Abstract
Background & Aims
Hepatic de-differentiation, liver development, and malignant transformation are processes in which the levels of hepatic S-adenosylmethionine (SAMe) are tightly regulated by two genes, MAT1A and MAT2A. MAT1A is expressed in the adult liver, whereas MAT2A expression is primarily extra-hepatic and is strongly associated with liver proliferation. The mechanisms that regulate these expression patterns are not completely understood. In silico analysis of the 3′ untranslated region of MAT1A and MAT2A revealed putative binding sites for the RNA-binding proteins AUF1 and HuR, respectively. We investigated the post-transcriptional regulation of MAT1A and MAT2A by AUF1, HuR and methyl-HuR in the aforementioned biological processes.
Results
During hepatic de-differentiation, the switch between MAT1A and MAT2A coincided with an increase in HuR and AUF1 expression. SAMe treatment altered this homeostasis by shifting the balance of AUF1 and methyl-HuR/HuR, which was identified for the first time as an inhibitor of MAT2A mRNA stability. We also observed a similar temporal distribution and a functional link between HuR, methyl-HuR, AUF1, and MAT1A and MAT2A during the fetal liver development. Immunofluorescent analysis revealed increased levels of HuR and AUF1, and a decrease in methyl-HuR levels in human livers with hepatocellular carcinoma (HCC).
Conclusions
Our data strongly support a role for AUF1 and HuR/methyl-HuR in liver de-differentiation, development and human HCC progression through the post-translational regulation of MAT1A and MAT2A mRNAs.
Keywords: HuR, AUF1, MAT1A, MAT2A
INTRODUCTION
Methionine adenosyltransferase (MAT) catalyzes the synthesis of S-adenosylmethionine (SAMe), the principal methyl donor and a key regulator of hepatocyte proliferation and differentiation (1). In mammals, two genes encode this enzyme, MAT1A and MAT2A. MAT1A expression is associated with high SAMe levels and represents an excellent adult liver marker (1). MAT2A is related to lower SAMe levels and a more de-differentiated phenotype; it is predominantly expressed in fetal liver, where it is progressively replaced by MAT1A as development proceeds. MAT2A expression is upregulated during hepatocyte de-differentiation in liver regeneration (2,3), human hepatocellular carcinoma (HCC) and cultured liver cancer cells (3,4,5). Thus, MAT1A expression is related to more restrained cell growth whereas MAT2A expression is strongly associated with rapid growth and de-differentiation. MAT1A deficient mice, with low SAMe levels, have a predisposition to liver injury and overexpress cell proliferation related genes, including MAT2A (6,7). Overall, these suggests that the MAT1A and MAT2A expression patterns are tightly regulated in the liver, according to tissue type and differentiation stage, and that deregulation occurs during disease and malignant transformation. The mechanisms underlying these processes remain unknown.
Previous reports suggest that MAT1A and MAT2A expression is associated with a specific pattern of promoter methylation and histone acetylation (8). Moreover, methionine conversion into SAMe, regulates MAT2A expression at the level of mRNA turnover (9). RNA-binding proteins regulate the turnover by recognizing specific RNA sequences (10, 11). The Hu/elav proteins (human embryonic lethal abnormal vision) are the best known RNA-binding proteins that selectively recognize and bind to AU-rich elements (AREs). HuR, a member of the Hu/Elav family, is a ubiquitously expressed protein (12) that is predominantly (>90%) localized in the nucleus of unstimulated cells, but becomes cytoplasmic in response to proliferative and stress stimuli, increasing the half-life and/or modulating the translation rate of target mRNAs (13,14). In contrast, a number of other RNA-binding proteins, including TTP, BRF1, KSRP and the AU-rich RNA binding factor 1 (AUF1) function as destabilizers of target mRNAs (15). HuR and AUF1 target mRNAs encode for mitogenic, immune and stress response, and cell cycle regulatory proteins (c-fos, interleukins, hsp70, cyclin D1, and cdc25) (16) and both have been implicated in carcinogenesis (17, 18).
We investigated whether these two RNA-binding proteins regulate the expression of the MAT genes during liver de-differentiation, hepatic proliferation and malignant transformation. We found that HuR associated with the MAT2A 3′UTR, enhanced MAT2A mRNA stability and steady-state levels, while AUF1 associated with the MAT1A 3′UTR, decreased MAT1A mRNA stability and steady-state abundance. In addition, we describe a novel function of methyl-HuR as a destabilizer of MAT2A mRNA. Finally, we observed a specific expression pattern of HuR and AUF1 mRNAs in correlation with MAT1A and MAT2A mRNA levels. These findings suggest that HuR/methyl-HuR and AUF1 are important regulators of hepatic SAMe levels during de-differentiation, development, liver proliferation and carcinogenesis by controlling the switch between MAT1A and MAT2A expression.
MATERIALS AND METHODS
Human samples
Surgically resected liver tumor specimens from 22 liver cirrhotic patients with HCC (Hepatitis C (n=10), alcoholic steatohepatitis (n=10), and non-alcoholic steatohepatitis (n=2)) along with 4 normal liver biopsies were examined. Informed consent to all clinical investigations, which were performed in accordance with the principles embodied in the Declaration of Helsinki, was provided. The institutional review board of Hospital Clínic de Barcelona (Barcelona) approved the protocol.
Mouse and rat fetal liver samples
Liver samples were harvested and snap-frozen for subsequent analyses from the different animals: eight-month-old male GNMT-KO (knockout) (8) and wild-type (C57BI/6J) mice, embryonic day 16 (E16), 18 (E18), post-natal day 1 (P1), 5 (P5) and 3 months old Wistar rats. All animals were treated according to international IACUC standards.
Isolation and culture hepatocytes
Hepatocytes were isolated from male Wistar rats (200g) as described previously (22). Adhered cells were maintained in MEM with 5% fetal bovine serum (FBS), and incubated with the test compounds.
RNA interference
SAMe-Deficient cells and H4IIE hepatoma cell lines were transfected with HuR, AUF1 and control siRNA designed and synthesized by either Qiagen or SIGMA (35).
Cloning of 3′UTR of mouse HuR cDNA and plasmid construct
cDNA from SAMe-D cells served as a template for PCR amplification. A 1400-bp fragment was obtained, purified by Qiaquik gel extraction (Qiagen) and cloned into the pEGFP-C2 vector (CLONTECH). The resulting plasmid was verified by sequencing.
Transient transfection of MLP29 cells
The mouse cell line MLP29 was grown in DMEM containing 10% FBS. For transfection assays, cells were plated into 6 multi-well dishes and 2 μg of pEGFP-C2-3′UTR or pEGFP-C2 were transfected using Lipofectamine 2000 (Invitrogen). 20 h later, cells were treated with SAMe (4 mM) for 4 h and lysed with RIPA buffer.
