Abstract
Attentional control involves the ability to allocate preparatory attention to improve subsequent stimulus processing and response selection. There is behavioral evidence to support the hypothesis that increased expectancy of stimulus and response conflict may decrease the subsequent experience of conflict during task performance. We used a cued Flanker and event-related fMRI design to separate processes involved in preparation from those involved in resolving conflict, and to identify the brain systems involved in these processes as well as the association between preparatory activity levels and activity related to subsequent conflict processing. Our results demonstrate that preparatory attentional allocation following a cue to the upcoming level of conflict is mediated by a network involving Dorsolateral Prefrontal Cortex (DLPFC) and the Intraparietal Sulcus (IPS). Informed preparation for conflict processing was associated with decreased Anterior Cingulate Cortex/preSupplementary Motor Area (ACC/preSMA) and IPS activity during the flanker target presentation, supporting their roles in conflict processing and visuospatial attention during the flanker task. Ventrolateral Prefrontal Cortex/Orbitofrontal Cortex (VLPFC/OFC) was active when specific strategic task rule and outcome information was available.
Introduction
Understanding attentional control is fundamental to understanding how the brain produces unified, voluntary behavior. Attentional control is the goal-driven allocation of attention toward the processing of task-appropriate stimuli and responses, and away from distracting stimuli and thoughts. Attentional control also involves the ability to monitor performance in relation to task demands, and to adjust the allocation of attention when necessary. These processes are mediated by a large-scale network involving frontal and parietal systems that interact with sensory and motor areas (e.g. Mesulam 1981; Posner and Petersen 1990; Posner & Raichle, 1994; Knight et al., 1999; Banich et al, 2000; Miller and Cohen, 2001). This network is particularly engaged when we experience competition, or “conflict”, between the processing of task-relevant information and items that distract us, such as on the Flanker task, in which subjects must identify the direction of the center arrow in incongruent (> > < > >) or congruent (< < < < <) stimulus arrays (Eriksen and Eriksen, 1974). Botvinick et al (2001) have proposed that Anterior Cingulate Cortex at the border of the pre-Supplementary Motor Area (ACC/preSMA) is generally responsible for monitoring performance in order to detect cognitive and behavioral conditions with potential negative outcomes, and signaling this state to other regions in the attentional control network, such as Dorsolateral Prefrontal Cortex (DLPFC), which then increase attention or change behavior (Cohen et al., 2000; Kerns et al., 2004; Botvinick et al. 1999; Carter et al., 1998; Carter et al., 2000; Durston et al., 2003, Milham et al., 2003). Kerns et al. (2004) have demonstrated that on trials in which conflict-related ACC/preSMA activity is higher, DLPFC activity on the next trial is higher, supporting the hypothesis that detection of increased conflict by ACC leads to increased allocation of attention, mediated by DLPFC. From these models, it then follows that an increase in the allocation of attention in preparation for conflicting stimuli should reduce the experience of conflict when those stimuli occur, thereby reducing activity in conflict processing regions.
There is behavioral evidence to support the hypothesis that increased expectancy of conflict may decrease the subsequent experience of conflict during task performance (Gratton et al., 1992). Evidence from fMRI research has shown that when the probability of incongruent (conflicting) stimuli is high in a block of trials of a Flanker task, DLPFC activity is higher, ACC/preSMA activity is lower, and response times are faster, than when the probability of incongruent stimuli in the block is low (Casey et al., 2000). Similarly, other studies have shown that when an incongruent trial is preceded by an incongruent trial, DLPFC activity is higher and ACC/preSMA activity is lower than when an incongruent trial is preceded by a congruent trial (Botvinick et al., 1999; Kerns et al., 2004, Durston et al., 2003; but see Mayr et al., 2003). Thus, it appears that DLPFC activity increases when the expectation of conflict is high but the experience of conflict is low, whereas ACC activity increases when expectation of conflict is low and experience of conflict is high. These results lead to the hypothesis that different levels of expectation invoke changes in the allocation of attention, which are mediated by DLPFC, in preparation for the expected conflict level, and that it is this preparatory allocation of attention that is associated with the subsequent alteration in conflict processing, mediated by ACC/preSMA. In these previous designs, the differences in preparatory allocation of attention as a result of expectancy are inferred from differences in activity during Flanker conflict processing. In order to directly determine how expectation of conflict engages preparatory attention activity, and whether this activity influences subsequent conflict processing, we used a cued Flanker task to separate these processes. In our modified version of the Flanker task, subjects were either cued to the level of conflict prior to every Flanker trial (high or low), or received a neutral cue that did not provide advance information about conflict in the subsequent trial We specifically tested the hypothesis that expectation for conflict leads to increased preparatory allocation of attention, mediated by DLPFC, and that increased preparatory attention results in subsequent decreases in the level of experienced conflict during incongruent target processing, mediated by ACC/preSMA.
We further hypothesized that parietal regions, in addition to DLPFC and ACC/preSMA, would play a role in preparatory attention and conflict processing, particularly under the visuospatial demands of the Flanker task. Activity in parietal cortex and modulations of downstream sensory representations have been widely implicated in preparatory attention, especially for visuospatial tasks (Kastner et al., 1999; Luks and Simpson, 2004; Yantis et al., 2002; Hopfinger et al., 2000; Corbetta et al., 2000; Cabeza and Nyberg, 2000), and inferior parietal activity has been reported after response conflict (van Veen et al., 2001; Carter et al., 2000; Bunge et al., 2002). If parietal cortex is involved in the deployment of attention and visuospatial processing that is necessary to correctly perform the Flanker task, it should display increased activity during cued preparatory deployment of attention, and then decreased activity during conflict-related processing of the Flanker targets.
