Abstract
Background
Changes in levels of estradiol and progesterone that occur with the transition to reproductive senescence may influence nociception or affect.
Objective
To ascertain whether nociceptive and affective processes change with reproductive senescence, this study examined pain and anxiety-like behaviors in middle-aged female rats that were reproductively competent, transitioning to reproductive senescence, or reproductively senescent.
Methods
Middle-aged (12–14 months old) female rats (N = 46) were tested in the following tasks to assess pain and anxiety-like behavior: tail flick, elevated plus maze, elevated zero maze, mirror maze, Vogel punished drinking, and defensive burying. For the tail-flick task, the latency for rats to move their tail from a heat source, as an indication of pain sensitivity, was determined. In the elevated plus and elevated zero mazes, the time spent on the open arms or quadrants, respectively, were determined as measures of reduced anxiety behavior. In the mirror maze, the time spent in the mirrored portion of the chamber was used as an indicator of anxiety-like responding. In the Vogel task, the number of punished licks made was determined as a measure of reduced anxiety-like behavior. In the defensive burying task, the duration spent by rats burying an electrified prod postfootshock was utilized as an index of anxiety-like responding. All rats were experimentally naive, retired breeders from our colony and had not had a litter or been lactating for 1 to 4 weeks before behavioral testing.
Results
Although tail-flick latencies were not significantly different among rats that were reproductively competent or senescent, reproductively competent rats had less anxiety-like behavior in the elevated plus maze (more time spent on the open arms: F2,43 = 5.93; P < 0.01), elevated zero maze (more time spent on the open quadrants: F2,43 = 4.62; P = 0.01), and Vogel punished drinking task (more punished licks made: F2,43 = 3.76; P = 0.03). There were no statistically significant differences in the mirror maze and defensive burying task.
Conclusion
In this study of adult female rats, nociceptive behavior did not vary significantly with reproductive senescence, but anxiety-like behavior of rats did.
Keywords: estrogen, progesterone, anxiety, affect, aging, menopause
INTRODUCTION
Profound sex differences in pain and other stress-related somatic complaints/disease states suggest that ovarian steroids, such as estradiol and progesterone, may modulate these effects. Compared with men, women are more likely to be affected with pain and stress-related somatic complaints/disease states (eg, fibromyalgia, systemic lupus erythematosus, migraine, chronic pain).1–5 Among women aged 20 to 59 years, lifetime differences in hormone exposure, such as younger age at menarche, increased parity, past experience with irregular menstrual cycling, or hysterectomy, as well as duration of oral contraceptive use and estradiol-based hormone therapies during menopause, are associated with differences in chronic musculoskeletal pain.6 Negative physical symptoms are observed with aging/menopause.7,8 Thus, these data indicate the likely role of ovarian steroids in altering pain processes across the lifespan of women.
Anxiety and mood disorders disproportionately affect women compared with men. More women than men are diagnosed with anxiety disorders, such as social anxiety, phobias, posttraumatic stress disorder, general anxiety disorder, and mood disorders, particularly unipolar depression.9–12 In young women, these gender differences may be related to cyclical changes in ovarian steroids (eg, estradiol, progesterone), such as those observed with changes in mood across the menstrual cycle or with pregnancy and parturition (ie, premenstrual syndrome, premenstrual dysphoric disorder, postpartum depression).13 Increases in depressed mood or anxiety are some of the most cited psychological symptoms of menopause,14 and incidence of anxiety and depression disorders, which are often comorbid in women, increases during meno-pause, and particularly in postmenopause.15–19
Further investigation of how changes in ovarian function with aging/menopause may alter affect and pain, and of a possible interaction of these processes, is warranted. In addition to clear psychological sequelae, painful physical symptoms are common clinical features of anxiety and depression disorders, and of premenstrual syndromes as well.20–22 This comorbidity can negatively affect health-related quality of life and the severity, diagnosis, and treatment of the psychological disturbance. For instance, migraine is commonly comorbid with major depressive disorder, and there is a positive relationship between the pain associated with migraine, the severity of depression symptoms, and the decreased likelihood of favorable response to treatment.23–29 It is important to consider the role of ovarian steroids in affect as well as pain, in which there is likely a bidirectional relationship. Therefore, animal models may be better suited for investigations of the role of ovarian steroids for both pain and affective processes.
Research using rodent models supports the concept that ovarian hormones influence nociception. There are changes in nociception across the estrous cycle and with steroid administration.30 Rats have higher latencies to move their tail or paws away from a heat source, which is a commonly used behavioral task of pain sensitivity, when levels of estradiol and progestins are naturally high (during proestrus, pregnancy).31–33 In a model of pelvic pain syndrome, endogenous estradiol levels can modulate cross-organ sensitization between the uterus and the urethra.34 Severity of vaginal hyperalgesia due to surgically induced endometriosis is increased in middle-aged rats that are reproductively senescent.35 Surgical extirpation and ovarian replacement studies also support a role for estradiol and progesterone in modulating nociception.36–42 Thus, ovarian steroids may play a role in altering nociception in young adult rodents.
There are sex differences and a contributory role of estrous-cycle stage in anxiety- and depression-like behavior. Rats in proestrus have reduced anxiety- and depression-like behavior compared with rats in diestrus or male rats.33,43 Furthermore, post-partum decline in endogenous ovarian steroids is associated with increased depression-like behavior in rats.44 There is a similar pattern of effects for reduced expression of anxiety- or depressive-like behavior if rats are ovariectomized and primed with estradiol and progesterone to mimic proestrous levels, compared with rats that are ovariectomized and administered placebo vehicle.13,40 Thus, ovarian steroids alter anxiety- and depression-like behavior of young adult rodents.
