Abstract
Chronic lymphocytic leukemia (CLL) is characterized by a monoclonal expansion of mature B-lymphocytes. Mutational status of the immunoglobulin variable heavy chain region (IGHV) gene stratifies CLL patients into two prognostic groups. We performed microarray analysis of CLL cells using the Agilent platform to detect the most important gene expression differences regarding IGHV status in CLL cells. We analyzed a cohort of 118 CLL patients with different IGHV mutational status and completely characterized all described prognostic markers using expression microarrays and quantitative real-time RT-PCR (reverse transcription PCR). We detected lymphocyte-activation gene 3 (LAG3) as a novel prognostic marker: LAG3 high expression in CLL cells correlates with unmutated IGHV (P < 0.0001) and reduced treatment-free survival (P = 0.0087). Furthermore, quantitative real-time RT-PCR analysis identified a gene-set (LAG3, LPL, ZAP70) whose overexpression is assigned to unmutated IGHV with 90% specificity (P < 0.0001). Moreover, high expression of tested gene-set and unmutated IGHV equally correlated with reduced treatment-free survival (P = 7.7 * 10−11 vs. P = 1.8 * 10−11). Our results suggest that IGHV status can be precisely assessed using the expression analysis of LAG3, LPL, and ZAP70 genes. Expression data of tested markers provides a similar statistical concordance with treatment-free survival as that of the IGHV status itself. Our findings contribute to the elucidation of CLL pathogenesis and provide novel prognostic markers for possible application in routine diagnostics.
B-cell chronic lymphocytic leukemia (CLL) is a lymphoproliferative disorder with a highly variable clinical outcome, characterized by clonal expansion of mature B-cells expressing cell surface antigens CD5, CD23, and CD27, and low levels of surface Ig.1,2 There are several independent prognostic factors used in prediction of clinical outcome of CLL disease. Apart from traditional Rai3 and Binet4 staging, lymphocyte doubling time, morphology, immunophenotype, β-2-microglobulin or lactate dehydrogenase, cytogenetics, and molecular markers assume an important place. There are four most common recurrent genomic alterations with prognostic significance detectable in CLL cases5: deletion 13q has a favorable clinical outcome compared with normal karyotype, deletions 17p, and 11q, and trisomy 12 are negative prognostic markers. The mutational status of the immunoglobulin variable heavy chain region (IGHV) gene is one of the most important molecular prognostic factors in CLL. The presence of unmutated IGHV gene identical with the germ line sequence by more than 98% is associated with a worse prognosis and shorter survival.6,7 The reason for the close correlation of the IGHV mutational status and different clinical course remains a matter of intense debate. The presence of somatic hypermutation in IGHV in half of CLL cases led to the theory that CLL cases with unmutated IGHV emerged from naive B-cells meaning that these cells are not antigen-experienced. On the other hand, CLL with mutated IGHV were supposed to develop from post-germinal center (GC) B-cells. This hypothesis was consequently rejected with detailed immunophenotypic studies8 and also with gene expression profiling using high-throughput approaches.9,10,11,12,13 Moreover identification of a physiological analogue to CLL cells remains unsuccessful.
