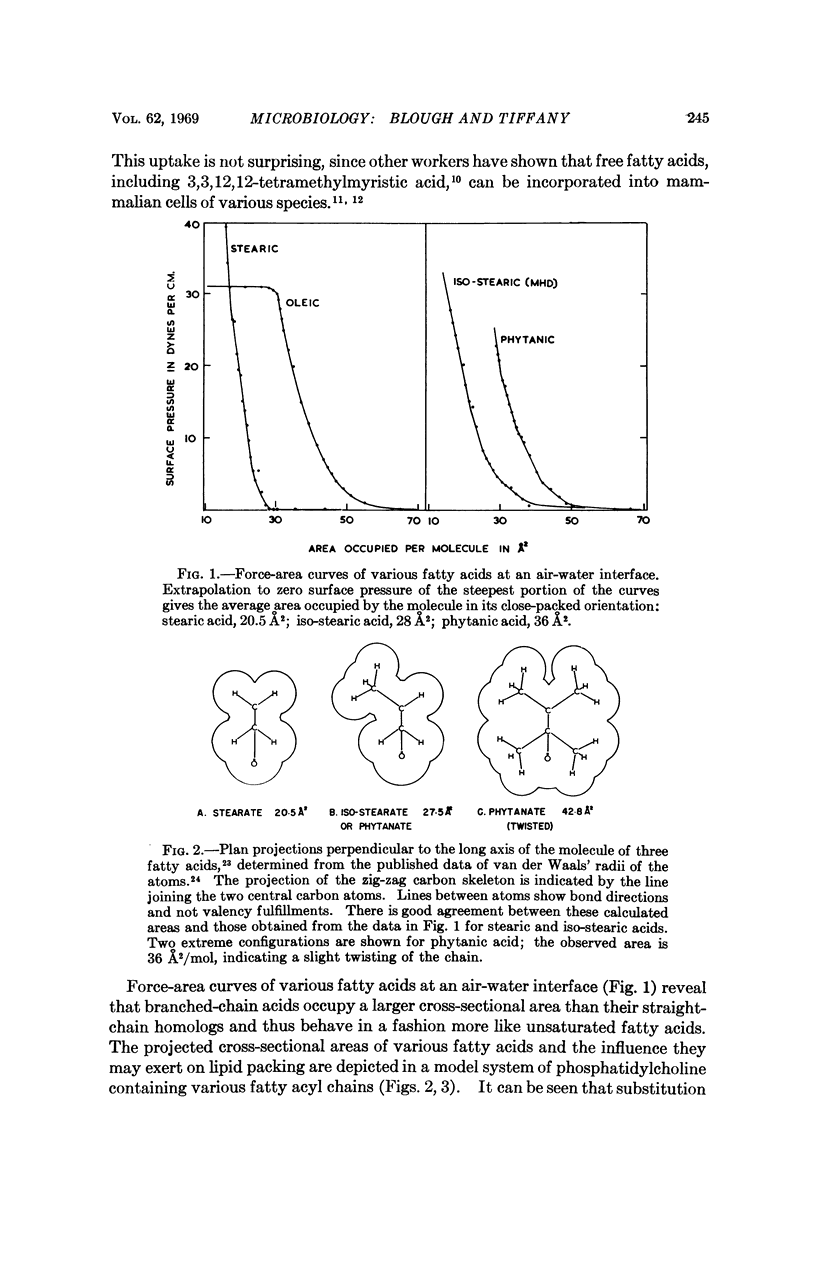
Abstract
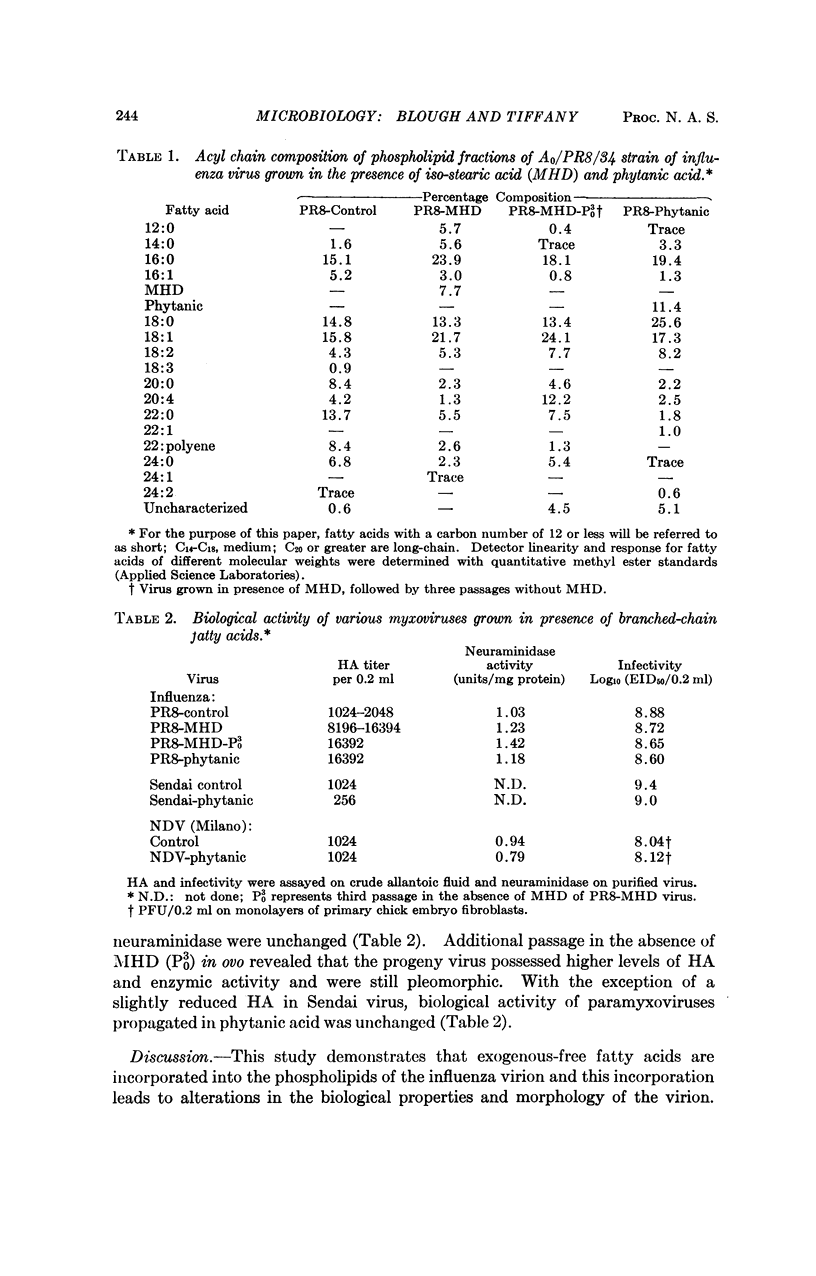
The incorporation of free fatty acids into myxoviruses was shown, using branched-chain fatty acids as molecular markers. The presence of isostearic or phytanic acid was detected by gas-liquid chromatography in the phospholipid fraction of the A0/PR8/34 strain of influenza virus. Uptake of free fatty acids into the virus varied from 8 to 11 per cent and was accompanied by a shift in the fatty acid profile. Infected allantoic fluids from eggs treated with branched-chain acids possessed higher hemagglutinin activity when compared to fluids infected under normal conditions. Attempts to detect branched-chain acids in Sendai virus were unsuccessful. Shifts in acyl chain composition persisted after three passages of modified viruses in the absence of branched-chain acids. Force-area curves at an air-water interface revealed the cross-sectional area of branched-chain acids to be greater than their straight-chain homologs. It is suggested that hydrophobic interactions can alter the configuration of envelope proteins. Such changes may have an important role in the selection of fragments of influenza viral genome and can conceivably alter viral genotype.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson A. A. On the orientation of lipids in chloroplast and cell membranes. J Am Oil Chem Soc. 1966 May;43(5):265–270. doi: 10.1007/BF02609671. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Lawson D. E. The lipids of paramyxoviruses: a comparative study of Sendai and Newcastle disease viruses. Virology. 1968 Oct;36(2):286–292. doi: 10.1016/0042-6822(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Blough H. A. Selective inactivation of biological activity of myxoviruses by glutaraldehyde. J Bacteriol. 1966 Jul;92(1):266–268. doi: 10.1128/jb.92.1.266-268.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough H. A., Weinstein D. B., Lawson D. E., Kodicek E. The effect of vitamin A on myxoviruses. II. Alterations in the lipids of influenza virus. Virology. 1967 Nov;33(3):459–466. doi: 10.1016/0042-6822(67)90121-3. [DOI] [PubMed] [Google Scholar]
