Abstract
Mycobacterium avium subsp. paratuberculosis is a pathogen which causes a debilitating chronic enteritis in ruminants. Unfortunately, the mechanisms that control M. avium subsp. paratuberculosis persistence during infection are poorly understood and the key steps for developing Johne's disease remain elusive. A proteomic analysis approach, based on one dimensional polyacrylamide gel electrophoresis (SDS-PAGE) followed by LC-MS/MS, was used to identify and characterize the cell wall associated proteins of M. avium subsp. paratuberculosis K10 and an cell surface enzymatic shaving method was used to determine the surface-exposed proteins. 309 different proteins were identified, which included 101 proteins previously annotated as hypothetical or conserved hypothetical. 38 proteins were identified as surface-exposed by trypsin treatment. To categorize and analyze these proteomic data on the proteins identified within cell wall of M. avium subsp. paratuberculosis K10, a rational bioinformatic approach was followed. The analyses of the 309 cell wall proteins provided theoretical molecular mass and pI distributions and determined that 18 proteins are shared with the cell surface-exposed proteome. In short, a comprehensive profile of the M. avium subsp. paratuberculosis K10 cell wall subproteome was created. The resulting proteomic profile might become the foundation for the design of new preventive, diagnostic and therapeutic strategies against mycobacterial diseases in general and M. avium subsp. paratuberculosis in particular.
Introduction
Mycobacterium avium subsp. paratuberculosis is a member of the M. avium complex, next to three other subspecies M. avium subsp. hominissuis, Mycobacterium avium subsp. avium and M. avium subsp. silvaticum and the species M. intracellulare. M. avium subspecies hominissuis and M. intracellulare are widely distributed in the environment and also inhabit healthy animal and human intestines, but do not usually cause disease unless the host is debilitated or immunocompromised. M. avium subsp. paratuberculosis, in contrast, is a pathogen which causes a debilitating chronic enteritis in ruminants[1] and has been implicated in Crohn's disease in humans [2]. Unfortunately, the mechanisms of virulence that control M. avium subsp. paratuberculosis persistence during infection are poorly understood and the key steps for developing paratuberculosis remain elusive. The current challenge is to identify elements that are essential for virulence and survival of the bacterium during infection, especially those that influence the immune responses against M. avium subsp. paratuberculosis.
A characteristic feature of mycobacteria is the thick, waxy cell wall, a highly impermeable outer surface, which enables mycobacteria to survive in extreme environmental conditions and the presence of antibiotics. This cell wall contains 60% lipid, which confers on it the properties of acid fastness (the ability to resist decolorization by acidified alcohol), hydrophobicity, and increased resistance to chemicals (e.g. chlorine) and physical processes (e.g. pasteurization)[3].
Bacterial surface proteins play a fundamental role in the interaction between the bacterial cell and its environment [4-6]. They are involved in adhesion to and invasion of host cells, in sensing the chemical and physical conditions of the external milieu and sending appropriate signals to the cytoplasmic compartment, in mounting defenses against host responses and in toxicity. In this study, we also aimed to identify surface-exposed proteins of M. avium subsp. paratuberculosis K10 using a proteolytic digest of the bacterial surface followed by mass spectrometry. In previous studies, this enzymatic 'shaving' technique resulted in the identification of many surface exposed proteins [7-9].
The goal of this study was to comprehensively identify all cell wall associated and cell surface exposed proteins of M. avium subsp. paratuberculosis K10 to support vaccine development and pathogenesis studies.
Materials and methods
Bacterial strain and growth conditions
M. avium subsp. paratuberculosis K10 was grown in Middlebrook 7H9 broth (Becton Dickinson, Oakville, ON, Canada) supplemented with 0.5% glycerol, 0.05% Tween 80, 2 μg/ml of mycobactin J (Allied Monitor, Fayette, MO, US), and 10% oleic acid albumin dextrose complex (OADC, Becton Dickinson) until mid-exponential growth phase. The culture was harvested by centrifugation for 10 min at 10 000 × g at 4°C and washed three times with ice-cold phosphate buffered saline (PBS) (pH7.4). The pelleted cells were frozen at -80°C until needed.
Cell wall proteins preparation
The extraction of cell wall proteins from M. avium subsp. paratuberculosis K10 was carried out according to Mandana et al. with minor modification [10]. Cells were harvested at 4400 × g and washed with NaCl solution (0.16 M). The weight of wet cells was determined and for each gram of bacteria one ml lysis buffer (0.05 M potassium phosphate, 0.022% (v/v) β-mercaptoethanol, pH 6.5) was added. Lysozyme (Roche, Mississauga, ON, Canada) was added to the cells to a final concentration of 2.4 mg/ml. The cells were then incubated at 37°C for 2 h. Subsequently, cells (maintained in screw cap Eppendorf tubes) were disrupted with a bead beater (Biospec products, USA) for 4-6 times (1.5 min each time, ice cool down at intervals). The lysates were subjected to a low speed centrifugation at 600 × g to remove unbroken cells. Centrifugation was repeated 3 to 5 times for 40 min at 22,000 × g to pellet the cell walls. All pellets were resuspended and pooled. A second cell lysis, equal to the first, was performed on the pooled pellet. A single centrifugation at 22,000 × g gave the pellet of cell wall fraction. The pellet was resuspended in PBS buffer and centrifugated at 22,000 × g, then stored frozen at -80°C.
Bacterial surface digestion
Procedure was carried out according to Guido Grandi et al[7] with some modifications. Bacteria were harvested from culture at an OD600 of 0.4 (exponential phase) by centrifugation at 3,500 × g for 10 min at 4°C, and washed three times with PBS. Cells were resuspended in one-hundredth volume of PBS containing 40% sucrose (pH 7.4). Digestions were carried out with 20 mg proteomic grade trypsin (Sigma-Aldrich, Oakville, ON, Canada) in the presence of 5 mM DTT, for 30 min at 37°C. A control experiment in parallel was carried out. Briefly, we incubated M. avium subsp. paratuberculosis K10 cells in the "trypsin shaving" incubation buffer without trypsin for 2 hours. The digestion mixtures were centrifuged at 3,500 × g for 10 min at 4°C, and the supernatants (containing the peptides) were incubated at 37°C for around 12~14 hrs for full digestion after being filtered using 0.22 μm pore-size filters (Millipore, Etobicoke, ON, Canada). Protease reactions were stopped with formic acid at 0.1% final concentration. Peptide fractions were concentrated with a Speed-vac centrifuge (Savant), and kept at -20°C until further analysis.
