Abstract
P3 amplitude reduction (P3-AR) is associated with biological vulnerability to a spectrum of externalizing disorders, such as ADHD, conduct disorder, and substance use disorders. P3, however, is generally characterized as a broad activation involving multiple neurophysiological processes. One approach to separating P3-related processes is time-frequency (TF) analysis. The current study used a novel PCA-based TF analysis method to investigate relationships between P3, its associated TF components, and externalizing in a community-based sample of adolescent males. Results showed that 1) alone, P3 and each TF-PCA derived component could successfully discriminate diagnostic groups from controls, and 2) delta components in specific time ranges accounted for variance beyond that accounted for by P3. One delta component was associated with all diagnostic groups, suggesting it may represent a more parsimonious endophenotype for externalizing than P3-AR.
Keywords: P3, externalizing, substance use disorder, ADHD, conduct disorder, oppositional defiant disorder, time-frequency, PCA, delta, theta
Introduction
Reduced amplitude of the P3 event-related potential (ERP) has long been associated with alcoholism and familial risk of developing alcoholism (Begleiter, Porjesz, Bihari, & Kissin, 1984; Hill, 2004; 1994). This association between P3 amplitude reduction (P3-AR) and alcoholism, however, has recently been extended to encompass a spectrum of disorders characterized by behavioral disinhibition. In addition to alcoholism, this disinhibition spectrum includes disorders such as conduct disorder, attention-deficit/hyperactivity disorder, oppositional defiant disorder, and substance use disorders (Bauer & Hesselbrock, 2003; Iacono, Carlson, Malone, & McGue, 2002; Justus, Finn, & Steinmetz, 2001). Large-scale epidemiological studies with twins have shown that the common comorbidity among these disorders can be accounted for by an underlying “externalizing” factor that is highly heritable (Kendler, Prescott, Myers, & Neale, 2003; Krueger et al., 2002; Young, Stallings, Corley, Krauter, & Hewitt, 2000). Recently, P3-AR was shown to be associated with this externalizing factor (Patrick et al., 2006), and this association is accounted for by shared genetic effects (Hicks et al., 2007). These findings support the hypothesis that P3-AR is an endophenotype for general vulnerability to the spectrum of externalizing disorders, rather than for any one disorder specifically.
With regard to psychological disorders, an endophenotype is a measurable trait intermediate between the clinical manifestation of the disorder and the genes underlying the disorder (Gottesman & Gould, 2003). The endophenotype, then, is putatively a less complex correlate of the disorder that is closer to gene action, and that can aid in discovering the disorder’s genetic etiology. While P3-AR has been associated with a general vulnerability to externalizing, its utility as an endophenotype has only been tested with regard to alcohol use disorders. Visual P3 amplitude itself has shown significant linkage on chromosomes 2, 5, 6, 13, and 17 (Begleiter et al., 1998; Porjesz et al., 2002; Porjesz et al., 2005), and an association between P3 amplitude and the dopamine receptor A1 allele has been found (Hill et al., 1998). With respect to alcohol use, there is evidence that particular genetic loci affecting P3 amplitude also influence risk of alcohol dependence. Williams et al. (1999) found that reduced P3 amplitude and an alcoholism diagnosis were jointly linked to a region on chromosome 4 near the alcohol dehydrogenase gene (ADH3), and Hill et al. (1998) found an association between lower P3 amplitude and presence of the A1 allele in children from alcoholic families.
While these studies have demonstrated P3-AR’s potential as an endophenotype for alcoholism, there has long been evidence that multiple processes compose the P3 (e.g. Dien, Spencer, & Donchin, 2003; Mantini, Corbetta, Perrucci, Romani, & Del Gratta, 2009), suggesting that conventional P3 measures (e.g. peak amplitude) may not be an optimal representation of the processes involved in P3-AR. An emerging approach to this problem is time-frequency (TF) decomposition, which has been used to show that ERP activity during the P3 can be characterized by two primary TF components: theta (3–7 Hz) and delta (0–3 Hz) (Basar-Eroglu, Basar, Demiralp, & Schurmann, 1992; E. M. Bernat, Malone, Williams, Patrick, & Iacono, 2007; Demiralp, Ademoglu, Istefanopulos, Basar-Eroglu, & Basar, 2001; Jones et al., 2006; Yordanova, Devrim, Kolev, Ademoglu, & Demiralp, 2000). Theta in the P3 window has been attributed to frontal neural generators, and has been considered to index focused attention and memory encoding processes (Basar-Eroglu et al., 1992; Klimesch, 1999; Yordanova et al., 2000). P3-related delta, which tends to be parietally maximal, has been considered to index signal matching, decision-making, and memory updating (Basar-Eroglu et al., 1992; Karakas, Erzengin, & Basar, 2000).
From a signal processing perspective, TF approaches can offer a complete representation of activity in averaged time-domain signals typically used to measure P3, and thus have the potential to supplant current time-domain P3 measures and advance the utility of EEG/ERP data in this area. However, several obstacles have hampered adoption of TF approaches as a general replacement for standard time domain approaches to measuring P3. First, most TF methods do not directly target condition average waveforms, the level of analysis used in the vast majority of EEG/ERP studies of P3. Instead, applications have been more focused on information not available in condition averages, to make inferences about oscillatory dynamics in trial-level data or high-frequency activity (e.g. gamma activity, 30–50 Hz). Another obstacle has been the wavelet TF transform, which is the most widely used, but which lacks important properties such as uniform TF resolution and accurate representation of the energy in the signal. Particularly relevant is that wavelets do not generally provide good time support for activity at lower frequencies (e.g. below 3 Hz), where a majority of energy is located in the time region containing standard time-domain EEG/ERP components such as the P3. Finally, because TF transforms add a dimension to the signal representation (TF versus time or frequency alone), the complexity and amount of data is greatly increased, creating a need for new data reduction techniques. The approaches taken in the current report address these problems by utilizing more advanced TF representation algorithms (Cohen’s class reduced interference distribution (RID: Cohen, 1995) as opposed to wavelets), and implementing an effective TF data reduction technique based on the widely-understood statistical technique of principal components analysis (E.M. Bernat, Williams, & Gehring, 2005). For example, in recent work we presented evidence that the time-frequency principal components analysis (TF-PCA) approach can disentangle overlapping theta and delta processes, and produce better measures of the relevant processes in a feedback task typically indexed with time-domain feedback related-negativity (FRN) and P3 components (E.M. Bernat, Nelson, Holroyd, Gehring, & Patrick, 2008). In that report, the TF-PCA approach revealed statistically independent theta and delta processes, which were summed together in time-domain measures, creating confounded ERN and P3 measures that contained mixtures of the processes indexed separately by the TF measures. For all of these reasons, TF measures may provide a more optimal representation of the activity contained in the signals, and thus components measured in this way may ultimately serve as more parsimonious endophenotypes for externalizing than P3-AR.