- Deusberg P. H., Robinson W. S. On the structure and replication of influenza virus. J Mol Biol. 1967 May 14;25(3):383–405. doi: 10.1016/0022-2836(67)90193-3. [DOI] [PubMed] [Google Scholar]
- ELSBACH P. COMPARISON OF UPTAKE OF PALMITIC, STEARIC, OLEIC AND LINOLEIC ACID BY POLYMORPHONUCLEAR LEUKOCYTES. Biochim Biophys Acta. 1964 Feb 24;84:8–17. doi: 10.1016/0926-6542(64)90095-2. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., TISDALE H. D., CRIDDLE R. S., BOCK R. M. The structural protein and mitochondrial organization. Biochem Biophys Res Commun. 1961 May 15;5:81–84. doi: 10.1016/0006-291x(61)90085-7. [DOI] [PubMed] [Google Scholar]
- Guzzo A. V. The influence of amino-acid sequence on protein structure. Biophys J. 1965 Nov;5(6):809–822. doi: 10.1016/S0006-3495(65)86753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G. K. Genetic recombination with Newcastle disease virus, polioviruses, and influenza. Cold Spring Harb Symp Quant Biol. 1962;27:303–309. doi: 10.1101/sqb.1962.027.001.028. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Laurell S. Separation and characterization of phytanic acid-containing plasmatriglycerides from a patient with Refsum's disease. Biochim Biophys Acta. 1968 Jan 10;152(1):75–79. doi: 10.1016/0005-2760(68)90009-x. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., MOORE D. H. Structure and development of viruses observed in the electron microscope. III. Influenza virus. J Exp Med. 1956 Aug 1;104(2):171–182. doi: 10.1084/jem.104.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. Studies on influenza virus ribonucleic acid. Virology. 1967 Mar;31(3):523–531. doi: 10.1016/0042-6822(67)90234-6. [DOI] [PubMed] [Google Scholar]
- SPECTOR A. A., STEINBERG D., TANAKA A. UPTAKE OF FREE FATTY ACIDS BY EHRLICH ASCITES TUMOR CELLS. J Biol Chem. 1965 Mar;240:1032–1041. [PubMed] [Google Scholar]


