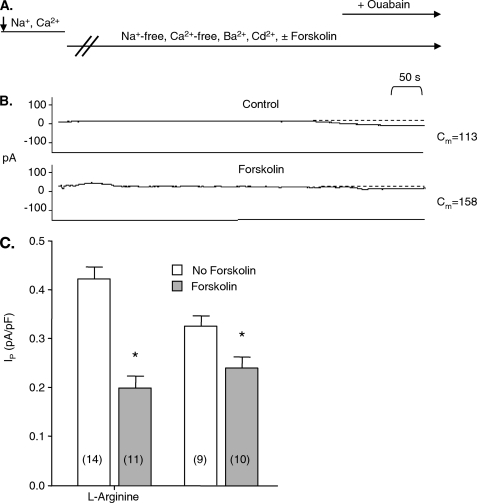
The forskolin-induced increase in glutathionylation of the β1 subunit of the Na+-K+ pump in cardiac myocytes shown in Fig. 1 is expected to cause pump inhibition (3) and is, therefore, difficult to reconcile with the pump stimulation widely reported to be mediated by cAMP-dependent pathways. We examined the effect of forskolin on Ip measured in myocytes with the whole-cell patch clamp technique. With this technique, the intracellular compartment is perfused with patch pipette solutions, and the concentration of some low molecular weight substances is expected to be altered. l-Arginine is of particular interest as either a decrease or an increase in its concentration may promote oxidation; a reduction in l-arginine levels may uncouple nitric-oxide synthase to preferentially synthesize superoxide rather than NO. Conversely, NO synthesized from supplemental l-arginine may combine with superoxide to form the highly oxidant species peroxynitrite. To minimize risk of an experimental artifact resulting from alterations in intracellular l-arginine concentrations, initial experiments were performed with and without l-arginine included in patch pipette solutions. In a first series of experiments we included 10 μmol/liter l-arginine in pipette solutions, a concentration ∼10-fold above the KD for relevant nitric-oxide synthase isoforms (19). The myocytes were superfused with forskolin-free “standard” Na+- and Ca2+-containing Tyrode solution, whereas the whole-cell configuration was established. We then switched to a nominally Na+-free superfusate to rule out influx of Na+ that might cause secondary pump stimulation. This superfusate was also nominally Ca2+-free. It contained forskolin or was forskolin-free in control experiments. Fig. 3A shows the timing of changes in the composition of superfusates. Fig. 3B shows currents of a control myocyte and a myocyte exposed to forskolin. Ip was smaller for the myocyte exposed to forskolin than for the control myocyte. Fig. 3C shows that forskolin induced a statistically significant decrease in mean Ip. An independent set of experiments was performed using an identical protocol with the exception that pipette solutions did not contain l-arginine. Results are included in Fig. 3C. In agreement with our previous study (19), Ip in control experiments was smaller than in experiments performed using patch pipettes containing l-arginine. However, forskolin again significantly reduced Ip. Holding currents after Na+-K+ pump blockade with ouabain in both sets of experiments were very small and similar for control myocytes and myocytes exposed to forskolin, consistent with use of the calculated equilibrium potential for chloride as the test potential. Although qualitatively similar, the forskolin-induced decrease in Ip appeared larger when l-arginine was included in pipette solutions. To avoid a bias in favor of Na+-K+ pump inhibition that we expected from the β1 subunit glutathionylation and to facilitate comparisons with previous studies, we used patch pipette solutions without added l-arginine in all subsequent experiments.
FIGURE 3.
Effect of forskolin on Na+-K+ pump current in myocytes. Panel A shows the timing of changes in the composition of superfusates. The arrow on the left side of the panel indicates establishment of the whole-cell configuration and, hence, perfusion of the intracellular compartment with pipette solution. The switch from a Ca2+-containing, forskolin-free solution in the tissue bath to a nominally Ca2+-free solution containing Ba2+, Cd2+, and forskolin and the switch to a solution also containing ouabain (the arrow on the right side of the panel) are shown. Panel B shows examples of holding currents. Large changes in the currents that occur the first 1–2 min after the switch to Na+- and Ca2+-free Ba2+- and Cd2+-containing superfusate before currents stabilize are not shown. Stable holding currents before exposure of myocytes to ouabain are important for the measurement of Ip. Stability of the currents as well as the ouabain-induced shift in them was identified from the read-out of an electronic cursor. Cm indicates membrane capacitance in picofarads (pF). Panel C shows the mean Ip normalized for membrane capacitance. The pipette solution contained l-arginine where indicated. The numbers of myocytes in each group are indicated in parentheses. The asterisk indicates a significant difference between the means of Ip.