Sample digestion
Protein sample was separated by 12.5% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE), run for 1 h at 30 W, then for 4.5 h at 180 W. The gels were coomassie stained and the lane corresponding to the cell wall proteins was cut into 6 equal pieces. The gel pieces were individually in-gel digested as described previously with some modifications [11]. Briefly, after in-gel digestion using trypsin, the digested solution was transferred into a clean 0.6 ml tube. Fifty microliters of 50% acetonitrile (ACN)/5% formic acid (FA) was added to the gel pieces and sonicated for 30 min. This extraction procedure was repeated three times, and a total of 150 μl of extracts was collected. All extracts were pooled and concentrated to less than 10 μl using an SPD 2010 SpeedVac system (Thermo Electron, Waltham, MA). Thereafter, the sample was diluted with 0.1% FA in HPLC water to 100 μL for direct LC-MS/MS analysis or reconstituted with trifluoroacetic acid (TFA) to a final concentration of 0.1% and subjected to sample cleanup steps using C18 ZipTips (Millipore) prior to LC-MS/MS analysis. The C18 ZipTips were conditioned with 100% ACN and then equilibrated three times with 0.1% TFA. The peptides were bound to the ZipTip pipet tip by aspirating and dispensing the sample for at least 15 cycles, washed with 0.1% TFA, and eluted by 20 μL of elution buffer (75% ACN, 0.1% TFA).
Protein identification by LC-MS/MS
Digests were analyzed using an integrated Agilent 1100 LC-ion-Trap-XCT-Ultra system fitted with an Agilent ChipCube source sprayer. Injected samples were first trapped and desalted on a Zorbax 300 SB-C18 Precolumn (5 μm, 5 × 300-μm inside diameter; Agilent) for 5 min with 0.2% formic acid delivered by the auxiliary pump at 0.3 μl/min. The peptides were then reverse eluted from the trapping column and separated on an analytical Zorbax 15 cm-long 300SB-C18 HPLC-Chip 0.3 μl/min. Peptides were eluted with a 5-45% acetonitrile gradient in 0.2% formic acid over a 50 min interval. Data-dependent acquisition of collision-induced dissociation MS/MS was utilized, and parent ion scans were run over the mass range m/z 400 -2,000 at 8,100. For analysis of LC-MS/MS data, Mascot searches used the following parameters: 1.4 Da MS error, 0.8 Da MS/MS error, 1 potential missed cleavage, and variable oxidation (Methionine) [12].
Protein identification
Data files from the chromatography runs were batch searched against the M. avium subsp. paratuberculosis K10 proteome database using the SEQUEST algorithm16 contained within Bioworks v3.1 software[13]. Inclusion of identified proeins was based on minimum cross-correlation coefficients (Xcorr) of 1.9, 2.2, and 3.75 for singly, doubly, and triply charged precursor ions respectively and a minimum ΔCn of 0.1 were both required for individual peptides. For false positive analysis, a decoy search was performed automatically by choosing the Decoy checkbox on the search form.
Physicochemical characteristics and subcellular localization of the identified proteins
The full set of M. avium subsp. paratuberculosis K10 ORFs was downloaded from the NCBI databases, including 4399 genes. The codon adaptation indices (CAI) and hydrophilicity of the proteins were calculated with the standalone version of the software program CodonW (John Peden, http://bioweb.pasteur.fr/seqanal/interfaces/codonw.html). The TMHMM 2.0 program, based on a hidden Markov model http://www.cbs.dtu.dk/services/TMHMM/, was used to predict protein transmembrane topology[14]. The protein functional family was categorized according to the TubercuList http://genolist.pasteur.fr/TubercuList/.
Results
High-throughput identification of cell wall proteins with SDS-PAGE + LC-MS/MS
To avoid false-positive hits, we applied strict criteria for peptide and proteins identification. Additional file 1 shows detailed information about the identified proteins. In total, 309 unique proteins were identified, which included 101 proteins previously annotated as hypothetical or conserved hypothetical. Orthologues of the coding genes were found in M. avium subsp. hominissuis after blast searching the full genomic sequence using NCBI blast engine
Hydrophobicity analysis of the identified cell wall proteins
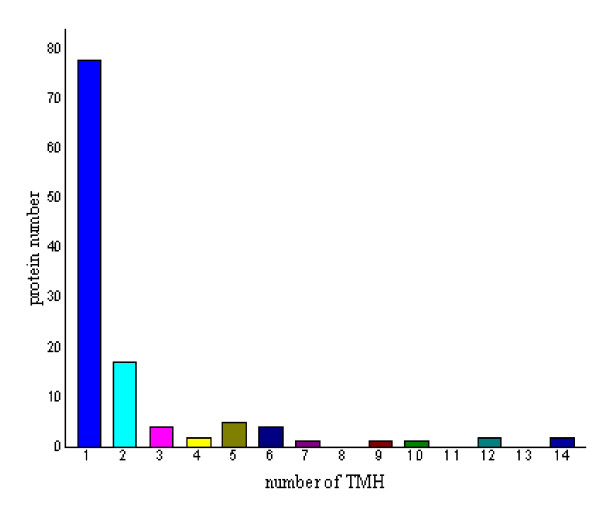
Potential cell wall associated proteins with 1-15 TMHs were assigned using the software TMHMM 2.0 program against the M. avium subsp. paratuberculosis K10 protein sequence database (excluding the possible signal sequences). In our study, 120 proteins (38.83%) were identified to have at least 1 transmembrane domain. The predicted TMH numbers of these proteins ranged from 1 to 14, 18 proteins contained two TMHs and 25 proteins (8.09%) with three or more TMHs. The profile of TMH in cell wall proteins of M. avium subsp. paratuberculosis K10 is very similar to previous reports about TMH in M. tuberculosis cell wall proteome[15]. The distribution of these TMHs is shown in Fig. 1. Among the 309 cell wall proteins identified, it is very interesting to find that there are 157 designated as cytoplasmic, 85 proteins have an unassigned location and 67 proteins are designated as cell wall related when analyzed by PSORTb location predictions.
Figure 1.
The distribution of of Transmembrane helices (TMH) as predicted by using the TMHMM 2.0 2.0 software identified in the Mycobacterium avium subsp. paratuberculosis K10 cell wall proteome.
