Abstract
The generation cycle of an established line of rat hepatoma cells (HTC cells) was studied using synchronized cell techniques. Dexamethasone phosphate (Dex), a synthetic adrenal corticosteriod which induces tyrosine aminotransferase (TAT) in HTC cells randomly distributed in the cell generation cycle, did not affect the durations of the various phases of the cycle. The activities of TAT and several dehydrogenases, and the rates of general protein and RNA synthesis, were studied throughout the cycle.
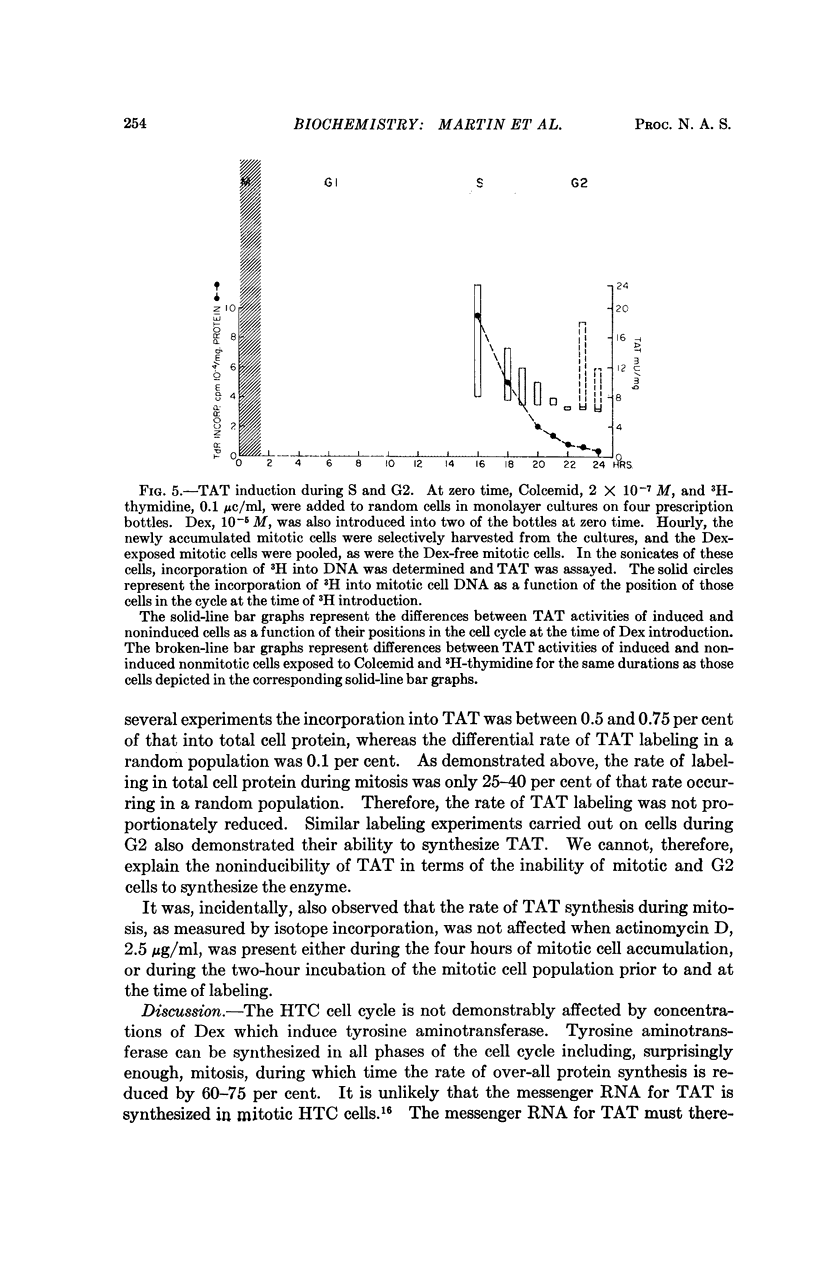
Dex, at 10-5 M, when added to a synchronized cell population in the latter two thirds of G1 phase or anywhere in the S phase, induces TAT. During the period made up of G2, M, and early G1, Dex does not induce TAT. However, radioisotope incorporation into specifically immunoprecipitated TAT continues during mitosis, when general protein and RNA synthesis are decreased.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Granner D. K., Hayashi S., Thompson E. B., Tomkins G. M. Stimulation of tyrosine aminotransferase synthesis by dexamethasone phosphate in cell culture. J Mol Biol. 1968 Jul 28;35(2):291–301. doi: 10.1016/s0022-2836(68)80025-7. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R., Ruddle F. H. Cyclic changes in enzyme activity in synchronized mammalian cell cultures. Science. 1968 Feb 9;159(3815):634–636. doi: 10.1126/science.159.3815.634. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., SANDERS P., PETERSEN D. LIFE CYCLE ANALYSIS OF MAMMALIAN CELLS. II. CELLS FROM THE CHINESE HAMSTER OVARY GROWN IN SUSPENSION CULTURE. Biophys J. 1964 Nov;4:441–450. doi: 10.1016/s0006-3495(64)86794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., STEFFEN J. LIFE CYCLE ANALYSIS OF MAMMALIAN CELLS. I. A METHOD FOR LOCALIZING METABOLIC EVENTS WITHIN THE LIFE CYCLE, AND ITS APPLICATION TO THE ACTION OF COLCEMIDE AND SUBLETHAL DOSES OF X-IRRADIATION. Biophys J. 1963 Sep;3:379–397. doi: 10.1016/s0006-3495(63)86828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S. E. RNA synthesis in synchronously growing populations of HeLa S3 cells. II. Rate of synthesis of individual RNA fractions. J Cell Physiol. 1968 Feb;71(1):95–104. doi: 10.1002/jcp.1040710111. [DOI] [PubMed] [Google Scholar]
- Showacre J. L., Cooper W. G., Prescott D. M. Nucleolar and nuclear RNA synthesis during the cell life cycle in monkey and pig kidney cells in vitro. J Cell Biol. 1967 May;33(2):273–279. doi: 10.1083/jcb.33.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERASIMA T., TOLMACH L. J. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp Cell Res. 1963 Apr;30:344–362. doi: 10.1016/0014-4827(63)90306-9. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Thompson E. B., Hayashi S., Gelehrter T., Granner D., Peterkofsky B. Tyrosine transaminase induction in mammalian cells in tissue culture. Cold Spring Harb Symp Quant Biol. 1966;31:349–360. doi: 10.1101/sqb.1966.031.01.045. [DOI] [PubMed] [Google Scholar]