Several gene expression profiling studies have focused on comparison of cells with mutated and unmutated IGHV.9,10,13 Surprisingly the differences between gene expression of CLL cells with mutated and unmutated IGHV were very low and the expression signatures did not correspond to any known physiological counterpart of B-cells in humans. However there were defined genes with slightly different gene expression between these two prognostic groups. Such studies also tried to establish a set of simply detectable markers that could help to improve stratification of newly diagnosed patients or even replace the analysis of IGHV mutational status. Using microarray approaches, ZAP70 (ζ-chain associated protein kinase 70 kDa) was defined as a new prognostic marker,9 and higher expression of this tyrosine kinase in CLL cells correlates to some extent with unmutated IGHV and worse prognosis. A lot of effort has been devoted to standardization of ZAP70 routine quantification14 and correlation between ZAP70 expression and IGHV mutational status concluding that high expression of ZAP70 is strongly associated with a worse prognosis independently of the IGHV mutational status.15
Vasconcelos et al16 in their microarray study combined two independent prognostic factors, ie, IGHV mutational status and Binet staging. Using a comparison between the extreme ends of the disease spectrum—stable and IGHV-mutated versus progressive and IGHV-unmutated CLL—they confirmed a slight difference in the gene expression underlying these two entities. In a subsequent study,17 the expression ratio of LPL (lipoprotein lipase) to ADAM29 (a disintegrin and metalloproteinase domain 29) has been established as the best predictor for prognosis and a potential surrogate marker for IGHV status. However, a confirmatory study identified LPL expression itself as a better prognostic factor compared with the LPL/ADAM29 ratio.18 Furthermore it was reported that the LPL expression represents the best survival predictor, being as good as IGHV mutational status itself and better than ZAP70 expression monitoring.19,20 The fact that LPL is not expressed in any other blood cells but the IGHV unmutated CLL lymphocytes represents a major advantage.21,22 Although LPL seems to be a superior surrogate marker for IGHV mutational status and a predictor of poor prognosis, some other genes have also been correlated with high-risk CLL and shorter survival. For example, high expression of CLLU1 (chronic lymphocytic leukemia up-regulated 1),23 SEPT10 (septin 10),22 AICDA (activation-induced cytidine deaminase),24 and a low expression of TCF7 (transcription factor 7)16 were detectable in the CLL cells with unmutated IGHV.
In our study, we performed a microarray analysis using the Agilent expression arrays in contrast to majority of similar studies that used mainly Affymetrix platform. The Agilent microarrays used detected expression of approximately 22,000 human genes and due to the different probe design were able to provide additional information to already used Affymetrix arrays. We correlated gene expression with IGHV mutational status and identified lymphocyte-activation gene 3 (LAG3, CD223) as a novel marker of CLL cases with unmutated IGHV. This gene has not been previously published in connection with CLL and IGHV mutational status. We tested expression level of LAG3 in correlation with worse prognosis on a cohort of 118 CLL patients using quantitative real time RT-PCR. Moreover, we also validated a set of seven most frequently studied molecular markers alone and in different combinations (LPL, ZAP70, AICDA, BCL2, CLLU1, SEPT10, and TCF7) on the same cohort of CLL patients. Finally, we selected a set of three genes (LAG3, LPL, and ZAP70) with the best statistical prognostic value compared with IGHV mutational status itself.
Materials and Methods
Blood Samples
Heparinized peripheral blood samples were collected from 118 CLL patients with informed consent. Samples originated from previously untreated patients (70 males and 48 females) with characterized IGHV mutational status (59 unmutated vs. 59 mutated) and a median age of 63 years (range 42 to 82 years). Cytogenetic data of analyzed patients are summarized in Table 1.
Table 1.
Summary of Cytogenetic Data
| Cytogenetics Number of patients |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13q− |
11q− |
||||||||||||
| IGHV Number of patients | No aberration | Alone | Plus 11q− | Plus 12+ | Plus 17p− | Plus 11q− and 12+ | Alone | Plus 12+ | Plus 17p− | Plus 12+ and 17p− | 12+ Alone | 17p− Alone | All aberrations |
| 59 mutated | 17 | 33 | 1 | 2 | 1 | 1 | 1 | − | − | − | 3 | − | − |
| 59 unmutated | 10 | 13 | 9 | 1 | 2 | − | 8 | 1 | 1 | 1 | 9 | 2 | 2 |
Sample Preparation
CLL cells were separated from peripheral blood using RosetteSep B Cell Enrichment Kit (StemCell Technologies, Vancouver, Canada) according to manufacturer's instructions. Samples with CLL cells content higher than 95% were used for further analysis (assessed by flow-cytometry). Total RNA was isolated with RNeasy Mini Kit and digested with DNase I (Qiagen, Hilden, Germany) according to manufacturer's instructions.