Although studies in people and animals provide the rationale for further investigation of how changes in pain may influence affect and vice versa, few studies to our knowledge have investigated the role of reproductive competence on affective or pain processing in animal models. To address this question further, we investigated nociception and anxiety-like behavior among same-aged rats with varying degrees of reproductive viability, which would be associated with changes in ovarian function (ie, estropause). We hypothesized that nociceptive and anxiety-like behaviors would be altered in middle-aged female rats that were categorized as either reproductively competent with normal reproductive function, transitioning to reproductive senescence with variable and lower reproductive function, or reproductively senescent based on their estrous cyclicity, fertility, and fecundity.
MATERIALS AND METHODS
These methods were preapproved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY.
Animals and Housing
Middle-aged (12–14 months old) female Long-Evans rats (N = 46) were obtained at 2 months of age from, or through in-house breeding from rats originally obtained from, Taconic Farms (Germantown, New York). Rats were experimentally naive and had been breeders in our colony since the onset of puberty. Rats were group housed (3–4 per cage) in polycarbonate cages (45 × 24 × 21 cm) in a temperature-controlled room (~21°C) in the Laboratory Animal Care Facility core in The Life Sciences Research Building at The University at Albany-SUNY. Rats were maintained on a 12/12-hour reversed light cycle, with lights off at 8:00 AM. Throughout the study, rats had ad libitum access to rodent chow and tap water in their home cages.
Reproductive Status Characterization
We investigated nociceptive and anxiety-like behavior among same-aged rats with varying degrees of reproductive viability, which would be associated with changes in ovarian function (ie, estropause). All rats were retired breeders from our colony and had not had a litter or been lactating for 1 to 4 weeks before behavioral testing. Because they were age matched and had similar parity, differences due to current ovarian/reproductive status could be assessed in the present experiment. Although rats had their estrous-cycle phase determined daily, the behavioral data collected could not be appropriately analyzed to reveal whether there were differences due to estrous-cycle effects (which are obvious in young, reproductively competent rats), given that the majority of rats were not regularly cycling and the number of animals per experimental group was low. Aged rodents do not show exactly the same type of gradual decline in ovarian function observed in the menopause of women, but there is a decline in reproductive function with aging.45
From ~55 days of age, rats had their estrous-cycle phase determined by examination of vaginal cytology, per previously published methods.46,47 On a daily basis, the proportion of leukocytes and cornified and nucleated epithelial cells in the vaginal smears collected from rats were briefly analyzed using light microscopy to determine the phase of the cycle.33,46,47 In maintenance of our breeding colony, rats were mated when receptive. Differences in cyclicity were noted at ~10 months of age, and rats were then monitored closely for cyclicity as well as the number of successful pregnancies after mating (fertility). The number of pups per litter was determined as an index of fecundity. Rats were then classified as reproductively competent, transitioning to reproductive senescence, or reproductively senescent based on these measures.48 Reproductively competent rats (ie, “preestropause”) had regular 4- to 5-day cycles, an average of ~90% successful pregnancies resulting in parturition, and an average fecundity of ≥10 pups/litter (n = 14). Rats that were transitioning to reproductive senescence (ie, “periestropause”) had irregular cycles, an average of ~65% successful pregnancies resulting in parturition, and an average fecundity of <10 pups/litter (n = 19). Rats were considered to be reproductively senescent (ie, “postestropause”) if they had irregular cycles, an average of ~40% successful pregnancies, and <10 pups/litter (n = 13).
Behavioral Testing
Rats were tested in behavioral tasks by an investigator blinded to the hypothesized outcome of the study. Data were simultaneously collected by the investigator and a video-tracking system (Any-maze; Stoelting, Inc., Wood Dale, Illinois), which were 95% concordant. Although the data collected with these 2 methods were nearly identical, the hand-collected data by the observer were used for statistical analyses (because data that were not congruent between the 2 collection measures were due to technical issues with the video-tracking system or computer, rather than to the actions of the investigator). Rats were tested in brightly lit testing rooms in the Laboratory Animal Care Facility core. Rats were tested once in each task, in 1 task per day, in the same order, to minimize potential exposure to anxiety tasks, which can activate the hypothalamic-pituitary-adrenal axis (HPA) and thereby alter subsequent nociceptive responding or responding in other anxiety tasks. To minimize the influence of diurnal fluctuations in HPA activity, rats were tested at the same time of day, in the early phase of their dark cycle.
Rationale for Tasks Utilized
It was important to utilize anxiety tasks with different stimuli to account for potential differences in the physical health of middle-aged rats transitioning to reproductive senescence, which may have altered motor behavior or physical strength. If a similar pattern of effects is observed across several tasks that have different physical demands and use different anxiogenic stimuli, this suggests that the differences observed are likely due to changes in reproductive status with aging, rather than nonspecific physical changes.
Tail-Flick Task
The tail-flick task was performed using previously described methods.49 Briefly, rats were covered with a towel and placed on the platform of the tail-flick apparatus (San Diego Instruments Inc., San Diego, California), and held in place while the distal region of their tails were placed flat against the radiant heat source. The mean latencies of 3 consecutive tail-flick trials were used as an index of nociception (maximum latency was 10 sec for each trial).