RNA isolation and real-time PCR (qPCR)
Total RNA from liver tissue or hepatocytes were isolated with Trizol (Invitrogen) and purified with RNeasy Mini kit (Qiagen). PCR was performed using BioRad iCycler thermocycler. Ct values were analyzed as described (23) and normalized to the housekeeping transcripts (24).
Total, cytosolic and nuclear protein isolation
Extraction of total protein, cytosolic and membrane lysates from liver tissue and primary hepatocytes has been described previously (25).
Western blotting
Proteins were size-fractionated by SDS-PAGE, transferred onto nitrocellulose membranes and were incubated with antibodies recognizing HuR (Santa Cruz Biotechnology), AUF1 (BD Pharmigen), MAT II (MAT2A (AbCam)), MATI/III (MAT1A (6)), GFP (Roche), β-actin or GAPDH (Sigma-Aldrich), and methyl-HuR (29). Following incubation with secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology), proteins were detected by Western Lightning Chemiluminescence Reagent (PerkinElmer). Ponceau S staining was performed to ensure equal loading.
RNP IP
For the immunoprecipitation of endogenous ribonucleoprotein complexes (15), whole-cell extracts were processed and analyzed as previously described (26).
Analysis of polysomes
Untreated or SAMe-treated rat hepatocytes (5×106 cells) were cultured for 24 h, then incubated with 0.1 mg/ml cycloheximide for 10 min. Cytoplasmic extracts were fractionated and collected through sucrose gradients (26). The RNA of each fraction was isolated with Trizol (Invitrogen) and RT-qPCR analysis was performed. Protein from each fraction was precipitated with 10% TCA and resuspended in sample buffer for Western blotting.
Biotin pull-down assay
For in vitro synthesis of biotinylated transcripts (10), total cDNA was used as template for PCR reactions as described (26). Biotin pull-down assays were carried out as described elsewhere (15) and bound proteins analyzed by Western blotting.
Immunofluorescence
Paraffin sections (5-μm thick) of formalin-fixed liver samples were rehydrated, subjected to antigen retrieval in 10 mM sodium citrate buffer (pH 6.0), and avidin-biotin blocked before incubation with primary antibodies (anti-HuR, 1:100; anti-AUF1, 1:100; anti-methyl-HuR, 1:1000) followed by incubation with corresponding secondary antibodies. For quantification of immunofluorescence, images were acquired using 20× or 40× objectives with consistent exposure times for each section. The immunofluorescence intensity of approximately 50 cells from random fields for each sample was quantified using ImageJ software (public domain software; rsb.info.nih.gov/ij/) and expressed as relative immunofluorescence intensity. To minimize variations in measurements, all specimens were immunolabelled at the same time and under identical conditions.
Statistical analysis
All experiments were performed in triplicate with data expressed as means ± SEM. Representative blots are shown. Statistical significance was estimated with Student’s t test. A p value < 0.05 was considered significant.
RESULTS
MAT2A and MAT1A mRNAs levels are stabilized by HuR- and AUF1 respectively
Hepatocyte growth factor (HGF) upregulates MAT2A mRNA levels (27). HGF and AICA Riboside (AICAR), activators of AMP-activated protein kinase (AMPK), exert a proliferative response in rat hepatocytes by regulating HuR localization and, consequently the stability of mRNAs encoding proteins involved in cell cycle proliferation (25). SAMe inhibits this effect by blocking AMPK activation and maintaining HuR in the nucleus.
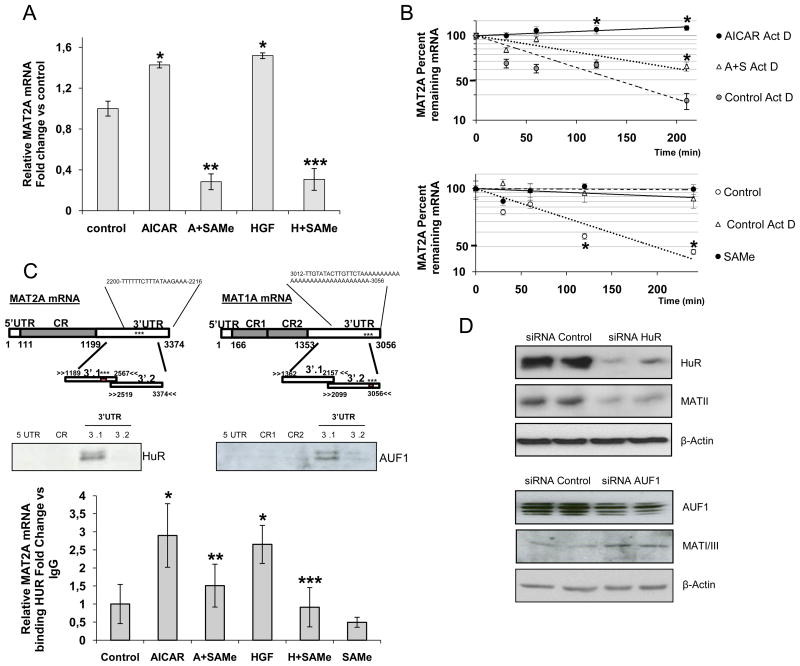
We found that AICAR and HGF upregulated the expression of MAT2A mRNA and this effect was blocked by SAMe (Figure 1A). Using actinomycin D (ActD), we determined that the half-life of the MAT2A mRNA was increased by AICAR (t1/2 >200 min) compared to ActD alone (t1/2 ≅77 min), and that the addition of SAMe limited this stabilization (AICAR+SAMe, t1/2 ≅173 min) (Figure 1B), suggesting that SAMe decreased MAT2A mRNA stability These data are consistent with previous reports demonstrating a downregulation in the half-life of MAT2A mRNA in a HCC cell line after SAMe treatment (28). AICAR treatment did not significantly change MAT1A mRNA levels, whilst SAMe treatment (t1/2 ≅1032 min) or ActD (t1/2 ≅693 min) increased the half-life of MAT1A mRNA compared to non-treated hepatocytes (t1/2 ≅87 min), suggesting that SAMe increased MAT1A mRNA stability.
Figure 1.