Indeed, some recent efforts have shifted focus away from time-domain P3 amplitude and toward TF measures of ERP activity. Recent studies, for example, have revealed an association between P3-related delta and theta and alcoholism. Power in these frequency ranges was lower in adult alcoholics (Jones et al., 2006) and in high-risk adolescent and adult offspring of alcoholics (Kamarajan et al., 2006; Rangaswamy et al., 2007). Delta and theta bands were also shown to provide unique information to discriminate between alcoholic and control groups (Jones et al., 2006). Further, significant associations between event-related activity in these bands and genes implicated in alcohol dependence and related disorders have been found. P3-related delta and theta activity has been linked to CHRM2, a cholinergic muscarinic receptor gene on chromosome 7 (Jones et al., 2004; Porjesz & Rangaswamy, 2007). CHRM2 has been associated with higher cognitive processing and IQ (Comings et al., 2003; Dick et al., 2007; Gosso et al., 2007), and has been implicated in alcohol and drug dependence (Luo et al., 2005; Wang et al., 2004), and, most recently, in externalizing disorders (Dick et al., 2008). While there is yet no study of pleiotropic effects (i.e. shared genetic influences by CHRM2 on both alcoholism and P3-related delta and theta activity), these findings demonstrate the promise of event-related delta and theta as endophenotypes of alcoholism. Left unanswered, however, is whether this promise extends to other disorders that compose the externalizing psychopathology spectrum.
The present investigation is a direct extension of Iacono et al. (2002), in which we demonstrated an association between P3-AR and specific externalizing spectrum disorders (Iacono et al., 2002; also see Patrick et al., 2006). The present study extends the P3 findings of Iacono et al. by using the same subjects to examine the association between the TF components and P3 amplitude in individuals diagnosed with any one of six disorders falling in this spectrum. This is the first study employing the TF-PCA method to examine these relationships, and to do so using a population-based sample, thus allowing greater generalizability of the findings. Probit regression was used to determine the abilities of P3 amplitude and each TF component’s amplitude to differentiate adolescent males with an externalizing disorder from those with no disorder. It was hypothesized that TF components would show reduced amplitude in externalizing disorders compared to a control group. Further, these components would be able to independently differentiate the two groups by accounting for unique variance above and beyond that accounted for by P3 amplitude.
Methods
Subjects
Subjects were 506 male youths (228 twin pairs and 50 unmatched twins; Mean age = 17.5 years, SD = 0.4; range 16.6 – 18.3 years) from the older cohort of the Minnesota Twin Family Study (MTFS), a longitudinal and epidemiological study investigating the development of substance use disorders and related psychopathology. Subjects were identified from birth records as twins born between January 1, 1972 and December 31, 1978. A comprehensive description of the MTFS is found in Iacono & McGue (2002). Consistent with demographics of the state of Minnesota at the time the twins were born, nearly all (99%) were Caucasian. All subjects and their parents gave written informed assent or consent as appropriate.
Diagnostic Assessment
Trained clinical interviewers administered structured in-person interviews with the twins and their parents independently. Members of each twin-pair were interviewed concurrently by separate interviewers. Lifetime presence of DSM-III-R substance abuse and conduct disorders was assessed via a revised version of the Diagnostic Interview for Children and Adolescents (DICA; (Reich, 2000)) and an expanded version of the Substance Abuse Module from the Composite International Diagnostic Interview (Robins, Babor, & Cottler, 1987). Mothers reported on their twin sons through interviews using the parent version of the DICA (Reich, 2000). A DSM-III-R diagnosis was assigned on the basis of a consensus, “best-estimate” approach (Leckman, Sholomskas, Thompson, Belanger, & Weissman, 1982) combining mother and son interview data. A lifetime study diagnosis was given if either all DSM-III-R symptom criteria were met (definite certainty level) or all criteria but one were met (probable certainty level). Because a single symptom is sufficient for a diagnosis of substance abuse, all substance abuse cases were of definite certainty.
Psychophysiological Assessment
A rotated-heads visual oddball task (Begleiter et al., 1984) was used. Subjects viewed 240 stimuli consisting of either an oval (two-thirds of trials – “standards”) or a superior view of a stylized head (one-third of trials – “targets”), in which a nose and one ear were depicted on the oval. Subjects were required to respond to target trials by pressing a button on either the left or right armrest of their chair, corresponding to the side of the head on which the ear appeared. On half the target trials the nose pointed up (such that the left ear appeared on the left side of the screen; an easy discrimination), while on the other half of target trials the head was rotated 180° so that the nose pointed down (left ear appeared on the right side of the screen; a hard discrimination). Stimulus duration was 98 ms, and the inter-trial interval, during which subjects fixated on a dot in the center of the screen, varied randomly between 1 and 2 seconds.
Electroencephalographic (EEG) data acquisition
A Grass model 12A Neurodata Acquisition System recorded EEG and electrooculographic (EOG) data at a sampling rate of 256 Hz and filtered from 0.01 – 30 Hz (6 dB/octave rolloff). EEG, referenced to linked earlobes, was recorded from three parietal electrodes: on the midline at Pz, and over left and right hemispheres at P3 and P4, respectively. EOG was recorded using a pair of biopotential electrodes arranged in a transverse montage, one electrode placed superior to the eye and the other at the outer canthus. Impedances were kept below 5 kΩ for EEG and below 10 kΩ for EOG. Trials consisted of 2 s of data, including a 500 ms prestimulus baseline. Target trials were repeated if the subject failed to respond or the analog-to-digital converter’s limits were exceeded. Two standard trials were presented before each repeated target trial to maintain a constant proportion of target and standard trials. Trials repeated more than twice were excluded from averaging.
EEG data processing and reduction
Blinks and other ocular artifacts were corrected using the method of Gratton, Coles, and Donchin (1983). Trials with activity >100 µV were excluded from further processing. Averaged target waveforms were constructed separately for the easy and hard target conditions at each electrode site. P3 amplitude was defined as the point between 280 and 600 ms at which amplitude of the average waveform was maximal. Although there are different ways to capture P3 amplitude, we adopted this peak-in-window approach because a survey of studies exploring P3-AR’s relationship to externalizing spectrum disorders showed this to be the method of choice (Bauer & Hesselbrock, 2003; Brigham, Herning, & Moss, 1995; Chen et al., 2007; Enoch, White, Waheed, & Goldman, 2008; Hill, Locke, & Steinhauer, 1999; Hill, Muka, Steinhauer, & Locke, 1995; Iacono et al., 2002; Jones et al., 2006; M. S. Kim, Kim, & Kwon, 2001; Maurage et al., 2007; O’Connor, Bauer, Tasman, & Hesselbrock, 1994; Polich & Ochoa, 2004; Prabhu et al., 2001; Rangaswamy et al., 2007; Reese & Polich, 2003).Thus, our aim was to determine what TF analysis adds to the information yield derived from studies that have successfully identified P3-AR when measured this way.
P3 amplitude was highly correlated between the three sites (r=0.87 for Pz-P3 and Pz-P4), as well as between the easy and hard target conditions (r=0.89). Further, previous analyses have shown that associations between P3 amplitude and the externalizing factor do not differ as a function of these three electrode sites (Hicks et al., 2007; Patrick et al., 2006). Therefore, to simplify presentation, current analyses were performed on ERPs only from the Pz electrode and averaged over easy/hard target conditions.