Elevated Plus Maze
Rats were tested in an elevated plus maze, which was constructed of 4 matte black arms (2 arms were “open” and 2 were enclosed by walls 30 cm in height), 49 cm long and 10 cm wide, elevated 50 cm off the ground, as previously described.50 After placement in the junction of the 4 arms, and facing out onto the open arm, the time spent and entries made on the open and closed arms were recorded during the 5-minute test period. A longer duration spent on the open arms indicated reduced anxiety-like behavior.
Elevated Zero Maze
The elevated zero maze consisted of a matte black circular platform (110 cm outer diameter with 10-cm-wide deck path), equally divided into 4 quadrants (2 enclosed by walls that were 27 cm in height and 2 that were “open”), elevated 65 cm above the ground. Rats were placed in a closed quadrant facing toward the center of the quadrant, and the time spent and entries made in the open and closed quadrants during the 5-minute test were recorded.51 Other exploratory behaviors in this task, such as head dips and stretch–attend postures, were recorded. A longer duration spent on the open quadrants indicated reduced anxiety-like behavior.
Mirror Maze
The mirror maze consisted of an open field chamber (76 × 57 × 35 cm) with 4 mirrored walls connected to a nonmirrored alleyway (57 × 12.5 × 35 cm), as modified from the apparatus utilized in mice.52 Rats were placed inside a nonmirrored alleyway at the beginning of the task. The total time spent in and entries made to the mirrored part of the chamber during this 5-minute test by rats were recorded. A longer duration of time spent in the mirrored part of the chamber was utilized as a measure of reduced anxiety behavior.
Vogel Punished Drinking Task
Rats were water deprived in their home cages for 18 hours. Rats were then placed in the clear Plexiglas Vogel chamber with a metal grid floor (44 × 22 × 20 cm).51 The chamber had an electrified water bottle suspended from the top and it was connected to a computer interface (Anxio-meter, Columbus Instruments, Columbus, Ohio), which automatically recorded the number of licks made and shocks received by the rat, with 1 shock being administered for every 20 licks made. This test began after the rat made an initial 20 licks and received its first shock (0.3 mA for 2 sec), and lasted for 3 minutes. The Vogel task is considered a classic anxiety task of conflict responding. It must be noted that some differences in this task may be related to tolerance to noxious or painful stimuli; however, the shock stimuli utilized in this task are minimal. In other tasks in which greater shock stimuli are used, such as the inhibitory avoidance or conditioned fear task, flinch-jump ratings (where 1 = flinch, 2 = one paw lifted from floor, 3 = two paws lifted from floor, and 4 = jump) are typically <3.5. In the Vogel task, rats in our laboratory would typically receive a flinch-jump score of <1, suggesting few to no differences in pain tolerance in this task.
Defensive Burying Task
Rats were tested in the defensive burying task utilizing previously published methods.51 Briefly, rats were placed in a clear Plexiglas chamber (26.0 × 21.2 × 24.7 cm), with bedding 10 cm in depth, and a small pedestal (2.5 cm in diameter, 10.0 cm in height) wrapped by wires connected to a shock source (Lafayette Model A615B, Lafayette, Indiana) set to deliver 6.66 mA of unscrambled shock, in the corner of the chamber. The shock source was turned on when the rats were introduced to the chamber. The latency to touch the shock prod and receive a single footshock, which was terminated by the rat's withdrawal of its paw from the pedestal, was recorded. The time spent burying the pedestal with the woodchip shavings in the chamber during the 15-minute test in response to the footshock received was recorded and used as a measure of anxiety-like responding in this task.
Statistical Analyses
Behavioral data of rats were analyzed using multiple, 1-way analyses of variance to determine the effects of reproductive status on performance. If there were significant main effects, Fisher's protected least significant difference post hoc tests were performed to determine group differences. The α level for statistical significance was P < 0.05.
RESULTS
Reproductive Status, Nociception, and Anxiety-Like Behaviors in Tasks Tail-Flick Task
Although reproductively competent rats had higher latencies (mean [SEM], 6.5 [0.6] sec) to move their tail away from a heat source in the tail-flick task compared with rats in transition (5.8 [0.6] sec) or those that were reproductively senescent (5.6 [0.8] sec), there was no main effect of reproductive status for tail-flick latencies (P = NS).
Elevated Plus Maze
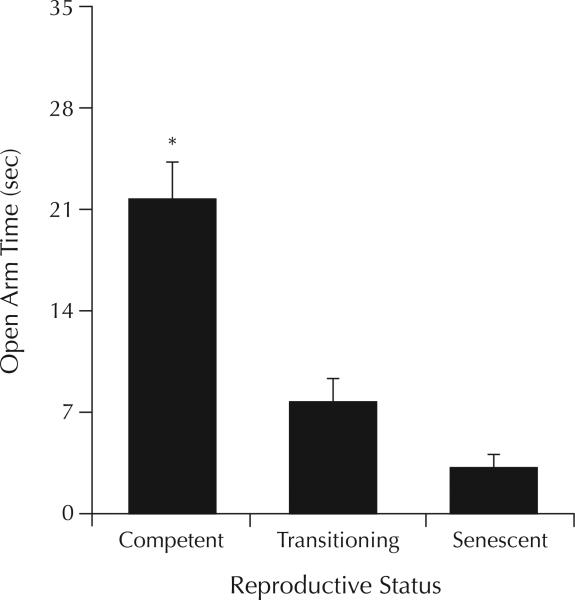
There was a main effect of reproductive status for time spent by rats on the open arms of the elevated plus maze (F2,43 = 5.93; P < 0.01). Post hoc tests found that reproductively competent rats spent significantly more time on the open arms than did rats that were transitioning to senescence or were reproductively senescent (both, P < 0.01) (Figure 1). There were no significant group differences for open arm or total entries made in this task (Table I).