HuR stabilizes MAT2A mRNA and AUF1 destabilizes MAT1A mRNA. A) Analysis of MAT2A mRNA from rat hepatocytes treated with AICAR (2 mM), AICAR+SAMe (4 mM), HGF (40 ng/ml) or HGF+SAMe for 4 h. Treatments were carried out in triplicate (p<0.05, *AICAR or HGF versus control, **SAMe+AICAR versus AICAR, ***SAMe+HGF versus HGF). B) After AICAR, SAMe or AICAR+SAMe treatments, rat hepatocytes were incubated with Actinomycin D (2 μg/ml) for 4 h. The levels of MAT2A and MAT1A mRNAs were normalized to GAPDH mRNA and represented on semi-logarithmic scale. Top graph *p<0.05, AICAR Act D or A+S Act D versus Act D, Bottom graph *p<0.05, SAMe versus control. C) Upper panel: Schematic representation of MAT2A and MAT1A mRNA, showing the biotinylated transcripts [5′UTR, Coding Region (CR), 3′UTR] and the predicted HuR and AUF1 motifs. Western blots demonstrate association between HuR or AUF1 with biotinylated MAT2A and MAT1A fragments. Biotin pull-down assays were carried out in triplicates using rat hepatocyte lysates. Bottom panel: RNP-IP analysis of MAT2A mRNA bound to HuR after SAMe, AICAR, AICAR+SAMe, HGF and HGF+SAMe treatments. The enrichment was calculated from triplicates (p<0.05, *AICAR or HGF versus control, **SAMe+AICAR versus AICAR, ***SAMe+HGF versus HGF). D) Three days after siRNA transfection, SAM-D and H4IIE cells were harvested to monitor the protein expression of HuR and MAT2A, or AUF1 and MAT1A, respectively. Western blots are representative of 3 independent experiments.
To directly assess the functional role of the 3′UTR in the stability of MAT2A mRNA, this region was cloned into the expression plasmid pEGFP-C2 and transfected into the MLP29 mouse cell line. After 24 h in culture, GFP protein expression from pEGFP-C2-3′UTR mRNA was lower than that expressed by pEGFP-C2 mRNA alone. SAMe treatment decreased the expression of pEGFP-C2-3′UTR mRNA, while having no effect on pEGFP-C2 mRNA alone (Figure S1). These data suggest that the 3′UTR of MAT2A mRNA is responsible for destabilizing GFP mRNA and sensitizing the MLP29 cells to SAMe treatment.
In silico analysis of the MAT2A and MAT1A 3′UTRs revealed one computationally predicted hit for an HuR-binding motif in MAT2A mRNA at position 2200 and another for AUF1 in MAT1A mRNA at position 3012 (Figure 1C; the star represents the position of the predicted motifs). No AUF1 or HuR binding sites were found in the 3′UTR of MAT2A and MAT1A respectively. To test the possibility that MAT2A and MAT1A mRNAs were associated with HuR and AUF1 respectively, we used biotinylated transcripts spanning different mRNA regions (Figure 1C) and incubated them with hepatocyte lysates. The interaction was assessed by biotin pull-down assays followed by Western blot analysis. As shown in Figure 1C (bottom panels), HuR only formed complexes with the 3′UTR-MAT2A, having a stronger association with 3′ (1) UTR-MAT2A than with 3′ (2) UTR-MAT2A. No interaction was observed with the 3′UTR-MAT1A (not shown). Biotinylated GAPDH 3′UTR was used as a negative control and no signal was detected (data not shown). In the case of AUF1, complexes were observed only with the 3′UTR-MAT1A, specifically with the 3′ (1) UTR-MAT1A although the computationally predicted site was in the 3′ (2) UTR. In silico predictions are not always biological hits, most likely because other RNA-binding proteins have greater affinity for the predicted site. Finally, we analyzed whether HGF, AICAR and SAMe regulated the affinity of HuR for MAT2A mRNA by ribonucleoprotein immunoprecipitation (RNP-IP) assays (Figure 1C, lower panel). The results showed that AICAR and HGF increased the binding of HuR to MAT2A mRNA, while SAMe treatment prevented the formation of this complex. Silencing of HuR markedly decreased the expression of MAT II (66%), the protein encoded by MAT2A (Figure 1D). In contrast, silencing of AUF1 markedly decreased the expression of MAT I/III (37%), the protein encoded by MAT1A mRNA (Figure 1D).
Coordinated expression of MAT2A and MAT1A, and their respective regulators, HuR and AUF1 during de-differentiation of cultured hepatocytes
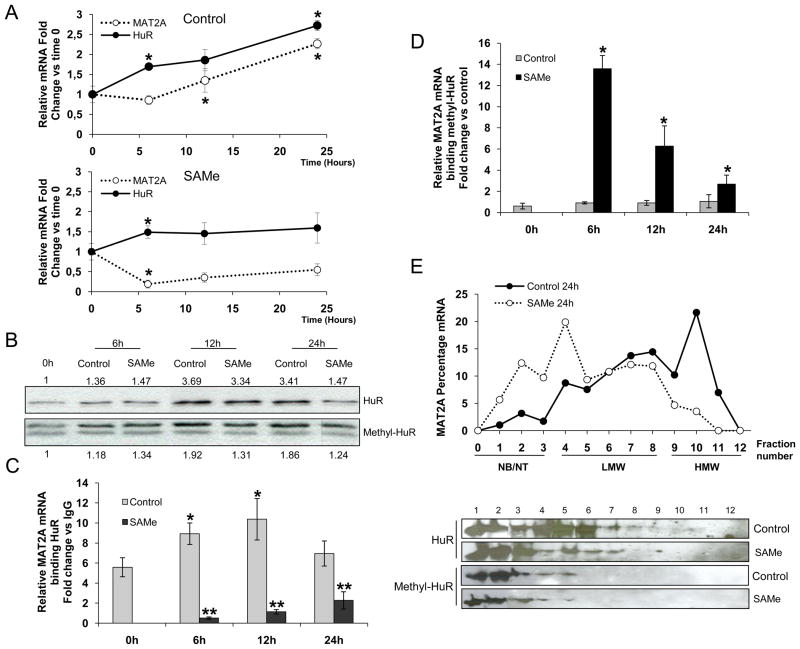
De-differentiation of cultured primary hepatocytes results in a switch in expression from MAT1A to MAT2A, an effect that is blocked by SAMe supplementation, which maintains the adult phenotype (29). We found that, similar to MAT2A, HuR mRNA levels also increased during culture, an effect also blocked by SAMe treatment (Figure 2A upper and lower panel). Previous reports have implicated the methylation of arginine 217 in modulating HuR levels (26). We analysed HuR and methyl-HuR protein levels in cultured hepatocytes, with or without SAMe treatment, over time and found that HuR levels remained stable at 6 and 12 h in the presence of SAMe and decreased at 24 h,. Methyl-HuR levels, however, changed only slightly during this de-differentiation process. (Figure 2B).
Figure 2.