Time-frequency PCA decomposition
The time-frequency PCA decomposition (TF-PCA) method is detailed in Bernat et al. (2005, also see E. M. Bernat et al., 2007). Here, the primary features are outlined. The data handling and decomposition steps were carried out in Matlab (version 6.5, Mathworks, Inc.) using a generalized set of scripts developed for this purpose1. All TF transforms were computed using Cohen’s class RID transform. TF transforms were created using the entire, baseline corrected (−500 to −10 ms), 2 s epoch to allow for rejection of edge effects from the transform. PCA was then performed on the resulting TF surfaces to decompose the surfaces into TF components. PCA applied to TF energy much resembles its application to signals in the time or frequency domain. First, TF surfaces are rearranged into vectors, recasting the TF energy into a matrix with subjects in rows (or trials if one were performing trial-level decomposition) and time-frequency energy points in columns. Then, the covariance matrix is decomposed, varimax rotation is applied to maximize simple structure, and the component vectors are rearranged back into surfaces representing each TF-PCA component’s matrix of rotated component loadings for each TF point. The number of components to extract was determined by inspecting the scree plot of singular values, representing the relative variance accounted for by each component, for a break, or elbow. Finally, each subject’s TF surface is weighted using the extracted TF-PCA components. To weight the original TF data, each time-frequency point is multiplied by the corresponding point in the matrix of rotated loadings for each component. This produces weighted data surfaces, for each subject for each TF-PCA component, whose data points represent energy in units weighted by the component loadings. For statistical analyses, component scores representing the peak energy on the weighted TF data surface (i.e. the time-frequency point with the highest energy) was used. This method allowed comparison of analogous measures from the TF (peak energy) and time (peak P3 amplitude) domains.
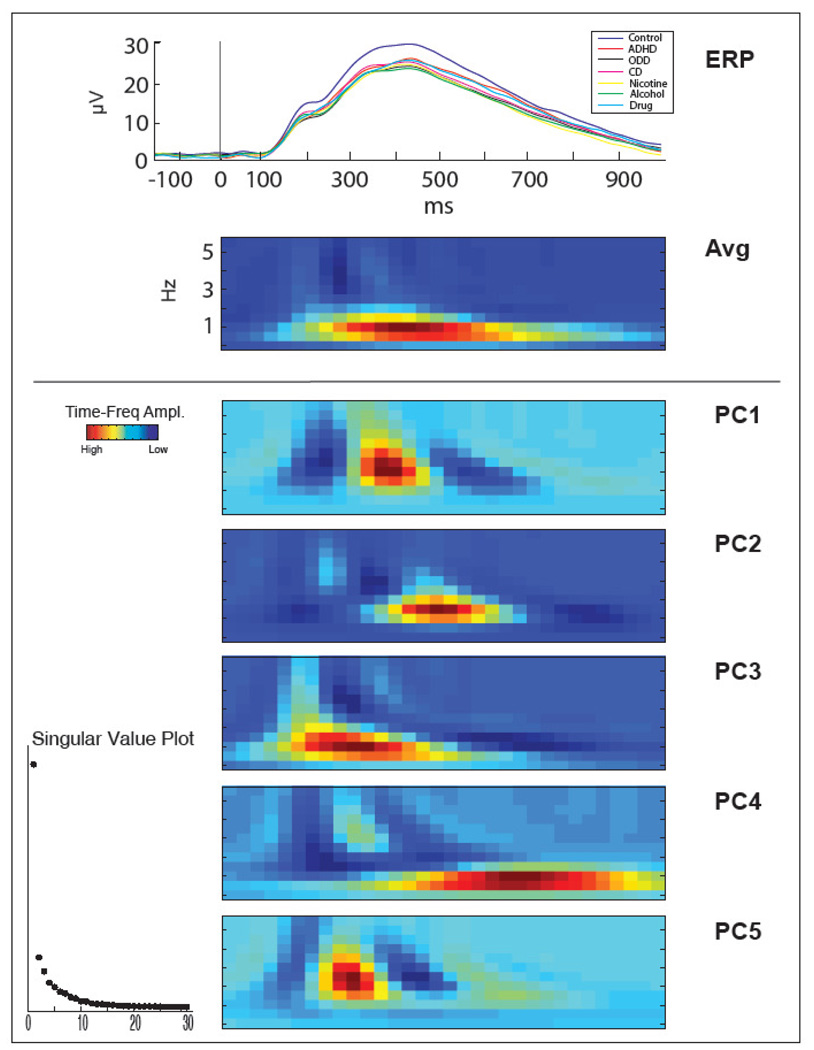
Decompositions were performed using averaged data, which enhanced brain activity that was consistently phase-locked to the stimulus, while attenuating non-phase-locked (e.g. induced) activity. This method provided the most direct parallel to the extant body of research on P3, thus allowing greater comparability to relevant P3 findings. Further, given our goal of exploring TF components as potential endophenotypes for externalizing, we sought to extend our previous findings by focusing current analyses on the same group of subjects used in Iacono et al. (2002), which characterized the relationship between P3-AR and externalizing disorders, and by utilizing the TF-PCA method of Bernat et al. (2007), which characterized the TF components associated with the P3 ERP. Therefore, to optimize the signal to noise ratio and stability of the components in the PCA decomposition, the TF-PCA was carried out using the 17-year-old sample described in Bernat et al. (N=2068), which included the subjects used in the current report, supplemented by the addition of 17-year-olds whose data have more recently become available (providing a total N=2084). Decompositions were performed on a frequency range of 0 – 5.75 Hz and a time range from stimulus onset (0 ms) to 1000 ms post-stimulus. Broader-range decompositions, which included frequencies through the upper range of alpha (0–12 Hz) were also performed; however, TF transforms of the averaged data predictably yielded no components in the upper frequency range. The present range was chosen, then, to achieve the best resolution decomposition of the frequency range within which there was activity of interest. Figure 1 shows the TF components for the group of 506 subjects in the current report, weighted by the PCA-derived component loadings from the larger sample (also see Results section for a more detailed description of the TF-PCA components).
Figure 1. Time-Frequency PCA (TF-PCA) Decomposition.
Time-frequency components for the group of 506 subjects in the current report, weighted by the PCA-derived component loadings from the larger sample (see Methods for a detailed description). Grand-averaged time (ERP) and time-frequency (Avg) plots are presented at the top. ERPs (from −150ms – 1000ms; stimulus onset at 0ms) are presented separately for each diagnostic group. The five time-frequency components (PCs 1–5) retained from the principal components analysis decomposition are presented below the grand averages. For all time-frequency plots, x-axis is time from stimulus onset (0 ms) to 1000 ms, and y-axes range from 0 – 5.75 Hz. Components are numbered (1–5: highest to lowest) based on the amount of variance for which they account in the varimax-rotated solution. Scree plot contains singular values (units not relevant) for the largest 30 components, depicting the relative variance accounted for by each component.
Statistical Analysis
To investigate the relationship between P3 amplitude, each TF component, and externalizing disorders, subjects were divided into diagnostic groups based on their clinical diagnoses at study intake. Diagnostic groups were: conduct disorder (CD; N=184), attention-deficit/hyperactivity disorder (ADHD; N=45), oppositional defiant disorder (ODD; N=87), nicotine dependence (ND; N=68), alcohol abuse/dependence (AAD; N=95), and illicit drug abuse/dependence (DAD; N=35; which included amphetamines, cannabis, cocaine, hallucinogens, inhalants, opioids, PCP, and sedatives). Group assignment was made without consideration of possible comorbid diagnoses, producing representative samples of individuals with each diagnosis. A control group was also formed, composed of those 71 subjects who were free of any psychiatric disorders and free of paternal risk for substance use disorders (i.e. their father and his first-degree male relatives had no history of serious substance abuse problems, determined by structured interviews with subjects’ fathers and mothers using the Substance Abuse Module, and a composite interview from the Family History-Research Diagnostic Criteria (Andreasen, Endicott, Spitzer, & Winokur, 1977) and Family Informant Schedule and Criteria (Mannuzza, Fyer, Endicott, & Klein, 1985)).