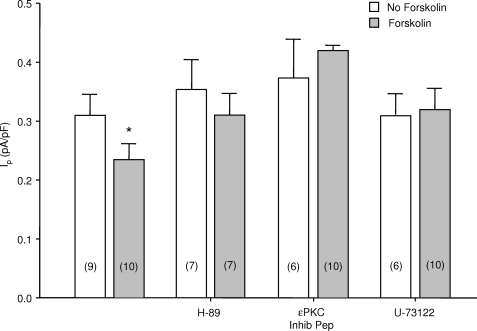
Effect of H-89 and ϵPKC Inhibitory Peptide on the Forskolin-induced Na+-K+ Pump Inhibition
We next included 500 nmol/liter H-89, an inhibitor of PKA, in patch pipette solutions and exposed myocytes to superfusate with or without forskolin. Fig. 4 shows that H-89 abolished the decrease in Ip. Because cross-talk between the ϵPKC and PKA has been reported (25), we included 100 nmol/liter ϵPKC inhibitory peptide in patch pipette solutions (26). It abolished the forskolin-induced decrease in Ip. PKA-dependent activation of PKC is mediated by phospholipase C (PLC) in non-cardiac tissues (25, 27, 28). We, therefore, included the PLC inhibitor U-73,122 in pipette solutions in a concentration of 1 μmol/liter. This abolished the forskolin-induced decrease in Ip as shown in Fig. 4.
FIGURE 4.
Effects of inhibitors of PKA, ϵPKC and PLC on forskolin-induced Na+-K+ pump inhibition. Myocytes were perfused with pipette solutions containing H89, ϵPKC inhibitory peptide, or U-73122 as indicated. The data from myocytes not exposed to inhibitors, previously presented in Fig. 1, is included for reference. The numbers of myocytes in each group are indicated in parentheses. The asterisk indicates a significant difference compared with control. pF, picofarads.
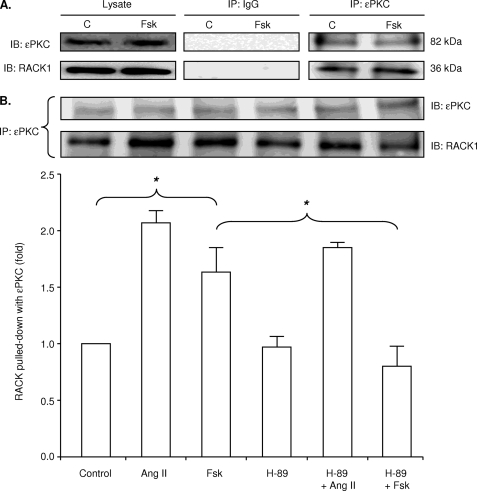
Functional studies in myocytes internally perfused with patch pipette solution supported a role for ϵPKC in forskolin-induced pump inhibition. We examined if forskolin increases co-immunoprecipitation of ϵPKC with ϵRACK in intact myocytes. Ang II, a known activator of ϵPKC in cardiac myocytes (4), was used as a positive control. Myocytes were exposed to 100 nmol/liter forskolin, 100 nmol/liter Ang II, or vehicle control solutions for 15 min. The lysate was immunoprecipitated with antibodies to ϵPKC, and the precipitate was immunoblotted with antibodies to ϵRACK. Fig. 5 shows that both Ang II and forskolin induced an increase in co-immunoprecipitation of ϵPKC with ϵRACK. Preincubation of myocytes with 500 nmol/liter H-89 prevented the forskolin-, but not the Ang II-induced increase in co-immunoprecipitation. These results suggest activation of ϵPKC is sensitive to H-89, consistent with the functional effects of both H-89 and the ϵPKC inhibitory peptide to abolish the forskolin-induced decrease in Ip shown in Fig. 4.