Materials and Methods
Subjects
Eleven subjects (8 male, 3 female) completed this study. One additional subject was unable to complete the study due to discomfort in the MR scanner. These subjects were 25–40 years old, right-handed, with graduate or post-graduate education levels. All had normal or corrected-to-normal vision, and had no reported history of head trauma, psychiatric or neurological disorders. All subjects gave written informed consent and were screened for MRI eligibility in accordance with guidelines established by the UCSF Committee on Human Research.
Task Design
This study used an event-related design with two experimental conditions segregated into separate MR acquisition runs within the same MR session: an informatively cued condition and a neutrally cued condition (Figure 1). In the informatively-cued condition, each trial consisted of a 500 msec cue, followed 2–5 seconds later by a 150 msec target. This interstimulus interval duration was randomly jittered across trials in .5 second increments (weighted to include twice as many 2–3 second increments as 3.5–5 second increments). The cue consisted of the word Easy or Hard (for low and high conflict trials, respectively, 100% valid) in 24 point font. The target consisted of 5 arrows, with the 4 flanker arrows in either the same direction as the center arrow (> > > > >) (low conflict) or in the opposite direction (< < > < <) (high conflict). After the target offset, the screen remained blank during a 5.5–7.5 second pause before the next trial began (again, randomly jittered in .5 second increments). Subjects were instructed to identify the direction of the center arrow, and make a two-finger forced-choice response. Low and high conflict trials were randomly intermixed. In the neutrally-cued condition, the cue consisted of the word “prep” in 24 point for both low conflict and high conflict trials. Target presentation was the same as for informatively-cued trials. Subjects completed five runs of neutrally-cued trials and five runs of informatively-cued trials (30 trials per run), presented in a pseudorandom order.
Figure 1. Experimental Design of the Cued Flanker Task.
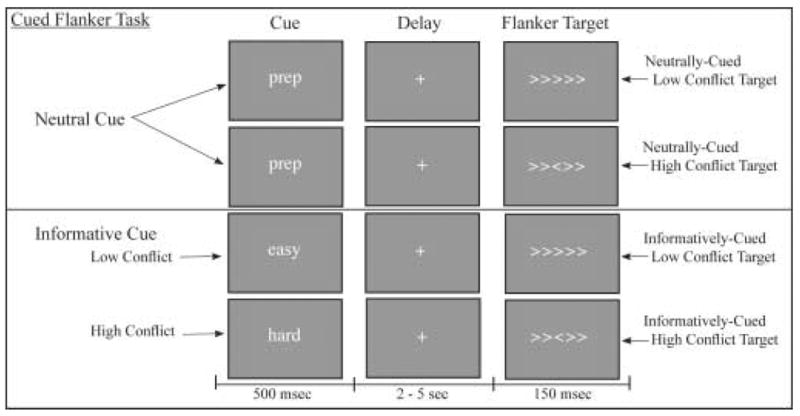
In the informatively-cued condition, the cue consisted of the word “easy” or “hard” presented for 250 msec, followed 2.5–5 seconds later by a 150 msec target. In the neutrally-cued condition, the cue always consisted of the word “prep”. The target consisted of 5 arrows, with the 4 flanker arrows in either the same direction as the center arrow (low conflict) or the opposite direction (high conflict). Subjects were instructed to identify the direction of the center arrow and make a forced-choice response. The neutral cue and informative cue conditions were presented in separate blocks of event-related trials. The inter-trial interval was 5.5 – 7.5 seconds.
Functional MRI
MR methods
Imaging was performed on a 1.5T General Electric Signa LX 8.3 scanner (Milwaukee, WI). Anatomical imaging consisted of a high-resolution T1-weighted rf-spoiled GRASS sequence (SPGR). Functional imaging consisted of blood-oxygen-level-dependent (BOLD) sensitive images acquired during performance of the experimental task, using a gradient-recalled echo-planar (EPI) sequence (TR=3 s; TE=50 ms; flip angle=60, matrix = 128 × 128, FOV = 26 × 26 cm, 19 slices, 5mm thickness, 1 mm gap).
Stimulus presentation and experiment control
Visual stimuli were generated with a Macintosh computer using PsyScope 1.2.5 software. Subjects used a mirror attached to the head coil to view stimuli back-projected onto a screen with an LCD projector. The experimental control software generated a data file containing recorded event times for all stimulus presentation events, subject response events, and scanner TTL synchronization pulses indicating EPI slice acquisition events.
Data Analysis
Behavioral accuracy and response time measures were collected for each subject during the fMRI sessions. To test for effects of cue and target conditions on performance, mean response times (RTs) for correct trials were submitted to a between-subjects repeated measures ANOVA with 2 factors: cue condition (neutral, informative) and target condition (low conflict, high conflict).
