The 1,3-dipolar cycloaddition reaction of azides and alkynes has been known for over 100 years and was studied extensively by Huisgen and co-workers in the 1960s.1 The resurgence of recent interest in the reaction has been stimulated by the discovery of the copper-catalyzed version of this process, the copper-catalyzed azide–alkyne cycloaddition (CuAAC).2 Copper catalysis increases the reaction rate by up to 107 and results in regioselective formation of 1,4-disubstituted triazoles. This reaction is generally recognized as the most striking example of click chemistry.3 What makes a click reaction so appealing is its application to label molecules of interest in complex biological samples without interferences with any other chemical functionalities.4 The most common catalyst systems for CuAAC employ water or alcohol solvents and use a Cu(II) salt in the presence of a reducing agent (often sodium ascorbate or metallic copper) to generate the required Cu(I) catalyst in situ. Cu(I) complexes can also be used directly, although the reaction often suffers from the formation of byproducts of red/ox processes catalyzed by copper and requires the addition of ligands to accelerate the cycloaddition.2
Microwave (MW) irradiation was efficiently applied to accelerate the azide/alkyne click reaction.5 Catalytic activity of metallic copper was established early on,6 and although the reaction times were long, the final product was clean, and the workup consisted of a simple removal of the copper turnings. Copper clusters7 have also been employed as precatalysts. Lipshutz et al. found that copper supported on charcoal (Cu(II)/C) was an efficient catalyst;8 Cu(I)/C was also successfully employed by Cintas et al. in MW-assisted protocols.9 Ultrasound (US) has been used to promote the CuAAC reaction as well. Sreedhar reported a sonochemical CuI-catalyzed synthesis of triazoles from terminal alkynes and alkyl/aryl azides, formed in situ.10 Worthy of mention are the efficient applications of copper nanoparticles as substitutes of bulk copper metal although their preparation involves an additional step.11 We studied this reaction under non-conventional conditions,9,12 namely, power US and MW, alone or combined. The specific advantages of US13 and MW14 in organic synthesis have been widely described. Using both simultaneously may be beneficial to the rates, yields, and selectivity of the reactions, as recent examples show.15
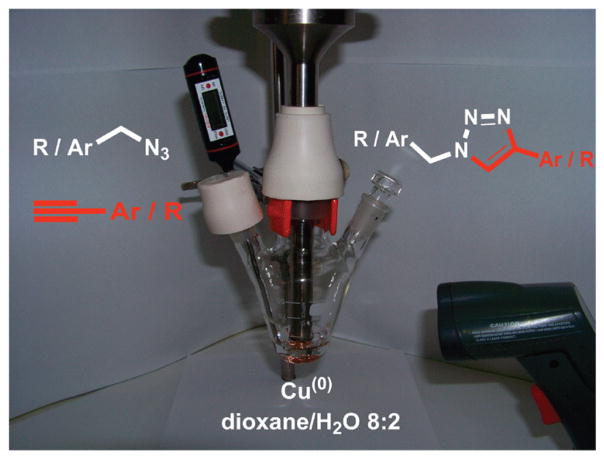
Here we describe a process in which metallic copper efficiently catalyzes azide–alkyne cycloadditions under US or simultaneous US/MW irradiation. Reactions involving metals represent the favorite domain of sonochemistry because US favors mechanical depassivation and enhances both mass transfer and electron transfer from the metal to the organic acceptor.16 Although a piece of metal placed inside a MW oven will lead to dangerous arcing, it is possible to perform organic reaction using well-dispersed fine metal particles in a polar high boiling solvent. This was first demonstrated by Whittaker and Mingos, who opened the doors to synthetic applications of metals under MW conditions.17 Besides the particle size, other important safety features are the following: (i) a moderate applied power (it is directly related to the electric field strength, decreasing the field strength decreases the risk of arcing, (ii) a low metal loading, and (iii) an efficient stirring to disperse the metal powder throughout the solvent. After several years of experiments under simultaneous US/MW irradiation18 by using every kind of solvents and solid catalysts, we can emphasize that this hybrid treatment is probably the most efficient technique to be applied to heterogeneous metal catalysis to reduce any risk of arcing. Combined US/MW irradiation in a single reaction vessel can be achieved inside a modified MW oven by inserting in it a non-metallic horn made of quartz, Pyrex, ceramic, or made from engineered plastics, such as PEEK (poly(etherether ketone)) or PTFE (poly(tetrafluoroethylene)).15 In a previous study, we found that the charcoal-supported CuAAC were strongly promoted by simultaneous US/MW irradiation, as well as by MW alone (with slightly lower yields).9 In this study, we experimented with the typical reaction of benzyl azide with phenylacetylene using metallic copper (fine turnings or wires) or Cu2O as catalysts. Figure 1 shows the typical setup for sonochemical reaction and the general scheme of the reaction, while results are summarized in Table 1.