IGHV Mutational Status Determination
IGHV mutational status was determined according to ERIC (European Research Initiative on CLL) recommendations.25 Briefly, total RNA was amplified by reverse transcription polymerase chain reaction (RT-PCR) using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) with heavy chain variable region (VH) family specific forward primers and common reverse heavy chain joining region (JH) primer. Clonal product was consequently sequenced. The nucleotide sequences were aligned with IMGT/V-QUEST. An unmutated status was assigned in case of ≤ 2% deviation from the germ line IGHV sequences.
Microarray Analysis
Ten samples with mutated and 10 samples with unmutated IGHV were randomly chosen from the studied cohort of 118 CLL patients for microarray analysis. Linear amplification of 1.2 μg total RNA (Low RNA Input Linear Amplification Kit, Agilent, Palo Alto, CA, modified protocol) including incorporation of aminoallyl-UTPs (Epicentre, Madison, WI) was performed and cRNA was consequently fluorescently labeled (Dy547-NHS-ester, Dy647-NHS-ester, Dyomics, Jena, Germany) and cohybridized with differently labeled reference RNA (Universal Human Reference RNA, Stratagene, Cedar Creek, TX) on Human 1A Arrays (Agilent). All patient samples were hybridized with the same reference. This reference was labeled with both dyes; part of the samples labeled with green and the other part with red fluorescent dye. Random dye assignment to the reference and patient samples controlled dye related bias. Maximizing the number of samples to control an interindividual variability was preferred over technical replication that would only help to estimate nonbiological sources of variation. With a certain number of arrays, the approach omitting dye-swap (technical replication) is beneficial in contrast to a dye-swap design.26,27 Microarray image analysis was performed using QuantArray software (PerkinElmer, Waltham, MA). Raw data were normalized in R and R Bioconductor28 and analyzed using MEV software29 and Significance Analysis of Microarrays 30 supervised algorithm. The microarray data has been deposited in ArrayExpress (http://www.ebi.ac.uk/microarray-as/ae/; accession no: E-TABM-696).
Quantitative Real Time RT-PCR of Selected Genes
cDNA synthesis from 500 ng total RNA was performed using SuperScript III Reverse Transcriptase with oligo(dT)12-18 primer (Invitrogen). Each sample was analyzed in triplicate using the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions (for list of used TaqMan Gene Expression Assays see Supplemental Table S1 at http://jmd.amjpathol.org). DNA amplification was detected using the 7300 Real Time PCR System (Applied Biosystems). Data were analyzed using the Sequence Detection System software version 1.3.1 (Applied Biosystems). Relative gene expression was normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. GAPDH was selected as a gene with the lowest variability among tested housekeeping genes on our set of samples.
Statistical Analyses
Statistical significance of differential expression between IGHV mutated and unmutated cases analyzed with quantitative real time (RT-PCR) was determined using the Wilcoxon rank sum test. A training set of 40 randomly chosen samples (20 with mutated and 20 with unmutated IGHV) was examined with series of threshold analysis to maximize the confidence of correct discrimination between cases with mutated and unmutated IGHV. Every possible combination of selected genes was tested to improve the discriminating potency of the gene expression analysis and determine the gene combination with the best predictive value. Every possible combination of threshold ΔCt values in the range 2 to 12 in increments of 0.1 was tested. The combination giving the best sensitivity and specificity values (Youden's index, for combinations with the same Youden's indexes, the one with highest sensitivity was preferred) was applied to the test set. A verifying set of 78 samples was consequently analyzed; sensitivity, specificity, positive prognostic value (PPV), negative prognostic value (NPV) was determined (Table 2). To further verify the performance of selected gene-set linear discriminant analysis was used to construct classifier. For linear discriminant analysis, the data set was divided into training group of 70 samples and testing group of 48 samples.
Table 2.