Figure 1.
Differences in time (mean [SEM] sec) spent on the open arms of the elevated plus maze by rats that were reproductively competent (n = 14), transitioning to reproductive senescence (n = 19), or reproductively senescent (n = 13). *P < 0.01 versus rats transitioning to, or in, reproductive senescence.
Table I.
Motor or exploratory measures for rats performing in an elevated plus maze, elevated zero maze, and mirror maze (N = 46). All values are noted as mean (SEM).*
| Elevated Plus Maze |
Elevated Zero Maze |
Mirror Maze |
|||||
|---|---|---|---|---|---|---|---|
| Reproductive Status | Total Arm Entries | Open Arm Entries | Total Quadrant Entries | Open Quadrant Entries | Head Dips | Stretch–Attend Postures | Mirrored Chamber Entries |
| Competent (n = 14) | 11.1 (1.2) | 2.8 (0.6) | 8.7 (1.7) | 4.3 (0.9) | 6.8 (1.0) | 1.8 (0.6) | 6.1 (0.4) |
| Transitioning (n = 19) | 9.6 (0.9) | 2.0 (0.5) | 7.5 (2.1) | 3.6 (1.0) | 5.0 (0.9) | 2.3 (0.5) | 5.9 (0.5) |
| Senescent (n = 13) | 9.2 (1.4) | 1.4 (0.7) | 4.5 (1.7) | 2.1 (0.9) | 4.8 (1.1) | 1.5 (0.4) | 6.6 (0.7) |
No statistically significant differences based on condition for the measures in these tasks.
Elevated Zero Maze
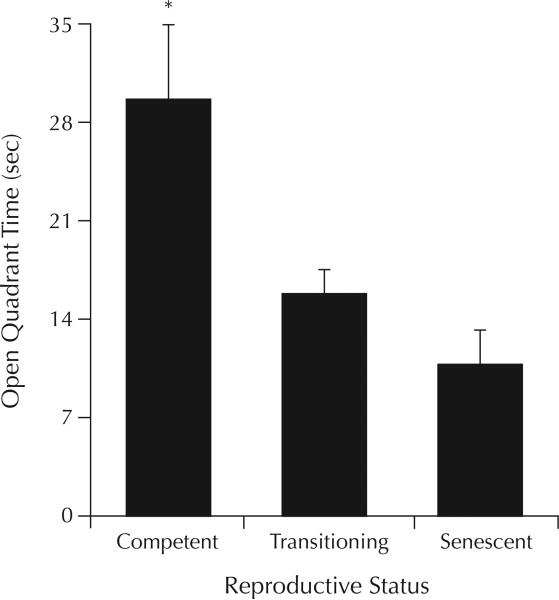
A pattern similar to the elevated plus maze was observed in the elevated zero maze (F2,43 = 4.62; P = 0.01). Post hoc tests found that reproductively competent rats spent significantly more time in the open quadrants than did rats that were transitioning to (P < 0.02) or in reproductive senescence (P < 0.01) (Figure 2). There were no significant group differences for open quadrant or total entries, head dips, or stretch–attend postures made in this task (Table I).
Figure 2.
Differences in time (mean [SEM] sec) spent on the open quadrants of the elevated zero maze by rats that were reproductively competent (n = 14), transitioning to reproductive senescence (n = 19), or reproductively senescent (n = 13). *P < 0.02 versus rats transitioning to, or in, reproductive senescence.
Mirror Maze
Reproductive status had no significantly different effects on the responses of rats in the mirror maze. However, compared with reproductively senescent rats, reproductively competent rats spent more time in the mirrored chamber in this task (Table II). There were no statistically significant differences between groups for entries made into the mirrored chamber (Table I).
Table II.
Differences in time (mean [SEM] sec) spent in the mirrored chamber of the mirror maze or burying an electrified prod by rats that were reproductively competent, transitioning to reproductive senescence, or reproductively senescent (N = 46).*
| Reproductive Status | Mirrored Chamber | Burying Prod |
|---|---|---|
| Competent (n = 14) | 127.1 (14.5) | 73.0 (21.4) |
| Transitioning (n = 19) | 98.6 (10.4) | 65.1 (19.8) |
| Senescent (n = 13) | 84.1 (16.2) | 141.2 (32.0) |
No statistically significant differences.
Vogel Punished Drinking Task
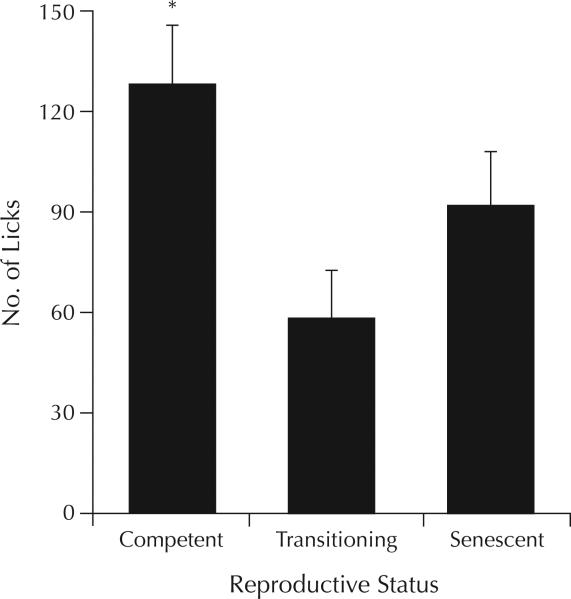
There was a main effect of reproductive status (F2,43 = 3.76; P = 0.03) for the behavior of rats in the Vogel task. Post hoc tests found that reproductively competent rats made significantly more licks on an electrified drinking bottle spout than did rats that were transitioning to, or in, reproductive senescence (P < 0.01) (Figure 3).