Figure 1.
General reaction scheme and setup for US-promoted reaction.
Table 1.
Synthesis of 1,2,3-Triazole from Benzylazide and Phenylacetylene (Dioxane/H2O 8:2, 70°C)
| no. | catalyst | method | time (h) | yield (%) |
|---|---|---|---|---|
| 1 | oil bath | 24 | 1a–4b | |
| 2 | US | 5 | 2a–2b | |
| 3 | CuSO4/ascorbic acid | oil bath | 0.5 | 78 |
| 4 | Cu2O | oil bath | 24 | 90 |
| 5 | Cu2O | US | 5 | 71 |
| 6 | Cu wires | oil bath | 24 | 52 |
| 7 | Cu wires | USc | 2 | 64 |
| 8 | Cu wires | US | 2 | 85 |
| 9 | Cu turnings | oil bath | 12 | 76 |
| 10 | Cu turnings | MW | 2 | 39 |
| 11 | Cu turnings | US | 1.5 | 89 |
| 12 | Cu turnings | USd | 2 | 25 |
| 13 | Cu turnings | MW/US | 1 | 95 |
| 14 | Cu turnings | MW/USe | 1 | 85 |
Isomer 1,4.
Isomer 1,5.
US 80W, without oil bath.
Depassivated metal.
Reaction carried out under nitrogen.
In all cases the temperature was strictly controlled at 70 °C by two different measurement systems: an IR-pyrometer and a thermocouple (under US), and an IR-pyrometer and an optic-fiber thermometer (under combined US/MW).
The US irradiation19 was combined with oil bath heating to reach the desired temperature. Because of the higher contact surface, thin copper turnings gave better results than common electrical wires. A portion of copper turnings was depassivated by washing with HCl and then with abundant distilled water. Entry 12 showed that its catalytic activity dropped, a result that supports our hypothesis that Cu(I) derives from the red/ox activated by sonication between the copper metal and the copper oxide on the surface. We suspect that oxygen dissolved in the solvent has certain effect on this equilibrium; in fact the same reaction carried out in a degassed solvent under nitrogen atmosphere gave a slightly lower yield (entry 14 vs 13). Reactions under conventional heating catalyzed by Cu2O powder gave excellent yields (entry 4), while under US required longer reaction times and gave somewhat lower yields (entry 5 vs 11). In the absence of any catalyst, a mixture of 1,4- and 1,5-disubstituted triazole isomers was obtained in 1–4% yield.
Preliminary results showed that an intense short sonication (10 min, 50 W) of the copper turnings in the solvent mixture sufficed to accelerate the reaction in comparison to mechanical stirring and conventional heating. The reaction rate increased further when US and MW irradiation was used simultaneously. Figure 2 shows the reaction setup for simultaneous US/MW irradiation in professional multimode oven, with the Pirex horn into the reaction vessel and a fiber optic thermometer to monitor the temperature.
Figure 2.
General setup for simultaneous US/MW irradiation.