Probit regression was then used to test the hypothesis that each TF component would uniquely and independently discriminate between those subjects with an externalizing disorder and those with no disorder, above and beyond P3 amplitude’s ability to do so. Probit regression is analogous to logistic regression (Amemiya, 1981) and thus is suitable for dichotomous outcome variables. We used a robust weighted least squares estimator in Mplus (ver. 4.2; Muthén & Muthén, 2007), which facilitated accounting for the non-independence of the twin-pairs’ observations in our sample, using Mplus’s method for deriving standard errors that are appropriately adjusted when data are nested in groups as with twin pairs. Two approaches were employed. First, univariate regression models, in which P3 peak amplitude and each TF component’s peak energy amplitude were entered into separate models, were used to determine the components’ individual relationships to each externalizing disorder. Second, bivariate regression models, in which P3 and each TF component were entered into the model together (i.e. a model including P3 and TF component 1, a model including P3 and TF component 2, etc.), were used to determine the ability of each TF component to uniquely discriminate diagnostic group membership in the presence of P3 (i.e. account for significant variance above and beyond that accounted for by P3). The significance of each univariate model was tested using a Z-score, derived from the ratio of the probit regression coefficient for each independent variable to its standard error. A chi-square difference test (df=1) was used to test for a significant difference between corresponding univariate and bivariate models (e.g. univariate model with TF component 1 only vs. bivariate model with P3 and TF component 1 together). A significant difference indicated that the univariate model did not adequately fit the data compared to the bivariate model, thus indicating that the added variable was accounting for a significant amount of variance. If the difference was not significant, the fit of the univariate model was deemed adequate, and it was concluded that the added variable was not accounting for any additional variance.
Results
Behavioral Performance
Reaction time and response accuracy (number of correct hits) measures were available for 494 of the subjects. T-tests revealed that none of the diagnostic groups differed significantly from the control group on reaction time in response to targets (all t <1.79). Due to the non-normal distribution of the response accuracy data (overall hit rate out of 80: M=78.64 hits, SD=1.90, Median=79.00), Mann-Whitney U tests were used to test for differences between the control group and each diagnostic group. With one exception, response accuracy did not significantly differentiate the control group from any diagnostic group. The only exception was the ADHD group, which averaged one fewer hit (M=78.07, SD=1.97, Median=79.00) than the control group (M=79.09, SD=0.94, Median=79.00) (U=989.50, z=2.94, p<.01). Given the results of no significant effects between groups for reaction time and little effect for response accuracy, these variables were not considered further.
P3 Latency
Since diagnostic group membership was not mutually exclusive (i.e. because comorbid diagnoses were allowed, the same person can appear in more than one diagnostic group), each group’s P3 latency was compared to the control group in separate t-tests. No diagnostic group’s P3 latency significantly differed from that of controls (all t <0.66; see bottom of Table 1 for P3 latencies of each group).
Table 1.
Mean (SD) peak amplitudes for P3 (µV) and each TF component (PCs 1–5; weighted energy units) and latency (ms) of P3 for each diagnostic group.
| Control (N=71) | CD (N=184) | ADHD (N=45) | ODD (N=87) | ND (N=68) | AAD (N=95) | DAD (N=35) | |
|---|---|---|---|---|---|---|---|
| P3 | 28.4 (9.5) | 24.6 (7.7) | 25.3 (6.3) | 23.4 (7.0) | 23.4 (6.3) | 23.2 (6.5) | 24.2 (7.7) |
| PC1 | 38.7 (26.7) | 30.1 (20.4) | 27.4 (16.5) | 26.8 (15.7) | 25.3 (12.0) | 25.5 (13.7) | 25.8 (13.5) |
| PC2 | 63.3 (44.7) | 44.8 (28.8) | 44.7 (23.6) | 40.9 (24.7) | 41.0 (19.7) | 38.9 (18.8) | 43.6 (24.7) |
| PC3 | 63.9 (42.7) | 45.8 (28.7) | 44.6 (25.4) | 39.9 (24.0) | 39.2 (19.6) | 39.4 (21.2) | 43.2 (24.7) |
| PC4 | 45.7 (34.1) | 31.7 (21.3) | 33.1 (18.5) | 29.1 (19.5) | 29.3 (16.5) | 28.3 (16.2) | 33.2 (21.4) |
| PC5 | 33.1 (21.8) | 26.0 (17.9) | 24.8 (15.6) | 22.8 (13.3) | 21.7 (11.3) | 21.9 (12.4) | 23.2 (12.4) |
| P3 Latency | 453.1 (47.6) | 448.4 (58.4) | 455.6 (60.1) | 447.6 (56.8) | 448.1 (53.2) | 448.3 (58.7) | 460.1 (60.5) |
CD=Conduct Disorder; ADHD=Attention Deficit/Hyperactivity Disorder; ODD=Oppositional Defiant Disorder
ND=Nicotine Dependence; AAD=Alcohol Abuse/Dependence; DAD=Illicit Drug Abuse/Dependence
Time-Frequency PCA Decomposition
Based on the scree plot, five principal components, accounting for 77.3% of the variance, were retained (see Figure 1). Components are ordered based on the amount of variance for which they account (highest to lowest) in the varimax-rotated solution. Principal Component 1 (PC1), with peak energy centered between 2 and 2.5 Hz, contains activity at the front edge of P3. Component 2 (PC2) contains activity around 1.5 Hz and is closest in time to the peak of P3. Component 3 (PC3; centered around 1 Hz) is a low-frequency delta component spanning the time-range of the P2-N2-P3 ERP complex. Component 4 (PC4) is the longest duration, lowest frequency component (centered between 0.5 and 1 Hz), and is consistent with the slow-wave after the P3. Finally, Component 5 (PC5) contains activity centered around 2.5 Hz, similar to Component 1; however, it occurs slightly earlier, beginning nearer in time to the N2 and lasting up to the peak of P3.
Table 1 shows mean peak amplitudes for P3 and each TF component for each diagnostic group. Multiple regression revealed that these TF components collectively account for nearly all of the variance in peak P3 amplitude (R2 =0.91; F(5,500)=983.51, p<.001). Additionally, each component had significant zero-order correlations with P3 and with each other (see Table 2)2. These results suggest that these PCA-derived TF components characterize brain activity in a complementary, yet more detailed, way than time-domain ERP measures.
Table 2.