Summary of quantitative real time RT-PCR Results
| Gene | Sensitivity % | Specificity % | PPV % | NPV % | Wilcoxon rank sum test |
|---|---|---|---|---|---|
| Set of three genes: LAG3, LPL, and ZAP70 | 86 | 90 | 89 | 87 | <2.2 * 10−16 |
| Set of two genes: LAG3 and ZAP70 | 79 | 80 | 83 | 78 | <2.2 * 10−16 |
| Set of two genes: LAG3 and LPL | 85 | 80 | 80 | 85 | <2.2 * 10−16 |
| Set of two genes: LPL and ZAP70 | 85 | 89 | 89 | 85 | <2.2 * 10−16 |
| LAG3 (lymphocyte activation gene 3) | 93 | 32 | 58 | 83 | 1.186 * 10−13 |
| LPL (lipoprotein lipase) | 88 | 80 | 81 | 87 | <2.2 * 10−16 |
| ZAP70 (ζ-chain associated protein kinase 70 kd) | 93 | 60 | 70 | 90 | 1.063 * 10−08 |
| AICDA (activation-induced cytidine deaminase) | 69 | 56 | 66 | 68 | 2.735 * 10−07 |
| BCL2 (B-cell-leukemia/lymphoma 2) | 74 | 62 | 67 | 69 | <2.2 * 10−16 |
| CLLU1 (chronic lymphocytic leukemia up-regulated 1) | 63 | 60 | 61 | 62 | <2.2 * 10−16 |
| TCF7 (transcription factor 7) | 84 | 80 | 82 | 83 | 1.544 * 10−11 |
| SEPT10 (septin 10) | 72 | 67 | 70 | 69 | 1.471 * 10−11 |
Expression of selected genes in CLL cells with different IGHV mutational status. Parameters calculated in relation to unmutated IGHV for tested genes. PPV, positive predictive value; NPV, negative predictive value.
Treatment-free survival (TFS) was evaluated as from the time of diagnosis to the beginning of CLL-related therapy using the Kaplan-Meier estimator, and statistical significance was calculated using the log-rank test.
All analyses were performed in R statistical environment.
Results
Our microarray study detected a set of ∼ 50 genes with different expression levels in CLL cells with different IGHV mutational status. Figure 1 shows the expression patterns of 20 microarray-tested CLL patients and a differentially expressed gene set, with analysis settings of median false discovery rate < 1%. Correlation of the reference RNA expression in between the individual arrays was characterized by Pearson's Correlation Coefficient r > 0.97.
Figure 1.
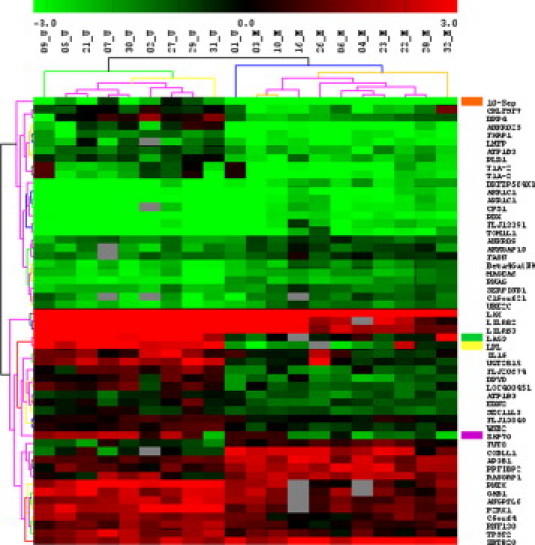
Significance Analysis of Microarrays supervised analysis of differently expressed genes. Expression profiles obtained using microarray analysis were divided into groups according to IGHV mutational status (M, mutated IGHV; n = 10 and U, unmutated IGHV; n = 10). Significant genes were selected with Significance Analysis of Microarrays and grouped into clusters. Gene coloring is based on normalized patient-to-reference RNA log2 ratios as shown at the top of the figure. Genes selected for further validation using quantitative real time RT-PCR are marked in colors on the side.