The catalysis with metallic copper was applied to a series of other substrates (Table 2), among which was the 6-monoazido-β-cyclodextrin (β-CD) both in the hydrophilic native form as well as in the lipophilic permethylated derivative (Scheme 1). The reaction with 1,3-bis(propargyloxy)benzene and excess benzylazide (entries 23–26) gave exclusively the monotriazole derivative in oil bath (23), and the bis-adduct under US and US/MW irradiation (25, 26). A mixture of both derivatives was obtained under MW irradiation.
Table 2.
Synthesis of 1,2,3-Triazole with Copper Turnings
| no. | reagentsa | method | time(h) | yield(%) |
|---|---|---|---|---|
| 15 | A/B | oil bath | 6 | 38 |
| 16 | MW | 3 | 21 | |
| 17 | US | 2 | 88 | |
| 18 | MW/US | 2 | 85 | |
| 19 | C/B | oil bath | 6 | 34 |
| 20 | MW | 3 | 16 | |
| 21 | US | 2 | 80 | |
| 22 | MW/US | 2 | 86 | |
| 23 | D/E | oil bath | 16 | 88 |
| 24 | MW | 3 | 41/37b | |
| 25 | US | 2 | 81c | |
| 26 | MW/US | 2 | 83c | |
| 27 | F/B | oil bath | 10 | 64 |
| 28 | MW | 3 | 55 | |
| 29 | MW/US | 2 | 80 | |
| 31 | G/B | oil bath | 6 | 56 |
| 32 | MW | 3 | 68 | |
| 33 | US | 2 | 81 | |
| 34 | MW/US | 2 | 87 |
A) 1-azidoctane; B) phenylacetylene; C) 1-azidoheptadecane; D) benzylazide; E) 1,3-bis(propargyloxy)benzene; F) 6-monoazido-β-CD; G) 6-monoazido-permethyl-β-CD.
Ratio % mono- and bis-triazole derivatives.
% bis-triazole.
Scheme 1.
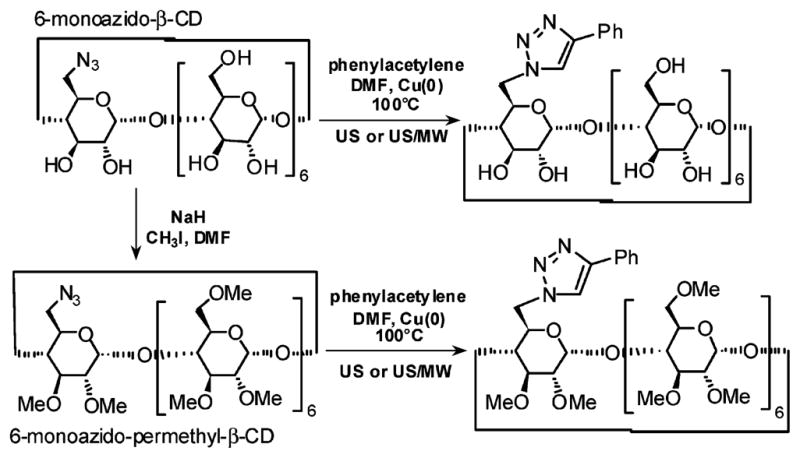
6-Monoazido-β-CD Transformations (Entries 27–34)
As reported in the literature, β-CD forms a stable copper ion-CD inclusion complex20 in which the two CD tori are joined by Cu(II) ions in a sandwich-type complex. In this metal-bridged complex, the CD secondary face which is masked21 is a strong limitation for further applications. The CD adducts from Cu(II)/sodium ascorbate catalyzed 1,3-dipolar cycloaddition showed a typical green-grayish color due to the complex with copper ions that require time-consuming purifications by means of competitive chelants. Although we would expect a great influence of water in radical formation under sonochemical conditions,22 the use of copper metal as a catalyst in DMF at 100 °C was extremely advantageous and provided very clean CD-triazole derivatives as white powder.23 This was probably due to the higher temperature and the better cavitation. The ICP-MS analysis of the crude products obtained from reactions catalyzed with metallic copper turnings, revealed only traces of the metal in the range of 3–6 ppb.