MAT2A expression during de-differentiation of cultured hepatocytes. A) Expression of MAT2A mRNA over a time course in rat hepatocytes in presence/absence of SAMe (4 mM) *p<0.05, time of treatment versus previous time. B) Representative western blots of HuR and methyl-HuR in rat hepatocytes during de-differentiation. HuR versus time 0 h and methyl-HuR versus time 0 ratios from densitometric analysis are presented; each assay was carried out in triplicate. C) The association of HuR with MAT2A mRNA was assayed by RNP-IP analysis using cytoplasmic fractions of rat hepatocytes incubated as described above. MAT2A mRNA was normalized to GAPDH mRNA in HuR-IPs and represented relative to the levels of MAT2A mRNA in IgG-IP samples. p<0.05, *time of treatment versus time 0 h, **SAMe versus control. D) RNP-IP analysis of MAT2A mRNA bound to methyl-HuR; the enrichment was calculated from triplicate samples, *p<0.05, SAMe versus control. E) Polysome gradient analysis in rat hepatocytes cultured for 24 h in presence/absence of SAMe. MAT2A mRNA levels were plotted as a percentage of the total MAT2A mRNA levels. The translational activity of each fraction is as follows: NB, not bound polysomes; NT, not translated, moderately translated (LMW, low-molecular-weight polysomes), and actively translated (HMW, high-molecular-weight polysomes) (upper panel). HuR and methyl-HuR protein in each fraction were analyzed by Western blot analysis. Each assay was carried out in triplicate.
To assess whether the two HuR forms bind MAT2A mRNA differentially, RNP-IP assays were performed over different time points in culture hepatocytes, with or without SAMe. As shown in Figure 2C, MAT2A mRNA was enriched in HuR-IP samples compared to control IgG-IP samples, with peak enrichment at 12 h. Interestingly, treatment with SAMe decreased the levels of MAT2A mRNA and largely suppressed the [HuR-MAT2A mRNA] RNP complexes (Figure 2A and Figure 2C). In contrast, methyl-HuR was bound to MAT2A mRNA only in the presence of SAMe (Figure 2D). This suggests that HuR and methyl-HuR have opposing effects on MAT2A regulation; HuR stabilizes MAT2A mRNA and methyl-HuR likely destabilizes MAT2A mRNA. Our results indicate that the ratio between HuR/methyl-HuR might function as a sensor mechanism to control specific targets like MAT2A mRNA during the de-differentiation process of hepatocytes.
To address the functional role of methyl-HuR in MAT2A mRNA destabilization, an HuR mutant was generated with lysine substitutions at the argine 217 methylation site [HuR(R217K)], and its interaction with MAT2A mRNA analyzed in MLP29 cells. We found that the association with HuR(WT) was constitutively lower in the presence of SAMe, while the association with HuR(R217K) remained unaltered (Figure S2). Furthermore, we failed to detect MAT2A mRNA in AUF1-IP samples from hepatocytes treated with SAMe (data not shown), suggesting that AUF1 is not involved in the methyl-HuR-mediated mRNA destabilization.
Finally, hepatocytes, cultured for 24 h in the presence or absence of SAMe, were used to prepare polysomes by sucrose gradient fractionation. MAT2A mRNA levels were elevated in the molecular-weight fractions 9 to 11 containing the actively translating polysomes of untreated cells. After SAMe treatment, MAT2A mRNA was localized to the lighter fractions (1 to 8), which had either very limited or no translational activity (Figure 2E upper panel). HuR protein was detected in fractions 1 to 8, while in SAMe-treated hepatocytes, HuR was more abundant in fractions characterized as having limited translational activity (1 to 3). Methyl-HuR signals were found almost exclusively in the low-molecular weight fractions irrespective of SAMe treatment (Figure 2E lower panel). Taken together, these data suggest that HuR-bound MAT2A mRNA is stabilized and actively translated during the de-differentiation of the hepatocytes, while in the presence of SAMe, only methyl-HuR remains colocalized with untranslated MAT2A mRNA.
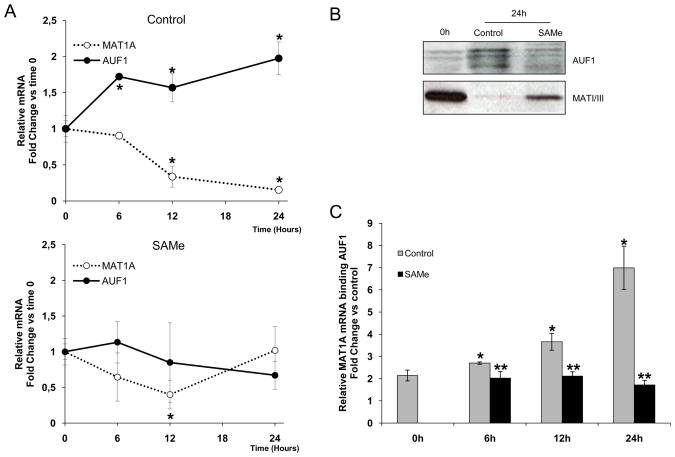
We also found that MAT1A mRNA and MATI/III protein decreased during the culture period, concomitant with an increase in AUF1 mRNA and protein levels (Figure 3A, upper panel, Figure 3B). This response was inhibited by SAMe treatment (Figure 3A, lower panel). AUF1-MAT1A mRNA RNP complexes also increased in a time-dependent manner and predictably, this effect was blocked by SAMe treatment (Figure 3C). These results suggest a functional link between AUF1 expression and MAT1A mRNA levels during the de-differentiation process, which is ablated by SAMe treatment.
Figure 3.
MAT1A expression during de-differentiation of cultured hepatocytes. A) MAT1A mRNA expression in rat hepatocytes at the indicated times, *p<0.05, time of treatment versus time 0 h. B) Representative Western blot analyses of AUF1 and MATI/III levels in rat hepatocytes undergoing de-differentiation; data are representative of 3 independent experiments. C) RNP-IP analysis of the association of AUF1 with MAT1A mRNA in cytoplasmic fractions of rat hepatocytes incubated as indicated. The enrichment of MAT1A mRNA in AUF1-IPs was calculated as described in Figure 2C. p<0.05, *time of treatment versus time 0 h, **SAMe versus control.
Role of HuR, methyl-HuR and AUF1 during liver development
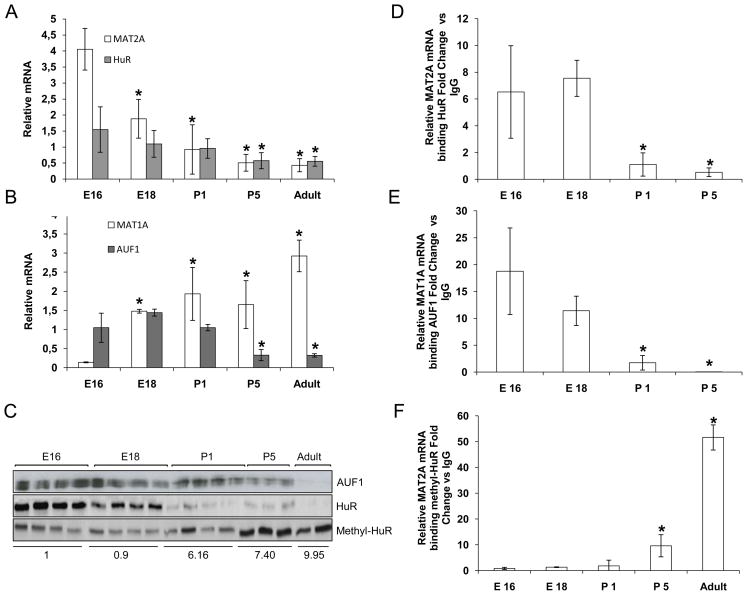
The aforementioned data raises the question of whether these two RNA-binding proteins are also involved in liver differentiation. In rats and humans, MAT1A is expressed only in the adult liver, while MAT2A is predominantly found in fetal liver with only minimal expression in the adult organ (2, 31). We examined the expression levels of MAT2A, MAT1A, HuR and AUF1 mRNAs in livers from fetuses (E16, E18), neonates (P1 and P5), and adult rats (3 months) (Figure 4A and 4B). MAT2A and HuR mRNA levels decreased during liver development to minimal levels in adult rats (Figure 4A). In contrast, MAT1A mRNA reached its maximum expression in adult liver, whereas AUF1 mRNA decreased as the rats aged (Figure 4B). There was a sharp decrease in AUF1 and total HuR protein levels with development, while methyl-HuR levels increased (Figure 4C). The methyl-HuR/HuR ratio revealed an increase of the methyl-HuR that correlated with an observed decrease in MAT2A mRNA levels.