Correlations (Pearson’s r) among P3 peak amplitude and each TF component’s peak energy amplitude, collapsed across groups.
| P3 | PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|---|
| PC1 | 0.78 | ||||
| PC2 | 0.90 | 0.84 | |||
| PC3 | 0.94 | 0.83 | 0.90 | ||
| PC4 | 0.87 | 0.65 | 0.92 | 0.85 | |
| PC5 | 0.75 | 0.90 | 0.73 | 0.85 | 0.60 |
Note: p≤.001 for all correlations
Probit Regression
Univariate models
Table 3 summarizes results of the univariate analyses, showing the Z-score and portion of variance explained by each component for each diagnostic group (represented by McKelvey & Zavoina’s pseudo-R2 , the measure of probit regression model performance that most closely approximates ordinary least squares regression’s measure of explained variance, R2 ; (Hagle & Mitchell, 1992; Veall & Zimmerman, 1994)). Results show that, with few exceptions, each component successfully discriminated diagnostic from control groups. The exceptions, however, were only marginally non-significant with p-values ≤ 0.1 (in ADHD and DAD: p=0.06 for PC4, p=0.07 for PC5; p=0.1 for P3 in ADHD). In all cases, significant discriminative ability was based on reduced amplitudes in the diagnostic group compared to the control group.
Table 3.
Univariate probit regression results of each component for each diagnostic group.
| CD |
ADHD |
ODD |
ND |
AAD |
DAD |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z | R2 | z | R2 | z | R2 | z | R2 | z | R2 | z | R2 | |
| P3 | 2.78 ** | 0.07 | 1.60 | 0.06 | 3.20 *** | 0.14 | 2.85 ** | 0.15 | 3.40 *** | 0.15 | 1.99 * | 0.09 |
| PC1 | 2.24* | 0.05 | 2.11* | 0.12 | 2.68** | 0.13 | 2.60** | 0.20 | 3.05 ** | 0.17 | 2.01 * | 0.18 |
| PC2 | 3.08 ** | 0.09 | 2.12* | 0.14 | 3.34*** | 0.18 | 2.83 ** | 0.23 | 3.45 *** | 0.27 | 2.17* | 0.15 |
| PC3 | 3.24*** | 0.09 | 2.34* | 0.13 | 3.60 *** | 0.20 | 2.94** | 0.25 | 3.48 *** | 0.22 | 2.13* | 0.15 |
| PC4 | 3.07** | 0.09 | 1.79 | 0.11 | 3.34*** | 0.17 | 2.74** | 0.20 | 3.42 *** | 0.22 | 1.76 | 0.09 |
| PC5 | 2.32* | 0.04 | 1.88 | 0.08 | 2.71 ** | 0.13 | 2.64** | 0.18 | 2.99 ** | 0.15 | 1.85 | 0.12 |
| P3(mean) | 2.78** | 0.07 | 1.69 | 0.06 | 3.24*** | 0.14 | 2.89** | 0.15 | 3.46*** | 0.16 | 2.03* | 0.08 |
| PC3-TD | 2.88** | 0.07 | 2.09* | 0.10 | 3.33*** | 0.17 | 2.80** | 0.21 | 3.32*** | 0.19 | 1.80 | 0.11 |
P3(mean)=mean amplitude within a 40ms window centered on the peak of P3
PC3-TD=mean amplitude in time-domain within the time range spanned by PC3
Note: df=1 for all tests; R2=McKelvey & Zavoina’s pseudo-R2
p≤.05
p≤.01
p≤.001
Results further suggest that each TF component performed at least as well as P3 in differentiating many of the diagnostic groups from controls. Inspection of Table 3 shows that the amount of variance in group membership explained by each TF component was equal to or exceeded that of P3 in most cases. Pseudo-R2 was greater in magnitude for TF components (maximum, 0.27; Median, 0.15) than for P3 amplitude (maximum, 0.15; Median, 0.11) in 26 out of 30 comparisons. Notably, PCs 2, 3, and 4, the delta frequency components, consistently accounted for greater portions of variance than did P3.
Bivariate models
Table 4 summarizes results of chi-square difference tests comparing the corresponding univariate and bivariate models. In all cases, combining the TF component and P3 into the model offered improved ability to discriminate diagnostic from control groups, as demonstrated by positive chi-square differences between the univariate and bivariate models. In most cases, however, this improved group discrimination was not significant, indicating that the addition of the second variable into the model offered no significant additional discriminative value over the univariate model. Further, neither the TF component nor P3 was a better discriminator; neither accounted for significant additional variance in the presence of the other. These results were not surprising given the high correlations between P3 and the TF components.
Table 4.
Results of chi-square difference tests comparing the corresponding univariate and bivariate probit regression models for each diagnostic group. †
| Components Tested | CD | ADHD | ODD | ND | AAD | DAD | |
|---|---|---|---|---|---|---|---|
| PC1 + P3 | |||||||
| P3 | 2.57 | 0.06 | 2.23 | 0.44 | 1.18 | 0.01 | |
| PC1 | 0.02 | 2.15 | 0.50 | 1.37 | 1.23 | 1.75 | |
| PC2 + P3 | |||||||
| P3 | 0.17 | 2.44 | 0.01 | 0.03 | 1.46 | 0.14 | |
| PC2 | 2.81 | 5.69 * | 1.67 | 1.95 | 5.97 * | 1.10 | |
| PC3 + P3 | |||||||
| P3 | 0.86 | 4.10 * | 1.51 | 2.94 | 2.22 | 0.97 | |
| PC3 | 4.58 * | 8.08 ** | 6.46 ** | 7.01 ** | 6.63 ** | 2.30 | |
| PC4 + P3 | |||||||
| P3 | 0.03 | 0.25 | 0.17 | 0.19 | 0.01 | 0.65 | |
| PC4 | 2.32 | 1.58 | 0.96 | 0.85 | 2.33 | 0.01 | |
| PC5 + P3 | |||||||
| P3 | 2.92 | 0.09 | 2.07 | 0.93 | 1.64 | 0.35 | |
| PC5 | 0.03 | 1.00 | 0.93 | 1.87 | 1.44 | 1.36 | |
| PC3 + P3(mean) | |||||||
| P3(mean) | 0.74 | 2.63 | 1.02 | 2.15 | 1.22 | 1.16 | |
| PC3 | 4.31* | 5.91* | 5.41* | 5.94* | 4.99* | 2.53 | |
| PCs + PC3-TD‡ | |||||||
| PC3-TD | 8.76* | 1.22 | 3.62 | 2.49 | 4.58 | 2.84 | |
| PCs | 6.47* | 1.03 | 2.18 | 1.68 | 3.61 | 2.55 | |
| PC3 + PC3-TD | |||||||
| PC3-TD | 0.15 | 0.11 | 0.05 | 0.23 | 0.01 | 0.34 | |
| PC3 | 4.54* | 3.55 | 8.39** | 7.08** | 8.45** | 2.94 | |
P3(mean)=mean amplitude within a 40ms window centered on the peak of P3
PCs=peak energies of PCs 1, 3, & 5, collectively
PC3-TD=mean amplitude in time-domain within the time range spanned by PC3
Note: df=1 for all tests, except ‡ df=2.
p ≤ .05
p ≤ .01
A significant result indicates that component accounted for a significant amount of variance in the bivariate model, beyond that accounted for by the other component. A non-significant result indicates that component added no significant discriminative information above that contained in the univariate model.
PC3 represented a notable exception to this trend. Despite its large correlation with P3, PC3 remained a significant determinant of group membership, across all externalizing diagnostic groups, when added to the model with P3. The chi-square difference was significant in all cases except the DAD group (p=0.13). Although this effect was not quite significant, PC3 showed the strongest effect of all the TF components in differentiating the small sample of DAD cases from controls. Further, P3’s contribution to group discrimination became non-significant when added to the model with PC3 for all diagnostic groups but ADHD. For ADHD, both PC3 and P3 amplitude made significant contributions, as the PC3+P3 bivariate model fit significantly better than either univariate model alone. In addition to PC3, PC2 was also able to significantly determine group membership in the presence of P3 for the ADHD and AAD groups.