Molecular mass and pI distributions of the identified cellwall proteins
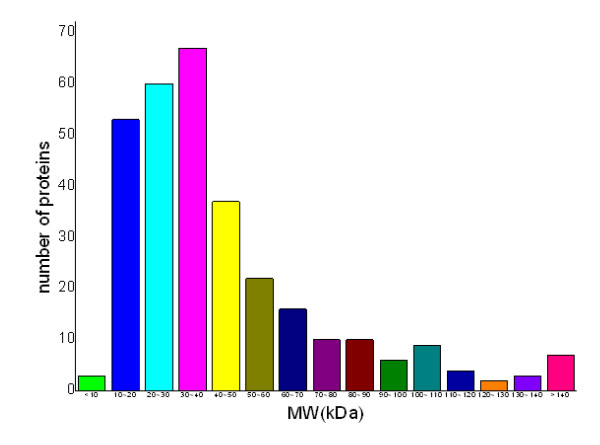
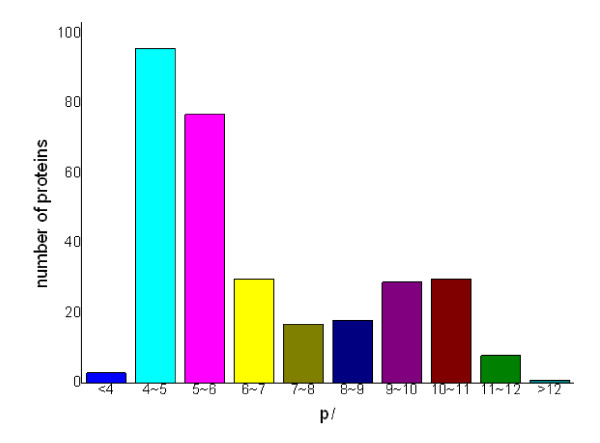
The theoretical Mr distribution of the identified cell wall proteins ranged from 2.92 kDa to 683.12 kDa. Moreover, proteins between Mr 10 and 50 kDa were in the majority, representing approximately 58.25% (180 out of 309) of all the identified cell wall proteins. Detailed distributions are shown in Fig. 2. The theoretical pI scores of the identified cell wall proteins ranged from 3.77 to 12.31. Detailed distributions are shown in Fig. 3. There are 39 proteins with pI scores over 10 and 15 proteins with Mr over 100 kDa. Taking GRAVY value into account, there will be at least 39 proteins beyond the general 2-DE separation limits. Additionally, there are 49 proteins with predicted signal peptide in the 309 identified cell wall proteins (Fig. 4A).
Figure 2.
The distribution of molecular mass (Mr) of the identified Mycobacterium avium subsp. paratuberculosis K10 cell wall proteome.
Figure 3.
The distribution of isoelectric points(PI values) of the identified Mycobacterium avium subsp. paratuberculosis K10 cell wall proteome.
Figure 4.
The distribution of proteins with asignalpeptide (SP)in (A) Mycobacterium avium subsp. paratuberculosis K10 cell wall proteome; (B) Mycobacterium avium subsp. paratuberculosis K10 cell surface-exposed proteome.
Analysis of functional groups in identified cell wall protein
Based on the Pasteur Institute functional classification tree http://genolist.pasteur.fr/TubercuList/, 309 identified proteins were distributed across eight of these functional groups (See table 1 for details). Most of the identified proteins were involved in intermediary metabolism and respiration (functional class 5, 23.95%), cell wall and cell process (21.04%) and conserved hypothetical proteins (17.48%). 62.47% of proteins were involved in the three major functional categories above. Many unexpected proteins such as the ribosomal proteins were found to be cell wall associated, which were also found in cell wall by previous research [7,15]. It is probable that these proteins interact tightly with the cell wall and join in cell envelop processes and would be potential significance in vaccine studies. Overlap between cytosolic, membrane and cell wall proteins in large scale proteomic studies is not uncommon. Additional studies are necessary to investigate the proteins with multiple cellular locations.
Table 1.
Functional classification of the identified Mycobacterium avium subsp. paratuberculosis K10 cell wall proteins according to Tubercurolist.
| Class | Function | Cell surface | Cell wall |
|---|---|---|---|
| 0 | Virulence, detoxification, adaptation | 7 | 17 |
| 1 | Lipid metabolism | 7 | 29 |
| 2 | Information pathways | 5 | 34 |
| 3 | Cell-wall and cell processes | 4 | 65 |
| 4 | PE and PPE proteins | 4 | 1 |
| 5 | Intermediary metabolism and respiration | 11 | 74 |
| 6 | Proteins of unknown function | 0 | 22 |
| 7 | Regulatory proteins | 1 | 13 |
| 8 | Conserved hypothetical proteins | 0 | 54 |
| Total | 39 | 309 | |
The fatty acid components are the most energetically expensive molecules to produce, and thus the regulation of fatty acid production is very tightly controlled to match the growth rate of cells. Mycolic acids are major and specific long-chain fatty acids of the cell envelope of several important human pathogens such as M. avium subsp. paratuberculosis, M. tuberculosis, M. leprae, and Corynebacterium diphtheriae. Their biosynthesis is essential for mycobacterial growth and represents an attractive target for developing new antituberculous drugs. In this study, 19 proteins related to lipid metabolism were identified as cell wall associated proteins, which include CmaA1(Mycolic acid synthase), CmaA2, FadE25_2, fadD32, fadA_1, FadB_1, fadD12_1, FadE3_2, FadD6, FadE24, FadE23, FadD29, fadA2, FadE20_3, Pks13, DesA1, DesA2, DesA3_2, fabG.
It is known for many bacterial species that there are tens of proteins required for cell division, for most of which exact functions are still unknown. In this study, the proteins related to cell division, ftsH, ftsZ, ftsX, ftsE, Wag31 (a homologue of the cell division protein DivIVA), PknA/PknB were identified as cell wall related proteins in this study.