Image analysis was performed on a Macintosh computer using MATLAB (Mathworks Inc.) and SPM2 software (www.fil.ion.ucl.ac.uk/spm). Prior to analysis, the functional images were converted to 3-D Analyze format volumes. The first four volumes in each run were discarded. Images were realigned to the 5th volume within each run to correct for motion artifacts using a 6-parameter rigid body affine transformation, and corrected for differences in slice acqusition timing. The resulting images were normalized to a standard stereotaxic space (Montreal Neurological Institute (MNI) Template) using a 12 parameter affine/non-linear transformation and spatially smoothed with a 8mm full-width half maximum isotropic Gaussian kernel. Image intensity was scaled to the mean global intensity of each timeseries. Data were submitted to an event-related General Linear Model analysis, fitting a reference hemodynamic response function (hrf) to an impulse response function (duration = 0 ms) for each event in the observed time-series data. The model included following cue conditions: Informative Easy cue, Informative Hard cue, Neutral cue, and the following target conditions: Informatively-cued low conflict targets, Informatively-cued high conflict targets, Neutrally-cued low conflict targets, and Neutrally-cued high conflict targets. Cues and Targets from incorrect trials were modeled separately. Contrasts of interest were performed on individual subject data. Second-level one-sample t-tests were performed on the combined individual results to create random-effect group analyses for each contrast (n=11). For each contrast at the group level, statistical parametric brain maps were generated that displayed the t-value (in signal intensity) of each voxel that met a threshold of p<.001 uncorrected for multiple comparisons. These images were overlaid onto SPM2’s single subject canonical T1 image in MNI space. We also calculated voxels that met a threshold of p<.05 corrected for multiple comparisons in four ROIs (ACC, Left DLPFC, Right DLPFC, Left IPS) using SPM2’s small volume correction (SVC) procedure. These ROIs were defined a priori as 30-mm-radius spheres centered at the locations of peak ACC and DLPFC activity reported by MacDonald et al. (2000) (4,0,47 and +/− 41,17,31 MNI space) and left IPS activity reported by Bunge et al. (2002) (−34,−60,34 Talairach space). Stereotaxic coordinates reported here were converted to approximate Talairach space from MNI coordinate space (www.mrccbu.cam.ac.uk/Imaging/mnispace.html).
Results
Behavioral Results
Accuracy in this task was near ceiling in all conditions. Mean percent correct (and standard errors) for neutrally-cued low conflict trials were 99% (1%), and for neutrally-cued high conflict trials were 92% (2%). Mean percent correct (and standard errors) for informatively-cued low conflict trials were 99% (1%) and for informatively-cued high conflict trials were 94% (2%). There were no significant main effects or interactions of cue or target conflict conditions. Mean response times (and standard errors) for neutrally-cued low conflict trials were 614 msec (68 msec), and for neutrally-cued high conflict trials were 871 msec (77 msec). Mean response times (and standard errors) for informatively-cued low conflict trials were 600 msec (28 msec) and for informatively-cued high conflict trials were 835 msec (70 msec). Subject response times were significantly faster for low conflict trials than high conflict trials (F(1,10) = 19.355, p<.001), and showed a trend towards faster times for informatively-cued trials relative to neutrally-cued trials (F(1,10) = 3.935, p = .075). There was no significant interaction between cue and target conflict conditions.
fMRI Activation During Cue Period
We first asked which brain regions showed greater activity in the informative cue condition during preparation for high conflict trials versus preparation for low conflict trials (i.e. Hard cues versus Easy cues). There were no significant differences in any frontal or parietal areas. We did observe significantly greater bilateral activity in lateral occipital cortex, in response to Hard cues relative to Easy cues (Table 1, Figure 2). Because Hard and Easy cues showed the same general pattern of activity in attentional control regions, we then compared these two informative conditions together relative to the neutral cue condition to investigate the regional activity during preparatory attention (i.e. informative cues vs. neutral cues).
Table 1.
Cue Period Activity
| Contrast | Area | Coordinates(x,y,z) | t value |
|---|---|---|---|
| Informative Hard Cues > | R Occipital | (36, −81, 8) | 5.02* |
| Informative Easy Cues | L Occipital | (−32, −83, 8)
(−32, −86, 4) |
4.50*
4.22* |
| Informative Cues (Hard + Easy) > | L DLPFC | (−46, 25, 36) | 3.70** |
| Neutral Cues | L IPS | (−22, −73, 22) | 4.06** |
| R VLPFC/OFC | (43, 12, −2)
(30, 36, −14) |
4.04*
3.59* |
|
| L VLPFC/OFC | (−34, 29, −1)
(−28, 26, −15) |
4.67*
4.55* |
p < .05 with a priori small volume correction for multiple comparisons.
p<.001 uncorrected for multiple comparisons. Given coordinates are local maxima > 8.0mm apart within the specified region. R = right, L = left, DLPFC = Dorsolateral Prefrontal Cortex, IPS = Intraparietal Sulcus, VLPFC/OFC = Ventrolateral Prefrontal Cortex/Orbitofrontal Cortex.
Figure 2. Informative Cue Period Activity in Occipital Cortex.
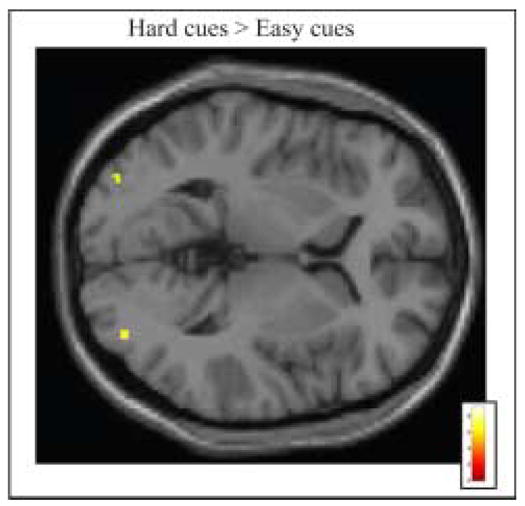
Group activation is significantly greater following Informative Easy cues than Informative Hard cues (p < .001 uncorrected for multiple comparisons, superimposed on SPM2’s canonical single subject T1 image). See tables for coordinates of active regions in this and all subsequent figures.