Figure 3.
Differences in number of licks (mean [SEM]) made to an electrified water bottle in the Vogel punished drinking task by rats that were reproductively competent (n = 14), transitioning to reproductive senescence (n = 19), or reproductively senescent (n = 13). *P < 0.01 versus rats transitioning to, or in, reproductive senescence.
Defensive Burying Task
Reproductive status had no significantly different effects on the responses of rats in the defensive burying task, but reproductively senescent rats spent more time burying the shock prod after footshock compared with reproductively competent rats and rats transitioning to reproductive senescence (Table II).
DISCUSSION
The hypothesis that reproductive status (defined by cyclicity, fertility, and fecundity) of rats would alter nociceptive or anxiety-like responses of rats was partially supported. Few differences were observed in nociception; rats that were reproductively competent had tail-flick latencies that were not significantly different from rats in transition to, or in, reproductive senescence. A different pattern emerged for anxiety-like responses. Reproductively competent rats had reduced anxiety-like behavior in the elevated plus maze, elevated zero maze, and Vogel punished drinking task compared with rats that were transitioning to, or in, reproductive senescence. Reproductively competent rats also spent more time in the mirrored chamber than did rats that were transitioning to, or in, reproductive senescence, but these effects were not statistically significant. In the defensive burying task, both reproductively competent rats and those transitioning to reproductive senescence spent less time burying the electrified prod post-footshock (ie, had reduced anxiety-like behavior) than did reproductively senescent rats, albeit these effects were not statistically significant. Thus, these data suggest that there may be changes in the anxiety-like behavior of rats transitioning to reproductive senescence that can be differentiated from changes in nociception.
Although only very modest differences in tail-flick latencies based on reproductive status were observed, it is clear that ovarian steroids modulate nociceptive processes in young cycling or ovariectomized rats. For example, administration of estradiol and progesterone to young ovariectomized rats has been found to increase latencies to respond to heat stimuli applied to the tail or paws, compared with vehicle administration.38–41,53 Ovarian steroids have been found to also modulate other nociceptive processes, such as those occurring in the pelvic regions, uterus, or vaginal canal.34,36,54 The present study's findings extend some of these results to suggest that some of these processes may be altered with aging or changes in reproductive status. Compared with reproductively senescent rats, middle-aged rats transitioning to estropause were found to have decreased endometriosis-induced vaginal hyperalgesia.35 These differences were observed among rats that were surgically manipulated to have hyperalgesia induced by surgical endometriosis; however, in the present study, we investigated nociception by determining the reflexive response to noxious heat stimuli in rats that were otherwise not manipulated. In our study, pain response was determined using 1 task, which employed a relatively innocuous stimulus, because a within-subjects approach was utilized and, therefore, it was less likely that hormonal modulation of responses on a nociceptive task would alter anxiety behavior or subsequent testing for pain sensitivity.55 Indeed, these data provide rationale for further investigation of the role of changes in reproductive status for nociception.
The present findings, that reproductively competent rats have reduced anxiety-like behavior compared with rats transitioning to, or in, reproductive senescence, are consistent with previous reports of the effects of decline in ovarian steroids, produced by ovariectomy, on affective behavior. Ovariectomy increased anxiety-like behavior, compared with that observed in young adult intact or proestrous rats,13 suggesting that ovarian steroids may mediate these effects. We, and others, have found that, compared with vehicle when administered to ovariectomized rats, estradiol increased time spent on the open arms of the plus maze, time spent on the open quadrants of the zero maze, and punished licks made in the Vogel punished drinking task.51,56–58 These results are not likely due to overall changes in motor and exploratory behavior, because there was little indication that these measures were significantly altered in the plus, zero, or mirror mazes. Thus, natural decline in ovarian function may have similar effects to those observed with surgical extirpation.
A similar pattern was observed in the present and in previous studies that investigated effects of natural fluctuations in ovarian steroids or exogenous administration of estradiol or progestins in young ovariectomized rats and aged intact female mice.13,51,52,59 This is interesting because the Vogel task is considered a conflict task and the anxiogenic stimuli are mild electric shocks, rather than open spaces in the elevated plus and zero mazes. The present findings in the defensive burying task further confirm these effects. There are estrous-cycle changes in the defensive burying task such that proestrous rats spend less time burying a prod that has delivered a mild footshock than do diestrous rats.33,60 Decline in progestins, particularly when abrupt, increases time spent burying in this task.61,62 Together, these data indicate that ovarian cessation, natural or surgical, can modify anxiety-like behaviors in approach–avoidance and conflict tasks. One question that was not addressed in this experiment was the potential mechanisms for these effects. In female rodents, there are clear antinociceptive and antianxiety effects of neurosteroids, such as 3α-hydroxy-5α-pregnan-20-one, which have actions via γ-aminobutyric acid (GABA) targets.33,37–40,43,63,64 Given that estradiol enhances conversion of progesterone to its neuroactive metabolites as well as amplifies the effects of neuroactive progestins to reduce pain sensitivity,38,65,66 future studies might examine whether some of the observed effects are due to the actions of neurosteroids in the brain. Older individuals generally are less responsive to GABAergic modulators67,68 and may have reduced responses to, or production of, neurosteroids.69–71 This may imply that older rodents in the present study were less sensitive to the modulating effects of (neuro)steroids for nociception, yet were responsive to effects on anxiety behavior if not reproductively senescent.