Surface exposed proteins
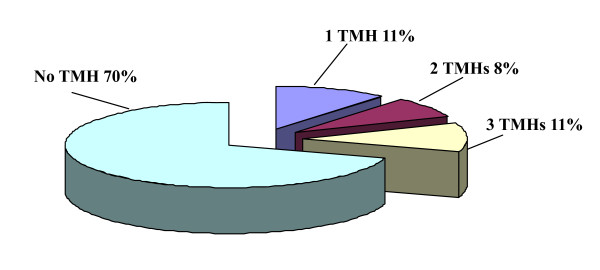
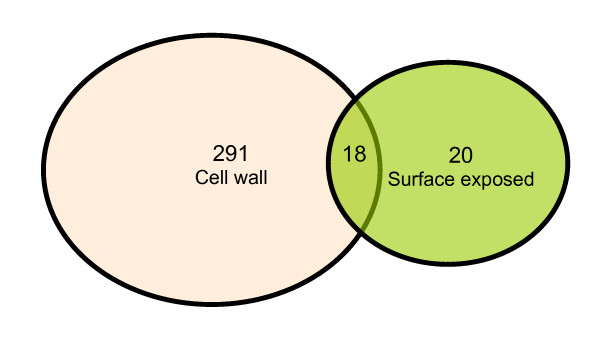
The integrity of the cells after trypsin treatment was confirmed by microscopy (live/dead staining) and cultivation methods, results of which confirmed the integrity of the cells (data not shown). Peptides released into the supernatant were collected to be fully digested with trypsin for 12~14 hrs, then concentrated and analyzed by LC-MS/MS. A total of 38 cell surface exposed proteins were successfully identified (as seen in table. 2). The predicted TMH numbers of these proteins ranged from 1 to 3, and 19% of which contained at least two TMHs. The distribution of these TMHs is listed in Fig. 5. 50% of the identified proteins have signal peptides (Fig 4B). As seen from Fig. 6, 18 proteins of 38 found surface-exposed proteins overlapped with the cell wall proteins, which include 3-oxoacyl-(acyl carrier protein) synthase II, acetyl-CoA acetyltransferase, acyl carrier protein, AhpD, AtpH, chaperonin GroEL, DesA2, DNA-directed RNA polymerase subunit alpha, elongation factor Tu, FadE24, FadE3_2, FixB, hypothetical protein MAP1563c, hypothetical protein MAP3007, hypothetical protein MAP3567, S-adenosyl-L-homocysteine hydrolase, SerA and Wag31. As seen from table. 3, among the 18 proteins that were identified as both the cell wall and cell surface proteins, there are two proteins (acyl carrier protein and S-adenosyl-L-homocysteine hydrolase) which are not found in the environmental M. smegmatis, five proteins (acyl carrier protein, AtpH, DesA2, hypothetical protein MAP1563c and hypothetical protein MAP3567) which are not found in Nocardia farcinica, a pathogenic member of the Actinomycetes, and nine proteins (acyl carrier protein, AhpD, AtpH, DesA2, FadE24, hypothetical protein MAP1563c, hypothetical protein MAP3007, hypothetical protein MAP3567 and Wag31) which are not found in Streptomyces coelicolor A3, a soil-dwelling member of the Actinomycetes.
Table 2.
Cell surface proteins identified by trypsin-shaving
| Genbank accession | Locus tag | gene name | Tuberculosis H37Rv homologue |
Functional classification | TMHs | pI | MW | Signal P (Y/N) |
Protein name |
|---|---|---|---|---|---|---|---|---|---|
| gi|41410034 | MAP3936 | Rv0440 | 0 | 4.60 | 56635.20 | N | chaperonin GroEL | ||
| gi|41410241 | MAP4143 | Tuf | Rv0685 | 2 | 4.96 | 43765.28 | N | elongation factor Tu | |
| gi|41409122 | MAP3024c | Rv2986c | 2 | 12.48 | 22187.24 | HupB | |||
| gi|41409749 | MAP3651c | FadE3_2 | Rv0215c | 1 | 1 | 6.12 | 44045.71 | N | FadE3_2 |
| gi|41408095 | MAP1997 | acpP | Rv2244 | 1 | 3.77 | 12483.25 | N | acyl carrier protein | |
| gi|33327135 | MAP3968 | hbhA | Rv0475 | 3 | 9.84 | 21534.4 | Y | heparin-binding hemagglutinin adhesin-like protein | |
| gi|41407220 | MAP1122 | mihF | Rv1388 | 2 | 11.02 | 20817.01 | Y | MIHF | |
| gi|13375557 | MAP1589c | ahpC | Rv2428 | 0 | 4.28 | 21566.2 | Y | alkylhydroperoxidase C | |
| gi|41407604 | MAP1506 | PPE26 | Rv1789 | 6 | 4.18 | 38588.55 | hypothetical protein MAP1506 | ||
| gi|41409460 | MAP3362c | sahH | Rv3248c | 7 | 4.72 | 54428.58 | N | S-adenosyl-L-homocysteine hydrolase | |
| gi|41407617 | MAP1519 | PPE30 | Rv1802 | 6 | 5.36 | 46020.98 | Y | hypothetical protein MAP1519 | |
| gi|41408796 | MAP2698c | DesA2 | Rv1094 | 1 | 4.62 | 31465.61 | N | DesA2 | |
| gi|41408096 | MAP1998 | KasA | Rv2245 | 1 | 1 | 4.84 | 43740.03 | N | 3-oxoacyl-(acyl carrier protein) synthase II |
| gi|41409938 | MAP3840 | dnaK | Rv0350 | 0 | 4.59 | 66830.67 | Y | molecular chaperone DnaK | |
| gi|41410362 | MAP4264 | groES | Rv3418c | 0 | 4.34 | 10772.12 | Y | co-chaperonin GroES | |
| gi|41409791 | MAP3693 | fadA2 | Rv0243 | 1 | 6.19 | 46844.14 | N | acetyl-CoA acetyltransferase | |
| gi|41407661 | MAP1563c | Rv1855c | 7 | 4.71 | 33359.95 | N | hypothetical protein MAP1563c | ||
| gi|41406496 | MAP0398c | Rv3676 | 9 | 10.24 | 24759.2 | Y | PROBABLE TRANSCRIPTIONAL REGULATORY PROTEIN | ||
| gi|41406994 | MAP0896 | sucC | Rv0951 | 7 | 4.74 | 40925.62 | Y | succinyl-CoA synthetase subunit beta | |
| gi|41407064 | MAP0966c | PPE61 | Rv3532 | 6 | 4.15 | 41549.19 | Y | hypothetical protein MAP0966c | |
| gi|41409131 | MAP3033c | SerA | Rv2996c | 7 | 4.6 | 54501.08 | N | SerA | |
| gi|41409105 | MAP3007 | Rv2971 | 7 | 4.27 | 30009.58 | N | hypothetical protein MAP3007 | ||
| gi|41409286 | MAP3188 | FadE24 | Rv3139 | 1 | 1 | 5.60 | 49533.15 | N | FadE24 |
| gi|41407088 | MAP0990 | eno | Rv1023 | 7 | 4.26 | 44929.34 | Y | phosphopyruvate hydratase | |
| gi|41407686 | MAP1588c | AhpD | Rv2429 | 0 | 4.73 | 18839.91 | N | AhpD | |
| gi|41407262 | MAP1164 | gap | Rv1436 | 7 | 5.05 | 35923.74 | Y | glyceraldehyde-3-phosphate dehydrogenase | |
| gi|41407987 | MAP1889c | Wag31 | Rv2145c | 3 | 4.38 | 28046.25 | N | Wag31 | |
| gi|41410331 | MAP4233 | rpoA | Rv3457c | 2 | 4.40 | 37699.63 | N | DNA-directed RNA polymerase subunit alpha | |
| gi|41410265 | MAP4167 | rpsC | Rv0707 | 2 | 10.71 | 29987.79 | Y | rpsC | |