FIGURE 5.
Effect of forskolin on co-immunoprecipitation of ϵPKC with RACK. Myocytes exposed to Ang II (positive control), forskolin, or vehicle control were lysed before co-immunoprecipitation. ϵPKC immunoprecipitate (IP) was immunoblotted (IB) for ϵPKC or RACK. The histogram shows the mean densitometric measurements of immunoblots from three experiments standardized to the value of control samples. Ang II induced an increase in ϵPKC/RACK co-immunoprecipitation that was insensitive to H-89. Forskolin also induced an increase in ϵPKC/RACK co-immunoprecipitation, but this was abolished by H-89. The asterisk indicates significant differences compared with control. C, control.
Oxidative Signaling Mediates the Forskolin-induced Decrease in Ip
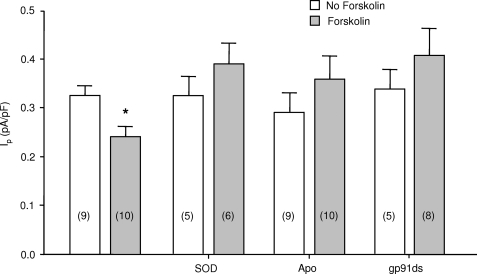
We next examined the role of NADPH oxidase and oxidant signaling in the forskolin-induced decrease in Ip. To examine the role of O2˙̄, we included 200 IU/ml SOD in patch pipette solutions and measured Ip after exposing the patch-clamped myocytes to superfusates containing forskolin or to control superfusates. Fig. 6 shows that SOD abolished the forskolin-induced decrease in Ip. In another series of experiments we inhibited NADPH oxidase. We exposed myocytes to 10 μmol/liter apocynin, included in the superfusate because it is readily membrane-permeable, or we used the gp91ds peptide to inhibit docking of p47phox and, hence, activation of NADPH oxidase. The gp91ds peptide is usually considered a low efficacy inhibitor when combined with the tat peptide that is used to facilitate transmembrane entry into intact cells (29). However, the whole cell patch clamp technique allowed us to directly perfuse the intracellular compartment with a tat-free compound. We included it in patch pipette solutions in a concentration of 10 μmol/liter, a concentration expected to prevent activation of NADPH oxidase (4). As shown in Fig. 6, forskolin-induced pump inhibition was abolished by apocynin or gp91ds peptide.
FIGURE 6.
Role of superoxide and NADPH oxidase in forskolin-induced Na+-K+ pump inhibition. Myocytes were perfused with pipette solutions containing SOD, apocynin (Apo), or the gp91ds peptide as indicated. The data from myocytes not exposed to inhibitors, previously presented in Fig. 1, is included for reference. Numbers of myocytes in each group are indicated in parentheses. The asterisk indicates a significant difference compared with control. pF, picofarads.
DISCUSSION
Na+-K+ pump inhibition mediated by cAMP-dependent pathways in cardiac myocytes was reported in early studies (8, 9), but many more recent studies have reported that these pathways mediate pump stimulation either by increasing maximal pump rate or by increasing pump affinity for intracellular Na+ (7, 11–16, 30). In our study, forskolin induced glutathionylation of the Na+-K+ pump β1 subunit. Because glutathionylation of the subunit is causally related to Na+-K+ pump inhibition (3), this is concordant with the forskolin-induced pump inhibition we demonstrated in functional studies.