We detected lipoprotein lipase (LPL) as the gene with the best differentiating potential. Identification of LPL in our experimental set of patients was in line with recently reported data.17,21 In concordance with previously published studies, we also confirmed the overexpression of ZAP709 and SEPT1022 in CLL cells with unmutated IGHV. Moreover, we detected the lymphocyte-activation gene 3 (LAG3), also known as CD223, as a novel independent marker of CLL cells harboring the unmutated IGHV gene.
Data acquired from microarray analysis was further validated using quantitative real time RT-PCR on 118 patients (including 20 patients initially microarray-tested). We analyzed the expression levels of LAG3, LPL, ZAP70, and SEPT10 that resulted from our microarray analysis, together with four other candidate genes selected from similar studies (AICDA, BCL2, CLLU1, and TCF7) to evaluate their prognostic relevance on our set of samples. Threshold values were set for each gene: 8.9 (ΔCt) for LAG3, 8.8 (ΔCt) for LPL, 5.4 (ΔCt) for ZAP70, 14 (ΔCt) for CLLU1, 14 (ΔCt) for AICDA, 12 (ΔCt) for SEPT10, 6 (ΔCt) for TCF7, and 2.2 (ΔCt) for BCL2. The values in the Table 2 present results obtained on the test set. Statistical significance of the quantitative real time RT-PCR data were analyzed using the Wilcoxon rank sum test and confirmed a strong association of high expression of LAG3, LPL, ZAP70, AICDA, BCL2, CLLU1, SEPT10, and low expression of TCF7 with unmutated IGHV (P < 0.0001 in all tested genes) summarized in Table 2. High expression of LAG3 alone correlates with the absence of IGHV mutations with 93% sensitivity. Relative gene expression levels of tested genes in groups with mutated and unmutated IGHV are presented as box plots (see Supplemental Figure S1 at http://jmd.amjpathol.org). We also tested the best gene combination to obtain a gene-set the expression of which has the highest correlation with IGHV mutational status. Our results showed that combined assessment of LPL, ZAP70, and LAG3 expression may correctly assign IGHV status with 90% specificity (P < 0.0001) and 86% sensitivity (P < 0.0001) (Table 2) with 89% positive predictive value. Linear discriminant analysis using the expression values of three best genes and IGHV mutational status trained on 70 samples correctly classified 84% of samples. The combination of three markers (LAG3 + LPL + ZAP70) provides higher sensitivity and specificity than any combination of two markers (ie, LAG3 + ZAP70, LAG3 + LPL, and LPL and ZAP70), as indicated in Table 2.
In addition to this, we correlated the expression of tested genes with TFS using the Kaplan-Meier estimator (Figure 2, A−F). Patients manifesting a high expression of LAG3, LPL, or ZAP70 required therapy significantly earlier than patients with a low expression of the tested genes. TFS median in patients with low LAG3 expression was not reached compared with a median of 50 months in cases with high expression (P < 0.0089) (Figure 2C). TFS median in patients with low LPL expression was 157 vs. 17 months in patients with high LPL expression (P < 0.001) (Figure 2B). TFS median in patients with low ZAP70 expression was not reached compared with 33 months in those with high ZAP70 expression (P < 0.001) (Figure 2D). The IGHV mutational status had TFS median of 13 months in unmutated cases and median not reached in mutated (P = 1.8 * 10−11) (Figure 2A), our tested gene-set (LPL and LAG3 together with ZAP70) showed TFS median of 157 months in cases with low vs. 15 months with high expression (P = 7.71 * 10−11) (Figure 2E). Furthermore, we compared the differences in TFS with respect to the number of overexpressed genes (Figure 2F). TFS median was not reached in arms with all three genes down-regulated or only one up-regulated (both P < 0.001). TFS median in cases with two genes up-regulated was 157 months (P < 0.001) in comparison with TFS median 15 months, in cases with all three genes up-regulated.
Figure 2.