| gi|41409159 | MAP3061c | fixA | Rv3029c | 7 | 4.37 | 28080.71 | Y | PROBABLE ELECTRON TRANSFER FLAVOPROTEIN (BETA-SUBUNIT) FIXA | |
| gi|41408326 | MAP2228 | fadE18 | Rv1933c | 1 | 6.18 | 38477.01 | Y | hypothetical protein MAP2228 | |
| gi|41410331 | MAP4233 | rpoA | Rv3457c | 2 | 4.40 | 37699.63 | N | DNA-directed RNA polymerase subunit alpha | |
| gi|41408552 | MAP2453c | AtpH | Rv1307 | 7 | 4.65 | 59985.27 | N | AtpH | |
| gi|41409103 | MAP3005c | Rv2969c | 3 | 5.77 | 26829.39 | Y | hypothetical protein MAP3005c | ||
| gi|41408378 | MAP2280c | clpP2 | Rv2460c | 0 | 4.76 | 23507.67 | Y | ATP-dependent Clp protease proteolytic subunit | |
| gi|41409665 | MAP3567 | Rv0148 | 7 | 5.62 | 30180.38 | N | hypothetical protein MAP3567 | ||
| gi|41407606 | MAP1508 | esxP | Rv2347c | 3 | 5.02 | 10977.18 | Y | hypothetical protein MAP1508 |
Figure 5.
Transmembrane helices (TMH) in the identified surface exposed proteins of Mycobacterium avium subsp. paratuberculosis K10.
Figure 6.
Venn diagram showing the overlap between the identified cell wall and cell surface exposed proteins.
Table 3.
Orthologues of the M. avium subsp. paratuberculosis proteins that were identified as both cell surface and cell wall associated in related organisms
| accession | Locus tag | Protein name | Orthologues (Y/N) | |||
|---|---|---|---|---|---|---|
| M. avium 104 | M. smegmatis | Nocardia farcinica | Streptomyces coelicolor A3 | |||
| gi|41408096 | MAP1998 | 3-oxoacyl-(acyl carrier protein) synthase II | Y | Y | Y | Y |
| gi|41409791 | MAP3693 | acetyl-CoA acetyltransferase | Y | Y | Y | Y |
| gi|41408095 | MAP1997 | acyl carrier protein | Y | N | N | N |
| gi|41407686 | MAP1588c | AhpD | Y | Y | Y | N |
| gi|41408552 | MAP2453c | AtpH | Y | Y | N | N |
| gi|41410034 | MAP3936 | chaperonin GroEL | Y | Y | Y | Y |
| gi|41408796 | MAP2698c | DesA2 | Y | Y | N | N |
| gi|41410331 | MAP4233 | DNA-directed RNA polymerase subunit alpha | Y | Y | Y | Y |
| gi|41410241 | MAP4143 | elongation factor Tu | Y | Y | Y | Y |
| gi|41409286 | MAP3188 | FadE24 | Y | Y | Y | N |
| gi|41409749 | MAP3651c | FadE3_2 | Y | Y | Y | Y |
| gi|41409159 | MAP3061c | FixB | Y | Y | Y | Y |
| gi|41407661 | MAP1563c | hypothetical protein MAP1563c | Y | Y | N | N |
| gi|41409105 | MAP3007 | hypothetical protein MAP3007 | Y | Y | Y | N |
| gi|41409665 | MAP3567 | hypothetical protein MAP3567 | Y | Y | N | N |
| gi|41409460 | MAP3362c | S-adenosyl-L-homocysteine hydrolase | Y | N | Y | Y |
| gi|41409131 | MAP3033c | SerA | Y | Y | Y | Y |
| gi|41407987 | MAP1889c | Wag31 | Y | Y | Y | N |
Discussion
In this study, cell wall proteins were first separated by SDS-PAGE according to their molecular weight followed by in-gel digested with trypsin into complex peptide mixture, and then the mixture was analyzed directly by LC-MS/MS. Subsequently, protein identifications were determined by database searching software [16]. Our experiments led to the identification of a much wider range of proteins in cell wall fraction than those identified using the conventional 2-DE based method[17] and can therefore be used as a comprehensive reference profile for Mycobacterium spp. cell wall proteomic studies. Additionally, the surface exposed proteome was identified by an enzymatic shaving technique. Two interesting observations result from the cell wall profile. Firstly, there is a discrepancy between the identified surface exposed proteins and the complete cell wall proteome. This is likely due to the loose association of these proteins with the cell wall which makes them prone to detachment. Indeed, some surface proteins are assumed to be attached to the cell wall in a non-covalent way and have been reported to be lost during mild standard manipulations[18,19]. Secondly, some proteins are not expected to be localized in the cell wall based on their annotated function. Till now, it is still unclear how proteins such as GroEL and elongation factor TU, leaving the bacterial cell, are retained on the cell surface and whether they have an additional function when associated with the cell wall different from their known function inside the bacterial cell. EF-Tu indeed was identified as a cell wall related protein in this study and has already been identified as cell wall protein in other studies [7]. It was found that only a small percentage of the proteins identified were classified as membrane bound by PSORTb in this study. The existing methods of subcellular localization have been developed for prokaryotic proteins mainly for bacterial proteins like PSORTb, PSLpred, CELLO, LOCtree, P-classifier, Gpos-ploc, GNBSL [20,21]. Not any method could correctly predict all proteins location. One of the challenges in subcellular localization is to predict location of proteins having multiplelocation [22]. It was reported that PSORTb version 2.0 correctly predicted 88% cytoplasmic, 81% integral membrane and 80% secretory proteins. PSORTb predicted only 18% membrane-attached into cytoplasmic membrane proteins and rest of them as unknown proteins[23].