We used the whole-cell patch clamp technique to examine functional effects of cAMP-dependent pathways on the Na+-K+ pump, as have most previous studies. If wide-tipped patch pipettes are used, the technique will in principle allow control of membrane voltage and the concentration of Na+-K+ pump ligands on both sides of the membrane in a largely intact cell. However, sources of error from transmembrane Na+ influx (31) or unstable base-line membrane currents can easily contaminate measurement of the small pump currents. Even when wide-tipped patch pipettes are used, agonist-induced Na+ influx and secondary pump stimulation can cause a large increase in the Ip that is measured (32). We used wide-tipped patch pipettes to optimize control of the intracellular milieu, and we used Na+-free superfusates to eliminate errors from transmembrane Na+ influx. Activation of the cAMP-dependent Cl− current might change the intracellular Cl− concentration and, hence, the electrochemical driving force for the Na+/K+/2Cl− co-transporter. Activation of the co-transporter in turn can alter the ouabain-sensitive current that defines Ip (32). We used the calculated equilibrium potential for Cl− as the test potential to eliminate a net change in cAMP-dependent Cl− channel fluxes. As indicated by the similar holding currents after superfusion of ouabain in experiments performed with and without forskolin, this objective was achieved.
Experimental activation of cAMP-dependent pathways in previous studies on the cardiac myocyte Na+-K+ pump has been achieved by exposing myocytes to a β adrenergic receptor agonist or by directly activating adenyl cyclase with exposure to forskolin. Receptor activation is the most physiologically relevant of the two approaches. However, available agonists have poor selectivity against the three different β adrenergic receptor subtypes (33), and the compounds used in some previous studies may have activated the β3 adrenergic receptor that is coupled to activation of nitric-oxide synthase. We have found the β3 adrenergic receptor mediates stimulation of the Na+-K+ pump in cardiac myocytes (5).5 We, therefore, used forskolin, as have most of the recent studies (7, 11, 12, 15, 16).
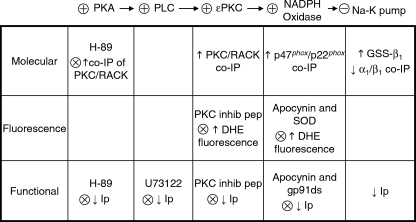
Data from functional Na+-K+ pump studies, studies on myocyte DHE fluorescence, and molecular studies implicated the pathway summarized at the top of Fig. 7. Although effects of cAMP-dependent pathways are classically attributed to PKA-mediated phosphorylation of effector proteins, the exchange protein directly activated by cAMP (Epac) has also become recognized as important. PKA and Epac can act independently of each other, in synergy or antagonistically (34). The effect of H-89 to block the forskolin-induced decrease in Ip and increase in co-immunoprecipitation of ϵPKC with RACK suggests a requirement for PKA activation but does not necessarily rule out a synergistic role of Epac.
FIGURE 7.
Summary of molecular, fluorescence, and patch clamp studies on effects of forskolin. Steps in the signaling pathway that are implicated are shown at the top with stimulation and inhibition indicated by + and −. Blocks of responses to forskolin are indicated below by ×. Glutathionylation of the β1 subunit is indicated by GSS-β1, and Na+-K+ pump current is indicated by Ip. co-IP, co-immunoprecipitation.
PLC β- and ϵ-isoforms can be activated downstream from PKA (25) or Epac (35), and the effect of U-73,122 to abolish the forskolin-induced decrease in Ip is consistent with a role for PLC activation in our study. An increase in diacylglycerol levels in turn may activate PKC. These results are also consistent with studies in the kidney showing PKA/PKC cross-talk-dependent Na+-K+ pump inhibition attributed to PLC activation (27).
The forskolin-induced increase in ϵPKC/RACK co-immunoprecipitation implicates PKC. The effect of the ϵPKC inhibitor peptide to abolish both an increase in DHE fluorescence and a decrease in Ip supports the functional role of PKC activation. PKC-dependent phosphorylation of the cytosolic NADPH oxidase subunit p47phox induces its translocation to the membranous p22phox subunit that is required for activation of NADPH oxidase (24). Consistent with NADPH oxidase activation, forskolin induced an increase in co-immunoprecipitation of p47phox with p22phox, and apocynin abolished the forskolin-induced increase in DHE fluorescence. The effect of the gp91ds peptide used to block docking of p47phox is also consistent with a downstream NADPH oxidase dependence of Na+-K+ pump inhibition. Because proximity to a source of oxidants is important for the specific oxidative modification of target proteins (36), the co-localization of the NADPH oxidase complex with the Na+-K+ pump we have reported previously (4) should facilitate glutathionylation of the pump β1 subunit and the functional equivalent of inhibition.