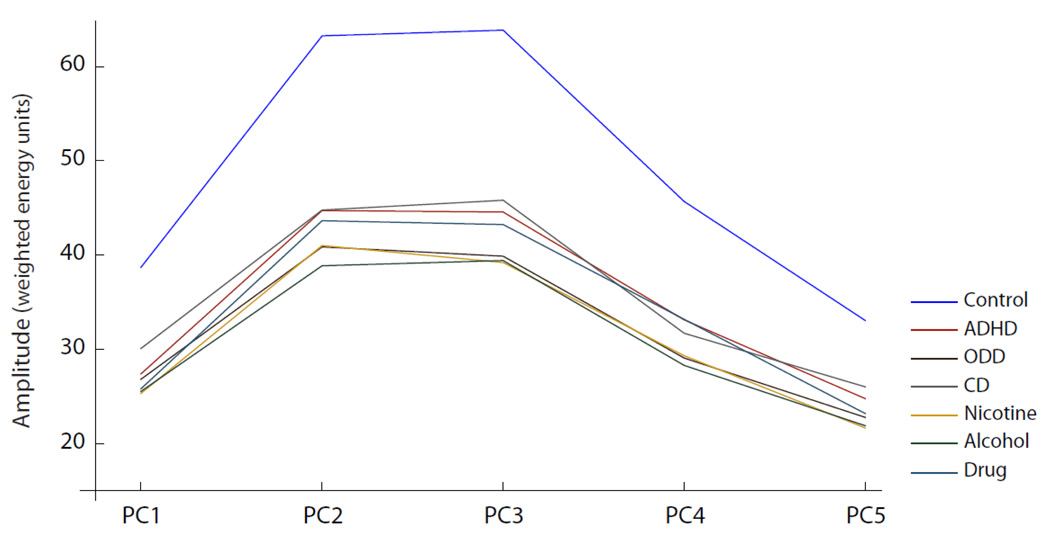
The similarity of results across diagnostic groups suggested that the profiles of TF activity are similar for these different facets of externalizing psychopathology. Similar TF profiles across groups supports previous findings (e.g. Krueger et al., 2002) of an externalizing factor underlying these disorders. As illustrated in the profile plot of energy amplitudes for each group (Figure 2), all diagnostic groups show comparable TF component profiles, with similarly reduced component amplitudes compared to the control group. To assess statistically whether amplitude of the TF components might be related to externalizing psychopathology, regardless of the specific diagnosis, we fit a common factor model to the (log-transformed) number of symptoms of each disorder. For drug dependence, we took the maximum number of symptoms of any of the eight classes of illicit substances. We included either PC2 or PC3 (given the significant results of the bivariate regression analyses) and allowed the component score to correlate with the common factor representing the shared variance among symptom counts of all disorders. Further, the variance unique to each disorder was allowed to correlate with each component, one at a time, to see if any such correlations were significant. For both PC2 and PC3, the correlation with the common factor was approximately r = −.20 and was highly significant, indicating that those higher in externalizing tendencies had smaller component scores. Correlations with the common factor were larger than the zero-order correlations with any of the symptom counts. Significant negative correlations between disorder-unique variance and the TF component would indicate that the component was associated with additional variance in the disorder not accounted for by what the disorder shares with the common factor. This was not the case for any of the disorders.
Figure 2. Time-Frequency Component Profiles.
Profile plot of peak energy amplitudes for each time-frequency component (PCs 1–5) for each diagnostic group.
Further Examination of the Relationship between TF-PCA and Time-Domain Measures
The results described above demonstrate that there is an advantage to characterizing the ERP signal in terms of its constituent TF components. P3-related delta activity, within particular time and frequency ranges, successfully differentiated those subjects with an externalizing spectrum disorder from those free of any disorder, above and beyond P3 peak amplitude’s ability to do so. P3 peak amplitude, however, while being the measure used in all the externalizing/substance use disorder studies we surveyed, may be more affected by noise and higher frequency activity than other measures (e.g. area or average amplitude). Therefore, to more comprehensively examine the relationship between externalizing, P3, and its TF components (PC3 specifically, given its unique relationship across externalizing spectrum disorders), we also performed follow-up analyses using additional time-domain measures.
P3 mean amplitude
A measure less commonly used to quantify P3 amplitude, but that may capture more P3 variance than does the peak, is to take the mean within some latency window. Therefore, we performed follow-up analyses using the mean amplitude within a latency window that was centered on each individual subject’s peak P3 amplitude. A 40 ms window was chosen to capture more P3 variance than does the peak, while excluding potential influence from earlier or later ERP activity. In the univariate probit regression model, mean P3 amplitude accounted for slightly more variance in group membership than did peak P3 amplitude for all diagnostic groups except DAD, for which it accounted for slightly less variance (differences between pseudo-R2 for P3 peak and P3 mean were all .01 or less; see Table 3). In the bivariate model with TF component PC3, results using mean P3 closely agreed with those using peak P3: PC3 still accounted for significant variance above and beyond that accounted for by P3 across diagnostic groups (again, not quite significant for DAD; see Table 4).
Mean ERP amplitude within the time range defined by TF-PC3
We additionally sought to demonstrate that the information contained in the TF-PCA components is the same as that contained in the condition average ERP time-domain measures – but characterized in a more optimal way. To this end, we extracted a new time-domain measure based on the TF-PCA optimized time window spanned by PC3 (given PC3’s unique association across externalizing groups). Probit regression analyses were performed for each diagnostic group comparing the activity in the time and TF domains residing within a time window defined by the time range spanned by PC3. As seen in Figure 1, the activity in PC3 lasts from 140–500 ms, spanning an interval containing the P2-N2-P3 ERP complex in the time domain and TF-PCA components 1, 3, and 5 in the TF domain. In the first set of analyses, the ability of the mean time-domain ERP amplitude within this time range to differentiate each diagnostic group from controls was compared to the ability of a model containing the peak energies, collectively, of the TF-PCA components that occurred within this same time range to do so (i.e. probit regression models containing either the time domain mean or PC 1, 3, and 5 peak energies were compared to a model containing all four of these measures). Thus, the collective time-domain information occurring in this time window was compared to the collective TF information occurring in this same window. First, this mean ERP measure alone accounted for slightly more variance in group membership than the traditional P3 peak amplitude, but less variance than did TF PC3 (see Table 3). Second, results show (see Table 4) that neither the mean ERP measure nor the group of TF-PCs within that time range accounted for any unique variance in group membership above and beyond the other (except the CD group, in which both measures accounted for the same, and some additional, variance). Thus, the same variance in group membership is accounted for by activity in both the time-domain and TF-PCA components residing in the time range defined by PC3.
A second set of analyses was performed to demonstrate the optimization of information gain gotten from the TF-PCA method. To this end, the discriminative ability of the mean ERP amplitude within this time range (spanned by TF-PC3) was compared to that of the mean energy of PC3 within the same time range, thus comparing analogous measures in the time and TF domains. Again, across externalizing groups, PC3 accounted for a significant amount of variance in group membership beyond that accounted for by the mean ERP measure (just short of significance for the ADHD and DAD groups, p=.059 & .086, respectively; see Table 4).