In this study, one PPE protein was identified in the cell wall fraction and four PPE proteins were identified in the cell surfaced exposed proteome. The names PE and PPE are derived from the motifs Pro-Glu and Pro-Pro-Glu, respectively, found in conserved domains near the N termini of these proteins. The PE and PPE gene families are highly expanded in the pathogenic members of this genus but show a conspicuous paucity in the nonpathogenic species. Although no precise function is known for any member of these families, members of the PE and PPE families have been linked to virulence [24,25] or have at least been shown to influence interactions with other cells [26].
It is known for many bacteria that there are tens of proteins required for cell division, most of which exact functions are still unknown. The proteins related to cell division, ftsH, ftsZ, ftsX, ftsE, Wag31 (a homologue of the cell division protein DivIVA), PknA/PknB were identified as cell wall related proteins in this study. The divIVA gene, which for the most part is confined to gram-positive bacteria, was first identified in Bacillus subtilis. Cells with a mutation in this gene have a reduced septation frequency and undergo aberrant polar division, leading to the formation of anucleate minicells[19,22,25]. A divIVA gene is also present in Streptomyces coelicolor[27] and in other actinomycetes, like Mycobacterium tuberculosis, where Wag31 (antigen 84) is proposed to be involved in cell shape maintenance[28]. FtsZ is a bacterial cytoskeletal protein that is essential for cell division many prokaryotes [29]. It has been shown to be a bacterial homolog of eukaryotic tubulin, based both on a low sequence identity and a striking structural similarity [30]. It appears to act at the earliest step in septation and is required through the final step of cytokinesis[31]. FtsE, in association with the integral membrane protein FtsX, is involved in the assembly of potassium ion transport proteins, both of which being relevant to the tubercle bacillus. Recently FtsE and FtsX have been found to localize to the septal ring in E. coli, with the localization requiring the cell division proteins FtsZ, FtsA, and ZipA but not FtsK, FtsQ, FtsL, and FtsI proteins, suggestive of a role for FtsEX in cell division. The receptor-like protein kinase PknB is encoded by the distal gene in a highly conserved operon, present in all actinobacteria, that may control cell shape and cell division. Genes coding for a PknB-like protein kinase are also found in many more distantly related gram-positive bacteria. It was demonstrated that the Ser/Thr protein kinase PknB is essential for sustaining mycobacterial growth and support the development of protein kinase inhibitors as new potential antituberculosis drugs[32].
The fatty acid components are the most energetically expensive molecules to produce, and thus the regulation of fatty acid production is very tightly controlled to match the growth rate of cells. Mycolic acids are major and specific long-chain fatty acids of the cell envelope of several important human pathogens such as Mycobacterium avium subsp. paratuberculosis, Mycobacterium tuberculosis, M. leprae, and Corynebacterium diphtheriae. Their biosynthesis is essential for mycobacterial growth and represents an attractive target for developing new antituberculous drugs. In this study, 19 proteins proteins related to lipid metabolism were identified as cell wall associated proteins, which include CmaA1(Mycolic acid synthase), CmaA2, FadE25_2, fadD32, fadA_1, FadB_1, fadD12_1, FadE3_2, FadD6, FadE24, FadE23, FadD29, fadA2, FadE20_3, Pks13, DesA1, DesA2, DesA3_2, fabG.
CmaA1 is a cis cyclopropanesynthetase which produces a distal cis cyclopropane ring in the alpha mycolate of M. smegmatis [33]. cmaA2 is the trans cyclopropane synthetase for both the methoxy and ketomycolates.
pks13 gene encodes condensase, the enzyme that performs the final condensation step of mycolic acid biosynthesis and is flanked by two genes, fadD32 and accD4, both of which have been indicated to play a role in the activation of the substrates of the condensase [34]. DesA1 is homologous to the plant stearoyl-ACP desaturase which introduce the first double bond in the saturated fatty acids, C16 and C18, the products of fatty acid biosynthesis. These fatty acids are then incorporated in the membrane glycerolipids, cuticular lipids and oilseeds of plants[35]. Involvement of these proteins in mycolic acid synthesis has been suggested based on sequence annotations[36] and structural characterization. However, experimental evidence regarding their functional roles are not presently available. FadE3_2 and FadE25_2 are enzymes involved in electron transport with acyl-CoA dehydrogenase activity. Such enzymes act at the first dehydrogenase step of the β-oxidation of fatty acids. A study of protein expression of M. avium engulfed by macrophages found that FadE2, a protein with 98% protein domain similarity to FadE3_2, was up-regulated[37]. It appears that these proteins are important in the utilisation of fatty acids as a carbon source and that they may have a direct correlation to mycobacterial replication, particularly within host macrophages.
A total of 18 identified proteins, HspR, DnaJ, DnaK, KatG, LprG, HtrA, PhoR, PMM, PepA, MmpL3, sdhA, ClpB, hbhA, HBHA, Tuf, groES, manB, DesA3_2, can be considered putative virulence factors as they have previously been suggested to play some role in virulence [38-52].
Conclusions
We have obtained a comprehensive picture of the M. avium subsp. paratuberculosis K10 cell wall protein repertoire, with an additional insight in the portion of these proteins that are cell surface exposed. With 309 distinct proteins identified, this study represents the first proteomic analysis of cell wall proteins of M. avium subsp. paratuberculosis K10. To our knowledge, this is also the first report of a SDS-PAGE-LC-MS/MS based proteomic approach, supported with cell surface enzymatic digestion, to localize proteins in the mycobacterial cell wall. Many of the cell wall-associated proteins found in this study are involved in cell division, lipid metabolism or are putative virulence factors. Therefore, they should be considered as new potential antigens for vaccine development to prevent M. avium subsp. paratuberculosis K10 infection.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ZH carried out the experiments, participated in the data analysis and drafted the manuscript. JDB conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Supplementary Material
summarization of the identified cell wall proteins of M. avium subsp. paratuberculosis. The data provided summarization of the identified cell wall proteins of M. avium subsp. paratuberculosis.
Contributor Information
Zhiguo He, Email: hzg78@hotmail.com.
Jeroen De Buck, Email: jdebuck@ucalgary.ca.