We observed significantly greater activity in left Dorsolateral Prefrontal Cortex (DLPFC) and in left Intraparietal Sulcus (IPS) following informative cues relative to neutral cues (Table 1). We also observed significantly greater activity in extensive regions of bilateral BA 47, which have been characterized as Ventrolateral Prefrontal Cortex (VLPFC) and as lateral OrbitoFrontal Cortex (OFC), extending into Anterior Insula (Table 1, Figure 3). There was no significant difference in Anterior Cingulate Cortex/pre-Supplementary Motor Area (ACC/pre-SMA) activity for informative versus neutral cues. To determine whether these results arose because ACC/pre-SMA was active but equivalent in the three cue conditions, we also compared each cue condition to the average baseline. No significant activation was observed in ACC/pre-SMA for any cue condition. However, relative to baseline, all three cue conditions activated a region of cingulate cortex posterior to the ACC/pre-SMA area typically activated in attentional control tasks. This area may be equivalent to Picard and Strick’s (2001) caudal cingulate zone, inferior to SMA (Table 2, Figure 4).
Figure 3. Cue Period Activity in Attentional Control Regions.
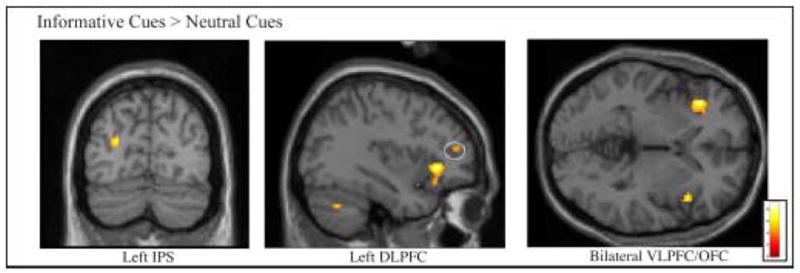
Group activation is significantly greater following Informative cues than Neutral cues in left Intraparietal Sulcus (IPS), left Dorsolateral Prefrontal Cortex (DLPFC), and bilateral Ventrolateral Prefrontal Cortex/Orbitalfrontal Cortex (VLPFC/OFC) (p<.001 uncorrected for multiple comparisons, superimposed on SPM2’s canonical single subject T1 image).
Table 2.
Cue Period Caudal Cingulate Zone (CCZ) Activity
| Contrast | Area | Coordinates(x,y,z) | t value |
|---|---|---|---|
| Hard Cue > Baseline | L CCZ | (−4, −11, 56) | 4.36* |
| Easy Cue > Baseline | L CCZ | (−2, −21, 40) | 7.14* |
| Neutral Cue > Baseline | L CCZ | (−4, −14, 30) | 8.39* |
p<.001 uncorrected for multiple comparisons. Given coordinates are local maxima > 8.0mm apart within the specified region.
Figure 4. Cue Period Activity in Caudal Cingulate Zone.
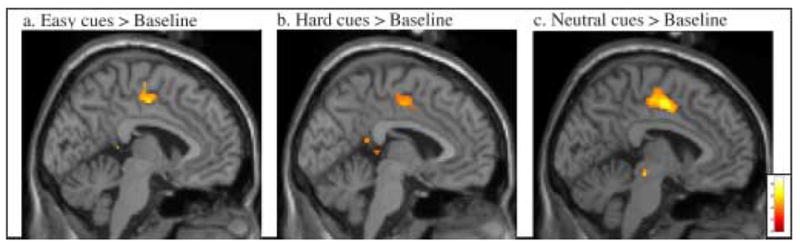
Group activation is significantly greater following a) Informative Easy, b) Informative Hard, and c) Neutral cues, relative to baseline (p < .001 uncorrected for multiple comparisons, superimposed on SPM2’s canonical single subject T1 image).
fMRI Activation during Target Period
Neutrally-Cued targets
In the target period of the flanker task, “conflict-related” processes are defined by significant increases in activation when contrasting the high conflict target condition to the low conflict target condition. We examined conflict-related activity in the neutrally-cued target conditions (that is, the difference in activity associated with high vs. low conflict target flanker stimuli, without informed preparatory deployment of attention). The results generally replicated the conflict-related activity observed in uncued versions of the flanker task in the literature. Bilateral ACC/pre-SMA, bilateral IPS, and right DLPFC were more active following high conflict targets relative to low conflict targets in the neutrally-cued condition (Table 3). We also observed significant bilateral activation in VLPFC/OFC following high conflict targets relative to low conflict targets in the neutrally-cued condition (Table 3, Figure 5).
Table 3.
Target Period Activity
| Contrast | Area | Coordinates(x,y,z) | t value |
|---|---|---|---|
| Informatively-Cued | R ACC/preSMA | (2, 19, 32) | 5.12** |
| High Conflict Targets > | (1, 18, 45) | 2.67** | |
| Low Conflict Targets | R DLPFC | (42, 44, 18) | 6.16** |
| (44, 42, 16) | 4.27** | ||
| L DLPFC | (−38, 44, 27) | 4.28** | |
| R IPS | (36, −71,11) | 5.15* | |
| L IPS | (−12, −72, 28) | 10.23** | |
| (−22, −59, 34) | 6.24** | ||
| Neutrally-Cued | R ACC/PreSMA | (2, 2, 39) | 6.65** |
| High Conflict Targets > | (10, 19, 34) | 6.12** | |
| Low Conflict Targets | L ACC/PreSMA | (−4, 4, 37) | 4.26** |
| R DLPFC | (40, 26, 24) | 5.01** | |
| R IPS | (38, −68, 38) | 7.00* | |
| (26, −76, 26) | 5.24* | ||
| L IPS | (−43, −48, 45) | 7.23** | |
| (−24, −75, 26) | 7.22** | ||
| R VLPFC/OFC | (34, 23, −5) | 6.36* | |
| (−41, 17, −6) | 5.34* |
p < .05 with a priori small volume correction for multiple comparisons.
p<.001 uncorrected for multiple comparisons. Given coordinates are local maxima > 8.0mm apart within the specified region. ACC/preSMA = Anterior Cingulate Cortex/preSupplementary Motor Area, other abbreviations as for Table 1.