In total, these follow-up analyses further demonstrated that the TF-PCA method represents the averaged event-related activity in a more comprehensive and detailed manner than do typical time-domain and TF analysis methods. Beyond delineating more refined time windows in which to focus analytic efforts, TF-PCA was able to parse the ERP signal into its constituent components – components with a unique relationship to externalizing spectrum disorders. In the present paper, the P3-related delta activity represented by PC3 remained a significant determinant of group membership when compared to a variety of measures, supporting its role as a potential endophenotype for externalizing.
Discussion
The present study extended previous findings of an association between P3-AR and externalizing psychopathology in a population-based sample of adolescents by employing a novel, data-driven time-frequency analysis method to decompose the averaged ERP data in a new way. The extracted time-frequency component measures accounted for almost all of the variance in the time-domain P3 measures, demonstrating that the relevant variance for measuring the P3-AR was well represented in the decomposition. Next, these P3-related TF components were shown to be associated with disinhibitory disorders in the externalizing spectrum. Further, delta activity in particular time ranges was associated with externalizing disorders above and beyond the association between these disorders and P3 amplitude, suggesting that these TF components may serve as more parsimonious endophenotypes for externalizing than P3-AR.
A primary reason the TF measures outperformed the time-domain P3 measure is that the time-region defined by the TF-PCA approach was more sensitive to differences due to externalizing. This stimulus-related activity was not apparent in the time-domain alone, and current time-domain measurement approaches offered no rational approach to selecting this window. In particular, approaches based on ranges of time or time-frequency activity (i.e. time-windows or TF regions of interest) are not data driven, and activity must be visually apparent or defined a priori. The most widely used time-domain data driven approach, PCA, generally produces separate measures for P2, N2, and P3 (see e.g. Chapman & McCrary, 1995; Dien et al., 2003; Spencer, Dien, & Donchin, 1999, 2001), rather than spanning that time range like the TF-PC3 from the current decomposition. The current results demonstrate that this new approach to ERP decomposition can extract time- and frequency-specific activity that is more sensitive to externalizing-related variance in the ERP than P3 peak amplitude, and offers support for the idea that TF-PCs from this approach may better represent processes relevant to these psychopathologies.
Of the five TF components extracted in the present study, two delta components were able to independently discriminate group membership, accounting for an increment in variance over that accounted for by P3 amplitude: PC2 for the ADHD and alcohol groups, PC3 across all diagnostic groups (just short of significance for the small DAD group). Further, these two components were associated with a common factor representing the shared variance among symptom counts of all disorders. PC2 corresponds closest in time to the P3b wave typically found in response to targets in the oddball task. PC3 spans the time range of the P2-N2-P3 ERP complex (and is significantly correlated with P2 amplitude, r=.53, p<.001, and the microvolt value associated with the trough of the N2 wave, r=.39, p<.001). PC3, then, may be most representative of the specific activity indexing signal matching, decision-making, and salience detection processes previously attributed to P3-related delta activity generally (Basar-Eroglu et al., 1992; Karakas et al., 2000; Knyazev, 2007). These cognitive processes have been implicated in ADHD, conduct disorder, and substance use disorders (Dom, De Wilde, Hulstijn, van den Brink, & Sabbe, 2006; Garon, Moore, & Waschbusch, 2006; Y. T. Kim, Lee, & Kim, 2006), further supporting the relationship between this delta component and externalizing.
Present results suggest that abnormalities in these two delta components, particularly PC3 with its unique relationship across disorders, may play an important role in the association between P3-AR and externalizing psychopathology. Previous findings have shown reduced power in P3-related delta activity to be associated with alcoholism and risk of developing alcoholism (Jones et al., 2006; Kamarajan et al., 2006; Rangaswamy et al., 2007). Further, some research has suggested that the activity revealed by time-frequency analyses may be closer to gene function than are clinical and cognitive measures (Begleiter & Porjesz, 2006). Thus, the aforementioned associations between P3-related delta and theta, alcohol and drug use disorders, and the CHRM2 gene (Dick et al., 2008; Jones et al., 2004; Luo et al., 2005; Porjesz & Rangaswamy, 2007; Wang et al., 2004) suggest a genetic component potentially relevant to the etiology of substance use disorders and externalizing psychopathology – a genetic component closely linked to the P3-related TF components found in the present study.
While the present study’s focus was on the relationship between P3-related activity and externalizing disorders, an important next step in the characterization of these components as endophenotypes is to investigate their heritability and association with familial risk for externalizing disorders. P3 amplitude has been shown to be strongly heritable (van Beijsterveldt & van Baal, 2002; Yoon, Iacono, Malone, & McGue, 2006), and the relationship between P3 and externalizing can be accounted for by shared genetic effects (Hicks et al., 2007). Future studies will utilize our large twin sample to extend these findings to the TF-PCA derived components associated with the P3 ERP. Given that topographic differences may also play a role in the relationship between P3, TF components, and externalizing (e.g. theta contributions to P3 tend to be frontally maximal, while delta contributions tend to be parietally maximal; (e.g. Basar-Eroglu et al., 1992; Jones et al., 2006)), future work will assess a broader range of electrode sites to capture any topographically-mediated variance.
The present results demonstrated that activity indexed by the P3 can be more optimally represented by multiple overlapping TF components. Further, these components accounted for externalizing-related variance including and beyond what is indexed by P3. These findings suggest that these TF measures may be more sensitive to variance related to these disorders, and contributes to the growing suggestion that TF representations of EEG/ERP may produce more optimal indices of underlying neurophysiological processes. Specifically, the measured delta activity during the early stages and peak of P3 (i.e. PC3) was the most sensitive index. Given findings of links between delta (and theta) and genes related to alcoholism and externalizing spectrum disorders (Dick et al., 2008; Porjesz et al., 2005), this delta activity may serve as a more parsimonious endophenotype for externalizing psychopathology than P3-AR itself.
Acknowledgments
This research was supported by grants DA 05147, DA 13240, DA 024417, and K08 MH080239-01A2 (Bernat) from the National Institutes of Health.
Footnotes
Scripts available upon request.
It is a common misconception that orthogonal components are necessarily uncorrelated (after rotation). In fact, it is not possible to preserve both orthogonality and independence (in the sense of uncorrelatedness) of components following rotation (Jolliffe, 1995). Lack of correlation among components can be preserved, but at the expense of loss of orthogonality. We instead opt for a normalization constraint (Kaiser’s) that scales the loadings vectors to unit length (rather than the components) and preserves orthogonality (thus increasing interpretability) while relaxing the requirement of lack of correlation.