In conclusion, a US or US/MW-enhanced, efficient and sustainable metallic copper-catalyzed Huisgen 1,3-dipolar regioselective cycloaddition of azides and alkynes has been developed. This method could be applied to the production of large libraries and combinatorial strategies; moreover, US and MW can now be implemented in flow systems,24 which should enable large-scale production. Recently click reactions have been successfully conducted in a modular flow reactor.25
The reaction performs well in aqueous media, avoiding any pretreatments and the addition of ligands or amines. The use of copper turnings as a heterogeneous catalyst represents a much cleaner approach for click reactions that may blossom into a plethora of applications in pharmaceutical and combinatorial chemistry, as well as in biological systems.
Supplementary Material
Acknowledgments
This work was supported by the University of Turin and the Regione Piemonte (NanoIGT Project, Converging Technologies 2007).
Footnotes
Supporting Information Available. Characterization data (1H and 13C NMR, EI-MS, IR and MP) of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.(a) Huisgen R. In: 1,3-Dipolar Cycloaddition Chemistry. Padwa A, editor. Wiley; New York: 1984. pp. 1–176. [Google Scholar]; (b) Padwa A. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 4. Pergamon; Oxford: 1991. pp. 1069–1109. [Google Scholar]
- 2.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 3.For reviews see: Bock VD, Hiemstra H, van Maarseveen JH. Eur J Org Chem. 2005:51–68.Wang Q, Chittaboina S, Barnhill HN. Lett Org Chem. 2005;2:293–301.Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n.Wu P, Fokin VV. Aldrichimica Acta. 2007;40:7–17.Hein CD, Liu XM, Wang D. Pharm Res. 2008;25:2216–2230. doi: 10.1007/s11095-008-9616-1.
- 4.Sletten EM, Bertozzi CR. Angew Chem, Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Munteanu M, Choi S, Ritter H. Macromolecules. 2008;41:9616–9623. [Google Scholar]; (b) van Dijk M, Nollet ML, Weijers PL, Dechesne AC, van Nostrum CF, Hennink WE, Rijkers DTS, Liskamp RMJ. Biomacromolecules. 2008;9:2834–2843. doi: 10.1021/bm8005984. [DOI] [PubMed] [Google Scholar]; (c) Vecchi A, Melai B, Marra A, Chiappe C, Dondoni A. J Org Chem. 2008;7316:6437–6440. doi: 10.1021/jo800954z. [DOI] [PubMed] [Google Scholar]
- 6.(a) Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV. J Am Chem Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]; (b) Gommermann N, Gehrig A, Knochel P. Synlett. 2005:2796–2798. [Google Scholar]
- 7.Pachòn LD, van Maarseveen JH, Rothenberg G. Adv Synth Catal. 2005;347:811–815. [Google Scholar]
- 8.Lipshutz BH, Taft BR. Angew Chem, Int Ed. 2006;118:8415–8418. [Google Scholar]
- 9.Cintas P, Martina K, Robaldo B, Garella D, Boffa L, Cravotto G. Collect Czech Chem Commun. 2007;72:1014–1024. [Google Scholar]
- 10.Sreedhar B, Surendra Reddy P. Synth Commun. 2007;37:805–812. [Google Scholar]
- 11.(a) Sarkar A, Mukherjee T, Kapoor S. J Phys Chem C. 2008;112:3334–3340. [Google Scholar]; (b) Park IS, Kwon MS, Kim Y, Lee JS, Park J. Org Lett. 2008;10:497–500. doi: 10.1021/ol702790w. [DOI] [PubMed] [Google Scholar]; (c) Alonso F, Moglie Y, Radivoy G, Yus M. Tetrahedron Lett. 2009;50:2358–2362. [Google Scholar]