Perfusion of the intracellular compartment using patch pipettes with particularly wide tips in our study might have altered signaling pathways. However, the concordance of results in voltage-clamped, internally perfused myocytes and intact myocytes studied with fluorescence and molecular techniques, summarized in Fig. 7, suggest that signaling domains are not readily susceptible to changes induced by myocyte perfusion. This may reflect compartmentalization of signaling and perhaps anchoring of key signaling molecules. Taken together, the results suggest the signaling pathway shown in Fig. 7 is plausible, and the consistency of results obtained with different techniques supports the overall validity of the study.
The time dependence of the forskolin-induced increase in glutathionylation shown in Fig. 1A suggests that effects are not sustained long term. This may reflect a cellular homeostatic response to activation of cAMP-dependent signaling that terminates a phosphorylation-dependent part of the signal as discussed previously (37). For the variable we measure here, mechanisms that inactivate the downstream oxidative signaling pathway or directly activate deglutathionylation may have additional effects on the time course. However, our in vitro study cannot accurately reflect the complex structural, mechanical, and neurohormonal in vivo milieu of the beating heart, and it will be important to determine the effects of cAMP-dependent signaling on Na+-K+ pump function and β1 subunit glutathionylation in vivo in future studies.
The effect of cAMP-dependent signaling on Na+-K+ pump function has important implications for our understanding of excitation-contraction coupling in the heart under physiological and pathophysiological conditions. In the normal heart, pump inhibition with an increase in adrenergic drive is expected to increase intracellular Na+ and, hence, Ca2+ levels and act in synergy with the well recognized positive inotropic effect of cAMP on Ca2+ entry and intracellular Ca2+ handling. However, although a modest increase in intracellular Na+ levels enhances contractility, the high levels typically seen in heart failure can have the opposite effect and are believed to contribute adversely to its phenotype (38, 39). It is of particular importance for the present study that adverse effects of NADPH oxidase-dependent redox signaling may also contribute (40). Efficacy of treatments that target dysregulation of adrenergic signaling in heart failure trials are consistent with an in vivo relevance of our results. Blockade of adenyl cyclase-coupled receptors by “β-blockers” may exert some of its benefit (41) by reducing oxidative stress, reversing Na+-K+ pump inhibition, and hence, reducing cellular Na+ overload. This contrasts with the long term detrimental effect of activation of cAMP-dependent signaling by a β1 adrenergic receptor agonist (42) or a phosphodiesterase III inhibitor (43) documented in clinical trials. The common role of PKC-dependent NADPH oxidase activation in both Ang II- and cAMP-induced Na+-K+ pump inhibition may contribute to the well established therapeutic synergy between angiotensin-converting enzyme inhibitors and β-blockers in heart failure (41). Their combined use is expected to inhibit the shared oxidative signaling pathway more effectively than either group of drugs used alone.
Acknowledgments
We acknowledge the facilities as well scientific and technical assistance from the staff at the NANO Major National Research Facility at the Electron Microscopy Unit, the University of Sydney. In particular, the assistance from Ellie Kable, and Professor Filip Braet was greatly appreciated. We also greatly appreciate advice from Dr. Ron Clarke about implications of Na+-K+ pump kinetics.
The work was supported by grants from the North Shore Heart Research Foundation and the National Health and Medical Research Council.
H. Bundgaard, C. Liu, A. Garcia, E. J. Hamilton, Y. Huang, K. K. Chia, S. H. Hunyor, G. A. Figtree, and H. H. Rasmussen, submitted for publication.
Results of molecular, DHE fluorescence, and patch clamp studies used in this study are summarized and compared in Fig. 7.
- Ang II
- angiotensin II
- PKC
- protein kinase C
- PKA
- protein kinase A
- PLMIp
- electrogenic Na+-K+ pump current
- RACK
- receptor for activated kinase
- DHE
- dihydroethidium
- SOD
- superoxide dismutase
- PLC
- phospholipase C
- Epac
- exchange protein directly activated by cAMP.