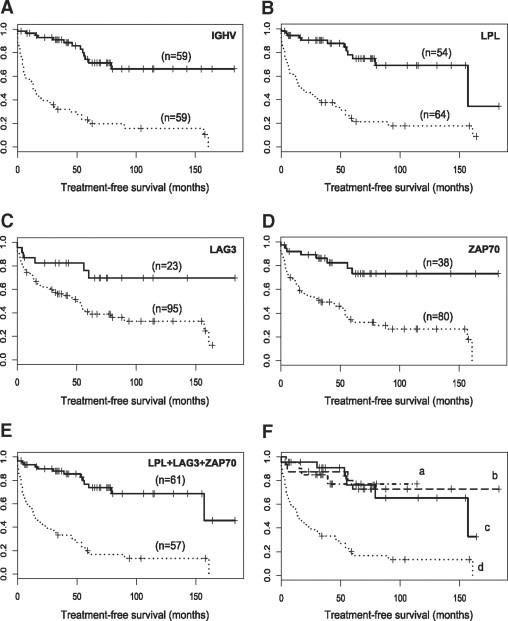
Kaplan-Meier curves for treatment-free survival (TFS) from diagnosis to onset of CLL-related therapy according to IGHV mutational status (A) and expression of tested genes (B, C, D, E, and F). Statistical significance was calculated using a log-rank test. A: Patients were grouped according to IGHV mutational status; the TFS median was not reached vs. 13 months in mutated and in unmutated arms, respectively (P < 0.001). Solid line corresponds to mutated IGHV; dotted line corresponds to unmutated IGHV. B: Patients were grouped according to LPL gene expression; the TFS median was 157 vs. 17 months in low and high expression arms, respectively (P < 0.001). Solid line corresponds to low expression; dotted line corresponds to high expression of LPL. C: Patients were grouped according to LAG3 gene expression; the TFS median was not reached vs. 50 months in low and high expression arms, respectively (P < 0.0089). Solid line corresponds to low expression; dotted line corresponds to high expression of LAG3. D: Patients were grouped according to ZAP70 gene expression; the TFS median was not reached vs. 33 months in low and high expression arms, respectively (P < 0.001). Solid line corresponds to low expression; dotted line corresponds to high expression of ZAP70. E: Patients were grouped according to combined 3-gene set expression (LPL + LAG3 + ZAP70); the TFS median was 157 vs. 15 months in low and high expression arms, respectively (P < 0.001). Solid line corresponds to low expression and dotted line corresponds to high expression of gene set. F: Patients were grouped according to combined 3-gene set expression (LPL + LAG3 + ZAP70) considering number of up-regulated genes; the TFS median was: (a) not reached in arm with all 3 genes down-regulated (n = 16), dash-and-dot line, P < 0.001, as compared with (d); (b) not reached in arm with 1 gene up-regulated (n = 23), dashed line, P < 0.001, as compared with (d); (c) 157 months in arm with 2 genes up-regulated (n = 22), solid line, P < 0.001, as compared with (d); and (d) 15 months in arm with all three genes up-regulated (n = 57), dotted line.