Another important consideration in interpreting the present findings is the potential role of stress responsiveness. Ovarian steroids can modulate HPA function72,73 and produce behavioral effects, such as stress-induced analgesia. Estradiol and progesterone administration increased tail-flick latencies of rats exposed to the odor of a predator,49 which was consistent with other reports that ovarian hormones modulated stress-induced analgesia.74–77 Yet another consideration is that physical or psychological stressors can alter the endocrine milieu of rats63 and, therefore, have the potential to subsequently alter these behaviors. That there were few differences in the tail-flick task across groups in the present study suggests that the electric-shock response effects in the defensive burying and Vogel tasks were not solely due to differences in nociceptive thresholds or to other physical changes that may have been associated with aging. It has been proposed that there may be different neural substrates that control reactions to psychologic versus physiologic stressors.78,79 Although a limitation was that HPA output was not assessed, it may be that there were differences in the neural circuitry that were altered with aging and reproductive senescence in the present study, as suggested by the different pattern of behavioral effects in anxiety and nociception tasks. Individual differences in reactions to stressors can be important determinants of health and may even underlie some of the sex/gender differences in psychologic and somatic disease states.1,80,81
CONCLUSIONS
In summary, the present study suggests that the natural decline in reproductive viability (ovarian function) may alter affective processing of female rats across a variety of tasks. Reproductively competent rats had less anxiety-like behavior in approach– avoidance (elevated plus, elevated zero, and mirror mazes) and conflict tasks, such as the Vogel punished drinking task, than did rats transitioning to, or in, reproductive senescence. In the defensive burying task, differences were noted in reproductively competent rats and those transitioning to reproductive senescence, in that they spent more time burying an electrified prod following footshock than did reproductively senescent rats. Importantly, despite clear hormonal modulation of, and comorbidity between, pain and affective disorders among people, changes in anxiety could be separated from effects on nociception in middle-aged rats transitioning to reproductive senescence in the present study.
ACKNOWLEDGMENTS
This research was supported in part by funding from the National Science Foundation (IBN03–16083) and the National Institute of Mental Health (MH0676980). The technical assistance of Alicia Babson and Irene Chin is greatly appreciated.
REFERENCES
- 1.Bradley LA. Pathophysiologic mechanisms of fibromyalgia and its related disorders. J Clin Psychiatry. 2008;69(Suppl 2):6–13. [PubMed] [Google Scholar]
- 2.Buskila D, Cohen H. Comorbidity of fibromyalgia and psychiatric disorders. Curr Pain Headache Rep. 2007;11:333–338. doi: 10.1007/s11916-007-0214-4. [DOI] [PubMed] [Google Scholar]
- 3.Lockshin MD, for the Mary Kirkland Center for Lupus Research Consortium Biology of the sex and age distribution of systemic lupus erythematosus. Arthritis Rheum. 2007;57:608–611. doi: 10.1002/art.22676. [DOI] [PubMed] [Google Scholar]
- 4.Verhaak PF, Kerssens JJ, Dekker J, et al. Prevalence of chronic benign pain disorder among adults: A review of the literature. Pain. 1998;77:231–239. doi: 10.1016/S0304-3959(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe F, Ross K, Anderson J, Russell IJ. Aspects of fibromyalgia in the general population: Sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22:151–156. [PubMed] [Google Scholar]
- 6.Wijnhoven HA, de Vet HC, Picavet HS. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain. 2006;22:717–724. doi: 10.1097/01.ajp.0000210912.95664.53. [DOI] [PubMed] [Google Scholar]
- 7.Hunter M, Battersby R, Whitehead M. Relationships between psychological symptoms, somatic complaints and menopausal status. Maturitas. 1986;8:217–228. doi: 10.1016/0378-5122(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 8.Vanwesenbeeck I, Vennix P, van de Wiel H. ‘Menopausal symptoms’: Associations with menopausal status and psychosocial factors. J Psychosom Obstet Gynaecol. 2001;22:149–158. doi: 10.3109/01674820109049967. [DOI] [PubMed] [Google Scholar]
- 9.Breslau N, Schultz L, Peterson E. Sex differences in depression: A role for preexisting anxiety. Psychiatry Res. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 12.Seeman MV. Psychopathology in women and men: Focus on female hormones. Am J Psychiatry. 1997;154:1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- 13.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander JL, Dennerstein L, Woods NF, et al. Neurobehavioral impact of menopause on mood. Expert Rev Neurother. 2007;7(Suppl 11):S81–S91. doi: 10.1586/14737175.7.11s.S81. [DOI] [PubMed] [Google Scholar]
- 15.Bebbington PE, Dunn G, Jenkins R, et al. The influence of age and sex on the prevalence of depressive conditions: Report from the National Survey of Psychiatric Morbidity. Psychol Med. 1998;28:9–19. doi: 10.1017/s0033291797006077. [published correction appears in Psychol Med. 1998;28:1253] [DOI] [PubMed] [Google Scholar]
- 16.Goodwin FK, Jamison KR. Manic-Depressive Illness. Oxford University Press; New York, NY: 1990. [Google Scholar]