REFERENCES
- 1.Blanco G., Mercer R. W. (1998) Am. J. Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- 2.Geering K. (2008) Curr. Opin. Nephrol. Hypertens. 17, 526–532 [DOI] [PubMed] [Google Scholar]
- 3.Figtree G. A., Liu C. C., Bibert S., Hamilton E. J., Garcia A., White C. N., Chia K. K., Cornelius F., Geering K., Rasmussen H. H. (2009) Circ. Res. 105, 185–193 [DOI] [PubMed] [Google Scholar]
- 4.White C. N., Figtree G. A., Liu C. C., Garcia A., Hamilton E. J., Chia K. K., Rasmussen H. H. (2009) Am. J. Physiol. Cell Physiol 296, C693–C700 [DOI] [PubMed] [Google Scholar]
- 5.Garcia A., Bundgaard H., Hamilton E., Liu C., Chia K. K., Figtree G. A., Rasmussen H. H. (2008) 12th International ATPase Conference, Aarhus, Denmark, August 5–10, 2008, abstract 157 [Google Scholar]
- 6.Therien A. G., Blostein R. (2000) Am. J. Physiol. Cell Physiol 279, C541–C566 [DOI] [PubMed] [Google Scholar]
- 7.Kockskämper J., Sendhoff K., Erlenkamp S., Bordusa F., Cerovsky V., Glitsch H. G. (2001) Pflugers Arch 441, 807–815 [DOI] [PubMed] [Google Scholar]
- 8.Gao J., Mathias R. T., Cohen I. S., Baldo G. J. (1992) J. Physiol. 449, 689–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J., Cohen I. S., Mathias R. T., Baldo G. J. (1998) Pflugers Arch. 435, 479–484 [DOI] [PubMed] [Google Scholar]
- 10.Despa S., Bossuyt J., Han F., Ginsburg K. S., Jia L. G., Kutchai H., Tucker A. L., Bers D. M. (2005) Circ. Res. 97, 252–259 [DOI] [PubMed] [Google Scholar]
- 11.Erlenkamp S., Glitsch H. G., Kockskämper J. (2002) Pflugers Arch. 444, 251–262 [DOI] [PubMed] [Google Scholar]
- 12.Silverman B. Z., Fuller W., Eaton P., Deng J., Moorman J. R., Cheung J. Y., James A. F., Shattock M. J. (2005) Cardiovasc. Res. 65, 93–103 [DOI] [PubMed] [Google Scholar]
- 13.Despa S., Tucker A. L., Bers D. M. (2008) Circulation 117, 1849–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossuyt J., Despa S., Han F., Hou Z., Robia S. L., Lingrel J. B., Bers D. M. (2009) J. Biol. Chem. 284, 26749–26757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller W., Howie J., McLatchie L. M., Weber R. J., Hastie C. J., Burness K., Pavlovic D., Shattock M. J. (2009) Am. J. Physiol. Cell Physiol 296, C1346–C1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kockskamper J., Erlenkamp S., Glitsch H. G. (2000) J. Physiol. 523, 561–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hool L. C., Whalley D. W., Doohan M. M., Rasmussen H. H. (1995) Am. J. Physiol. 268, C366–C375 [DOI] [PubMed] [Google Scholar]
- 18.Adachi T., Weisbrod R. M., Pimentel D. R., Ying J., Sharov V. S., Schöneich C., Cohen R. A. (2004) Nat Med 10, 1200–1207 [DOI] [PubMed] [Google Scholar]
- 19.White C. N., Hamilton E. J., Garcia A., Wang D., Chia K. K., Figtree G. A., Rasmussen H. H. (2008) Am. J. Physiol. Cell Physiol. 294, C572–C578 [DOI] [PubMed] [Google Scholar]
- 20.Kim Y. M., Guzik T. J., Zhang Y. H., Zhang M. H., Kattach H., Ratnatunga C., Pillai R., Channon K. M., Casadei B. (2005) Circ. Res. 97, 629–636 [DOI] [PubMed] [Google Scholar]