Figure 5. Target Period Activity in Attentional Control Regions.
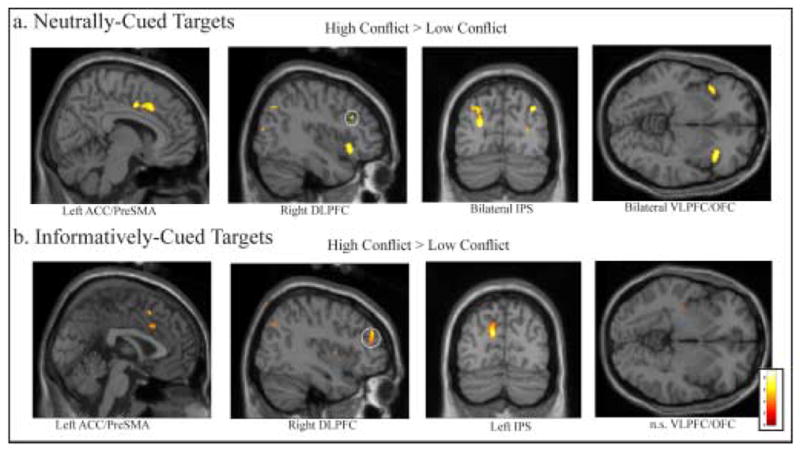
For a) Neutrally-cued targets, group activation is significantly greater for High Conflict targets than Low Conflict targets in left Anterior Cingulate Cortex/Pre-Supplementary Motor Area (ACC/PreSMA), right DLPFC, bilateral IPS, and bilateral VLPFC/OFC (abbreviations as for Figure 3). For b) Informatively-cued targets, group activation is significantly greater for High Conflict targets than Low Conflict targets in left ACC/Pre-SMA, right DLPFC, and left IPS (p < .001 uncorrected for multiple comparisons, superimposed on SPM2’s canonical single subject T1 image).
Informatively-Cued targets
Conflict-related activity in the informatively-cued target condition (i.e. activity to high conflict targets following Hard cues vs. activity to low conflict targets following Easy cues), showed activity in bilateral ACC/pre-SMA, right DLPFC, and left IPS. There was no significant conflict-related target activity in VLPFC/OFC following informative cues. Thus, the processing of conflict in the Flanker task significantly activated a common network of brain regions in both the informatively and neutrally-cued conditions, excepting VLPFC/OFC (Table 3, Figure 5).
To examine the interactive effects of preparation and conflict-related activity in this network, we compared conflict-related activity following informative vs. neutral preparation. We measured the interaction between the informatively-cued and neutrally-cued conditions and the high and low conflict conditions with the following contrasts; [(neutrally-cued high conflict targets – neutrally-cued low conflict targets) – (informatively-cued high conflict targets – informatively-cued low conflict targets)], and [(informatively-cued high conflict targets – informatively-cued low conflict targets) – (neutrally-cued high conflict targets – neutrally-cued low conflict targets)]. These contrasts revealed that the conflict-related target activity following informative cues, relative to neutral cues, was significantly reduced in ACC/pre-SMA and left IPS, indicating that preparation reduced the degree of conflict-related activity in these regions condition (Table 4). Conflict-related activity in right DLPFC was not significantly different in the informatively-cued condition versus the neutrally-cued. Additionally, Conflict-related activity was significantly greater in the informatively-cued condition versus the neutrally-cued condition in one region of right superior prefrontal cortex (Talairach coordinates: 32,26,47; t=4.45). Thus, conflict-related ACC/pre-SMA, IPS and VLPFC/OFC activity during presentation of flanker stimuli was reduced when the level of conflict was known and prepared for in advance, whereas the conflict-related activity in right DLPFC and right superior PFC during presentation of flanker stimuli did not show this pattern.
Table 4.
Effects of Preparation on Target Conflict Processing
Discussion
Can preparatory allocation of attention influence subsequent conflict processing? We investigated the brain systems that control these processes and the relationship between them by separating preparatory allocation of attention from conflict processing with a cued flanker design. Our results demonstrate that attentional allocation following a cue to conflict level is mediated by a network involving DLPFC and IPS. This informed preparation for conflict processing was associated with decreased ACC/preSMA and IPS activity during the flanker target presentation, supporting their roles in conflict processing and visuospatial attention during the flanker task. VLPFC/OFC was active when specific strategic task rule and outcome information was available.