Discussion
Expression analysis of different subgroups of CLL patients identified LAG3 as an important prognostic marker with possible relationship to CLL pathogenesis. LAG3 (CD223) was identified in 1990 by authors Triebel et al.31 This surface protein is related to CD4 molecule and physiologically expressed on T-cells and natural killer-cells after cell activation and not on resting peripheral blood lymphocytes.31 Surface LAG3 expression on activated human T-cells is up-regulated by interleukin IL-2, IL-7, and IL-12,32 and is detectable on all human T-cells 2 to 3 days after activation.33 Therefore LAG3 probably does not take part in induction phase of immune response but may play essential role during activation. Foa et al34 reported that IL-2 released by B-CLL T-lymphocytes may be used by the neoplastic B-cell clone expressing the IL-2 receptor. Decreased availability of IL-2 could therefore play part in some of the T-cell defects in B-CLL. Moreover, Huard et al35 reported that LAG3 down-regulates CD4+ antigen-specific T-cell proliferation through interaction with MHC II molecules and Workman and Vingali36 suggest that LAG3 functions as a negative regulator of T-cell homeostasis. These findings correlate with facts that total amount of T-cells in B-CLL is often increased and the ratio of helper CD4 to suppressor CD8 T-cells is in many cases reversed.37
During normal B-cell maturation naive B-cells enter the lymph node, are activated with antigen, form GCs inside the lymph node follicles and start to proliferate with the contribution of CD4+ helper T-cells. In this respect Kisielow et al38 reported an interesting finding that LAG3 can be also expressed on activated murine B-lymphocytes, but only on B-cells activated with T-cells. They suggested that LAG3 can serve as a marker of T-cell induced B-cell activation while the strength of B-cell activation could influence the formation of GCs.39 Activated extrafollicular B-cells form short-lived plasmablasts, enter GC reaction and differentiate into memory cells. Insufficient specific T-cell help together with strong B-cell activation lead to transient GC formation and extrafollicular differentiation of activated B-cells. Aberrant expression of activating markers on CLL cells like ZAP70 and LAG3 together with detectable expression of AICDA outside the germinal centers could therefore indicate improperly finished or prematurely terminated process of B-lymphocyte affinity maturation of CLL cells with unmutated IGHV.
Analysis of the IGHV mutational status as a prognostic marker in CLL has been widely used; however this process is time consuming and includes several steps. Proposed gene-set (LPL, ZAP70, and LAG3) detection enables fast testing of a large set of samples in only two-steps analysis. In addition, many laboratories introduce ZAP70 analysis as a part of routine CLL diagnostics and prognosis assessment. ZAP70 expression can be detected on mRNA level using quantitative real time RT-PCR or on protein level using flow-cytometry, but proper standardization of flow-cytometric analysis still remains controversial. ZAP70 is physiologically expressed in T-cells, depletion of these cells is crucial for correct quantitative real time RT-PCR analysis. Therefore the enlargement of a set of analyzed markers could increase the confidence of the prognosis assessment. We proved that combined expression analysis of tested surrogate markers (LPL, LAG3, and ZAP70) provides a similar statistical concordance with TFS as that of the IGHV status itself and could be potentially used in diagnostics.
Footnotes
Supported by research grants IGA MZ CR NR8448-3/2005, NR9293-3/2007, NS10439-3/2009 and research program MSMT CR MSM0021622430.
Supplemental material for this article can be found on http://jmd.amjpathol.org.
Web Extra Material
A-H. Relative expression of tested genes in groups with mutated and unmutated IGHV detected using qRT-PCR.
List of used TaqMan® Gene Expression Assays.
References