- 17.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004;161:2238–2244. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- 18.Weissman MM, Olfson M. Depression in women: Implications for health care research. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 19.Wittchen HU, Hoyer J. Generalized anxiety disorder: Nature and course. J Clin Psychiatry. 2001;62(Suppl 11):15–19. discussion 20–21. [PubMed] [Google Scholar]
- 20.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 21.Dickerson LM, Mazyck PJ, Hunter MH. Premenstrual syndrome. Am Fam Physician. 2003;67:1743–1752. [PubMed] [Google Scholar]
- 22.Gureje O. Comorbidity of pain and anxiety disorders. Curr Psychiatry Rep. 2008;10:318–322. doi: 10.1007/s11920-008-0051-0. [DOI] [PubMed] [Google Scholar]
- 23.Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66:17–22. doi: 10.1097/01.psy.0000106883.94059.c5. [DOI] [PubMed] [Google Scholar]
- 24.Fava M. Depression with physical symptoms: Treating to remission. J Clin Psychiatry. 2003;64(Suppl 7):24–28. [PubMed] [Google Scholar]
- 25.Fasmer OB. The prevalence of migraine in patients with bipolar and unipolar affective disorders. Cephalalgia. 2001;21:894–899. doi: 10.1046/j.1468-2982.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 26.Hung CI, Wang SJ, Hsu KH, et al. Risk factors associated with migraine or chronic daily headache in out-patients with major depressive disorder. Acta Psychiatr Scand. 2005;111:310–315. doi: 10.1111/j.1600-0447.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 27.Hung CI, Liu CY, Juang YY, Wang SJ. The impact of migraine on patients with major depressive disorder. Headache. 2006;46:469–477. doi: 10.1111/j.1526-4610.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 28.Hung CI, Weng LJ, Su YJ, Liu CY. Depression and somatic symptoms scale: A new scale with both depression and somatic symptoms emphasized. Psychiatry Clin Neurosci. 2006;60:700–708. doi: 10.1111/j.1440-1819.2006.01585.x. [DOI] [PubMed] [Google Scholar]
- 29.Oedegaard KJ, Fasmer OB. Is migraine in unipolar depressed patients a bipolar spectrum trait? J Affect Disord. 2005;84:233–242. doi: 10.1016/j.jad.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain. 2003;19:168–174. doi: 10.1097/00002508-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Frye CA, Bock BC, Kanarek RB. Hormonal milieu affects tailflick latency in female rats and may be attenuated by access to sucrose. Physiol Behav. 1992;52:699–706. doi: 10.1016/0031-9384(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 32.Frye CA, Cuevas CA, Kanarek RB. Diet and estrous cycle influence pain sensitivity in rats. Pharmacol Biochem Behav. 1993;45:255–260. doi: 10.1016/0091-3057(93)90116-b. [DOI] [PubMed] [Google Scholar]
- 33.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 34.Peng HY, Huang PC, Liao JM, et al. Estrous cycle variation of TRPV1-mediated cross-organ sensitization between uterus and NMDA-dependent pelvic-urethra reflex activity. Am J Physiol Endocrinol Metab. 2008;295:E559–E668. doi: 10.1152/ajpendo.90289.2008. [DOI] [PubMed] [Google Scholar]
- 35.Berkley KJ, McAllister SL, Accius BE, Winnard KP. Endometriosis-induced vaginal hyperalgesia in the rat: Effect of estropause, ovariectomy, and estradiol replacement. Pain. 2007;132(Suppl 1):S150–S159. doi: 10.1016/j.pain.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradshaw HB, Berkley KJ. Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas. 2002;41:157–165. doi: 10.1016/s0378-5122(01)00261-4. [DOI] [PubMed] [Google Scholar]
- 37.Frye CA, Duncan JE. Progesterone metabolites, effective at the GABA receptor complex, attenuate pain sensitivity in rats. Brain Res. 1994;643:194–203. doi: 10.1016/0006-8993(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 38.Frye CA, Duncan JE. Estradiol benzoate potentiates neuroactive steroids’ effects on pain sensitivity. Pharmacol Biochem Behav. 1996;53:27–32. doi: 10.1016/0091-3057(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 39.Frye CA, Lacey EH. Progestins influence performance on cognitive tasks independent of changes in affective behavior. Psychobiology. 2000;28:550–563. [Google Scholar]
- 40.Frye CA, Walf AA. Estrogen and/or progesterone systemically or to the amygdala can have anxiety, fear, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- 41.Gordon FT, Soliman MR. The effects of estradiol and progesterone on pain sensitivity and brain opioid receptors in ovariectomized rats. Horm Behav. 1996;30:244–250. doi: 10.1006/hbeh.1996.0029. [DOI] [PubMed] [Google Scholar]
- 42.Ren K, Weil F, Dubner R, et al. Progesterone attenuates persistent inflammatory hyperalgesia in female rats: Involvement of spinal NMDA receptor mechanisms. Brain Res. 2000;865:272–277. doi: 10.1016/s0006-8993(00)02267-8. [DOI] [PubMed] [Google Scholar]
- 43.Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- 44.Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Wise PM. Neuroendocrine modulation of the “menopause”: Insights into the aging brain. Am J Physiol. 1999;277:E965–E970. doi: 10.1152/ajpendo.1999.277.6.E965. [DOI] [PubMed] [Google Scholar]
- 46.Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. Neuroendocinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- 47.Long JA, Evans HM. The estrous cycle in the rat and its associated phenomena. Memories of University of California. 1922;6:1–148. [Google Scholar]
- 48.Frye CA, Paris JJ, Walf AA. Transition to reproductive senescence of middle-aged Long-Evans rats produces minimal effects on social interaction or lordosis, but attenuates proceptive, and enhances aggressive, behavior during a semi-naturalistic, paced mating test. Reproduction. In press. [Google Scholar]