Cue Period – Preparatory Allocation of Attention
Our cueing design enabled us to differentiate preparatory allocation of attention for specific levels of conflict from a neutral preparation state. Our results suggest a model of preparatory attention in which control regions are engaged when specific processing strategies can be executed, and the target regions of these control processes subsequently implement those processing strategies, exhibiting activity that differs according to the processing required. When preparatory cues were informative, rather than neutral, we observed greater preparatory activity in left DLPFC and left IPS regions. Consistent with the idea that preparatory allocation of attention occurs whenever the upcoming task demands are known (whether high or low), the activity in these control regions did not differ in response to cues to prepare for high versus low conflict Flanker conditions, suggesting that subjects selected and deployed a preparatory strategy to maximize performance for each conflict level. In other words, there can be equivalent amplitudes of DLPFC and IPS control activity for orchestrating sensory activity in preparation for two different task conditions, while the pattern (and amplitude) of activity in those controlled sensory areas will differ by condition. In this study, activity in occipital regions, likely to be the sensory targets of DLPFC and IPS control, was significantly different during preparation for high versus low conflict. This is in keeping with recent evidence for preparatory modulations of stimulus representations in visual cortex (e.g. Kastner et al., 1999; Desimone and Duncan, 1995; Luks and Simpson, 2004; Driver and Frackowiak, 2001; Hopfinger et al., 2000). Increased activity in left DLPFC is consistent with previous evidence for involvement of this area in maintaining task-relevant information and deploying top-down attention to modulate incoming sensory information accordingly (Banich et al., 2000; Milham et al., 2003, Cohen et al., 2000). IPS has been implicated in the deployment and sustaining of visuospatial attention (e.g. Corbetta and Shulman, 2002; Yantis et al., 2002; Beauchamp et al., 2001; Vandenberghe et al., 2001). Our finding of increased preparatory activation in left IPS for both Hard and Easy cue conditions suggests that IPS is involved in strategic adjustments of spatial attention in preparation for the two target Flanker conditions (perhaps broadening spatial attention for low conflict targets and narrowing spatial attention for high conflict targets). Other studies have reported greater DLPFC activation during preparation for a high conflict than low conflict task (MacDonald et al., 2000; Weissman et al., 2004). However, in those studies, subjects were cued to different task rules, with different difficulty levels, whereas in our study, the task rules remained the same, but the stimulus discrimination difficulty varied. Different task rules may require different degrees of DLPFC involvement. When our results are considered together with the literature, they suggest that the amount of preparatory activity in these frontal and parietal attentional control regions may not depend as much on the difficulty of the upcoming task as on the specificity with which task demands are known, and with which an attentional strategy can be deployed in advance of stimulus presentation to facilitate stimulus processing and response selection.
VLPFC/OFC Cue Period Activity
The most robust difference in activity that we observed between cue conditions was in a bilateral region of Brodmann’s Area 47, which has been labeled in the literature as the inferior end of VLPFC or the posterior end of OFC, with activity extending medially into adjacent anterior insula. Both the VLPFC literature and OFC literature ascribe task-rule mapping to this region of BA 47. VLPFC has been associated with rule learning and rule retrieval, including both simple stimulus-response-reward patterns, and more complex cue-task rule associations. This region has been activated in neuroimaging studies by rule-learning paradigms, task-shifting paradigms, and holding stimulus-response rules in working memory (e.g. Bunge, 2004; Hampshire and Owen, 2006; Crone et al., 2006; Brass and von Cramon, 2002). Lateral posterior OFC and adjacent anterior insula have been implicated in mapping the contingency relationship between outcomes (such as rewards, or emotions) and stimulus-response associations, and particularly in mapping changes in this relationship (Elliot et al., 2000; Happaney et al., 2004). Lateral OFC activity is observed when stimulus-response-outcome associations are remapped, especially for changes to a negative reward state, which typically result in inhibition of behavior in normal subjects (Lamar et al., 2004; Elliot et al., 2000, O’Doherty et al., 2003, Bechara et al., 2000; Arana et al., 2003; Gottfried et al., 2003). OFC and anterior insula may be necessary to instruct other regions within the attentional control system of changes in stimulus-response-outcome contingencies in order to direct behavior, especially when these changes are learned by feedback, rather than explicit instruction. More medially-located OFC regions have also been associated with the top-down control of visual sensory processing (Bar et al., 2006).
In this study, VLPFC/OFC activity was greater for informative cues than for neutral cues, and did not differ for Easy versus Hard cues. One interpretation for the VLPFC/OFC activity we observed during preparation for both high and low conflict flanker trials is that it may reflect the trial-by-trial mapping of the association between the cue, the anticipated task rule/strategy for each conflict level, and the emotional outcome associated with correct and incorrect task performance. It is possible that VLPFC/OFC may be responsible for mapping these outcome contingencies to the specific preparatory attentional strategies that are deployed in response to the informative cues. Perhaps in the neutral cue condition, because subjects are unable to deploy a specific preparatory strategy and anticipate a specific outcome, they do not engage this VLPFC/OFC function. A related alternative explanation may be that the VLPFC/OFC is particularly involved in mapping changes in anticipated or experienced outcomes. Because the Hard and Easy cues were randomly intermixed in the informative condition, the appropriate preparatory attention strategies and anticipated outcomes frequently changed from cue to cue. However, in the neutral cue condition, information about change in outcome is not available during the cue period. Thus it may be that VLPFC/OFC is activated by cues that indicate a trial-by-trial change from high to low conflict conditions, or low to high conflict conditions. This leads to the directly testable hypothesis that VLPFC/OFC activation will be greater for cues indicating a change in conflict level from the previous trial, relative to cues indicating a repetition of conflict level, although we do not have a sufficient number of trials to test this hypothesis with the present data.
ACC/pre-SMA Cue Period Activity
We did not observe any significant differences in ACC/pre-SMA activity during the cue period when comparing Hard versus Easy cues, or informative versus neutral cues, and also observed no significant ACC/pre-SMA activity in any of the three cue conditions relative to baseline. Thus, we found no evidence that preparing for high conflict stimuli resulted in significant anticipatory ACC/pre-SMA activity, or that any particular preparatory attention process activated ACC/preSMA. This is in contrast to Weissman et al. (2004), who reported greater ACC/pre-SMA activity in preparation for a harder task. In general, the literature reveals an inconsistent picture of ACC/pre-SMA activity during preparatory attention. Some studies have found cue period ACC/pre-SMA activity that varies according to cue condition (e.g. Posner and Raichle, 1994; Luks et al., 2002; Weissman et al., 2002; Brass and von Cramon, 2002) or is constant across different cue conditions (MacDonald et al., 2000), while several other papers report no ACC/pre-SMA activity during cue periods (e.g. Hopfinger et al., 2000; Corbetta et al., 2000; Giesbrecht et al., 2003). It has been suggested that ACC/preSMA is particularly responsive to conditions of response conflict, rather than stimulus conflict (van Veen et al., 2001). It may be that ACC/preSMA is active in preparation for targets if preparation for response selection is a useful strategy for minimizing subsequent response conflict. In the Flanker task, however, preparation for stimulus selection is arguably the most useful strategy, and, as we have discussed above, DLPFC and IPS activity during the preparatory period may serve to focus attention and spatial processing of the Flanker stimuli to minimize stimulus-level conflict (which in turn could reduce the probability for subsequent response conflict during the target period). Perhaps cued tasks that involve high levels of response conflict, but little stimulus-level conflict, will be more likely to evoke preparatory ACC/preSMA. The test of this hypothesis (as proposed by Ulrich Mayr, personal communication) would be to compare preparatory ACC/preSMA activity in our cued Flanker task to preparatory ACC/preSMA activity in a cued task in which the primary conflict for which subjects can prepare is response conflict. Findings of greater preparatory activity in the latter case would be consistent with observations of preparatory ACC/preSMA activity in studies which involve more response conflict than the Flanker task or which involve preparing one of two possible response mappings during the preparatory periods, such as in cued task-switching paradigms (e.g. Luks et al., 2002; Brass and von Cramon, 2002).