Acknowledgements
This work was financially supported by a grant of the Crohn's and Colitis Foundation of Canada.
References
- Wu CW, Schmoller SK, Bannantine JP, Eckstein TM, Inamine JM, Livesey M, Albrecht R, Talaat AM. A novel cell wall lipopeptide is important for biofilm formation and pathogenicity of Mycobacterium avium ssp. paratuberculosis. Microb Pathog. 2009;46(4):222–30. doi: 10.1016/j.micpath.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzoigwe JC, Khaitsa ML, Gibbs PS. Mycobacterium avium ssp. paratuberculosis as a cause of Crohn's disease. Epidemiol Infect. 2007;135(7):1057–68. doi: 10.1017/S0950268807008448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MT, Grant IR. Mycobacterium avium ssp. paratuberculosis and its potential survival tactics. Lett Appl Microbio. 2006;42:305–311. doi: 10.1111/j.1472-765X.2006.01873.x. [DOI] [PubMed] [Google Scholar]
- Lindahl G, Stalhammar-Carlemalm M, Areschoug T. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev. 2005;18:102–127. doi: 10.1128/CMR.18.1.102-127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Huang S, Zhang Q. Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes Infect. 2002;4:325–331. doi: 10.1016/S1286-4579(02)01545-9. [DOI] [PubMed] [Google Scholar]
- Niemann HH, Schubert WD, Heinz DW. Adhesins and invasins of pathogenic bacteria: a structural view. Microbes Infect. 2004;6:101–112. doi: 10.1016/j.micinf.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ortega MJ, Norais N, Bensi G, Liberatori S, Capo S, Mora M, Scarselli M, Doro F, Ferrari G, Garaguso I, Maggi T, Neumann A, Covre A, Telford JL, Grandi G. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat Biotechnol. 2006;24(2):191–197. doi: 10.1038/nbt1179. [DOI] [PubMed] [Google Scholar]
- Muskens J, van Zijderveld F, Eger A, Bakker D. Evaluation of the long-term immune response in cattle after vaccination against paratuberculosis in two Dutch dairy herds. Vet Microbiol. 2002;86(3):269–78. doi: 10.1016/S0378-1135(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Newton V, McKenna SL, De Buck J. Presence of PPE proteins in Mycobacterium avium subsp. paratuberculosis isolates and their immunogenicity in cattle. Vet Microbiol. 2009;135(3-4):394–400. doi: 10.1016/j.vetmic.2008.09.066. [DOI] [PubMed] [Google Scholar]
- Rezwan M, Lanéelle MA, Sander P, Daffé M. Breaking down the wall: fractionation of mycobacteria. J Microbiol Methods. 2007;68(1):32–9. doi: 10.1016/j.mimet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1(6):2856–60. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- Barinov A, Loux V, Hammani A, Nicolas P, Langella P, Ehrlich D, Maguin E, Guchte M van de. Prediction of surface exposed proteins in Streptococcus pyogenes, with a potential application to other Gram-positive bacteria. Proteomics. 2008;9(1):61–73. doi: 10.1002/pmic.200800195. [DOI] [PubMed] [Google Scholar]
- Wei C, Yang J, Zhu J, Zhang X, Leng W, Wang J, Xue Y, Sun L, Li W, Wang J, Jin Q. Comprehensive proteomic analysis of Shigella flexneri 2a membrane proteins. J Proteome Res. 2006;5(8):1860–5. doi: 10.1021/pr0601741. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hiddenMarkov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Forst CV, Dobos KM, Belisle JT, Chen J, Bradbury EM, Bradbury ARM, Chen X. Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Mol Biol Cell. 2005;16:396–404. doi: 10.1091/mbc.E04-04-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Yates JR III. The application of mass spectrometry to membrane proteomics. Nat Biotechnol. 2003;21:262–267. doi: 10.1038/nbt0303-262. [DOI] [PubMed] [Google Scholar]
- Ebanks RO, Chisholm K, McKinnon S, Whiteway M, Pinto DM. Proteomic analysis of Candida albicans yeast and hyphal cell wall and associated proteins. Proteomics. 2006;6(7):2147–56. doi: 10.1002/pmic.200500100. [DOI] [PubMed] [Google Scholar]
- Kocincova D, Sonden B, Mendonca-Lima L, Gicquel B, Reyrat JM. The Erp protein is anchored at the surface by a carboxy-terminal hydrophobic domain and is important for cell-wall structure in Mycobacterium smegmatis. Fems Microbiol Lett. 2004;231:191–196. doi: 10.1016/S0378-1097(03)00964-9. [DOI] [PubMed] [Google Scholar]
- Lichtinger T, Burkovski A, Niederweis M, Kramer R, Benz R. Biochemical and biophysical characterization of the cell wall porin of Corynebacterium glutamicum: The channel is formed by a low molecular mass polypeptide. Biochemistry. 1998;37:15024–15032. doi: 10.1021/bi980961e. [DOI] [PubMed] [Google Scholar]
- Guo J, Lin Y, Liu X. GNBSL: a new integrative system to predict the subcellular location for Gram-negative bacteria proteins. Proteomics. 2006;6:5099–5105. doi: 10.1002/pmic.200600064. [DOI] [PubMed] [Google Scholar]
- Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, Ester M, Brinkman FS. PSORTb v.2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2005;21:617–623. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- Chou KC, Shen HB. Euk-mPLoc: a fusion classifier for largescale eukaryotic protein subcellular location prediction by incorporating multiple sites. Journal of Proteome Research. 2007;6:1728–1734. doi: 10.1021/pr060635i. [DOI] [PubMed] [Google Scholar]
- Rashid M, Saha S, Raghava GPS. Support Vector Machine-based method for predicting subcellular localization of mycobacterial proteins using evolutionary information and motifs. BMC Bioinformatics. 2007;8:337. doi: 10.1186/1471-2105-8-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Miltner E, Wu M, Petrofsky M, Bermudez LE. A Mycobacterium avium PPE gene is associated with the ability of the bacterium to grow in macrophages and virulence in mice. Cell Microbiol. 2005;7:539–548. doi: 10.1111/j.1462-5822.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L, Federspiel NA, Falkow S. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science. 2000;288:1436–1439. doi: 10.1126/science.288.5470.1436. [DOI] [PubMed] [Google Scholar]