- 21.Hansen P. S., Buhagiar K. A., Gray D. F., Rasmussen H. H. (2000) Am. J. Physiol. Cell Physiol. 278, C546–C553 [DOI] [PubMed] [Google Scholar]
- 22.William M., Vien J., Hamilton E., Garcia A., Bundgaard H., Clarke R. J., Rasmussen H. H. (2005) J. Physiol. 565, 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Despa S., Bers D. M. (2007) Am. J. Physiol. Cell Physiol. 293, C321–C327 [DOI] [PubMed] [Google Scholar]
- 24.Park J. W., Hoyal C. R., Benna J. E., Babior B. M. (1997) J. Biol. Chem. 272, 11035–11043 [DOI] [PubMed] [Google Scholar]
- 25.Yao L., Fan P., Jiang Z., Gordon A., Mochly-Rosen D., Diamond I. (2008) Mol. Pharmacol. 73, 1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buhagiar K. A., Hansen P. S., Bewick N. L., Rasmussen H. H. (2001) Am. J. Physiol. Cell Physiol. 281, C1059–C1063 [DOI] [PubMed] [Google Scholar]
- 27.Gomes P., Soares-da-Silva P. (2002) Am. J. Physiol. Renal Physiol. 282, F1084–F1096 [DOI] [PubMed] [Google Scholar]
- 28.Gomes P., Soares-da-Silva P. (2004) Eur. J. Pharmacol. 488, 51–59 [DOI] [PubMed] [Google Scholar]
- 29.Bedard K., Krause K. H. (2007) Physiol. Rev. 87, 245–313 [DOI] [PubMed] [Google Scholar]
- 30.Bibert S., Roy S., Schaer D., Horisberger J. D., Geering K. (2008) J. Biol. Chem. 283, 476–486 [DOI] [PubMed] [Google Scholar]
- 31.Nakao M., Gadsby D. C. (1989) J. Gen. Physiol. 94, 539–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihailidou A. S., Buhagiar K. A., Rasmussen H. H. (1998) Am. J. Physiol. 274, C175–C181 [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann C., Leitz M. R., Oberdorf-Maass S., Lohse M. J., Klotz K. N. (2004) Naunyn Schmiedebergs Arch. Pharmacol. 369, 151–159 [DOI] [PubMed] [Google Scholar]
- 34.Cheng X., Ji Z., Tsalkova T., Mei F. (2008) Acta Biochim. Biophys. Sin. 40, 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oestreich E. A., Wang H., Malik S., Kaproth-Joslin K. A., Blaxall B. C., Kelley G. G., Dirksen R. T., Smrcka A. V. (2007) J. Biol. Chem. 282, 5488–5495 [DOI] [PubMed] [Google Scholar]
- 36.Winterbourn C. C. (2008) Nat. Chem. Biol. 4, 278–286 [DOI] [PubMed] [Google Scholar]
- 37.Feschenko M. S., Stevenson E., Sweadner K. J. (2000) J. Biol. Chem. 275, 34693–34700 [DOI] [PubMed] [Google Scholar]
- 38.Pieske B., Houser S. R. (2003) Cardiovasc. Res. 57, 874–886 [DOI] [PubMed] [Google Scholar]
- 39.Pieske B., Houser S. R., Hasenfuss G., Bers D. M. (2003) Cardiovasc. Res. 57, 871–872 [DOI] [PubMed] [Google Scholar]
- 40.Murdoch C. E., Grieve D. J., Cave A. C., Looi Y. H., Shah A. M. (2006) Curr. Opin. Pharmacol. 6, 148–153 [DOI] [PubMed] [Google Scholar]
- 41.Klein L., O'Connor C. M., Gattis W. A., Zampino M., de Luca L., Vitarelli A., Fedele F., Gheorghiade M. (2003) Am. J. Cardiol. 91, 18F–40F [DOI] [PubMed] [Google Scholar]
- 42.The Zamoterol in Severe Heart Failure Group (1990) Lancet 336, 1–6 [PubMed] [Google Scholar]
- 43.Packer M., Carver J. R., Rodeheffer R. J., Ivanhoe R. J., DiBianco R., Zeldis S. M., Hendrix G. H., Bommer W. J., Elkayam U., Kukin M. L. (1991) N. Engl. J. Med. 325, 1468–1475 [DOI] [PubMed] [Google Scholar]