Caudal Cingulate Zone Cue Period Activity
We did observe significant activation in all three cue conditions (relative to baseline) in a region of cingulate cortex that is distinct from and posterior to the dorsal ACC/pre-SMA region implicated in most attentional control tasks (Bush et al., 2000). The peak of this activation was approximately 1–3 centimeters posterior to the ACC/pre-SMA activation observed during target processing. This region may be the caudal cingulate zone, as identified by Picard and Strick (2001), which is implicated in manual behavior and premotor preparation. Similar activation was reported during the preparatory period in a study by Weissman et al. (2005). Because this area was active in all three cue conditions, with no significant differences between them, this activity may be involved in the preparation of the two possible motor responses (index and middle finger presses).
Target Period - Conflict Processing
In this study, the neutrally-cued condition reveals the unprepared state of conflict processing, and the informatively-cued condition reveals how conflict processing is adjusted by preparation. The results support our hypothesis that preparation for high conflict stimuli leads to more efficient target processing, thus reducing conflict and ACC/pre-SMA activity associated with conflict processing. The regional activity we observed in the neutrally-cued low and high conflict flanker conditions replicated previous uncued fMRI studies of flanker tasks (e.g. Botvinick et al., 1999; Casey et al., 2000). We observed significantly greater activity in bilateral ACC/pre-SMA, bilateral IPS, and right DLPFC for high conflict neutrally-cued flanker targets than for low conflict neutrally-cued flanker targets. Following informatively-cued preparation, conflict-related activity in bilateral ACC/pre-SMA and bilateral IPS was reduced, and no significant conflict-related activity was observed in VLPFC/OFC. Thus, on informatively-cued trials, increased preparatory activity in left DLPFC, left IPS and bilateral VLPFC/OFC was followed by decreased conflict-related target activity in bilateral ACC/pre-SMA, bilateral IPS and bilateral VLPFC/OFC, relative to neutrally-cued trials. Increased IPS activity during the informative cue period and subsequent decreased IPS activity during conflict-related target processing is consistent with a role for IPS regions in selective spatial attention, distractor suppression and the structuring of attended information (e.g. Corbetta and Shulman, 2002; Silver et al., 2005; Wojciulik and Kanwisher, 1999; Friedman-Hill et al., 2003; Cusack, 2005). When the targets appear, their sudden onset draws attention exogenously to the flanking stimuli, as well as to the task-relevant central stimulus. On high conflict trials, IPS activity may facilitate the visual processing of the central location and inhibit the flanking locations. On low conflict trials, IPS activity may facilitate processing at all locations. When trials are informatively-cued, this spatial attention deployment can occur during the cue period in anticipation of targets, leading to faster and more efficient processing of the stimuli when they appear, resulting in less response conflict, and thus less activity related to conflict detection and resolution, consistent with the trend in RTs. On neutrally-cued trials, the allocation of spatial attention to the central location occurs after the stimuli appear and the conflict level is detected, resulting in more response conflict, and more activity related to conflict resolution which may be achieved in part by adjusting the visual spatial focus of attention.
Conflict resolution following ACC/pre-SMA activity in Target Period
When the experienced level of conflict is high (such as in the neutrally-cued condition), how does the attentional control system resolve that conflict in order to maintain accurate performance? Botvinick et al. (2001) have proposed that ACC/preSMA monitors for response conflict during stimulus processing, and triggers DLPFC (directly or indirectly) to increase attention to the task-appropriate processes for correct response, within the same trial (Carter et al., 1998; Cohen et al., 2000, but see Mayr et al., 2003; Weissman et al., 2004; Critchley et al., 2005), and on the next trial (Kerns et al., 2004). In the present study, the level of conflict (as measured by performance and suggested by ACC/pre-SMA activity) was higher in the neutrally-cued condition than in the informatively-cued condition. Thus, areas that are involved in resolving conflict in response to ACC/pre-SMA activity should have greater activity on the neutrally-cued trials, when conflict was higher, than on the informatively-cued trials, when conflict was reduced. We found that conflict-related IPS activity was reduced when conflict was reduced, whereas DLPFC activity was not statistically different. These results suggest that in our study, IPS is more likely to be involved in the resolution of conflict than DLPFC. In the flanker task, parietal regions may be particularly involved in the readjustment of visuospatial attention to resolve this type of conflict, as described above, whereas in tasks which require different conflict resolution processes, different regions may respond to conflict detection. Alternatively, DLPFC may briefly initiate the readjustment of attention, and parietal cortex may follow with sustained (and more detectable) activity during completion of the task, consistent with the roles we have suggested for these regions during the informative cue period. The temporal resolution of fMRI is not optimal for identifying the dynamic interactions of these regions during target processing. Future electrophysiological studies (EEG and MEG) with source localization will certainly aid in addressing these questions.