Figure 4.
Role of HuR, methyl-HuR and AUF1 during liver development. A, B) mRNA expression of MAT2A, HuR, MAT1A and AUF1 in fetal livers (E16, E18), and livers from pups (P1 and P5) and adult rats (3 months), normalized to GAPDH mRNA, p<0.05, *ages of development versus E16. C) Levels of HuR, methyl-HuR and AUF1 as evaluated by Western blot analysis. Ponceau S staining was used as loading control (Supplemental Figure 3). The ratio of methyl-HuR/HuR was calculated, *p<0.05, ages of development versus E16. D, E) Binding of HuR or AUF1 to target mRNAs during liver development, as assessed by RNP-IP and real-time PCR analysis, *p<0.05, ages of development versus E16. F) RNP-IP analysis of MAT2A mRNA bound to methyl-HuR, enrichment represents the average from triplicate experiments, *p<0.05, ages of development versus E16.
A sharp decrease in the HuR-MAT2A mRNA RNP complexes was observed during the different stages of liver development (Figure 4D). There was a reduction in the levels of [AUF1-MAT1A mRNA] RNP complexes, which correlated with increased MAT1A mRNA levels (Figure 4E). Finally, an increase in the binding of methyl-HuR to MAT2A mRNA was observed with development (Figure 4F), suggesting that methyl-HuR might destabilize MAT2A mRNA and/or inhibit its translation. Taken together, these results strongly suggest that a balance between methyl-HuR, HuR, and AUF1 is required to regulate the levels of MAT2A and MAT1A mRNAs during liver differentiation.
Regulation of MAT2A in an in vivo model of chronic excess of hepatic SAMe
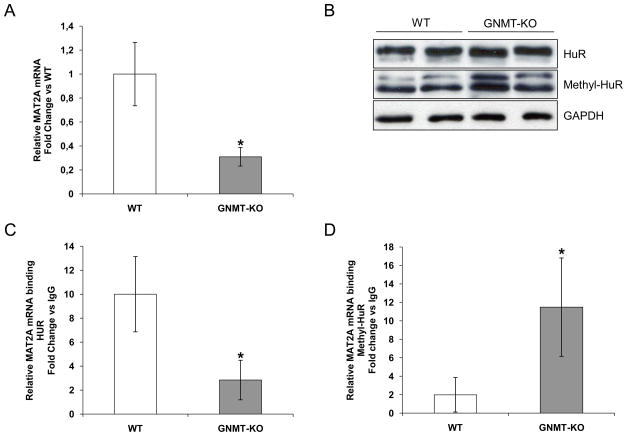
The maintenance of adequate hepatic SAMe levels for proper liver function is critical (32). GNMT is the enzyme responsible of the catabolism of SAMe. The GNMT KO mouse has a chronic excess of SAMe and spontaneously develops steatosis, fibrosis, and HCC (20). MAT2A mRNA levels were significantly lower in GNMT mutant livers compared to wild-type mice (WT) (Figure 5A) while methyl-HuR protein levels were significantly higher in GNMT mutant livers compared to WT animals (Figure 5B). In contrast, although SAMe levels were high, GNMT KO mice did not show reduced HuR levels, possibly due to the highly proliferative status of the livers in these mice (20). In addition, the amount of [HuR-MAT2A mRNA] RNP complexes was lower in KO mice compared to WTs, whereas [methyl-HuR-MAT2A mRNA] complexes were increased in KO animals (Figure 5C, 5D). The low levels of hepatic MAT2A mRNA observed in the GNMT KO mice could be due to an increase in the ratio of methyl-HuR/HuR, the reduction of [HuR-MAT2A mRNA] RNP complexes, and the enhanced levels of [methyl-HuR-MAT2A mRNA] complexes.
Figure 5.
The levels of MAT2A mRNA are regulated by HuR in GNMT KO mice. A) MAT2A mRNA expression in GNMT KO mice expressed as fold change versus WT (*p<0.05). B) Levels of HuR, methyl-HuR, and loading control GAPDH in total extracts. C) RNP-IP analysis of HuR binding to MAT2A mRNA. D) RNP-IP analysis of MAT2A mRNA bound to methyl-HuR, *p<0.05, GNMT KO versus WT.
HuR and AUF1 levels in human hepatocellular carcinoma
The switch between MAT1A and MAT2A genes was investigated in human hepatoma cell lines and HCC tissues resected from patients (33). Immunofluorescent analyses of healthy versus cancerous liver revealed that HuR and AUF1 were expressed at low levels in normal human liver samples, while liver tumors were strongly positive for HuR and AUF1 staining (Figure 6). In contrast, the signal for methyl-HuR was stronger in healthy livers than in livers containing tumors (Figure 6).
Figure 6.
HuR, methyl-HuR and AUF1 detection in human HCC. A) Representative immunofluorescence analysis of HuR, methyl-HuR and AUF1 protein in normal and human HCC samples. B) The relative immunofluorescence intensity in cancer tissues was calculated using Image J software and expressed as fold change of the relative immunofluorescence intensity in normal tissues. Data are representative of experiments realized in 22 HCC patients and 4 normal biopsies, and fold changes are significantly different (*p<0.05).
DISCUSSION
MAT is the sole enzyme responsible for SAMe synthesis, and the proper regulation of SAMe levels is critical for the maintenance of liver function (32, 1). In adult and quiescent hepatocytes, MAT1A is the gene expressed, encoding the isoform MATI/III. However, in fetal liver or during periods of proliferation and malignant transformation, it is the MATII isoform, encoded by the MAT2A gene, that predominates (2). In hepatocytes, SAMe controls development, de-differentation, and proliferation (2, 22, 25, 29). Our data indicate that HuR and AUF1 could be pivotal regulators of this process through their influence on the post-transcriptional expression of MAT2A and MAT1A. Specifically, we found correlative shifts between HuR and AUF1 abundance and MAT2A and MAT1A expression during these processes. In addition, the observation that SAMe regulates the balance between methyl-HuR and HuR, thereby modulating MAT2A mRNA levels, led us to propose that methyl-HuR could function as a destabilizer of MAT2A mRNA, potentially influencing hepatocyte proliferation.