- Brennan MJ, Delogu G, Chen YP, Bardarov S, Kriakov J, Alavi M, Jacobs WR. Evidence that mycobacterial PE_PGRS proteins are cell surface constituents that influence interactions with other cells. Infect Immun. 2001;69:7326–7333. doi: 10.1128/IAI.69.12.7326-7333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GR, Newton SM, Wilkinson KA, Humphreys IR, Murphy HN, Robertson BD, Wilkinson RJ, Young DB. The stress-responsive chaperone alpha-crystallin 2 is required for pathogenesis of Mycobacterium tuberculosis. Mol Microbiol. 2005;55:1127–1137. doi: 10.1111/j.1365-2958.2004.04450.x. [DOI] [PubMed] [Google Scholar]
- Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nat Rev Microbiol. 2003;1:97–105. doi: 10.1038/nrmicro749. [DOI] [PubMed] [Google Scholar]
- Bramhill D. Bacterial cell division. Annu Rev Cell Dev Biol. 1997;13:395–424. doi: 10.1146/annurev.cellbio.13.1.395. [DOI] [PubMed] [Google Scholar]
- Erickson HP. Atomic structures of tubulin and FtsZ. Trends Cell Biol. 1998;8:133–137. doi: 10.1016/S0962-8924(98)01237-9. [DOI] [PubMed] [Google Scholar]
- Ma X, Ehrhardt DW, Margolin W. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using the green fluorescent protein. Proc Natl Acad Sci USA. 1996;93:12998–13003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez P, Saint-Joanis B, Barilone N, Jackson M, Gicquel B, Cole ST, Alzari PM. The Ser/Thr protein kinase PknB is essential for sustaining mycobacterial growth. J Bacteriol. 2006;188(22):7778–84. doi: 10.1128/JB.00963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Lee RE, Besra GS, Belisle JT, Barry CE. 3rd Identification of a gene involved in the biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92(14):6630–6634. doi: 10.1073/pnas.92.14.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portevin D, de Sousa-D'Auria C, Montrozier H, Houssin C, Stella A, Lanéelle MA, Bardou F, Guilhot C, Daffé M. The acyl-AMP ligase FadD32 and AccD4-containing acyl-CoA carboxylase are required for the synthesis of mycolic acids and essential for mycobacterial growth: identification of the carboxylation product and determination of the acyl-CoA carboxylase components. J Biol Chem. 2005;280(10):8862–74. doi: 10.1074/jbc.M408578200. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman K, Rajagopalan P, Chandra N. Flux balance analysis of mycolic acid pathway: targets for anti-tubercular drugs. PLoS Comput Biol. 2005;1:e46. doi: 10.1371/journal.pcbi.0010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori L, Giannoni F, Bini L, Liberatori S, Frota C, Jenner P, Thoresen OF, Orefici G, Fattorini L. Induction of Mycobacterium avium proteins upon infection of human macrophages. Proteomics. 2004;4:3078–3083. doi: 10.1002/pmic.200300891. [DOI] [PubMed] [Google Scholar]
- Grandvalet C, de Crecy-Lagard V, Mazodier P. The ClpB ATPase of Streptomyces albus G belongs to the HspR heat shock regulon. Mol Microbiol. 1999;31:521–532. doi: 10.1046/j.1365-2958.1999.01193.x. [DOI] [PubMed] [Google Scholar]
- Gamer J, Multhaup G, Tomoyasu T, McCarty JS, Rudiger S, Schonfeld HJ, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor sigma32. EMBO J. 1996;153:607–617. [PMC free article] [PubMed] [Google Scholar]
- Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD. Role of LprG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol. 2004;52(5):1291–302. doi: 10.1111/j.1365-2958.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol. 2004;173(4):2660–8. doi: 10.4049/jimmunol.173.4.2660. [DOI] [PubMed] [Google Scholar]
- Stack HM, Sleator RD, Bowers M, Hill C, Gahan CG. Role for HtrA in stress induction and virulence potential in Listeria monocytogenes. Appl Environ Microbiol. 2005;71(8):4241–7. doi: 10.1128/AEM.71.8.4241-4247.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo Asensio J, Maia C, Ferrer NL, Barilone N, Laval F, Soto CY, Winter N, Daffé M, Gicquel B, Martín C, Jackson M. The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem. 2006;281(3):1313–6. doi: 10.1074/jbc.C500388200. [DOI] [PubMed] [Google Scholar]
- Kim SH, Ahn SH, Lee JH, Lee EM, Kim NH, Park KJ, Kong IS. Genetic analysis of phosphomannomutase/phosphoglucomutase from Vibrio furnissii and characterization of its role in virulence. Arch Microbio. 2003;180(4):240–50. doi: 10.1007/s00203-003-0582-z. [DOI] [PubMed] [Google Scholar]
- Hauser AR, Kang PJ, Engel JN. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27(4):807–18. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- Domenech P, Reed MB, Barry CE. Contribution of the Mycobacterium tuberculosis MmpL protein family to virulence and drug resistance. Infect Immun. 2005;73(6):3492–501. doi: 10.1128/IAI.73.6.3492-3501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci USA. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastanet A, Derre I, Nair S, Msadek T. clpB, a novel member of the Listeria monocytogenes CtsR regulon, is involved in virulence but not in general stress tolerance. J Bacteriol. 2004;186(4):1165–74. doi: 10.1128/JB.186.4.1165-1174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, Menozzi FD. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412(6843):190–4. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- Locht C, Hougardy J-M, Rouanet C, Place S, Mascart F. Heparin-binding hemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis. Tuberculosis. 2006;86:303–309. doi: 10.1016/j.tube.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Kunert A, Losse J, Gruszin C, Hühn M, Kaendler K, Mikkat S, Volke D, Hoffmann R, Jokiranta TS, Seeberger H, Moellmann U, Hellwage J, Zipfel PF. Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen binding protein. J Immunol. 2007;179(5):2979–88. doi: 10.4049/jimmunol.179.5.2979. [DOI] [PubMed] [Google Scholar]
- Lin YF, Wu MS, Chang CC, Lin SW, Lin JT, Sun YJ, Chen DS, Chow LP. Comparative immunoproteomics of identification and characterization of virulence factors from Helicobacter pylori related to gastric cancer. Mol Cell Proteomics. 2006;5(8):1484–96. doi: 10.1074/mcp.M600111-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
summarization of the identified cell wall proteins of M. avium subsp. paratuberculosis. The data provided summarization of the identified cell wall proteins of M. avium subsp. paratuberculosis.