References
- Amemiya T. Qualitative response models: A survey. J Econ Lit. 1981;19:1483–1536. [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol. 1992;13(2):161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: interactive effects on p300 amplitude and topography in male adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42(1):106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60(2):162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225(4669):1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, et al. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol. 1998;108(3):244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. Int J Psychophysiol. 2007;64(1):62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Holroyd CB, Gehring WJ, Patrick CJ. Separating cognitive processes with principal components analysis of EEG time-frequency distributions. Proc. SPIE. 2008;7074(70740S) [Google Scholar]
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clin Neurophysiol. 2005;116(6):1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Brigham J, Herning RI, Moss HB. Event-related potentials and alpha synchronization in preadolescent boys at risk for psychoactive substance use. Biol Psychiatry. 1995;37(12):834–846. doi: 10.1016/0006-3223(94)00218-R. [DOI] [PubMed] [Google Scholar]
- Chapman RM, McCrary JW. EP component identification and measurement by principal components analysis. Brain Cogn. 1995;27(3):288–310. doi: 10.1006/brcg.1995.1024. [DOI] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31(1):156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Cohen L. Time-frequency analysis. Englewood Cliffs, NJ: Prentice Hall; 1995. [Google Scholar]
- Comings DE, Wu S, Rostamkhani M, McGue M, Lacono WG, Cheng LS, et al. Role of the cholinergic muscarinic 2 receptor (CHRM2) gene in cognition. Mol Psychiatry. 2003;8(1):10–11. doi: 10.1038/sj.mp.4001095. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Basar-Eroglu C, Basar E. Wavelet analysis of oddball P300. Int J Psychophysiol. 2001;39(2–3):221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Kramer J, Wang JC, Hinrichs A, Bertelsen S, et al. Association of CHRM2 with IQ: converging evidence for a gene influencing intelligence. Behav Genet. 2007;37(2):265–272. doi: 10.1007/s10519-006-9131-2. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, et al. Using dimensional models of externalizing psychopathology to aid in gene identification. Arch Gen Psychiatry. 2008;65(3):310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Brain Res Cogn Brain Res. 2003;17(3):637–650. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, van den Brink W, Sabbe B. Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcohol Clin Exp Res. 2006;30(10):1670–1677. doi: 10.1111/j.1530-0277.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Waheed J, Goldman D. Neurophysiological and genetic distinctions between pure and comorbid anxiety disorders. Depress Anxiety. 2008;25(5):383–392. doi: 10.1002/da.20378. [DOI] [PubMed] [Google Scholar]
- Garon N, Moore C, Waschbusch DA. Decision making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa Gambling Task. J Atten Disord. 2006;9(4):607–619. doi: 10.1177/1087054705284501. [DOI] [PubMed] [Google Scholar]
- Gosso FM, de Geus EJ, Polderman TJ, Boomsma DI, Posthuma D, Heutink P. Exploring the functional role of the CHRM2 gene in human cognition: results from a dense genotyping and brain expression study. BMC Med Genet. 2007;8:66. doi: 10.1186/1471-2350-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hagle TM, Mitchell GE. Goodness-of-Fit Measures for Probit and Logit. American Journal of Political Science. 1992;36(3):762–784. [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, et al. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44(1):98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY. Trajectories of alcohol use and electrophysiological and morphological indices of brain development: distinguishing causes from consequences. Ann N Y Acad Sci. 2004;1021:245–259. doi: 10.1196/annals.1308.029. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Steinhauer SR. Absence of visual and auditory P300 reduction in nondepressed male and female alcoholics. Biol Psychiatry. 1999;46(7):982–989. doi: 10.1016/s0006-3223(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Zezza N, Kaplan B, Neiswanger K, Steinhauer SR, et al. Genetic association between reduced P300 amplitude and the DRD2 dopamine receptor A1 allele in children at high risk for alcoholism. Biol Psychiatry. 1998;43(1):40–51. doi: 10.1016/s0006-3223(97)00203-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muka D, Steinhauer S, Locke J. P300 amplitude decrements in children from families of alcoholic female probands. Biol Psychiatry. 1995;38(9):622–632. doi: 10.1016/0006-3223(94)00384-7. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59(8):750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Minnesota Twin Family Study. Twin Res. 2002;5(5):482–487. doi: 10.1375/136905202320906327. [DOI] [PubMed] [Google Scholar]
- Jolliffe IT. Rotation of principal components: Choice of normalization constraints. Journal of Applied Statistics. 1995;22(1):29–35. [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53(2):75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, et al. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006;117(10):2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, disinhibited personality, and early-onset alcohol problems. Alcohol Clin Exp Res. 2001;25(10):1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, et al. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59(7):625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000;285(1):45–48. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry Hum Dev. 2001;32(2):93–106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee SJ, Kim SH. Effects of the history of conduct disorder on the Iowa Gambling Tasks. Alcohol Clin Exp Res. 2006;30(3):466–472. doi: 10.1111/j.1530-0277.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev. 2007;31(3):377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111(3):411–424. [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39(8):879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Hum Mol Genet. 2005;14(16):2421–2434. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Fyer AJ, Endicott J, Klein DF. Family Informant Schedule and Criteria (FISC) New York: New York State Psychiatric Institute; 1985. [Google Scholar]
- Mantini D, Corbetta M, Perrucci MG, Romani GL, Del Gratta C. Large-scale brain networks account for sustained and transient activity during target detection. Neuroimage. 2009;44(1):265–274. doi: 10.1016/j.neuroimage.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Philippot P, Verbanck P, Noel X, Kornreich C, Hanak C, et al. Is the P300 deficit in alcoholism associated with early visual impairments (P100, N170)? An oddball paradigm. Clin Neurophysiol. 2007;118(3):633–644. doi: 10.1016/j.clinph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Bauer L, Tasman A, Hesselbrock V. Reduced P3 amplitudes are associated with both a family history of alcoholism and antisocial personality disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18(8):1307–1321. doi: 10.1016/0278-5846(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43(1):84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Ochoa CJ. Alcoholism risk, tobacco smoking, and P300 event-related potential. Clin Neurophysiol. 2004;115(6):1374–1383. doi: 10.1016/j.clinph.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115(1):55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, et al. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002;61(1–2):229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. ScientificWorldJournal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116(5):993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Prabhu VR, Porjesz B, Chorlian DB, Wang K, Stimus A, Begleiter H. Visual p3 in female alcoholics. Alcohol Clin Exp Res. 2001;25(4):531–539. [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, et al. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007;63(1):3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese C, Polich J. Alcoholism risk and the P300 event-related brain potential: modality, task, and gender effects. Brain Cogn. 2003;53(1):46–57. doi: 10.1016/s0278-2626(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39(1):59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Robins LN, Babor TF, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis: Authors; 1987. [Google Scholar]
- Spencer KM, Dien J, Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36(3):409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38(2):343–358. [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61(1–2):111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Veall MR, Zimmerman KF. Evaluating Pseudo-R-squareds for binary probit models. Quality & Quantity. 1994;28:151–164. [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- Williams JT, Begleiter H, Porjesz B, Edenberg HJ, Foroud T, Reich T, et al. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. II. Alcoholism and event-related potentials. Am J Hum Genet. 1999;65(4):1148–1160. doi: 10.1086/302571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HH, Iacono WG, Malone SM, McGue M. Using the brain P300 response to identify novel phenotypes reflecting genetic vulnerability for adolescent substance misuse. Addict Behav. 2006;31(6):1067–1087. doi: 10.1016/j.addbeh.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Devrim M, Kolev V, Ademoglu A, Demiralp T. Multiple time-frequency components account for the complex functional reactivity of P300. Neuroreport. 2000;11(5):1097–1103. doi: 10.1097/00001756-200004070-00038. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96(5):684–695. [PubMed] [Google Scholar]