There is great interest in understanding the genetic changes that occur during malignant transformation of the liver. Hepatocytes isolated from healthy rat livers lose the expression of their liver-specific genes when maintained in culture (34) and thus recapitulate certain characteristics of the transformation process. A switch between MAT1A/MAT2A mRNA occurs during the de-differentiation of cultured hepatocytes. SAMe prevented changes in the expression of these and other genes while maintaining homeostasis in one-carbon metabolism in functional hepatocytes (29). Our findings revealed that HuR and AUF1 mRNA levels were up-regulated in a time-dependent manner during the de-differentiation process. HuR became methylated in the presence of SAMe, and unexpectedly, the association of methyl-HuR with MAT2A mRNA appeared to promote the decay of MAT2A mRNA. Previous reports have considered HuR as a negative translational regulator in mammalian cells (30), and this effect of HuR is observed in cooperation with other RNA-binding proteins or microRNAs (35). In our studies, we focused on the consequences of the possible involvement of AUF1 in the decay of MAT2A mRNA associated with methyl-HuR in the presence of SAMe. However, RNP-IP analysis in the presence of SAMe showed no interaction, suggesting that AUF1 was not implicated in the loss of MAT2A mRNA.
Although many aspects of the regulation of HuR and methyl-HuR binding activities remain to be elucidated, a model emerges from our results whereby HuR promotes the accumulation of MAT2A mRNA, while methyl-HuR inhibits it. In the presence of high levels of SAMe, the balance between the two HuR forms ultimately determines the steady-state abundance of MAT2A mRNA. This mechanism is highly relevant in vivo, as HuR would be expected to influence the process of hepatocyte de-differentiation, leading to a loss of MAT homeostasis and impaired liver function, which might then enhance malignant transformation in the liver.
During embryonic development there is a switch in expression from MAT2A to MAT1A (2). During active liver proliferation, there is a reduction in HuR and an increase in methyl-HuR levels, thereby reducing MAT2A mRNA expression. In contrast, MAT1A mRNA levels showed an inverse correlation with AUF1 abundance, consistent with its decay-promoting function, its increased association with MAT1A mRNA during de-differentiation, and its reduced interaction in the presence of SAMe. Taken together, our results suggest a mechanism whereby the relative abundance of HuR, methyl-HuR, and AUF1 can drive the differentiation of hepatocytes, in a process dependent on SAMe levels. The preservation of MAT1A mRNA expression and MAT activity is a fundamental feature of healthy and differentiated hepatocytes. In this regard, AUF1 and HuR could be considered pivotal regulators of this essential biological process.
Finally, in human HCC, a switch from MAT1A mRNA to MAT2A mRNA expression facilitates cancer cell growth. Although a transcriptional regulation has been described for this switch in gene expression pattern (36), our results also suggest a post-transcriptional regulation of MAT2A and MAT1A mRNA levels by HuR, methyl-HuR, and AUF1 in HCC. HuR is commonly upregulated in most tumor types and the deregulation of AUF1 promotes tumorigenesis (17, 37). Normally, AUF1 is only found in very low levels in adult liver (38). We were able to detect the presence of both HuR and AUF1 in resected samples from HCC patients with different etiologies, whereas methyl-HuR was consistently found in low abundance. By contrast, in normal human control samples HuR and AUF1 were expressed at low levels, while methyl-HuR was expressed at high levels. Our results allow us to postulate that elevated levels of HuR and AUF1 and reduced expression of methyl-HuR observed in surgically resected HCC might be one of the hallmarks of the transformation of hepatocytes into cancer cells. Such an imbalance between HuR and AUF1 can underlie the deregulation of MAT2A/MAT1A homeostasis, the decrease in SAMe levels, and the proliferation of liver cancer cells.
In conclusion, our results provide a model for HuR, methyl-HuR and AUF1 function on the post-translational regulation of MAT2A and MAT1A mRNAs in a SAMe-dependent manner during essential biological processes like hepatocyte differentiation, de-differentiation, and malignant transformation (Figure 7). There is a similar temporal distribution of both RNA-binding proteins, suggesting that they regulate cell growth and differentiation through their opposing functions. Finally, the observation that methyl-HuR binds to MAT2A mRNA in correlation with its enhanced decay is a novel finding that points to a mechanism through which SAMe may regulate liver functionality. These results are significant as they reveal critical new insight into the molecular mechanisms underlying the switch between MAT1A and MAT2A expression, which is consistently observed during malignant hepatic transformation, and facilitates the development and progression of HCC.
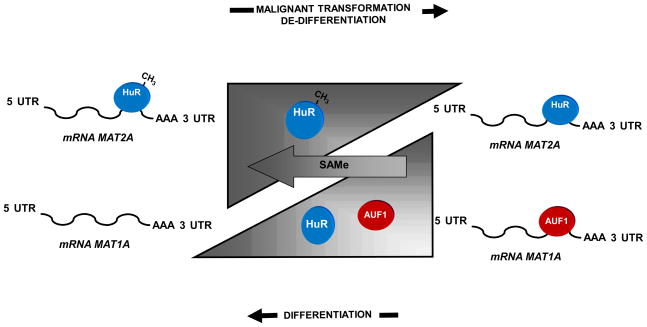
Figure 7.
Diagram of MAT2A, MAT1A and their post-translational regulators HuR, methyl-HuR, and AUF1 in hepatocyte de-differentiation, development and malignant transformation. Mature hepatocytes express high levels of MAT1A mRNA and low levels of AUF1, while MAT2A mRNA is in low abundance due to its negative regulator, methyl-HuR. During de-differentiation, the levels of AUF1 mRNA in hepatocytes increases and the ratio of methyl-HuR/HuR decreases. This leads to a switch from MAT1A to MAT2A mRNA expression. SAMe treatment of hepatocytes prevents these changes. Malignant transformation of hepatocytes is accompanied by similar expression patterns for AUF1, HuR and methyl-HuR, as well as for MAT1A and MAT2A mRNAs. During liver development, the opposite is observed, with decreased AUF1 levels and increased methyl-HuR/HuR ratios.
Supplementary Material
Supplemental Figure 1
The 3′UTR of MAT2A confers instability to GFP mRNA. The 3′UTR of MAT2A was cloned into the expression vector pEGFP-C2-3′UTR and the resulting construct was transfected in MLP29 cell line. Cells were treated with SAMe (4 mM) for 4 h. A) Western blot analysis of GFP expression. The results are representative of 3 independent experiments. B) Quantification of the expression levels for GFP and GFP-3′ UTR. There was no significant difference in GFP expression with or without SAMe; the decrease in protein expression was significant (p<0.05) when the cells were transfected with GFP-3′UTR.