VLPFC/OFC Target Period Activity
Our finding of VLPFC/OFC activity in the target period is consistent with a role for VLPFC/OFC in mapping strategic stimulus-response-outcome associations, and particularly mapping changes in these associations (Bunge, 2004; other VLPFC, Rolls et al., 2000; Elliot et al., 2000). We found greater VLPFC/OFC activity in response to high conflict flanker targets than to low conflict targets following neutral cues, but not informative cues. These results, together with the results from the cue period, suggest that when conflict-level information was available via informative cues, significant bilateral VLPFC/OFC activity was observed in the cue period, and when that information was first available during target processing (in the neutrally-cued condition), significant bilateral VLPFC/OFC activity was observed in the target period.
It is important to note that when conflict-level information is available in the cue period, the pattern of VLPFC/OFC activity is not exactly the same as the pattern when conflict-level information is available in the target period. In the target period, VLPFC/OFC activity was significantly greater for high conflict stimuli than for low conflict stimuli. In the cue period, VLPFC/OFC activity was statistically equivalent while preparing for high and low conflict trials. If VLPFC/OFC is mapping the relationship between conflict-level and outcomes alone, that mapping should be roughly equivalent whenever that conflict-level information becomes available. If VLPFC/OFC represents an association between different conflict levels, spatial attention strategies, and their associated outcomes, there will be differences in the patterns of activity in the cue period and in the target period. In the cue period, subjects have the information and time (2–5 seconds) to prepare strategically for both high and low conflict stimuli, and apparently do so. In the neutrally-cued target period, when subjects differentiate high and low conflict targets while in the process of resolving and responding to them, there may be more strategic attentional deployment in the high conflict condition than the low conflict condition, and thus a greater need to activate strategic task rule and outcome representations. The similarity of IPS and VLPFC/OFC patterns across cue and target periods further supports our hypothesis that VLPFC/OFC may be mapping the relationship between the strategic attentional deployment by IPS in response to specific stimuli and anticipated outcomes.
Acknowledgments
This research was supported by NIH grants: MH64295 (TLL), NS45171 (GVS), and NS27900 (GVS). Address correspondence to Tracy L. Luks, Department of Radiology, University of California San Francisco, San Francisco CA 94143-0946. Email: Tracy.Luks@radiology.ucsf.edu
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. Journal of Neuroscience. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z, Barad V, Gullett D, Shah C, Brown C. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Cognitive Brain Research. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassman KS, Ghurman AS, Boshyan J, Schmind AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E. Top-down facilitation of visual recognition. PNAS. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cerebral Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posnerm M. Cognitive and emotional influences in cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bunge SA. Using rules to select actions: a review of evidence from cognitive neuroscience. Cognitive, Affective and Behavioral Neuroscience. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: Who’s in control? Nature Neuroscience. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cerebral Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Cusack R. The intraparietal sulcus and perceptual organization. Journal of Cognitive Neuroscience. 2005;17:641–651. doi: 10.1162/0898929053467541. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Reviews in Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Driver J, Frackowiak RS. Neurobiological measures of human selective attention. Neuropsychologia. 2001;39:1257–1262. doi: 10.1016/s0028-3932(01)00115-4. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neuroscience. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: An event-related fMRI study. NeuroImage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, Watts R, Ulug AM, Casey BJ. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. NeuroImage. 2003;20:2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Elliot R, Friston KJ, Dolan RJ. Dissociable neural responses associated with reward, punishment and risk-taking behaviour. Journal of Neuroscience. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Friedman-Hill SR, Robertson LC, Desimone R, Ungerleider LG. Posterior parietal cortex and the filtering of distractors. Proceedings of the National Academy of Sciences. 2003;100:4263–4268. doi: 10.1073/pnas.0730772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. NeuroImage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cerebral Cortex. 2006 doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Happaney K, Zelazo PD, Stuss DT. Development of orbitofrontal function: Current themes and future directions. Brain and Cognition. 2004;55:1–10. doi: 10.1016/j.bandc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliot R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. NeuroReport. 2002;13:2453–2457. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stener VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. NeuroImage. 1999;9:269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychologica. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Lamar M, Swenson R, Kaplan E, Libon DJ. Characterizing alterations in executive functioning across distinct subtypes of cortical and subcortical dementia. Clinical Neuropsychology. 2004;18:22–31. doi: 10.1080/13854040490507127. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WJ. Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. NeuroImage. 2002;17:792–802. [PubMed] [Google Scholar]
- Luks TL, Simpson GV. Preparatory Deployment of Attention to Motion Activates Higher Order Motion Processing Brain Regions. NeuroImage. 2004;22:1515–1533. doi: 10.1016/j.neuroimage.2004.04.008. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsalateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. NeuroImage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An Integrative Theory of Prefrontal Cortex Function. Annual Reviews Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortex. Journal of Neuroscience. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Current Opinion in Neurobiology. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Raichle ME. Images of Mind. New York: Scientific American Library; 1994. [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. Journal of Neurophysiology. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gitelman DR, Parrish TB, Mesulam MM. Location- or feature-based targeting of peripheral attention. NeuroImage. 2001;14:37–47. doi: 10.1006/nimg.2001.0790. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Woldorff MG, Hazlett CJ, Mangun GR. Effects of practice on executive control investigated with fMRI. Cognitive Brain Research. 2002;15:47–60. doi: 10.1016/s0926-6410(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross-modal distraction. Journal of Neuroscience. 2004;24:10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23:747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Sereces JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nature Neuroscience. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]