- 49.Walf AA, Frye CA. Anti-nociception following exposure to trimethylthiazoline, peripheral or intra-amygdala estrogen and/or progesterone. Behav Brain Res. 2003;144:77–85. doi: 10.1016/s0166-4328(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 50.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walf AA, Frye CA. ERβ-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 52.Frye CA, Sumida K, Dudek BC, et al. Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- 53.Forman LJ, Tingle V, Estilow S, Cater J. The response to analgesia testing is affected by gonadal steroids in the rat. Life Sci. 1989;45:447–454. doi: 10.1016/0024-3205(89)90631-0. [DOI] [PubMed] [Google Scholar]
- 54.Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distension in the rat. Pain. 1999;82:187–197. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 55.Ceccarelli I, Fiorenzani P, Massafra C, Aloisi AM. Repeated nociceptive stimulation induces different behavioral and neuronal responses in intact and gonadectomized female rats. Brain Res. 2006;1106:142–149. doi: 10.1016/j.brainres.2006.05.089. [DOI] [PubMed] [Google Scholar]
- 56.Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Nomikos GC, Spyraki C. Influence of oestrogen on spontaneous and diazepam-induced exploration of rats in an elevated plus maze. Neuropharmacology. 1988;27:691–696. doi: 10.1016/0028-3908(88)90077-9. [DOI] [PubMed] [Google Scholar]
- 58.Walf AA, Frye CA. Estradiol's effects to reduce anxiety and depressive behavior may be mediated by estradiol dose and restraint stress. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- 59.Carboni E, Wieland S, Lan NC, Gee KW. Anxiolytic properties of endogenously occurring pregnanediols in two rodent models of anxiety. Psychopharmacology (Berl) 1996;126:173–178. doi: 10.1007/BF02246353. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez-Guasti A, Picazo O. Changes in burying behavior during the estrous cycle: Effect of estrogen and progesterone. Psychoneuroendocrinology. 1992;17:681–689. doi: 10.1016/0306-4530(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 61.Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: A possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- 62.Saavedra M, Contreras CM, Azamar-Arizmendi G. Hernández-Lozano M. Differential progesterone effects on defensive burying and forced swimming tests depending upon a gradual decrease or an abrupt suppression schedules. Pharmacol Biochem Behav. 2006;83:130–135. doi: 10.1016/j.pbb.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 63.Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–219. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kavaliers M, Wiebe JP. Analgesic effects of the progesterone metabolite 3α-hydroxy-5α-pregnan-20-one, and possible modes of action in mice. Brain Res. 1987;415:393–398. doi: 10.1016/0006-8993(87)90228-9. [DOI] [PubMed] [Google Scholar]
- 65.Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5-α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- 66.Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 67.Bowie MW, Slattum PW. Pharmacodynamics in older adults: A review. Am J Geriatr Pharmacother. 2007;5:263–303. doi: 10.1016/j.amjopharm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Wikinski SI, Acosta GB, Gravielle MC, et al. Diazepam fails to potentiate GABA-induced chloride uptake and to produce anxiolytic-like action in aged rats. Pharmacol Biochem Behav. 2001;68:721–727. doi: 10.1016/s0091-3057(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 69.Barbaccia ML, Concas A, Serra M, Biggio G. Stress and neurosteroids in adult and aged rats. Exp Gerontol. 1998;33:697–712. doi: 10.1016/s0531-5565(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 70.Genazzani AR, Stomati M, Bernardi F, et al. Conjugated equine estrogens reverse the effects of aging on central and peripheral allopregnano-lone and beta-endorphin levels in female rats. Fertil Steril. 2004;81(Suppl 1):757–766. doi: 10.1016/j.fertnstert.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 71.Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: Gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- 72.Serova L, Rivkin M, Nakashima A, Sabban EL. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;75:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- 73.Serova LI, Maharjan S, Sabban EL. Estrogen modifies stress response of catecholamine biosynthetic enzyme genes and cardiovascular system in ovariectomized female rats. Neuroscience. 2005;132:249–259. doi: 10.1016/j.neuroscience.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 74.Bodnar RJ, Romero MT, Kramer E. Organismic variables and pain inhibition: Roles of gender and aging. Brain Res Bull. 1988;21:947–953. doi: 10.1016/0361-9230(88)90032-9. [DOI] [PubMed] [Google Scholar]
- 75.Mogil JS, Sternberg WF, Kest B, et al. Sex differences in the antagonism of swim stress-induced analgesia: Effects of gonadectomy and estrogen replacement. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 76.Ryan SM, Maier SF. The estrous cycle and estrogen modulate stress-induced analgesia. Behav Neurosci. 1988;102:371–380. doi: 10.1037//0735-7044.102.3.371. [DOI] [PubMed] [Google Scholar]
- 77.Sternberg WF. Sex differences in the effects of prenatal stress on stress-induced analgesia. Physiol Behav. 1999;68:63–72. doi: 10.1016/s0031-9384(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 78.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]