Supplemental Figure 2
HuR(R217K)-V5 binding to MAT2A mRNA is not altered in the presence of SAMe. The binding of HuR(WT)-V5 and HuR(R217K)-V5 to MAT2A was assayed by RNP IP in MLP29 cells treated with or without SAMe (4 mM). The levels of MAT2A mRNA were first normalized to the levels of GAPDH mRNA, and expressed relative to the levels of MAT2A mRNA in IgG IPs. *p<0.05, SAMe versus control.
Supplemental Figure 3
Ponceau S staining of fetal liver extracts. Ponceau S staining of the nitrocellulose membranes was performed to ensure equal loading of protein samples in fetal liver western blot experiments.
Supplemental Table 1
Raw data of RNP IP analysis of MAT2A mRNA bound to HuR using cytoplasmic fractions of rat hepatocytes treated with SAMe for 24 h. MAT2A mRNA levels were normalized to GAPDH mRNA levels, and calculated relative to the levels of MAT2A mRNA in IgG IP samples. Data are the average of 3 independent experiments performed in triplicate.
Supplemental Table 2
Raw data of RNP IP analysis of MAT2A mRNA bound to methylated HuR using cytoplasmic fractions of rat hepatocytes treated with SAMe for 24 h. MAT2A mRNA levels in methylated HuR IP samples were normalized to GAPDH mRNA levels in the same IP samples, and expressed relative to the levels of MAT2A mRNA in IgG IP samples. Data are the average of 3 independent experiments performed in triplicate.
Acknowledgments
Grant Support: NIH grants DK51719 (to S. C. L) and AT-1576 (to S.C.L., J.M.M. and M.L.M.-C)), SAF2005-00855, HEPADIP-EULSHM-CT-205 and ETORTEK-2008 (to J.M.M. and M.L.M.-C); Cátedra de Biomedicina de la Fundación BBVA grant number CAT06_002 (to R.H.F and J.M.M.); Program Ramon y Cajal del MEC and Fundacion “La Caixa” (to M.L.M.-C.). NIH grant support DK15289 (to C.W). NIA-IRP, NIH (M.G). Pilot funds from the NIDDK-USC Liver Center (to I.A.L-O.) Ciberehd is funded by the Instituto de Salud Carlos III.
Footnotes
None of the authors have conflict of interest to declare
Contributions
Mercedes Vázquez-Chantada. Study concept and design, acquisition of data, technical support. Analysis of the data.
David Fernández-Ramos. Study concept and design, acquisition of data, technical support. Analysis of the data.
Nieves Embade. Study concept and design, acquisition of data, technical support. Analysis of the data.
Nuria Martínez. Study concept and design, acquisition of data, technical support. Analysis of the data.
Marta Varela-Rey. Study concept and design, acquisition of data, technical support. Analysis of the data. Critical revision of the manuscript for important intellectual content.
Ashwin Woodhoo. Techinical support. Critical revision of the manuscript.
Zigmund Luka. Critical revision of the manuscript and material support.
Conrad Wagner. Critical revision of the manuscript and material support.
Paul P. Anglim. Material support.
Richard H. Finnell. Critical revision of the manuscript for important intellectual content.
Juan Caballería. Critical revision of the manuscript for important intellectual content and material support.
Ite A. Laird-Offringa. Critical revision of the manuscript for important intellectual content and material support.
Myriam Gorospe. Critical revision of the manuscript for important intellectual content and material support.
Shelly C Lu. Critical revision of the manuscript for important intellectual content.
José M Mato. Analysis and interpretation of data. Critical revision of the manuscript for important intellectual content.
M Luz Martínez-Chantar. Study concept and design. Analysis and interpretation of data. Writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mato JM, Corrales FJ, Lu SC, et al. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- 2.Gil B, Casado M, Pajares M, et al. Differential expression pattern of methionine adenosyltransferase isoenzymes during rat liver development. Hepatology. 1996;24:876–881. doi: 10.1002/hep.510240420. [DOI] [PubMed] [Google Scholar]
- 3.Cai J, Mao Z, Hwang JJ, et al. Differential expression of methionine adenosyltransferase genes influences the rate of growth of human hepatocellular carcinoma cells. Cancer Res. 1998;58:1444–1450. [PubMed] [Google Scholar]
- 4.Pascale RM, Marras V, Simile MM, et al. Chemoprevention of rat liver carcinogenesis by S-adenosyl-L-methionine: A long-term study. Cancer Res. 1992;52:4979–4986. [PubMed] [Google Scholar]
- 5.Shivapurkar N, Poirier LA. Tissue levels of S-adenosylmethionine and S- adenosylhomocysteine in rats fed methyl-deficiency, amino acid-defined diets for one to five weeks. Carcinogenesis. 1983;4:1051–1057. doi: 10.1093/carcin/4.8.1051. [DOI] [PubMed] [Google Scholar]
- 6.Lu SC, Alvarez L, Huang ZZ, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Chantar ML, Corrales FJ, Martinez-Cruz LA, et al. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 8.Torre L, López-Rodas G, Latasa MU, et al. DNA methylation and histone acetylation of rat methionine adenosyltransferase 1A and 2A genes is tissue-specific. Int J Biochem Cell Biol. 2000;32:397–404. doi: 10.1016/s1357-2725(99)00140-5. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Chantar ML, García-Trevijano ER, Latasa MU, et al. Methionine adenosyltransferase II beta subunit gene expression provides a proliferative advantage in human hepatoma. Gastroenterology. 2003;124:940–8. doi: 10.1053/gast.2003.50151. [DOI] [PubMed] [Google Scholar]
- 10.Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zubiaga AM, Belasco JG, Greenberg ME. The nonamer UUAUUUAUU is the key AU-rich sequence motif that mediates mRNA degradation. Mol Cell Biol. 1995;15:2219–2230. doi: 10.1128/mcb.15.4.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenan CM, Steitz JA, Hinman MN, et al. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65:3168–81. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Caldwell MC, Lin S, et al. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 2000;19:2340–2350. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Yang X, Cristofalo VJ, et al. Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence. Mol Cell Biol. 2001;21:5889–5898. doi: 10.1128/MCB.21.17.5889-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antic D, Keene JD. Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. Am J Hum Genet. 1997;61:273–278. doi: 10.1086/514866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Wagner BJ, Ehrenman K, et al. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993;13:7652–7665. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouble A, Grazide S, Meggetto F, et al. A new player in oncogenesis: AUF1/hnRNPD overexpression leads to tumorigenesis in transgenic mice. Cancer Res. 2002;62:1489–1495. [PubMed] [Google Scholar]