- 12.Appukkuttan P, Dehaen W, Fokin VV, Van der Eycken E. Org Lett. 2004;6:4223–4225. doi: 10.1021/ol048341v. [DOI] [PubMed] [Google Scholar]
- 13.Cravotto G, Cintas P. Chem Soc Rev. 2006;35:180–196. doi: 10.1039/b503848k. [DOI] [PubMed] [Google Scholar]
- 14.Kappe CO, Dallinger D, Murphree SS. Strategies, Instruments, and Protocols. Wiley-VCH; Weinheim, Germany: 2009. Practical Microwave Synthesis for Organic Chemists. [Google Scholar]
- 15.(a) Cravotto G, Cintas P. Chem–Eur J. 2007;13:1902–1909. doi: 10.1002/chem.200601845. [DOI] [PubMed] [Google Scholar]; (b) Cravotto G, Garella D, Calcio Gaudino E, Lévêque J-M. Chemistry Today. 2008;26:39–41. [Google Scholar]
- 16.Cintas P, Luche J-L. In: Synthetic Organic Sonochemistry. Luche J-L, editor. Plenum Press; New York: 1998. pp. 167–234. [Google Scholar]
- 17.Whittaker AG, Mingos DMP. J Chem Soc, Dalton Trans. 2000:1521–1526. [Google Scholar]
- 18.(a) Palmisano G, Bonrath W, Boffa L, Garella D, Barge A, Cravotto G. Adv Synth Catal. 2007;349:2338–2344. [Google Scholar]; (b) Cravotto G, Boffa L, Lévêque JM, Estager J, Bonrath W. Aust J Chem. 2007;60:946–950. [Google Scholar]; (c) Wu ZL, Ondruschka B, Cravotto G. Environ Sci Technol. 2008;42(21):8083–8087. doi: 10.1021/es8013375. [DOI] [PubMed] [Google Scholar]; (d) Domini C, Vidal L, Cravotto G, Canals A. Ultrason Sonochem. 2009;16:564–569. doi: 10.1016/j.ultsonch.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Sonication conditions. Probe equipped with a titanium horn (frequency 21.4 kHz, power 25 W).
- 20.Klufers P, Piotrowski H, Uhlendorf J. Chem–Eur J. 1997;3:601–608. [Google Scholar]
- 21.Fuchs R, Habermann N, Klüfers P. Angew Chem, Int Ed Engl. 1993;32:852–854. [Google Scholar]
- 22.Henglein A. Ultrason Sonochem. 1995;2:115–121. [Google Scholar]
- 23.Typical Experimental Procedure. All reactions were carried out in a 50 mL heavy walled pear-shaped two-neck flask with non-standard taper outer joint. In all cases the temperature was strictly monitored by two measurement systems: an IR pyrometer and a thermocouple (under US) or an IR pyrometer and an optical-fiber thermometer (under MW). The fine turnings of metallic copper (50 mol %, 100 mol %, for entries 27–34) were suspended in a mixture of dioxane/H2O 8:2 or in DMF (10 mL). The azido compound (1 mmol, if not otherwise stated) and the acetylenic derivative (1.05 mmol, 5.0 mmol for entries 27–34) were added, and the mixture was heated and/or irradiated with MW or MW/US as indicated in the tables. The reaction outcome was monitored by TLC or GC/MS until complete conversion of the starting material was observed; then the copper was filtered off on paper filter. After evaporation of the solvent under vacuum, the crude product was purified by flash-chromatography. The residual copper had a minimal loss of weight (5–7%) and can be directly reused maintaining almost the same catalytic activity for a couple of times.
- 24.Cravotto G, Di Carlo S, Curini M, Tumiatti V, Roggero C. J Chem Tech Biotech. 2007;82:205–208. [Google Scholar]
- 25.Smith CD, Baxendale IR, Lanners S, Hayward JJ, Smith SC, Ley SV. Org Biomol Chem. 2007;5:1559–1561. doi: 10.1039/b702995k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.