- 18.Blaxall BC, Dwyer-Nield LD, Bauer AK, et al. Differential expression and localization of the mRNA binding proteins, AU-rich element mRNA binding protein (AUF1) and Hu antigen R (HuR), in neoplastic lung tissue. Mol Carcinog. 2000;28:76–83. [PubMed] [Google Scholar]
- 19.Di Marco PN, Pearce PH, Oliver IT. Postnatal changes in blood glucose, phosphopyruvate carboxylase and tyrosine aminotransferase after normal birth and premature delivery in the rat. Biol Neonate. 1976;30:10. [Google Scholar]
- 20.Martínez-Chantar ML, Vázquez-Chantada M, Ariz U, et al. Loss of Glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neufeld DS. Isolation of rat liver hepatocytes. Methods Mol Biol. 1997;75:145–151. doi: 10.1385/0-89603-441-0:145. [DOI] [PubMed] [Google Scholar]
- 22.Vázquez-Chantada M, Ariz U, Varela-Rey M, et al. Evidence for LKB1/AMP-activated protein kinase/endothelial nitric oxide synthase cascade regulated by hepatocyte growth factor, S-adenosylmethionine, and nitric oxide in hepatocyte proliferation. Hepatology. 2009;49:608–617. doi: 10.1002/hep.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. doi: 10.1016/s0301-472x(02)00806-8. [DOI] [PubMed] [Google Scholar]
- 24.Huggett J, Dheda K, Bustin S, et al. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Chantar ML, Vazquez-Chantada M, Garnacho M, Latasa MU, Varela-Rey M, Dotor J, Santamaría M, Martínez-Cruz LA, et al. S-adenosylmethionine regulates cytoplasmic HuR via AMP-activated kinase. Gastroenterology. 2006;131:223–232. doi: 10.1053/j.gastro.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Park S, Kilburn B, Jelinek MA, et al. Lipopolysaccharide-induced methylation of HuR, an mRNA-stabilizing protein, by CARM1. Coactivator-associated arginine methyltransferase. J Biol Chem. 2002;277:44623–30. doi: 10.1074/jbc.M206187200. [DOI] [PubMed] [Google Scholar]
- 27.Pañeda C, Gorospe I, Herrera B, et al. Liver cell proliferation requires methionine adenosyltransferase 2A mRNA up-regulation. Hepatology. 2002;35:1381–91. doi: 10.1053/jhep.2002.32538. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Chantar ML, Latasa MU, Varela-Rey M, et al. L-methionine availability regulates expression of the methionine adenosyltransferase 2A gene in human hepatocarcinoma cells: role of S-adenosylmethionine. J Biol Chem. 2003;278:19885–19990. doi: 10.1074/jbc.M211554200. [DOI] [PubMed] [Google Scholar]
- 29.García-Trevijano ER, Latasa MU, Carretero MV, et al. S-adenosylmethionine regulates MAT1A and MAT2A gene expression in cultured rat hepatocytes: a new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 2000;14:2511–2518. doi: 10.1096/fj.00-0121com. [DOI] [PubMed] [Google Scholar]
- 30.Katsanou V, Papadaki O, Milatos S, et al. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell. 2005;19:777–789. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Liau MC, Chang CF, Belanger L, et al. Correlation of isozyme patterns of S-adenosylmethionine synthetase with fetal stages and pathological states of the liver. Cancer Res. 1979;39:162–169. [PubMed] [Google Scholar]
- 32.Martínez-López N, Varela-Rey M, Ariz U, et al. S-adenosylmethionine and proliferation: new pathways, new targets. Biochem Soc Trans. 2008;36:848–852. doi: 10.1042/BST0360848. [DOI] [PubMed] [Google Scholar]
- 33.Avila MA, Berasain C, Torres L, Martín-Duce A, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–914. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 34.Schuetz EG, Li D, Omiecinski CJ, Muller-Eberhard U, et al. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988;134:309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- 35.Suswam EA, Nabors LB, Huang Y, et al. IL-1beta induces stabilization of IL-8 mRNA in malignant breast cancer cells via the 3′ untranslated region: Involvement of divergent RNA-binding factors HuR, KSRP and TIAR. Int J Cancer. 2005;113:911–919. doi: 10.1002/ijc.20675. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Huang ZZ, Wang J, et al. The role of c-Myb and Sp1 in the up-regulation of methionine adenosyltransferase 2A gene expression in human hepatocellular carcinoma. FASEB J. 2001;15:1507–1516. doi: 10.1096/fj.01-0040com. [DOI] [PubMed] [Google Scholar]
- 37.Kang MJ, Ryu BK, Lee MG, et al. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008;135:2030–2042. 2042.e1–3. doi: 10.1053/j.gastro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Gouble A, Morillo D. Synchronous and regulated expression of two AU-binding proteins, AUF1 and HuR, throughout murine development. Oncogene. 2000;19:5377–5384. doi: 10.1038/sj.onc.1203910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1
The 3′UTR of MAT2A confers instability to GFP mRNA. The 3′UTR of MAT2A was cloned into the expression vector pEGFP-C2-3′UTR and the resulting construct was transfected in MLP29 cell line. Cells were treated with SAMe (4 mM) for 4 h. A) Western blot analysis of GFP expression. The results are representative of 3 independent experiments. B) Quantification of the expression levels for GFP and GFP-3′ UTR. There was no significant difference in GFP expression with or without SAMe; the decrease in protein expression was significant (p<0.05) when the cells were transfected with GFP-3′UTR.
Supplemental Figure 2
HuR(R217K)-V5 binding to MAT2A mRNA is not altered in the presence of SAMe. The binding of HuR(WT)-V5 and HuR(R217K)-V5 to MAT2A was assayed by RNP IP in MLP29 cells treated with or without SAMe (4 mM). The levels of MAT2A mRNA were first normalized to the levels of GAPDH mRNA, and expressed relative to the levels of MAT2A mRNA in IgG IPs. *p<0.05, SAMe versus control.
Supplemental Figure 3
Ponceau S staining of fetal liver extracts. Ponceau S staining of the nitrocellulose membranes was performed to ensure equal loading of protein samples in fetal liver western blot experiments.
Supplemental Table 1
Raw data of RNP IP analysis of MAT2A mRNA bound to HuR using cytoplasmic fractions of rat hepatocytes treated with SAMe for 24 h. MAT2A mRNA levels were normalized to GAPDH mRNA levels, and calculated relative to the levels of MAT2A mRNA in IgG IP samples. Data are the average of 3 independent experiments performed in triplicate.
Supplemental Table 2
Raw data of RNP IP analysis of MAT2A mRNA bound to methylated HuR using cytoplasmic fractions of rat hepatocytes treated with SAMe for 24 h. MAT2A mRNA levels in methylated HuR IP samples were normalized to GAPDH mRNA levels in the same IP samples, and expressed relative to the levels of MAT2A mRNA in IgG IP samples. Data are the average of 3 independent experiments performed in triplicate.