- 1.Küppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:1520–1529. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 3.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 4.Binet JL, Lepoprier M, Dighiero G, Charron D, D'Athis P, Vaugier G, Beral HM, Natali JC, Raphael M, Nizet B, Follezou JY. A clinical staging system for chronic lymphocytic leukemia: prognostic significance. Cancer. 1977;40:855–864. doi: 10.1002/1097-0142(197708)40:2<855::aid-cncr2820400239>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Döhner H, Stilgenbauer S, Döhner K, Bentz M, Lichter P. Chromosome aberrations in B-cell chronic lymphocytic leukemia: reassessment based on molecular cytogenetic analysis. J Mol Med. 1999;77:266–281. doi: 10.1007/s001090050350. [DOI] [PubMed] [Google Scholar]
- 6.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 7.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J, Lichtman SM, Schulman P, Vinciguerra VP, Rai KR, Ferrarini M, Chiorazzi N. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 8.Damle RN, Ghiotto F, Valetto A, Albesiano E, Fais F, Yan XJ, Sison CP, Allen SL, Kolitz J, Schulman P, Vinciguerra VP, Budde P, Frey J, Rai KR, Ferrarini M, Chiorazzi N. B-cell chronic lymphocytic leukemia cells express a surface membrane phenotype of activated, antigen-experienced B lymphocytes. Blood. 2002;99:4087–4093. doi: 10.1182/blood.v99.11.4087. [DOI] [PubMed] [Google Scholar]
- 9.Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, Yang L, Pickeral OK, Rassenti LZ, Powell J, Botstein D, Byrd JC, Grever MR, Cheson BD, Chiorazzi N, Wilson WH, Kipps TJ, Brown PO, Staudt LM. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, Freedman A, Inghirami G, Cro L, Baldini L, Neri A, Califano A, Dalla-Favera R. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med. 2001;194:1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stratowa C, Löffler G, Lichter P, Stilgenbauer S, Haberl P, Schweifer N, Döhner H, Wilgenbus KK. cDNA microarray gene expression analysis of B-cell chronic lymphocytic leukemia proposes potential new prognostic markers involved in lymphocyte trafficking. Int J Cancer. 2001;91:474–480. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1078>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Jelinek DF, Tschumper RC, Stolovitzky GA, Iturria SJ, Tu Y, Lepre J, Shah N, Kay NE. Identification of a global gene expression signature of B-chronic lymphocytic leukemia. Mol Cancer Res. 2003;1:346–361. [PubMed] [Google Scholar]
- 13.Haslinger C, Schweifer N, Stilgenbauer S, Döhner H, Lichter P, Kraut N, Stratowa C, Abseher R. Microarray gene expression profiling of B-cell chronic lymphocytic leukemia subgroups defined by genomic aberrations and VH mutation status. J Clin Oncol. 2004;22:3937–3949. doi: 10.1200/JCO.2004.12.133. [DOI] [PubMed] [Google Scholar]
- 14.Letestu R, Rawstron A, Ghia P, Villamor N, Boeckx N, Boettcher S, Buhl AM, Duerig J, Ibbotson R, Kroeber A, Langerak A, Le Garff-Tavernier M, Mockridge I, Morilla A, Padmore R, Rassenti L, Ritgen M, Shehata M, Smolewski P, Staib P, Ticchioni M, Walker C, Ajchenbaum-Cymbalista F. Evaluation of ZAP-70 expression by flow cytometry in chronic lymphocytic leukemia: a multicentric international harmonization process. Cytometry B Clin Cytom. 2006;70:309–314. doi: 10.1002/cyto.b.20132. [DOI] [PubMed] [Google Scholar]
- 15.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, Kay NE, Brown JR, Gribben JG, Neuberg DS, He F, Greaves AW, Rai KR, Kipps TJ. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–1930. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasconcelos Y, De Vos J, Vallat L, Rème T, Lalanne AI, Wanherdrick K, Michel A, Nguyen-Khac F, Oppezzo P, Magnac C, Maloum K, Ajchenbaum-Cymbalista F, Troussard X, Leporrier M, Klein B, Dighiero G, Davi F. French Cooperative Group on CLL: gene expression profiling of chronic lymphocytic leukemia can discriminate cases with stable disease and mutated Ig genes from those with progressive disease and unmutated Ig genes. Leukemia. 2005;19:2002–2005. doi: 10.1038/sj.leu.2403865. [DOI] [PubMed] [Google Scholar]
- 17.Oppezzo P, Vasconcelos Y, Settegrana C, Jeannel D, Vuillier F, Legarff-Tavernier M, Kimura EY, Bechet S, Dumas G, Brissard M, Merle-Béral H, Yamamoto M, Dighiero G, Davi F. French Cooperative Group on CLL: the LPL/ADAM29 expression ratio is a novel prognosis indicator in chronic lymphocytic leukemia. Blood. 2005;106:650–657. doi: 10.1182/blood-2004-08-3344. [DOI] [PubMed] [Google Scholar]
- 18.Nückel H, Hüttmann A, Klein-Hitpass L, Schroers R, Führer A, Sellmann L, Dührsen U, Dürig J. Lipoprotein lipase expression is a novel prognostic factor in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47:1053–1061. doi: 10.1080/10428190500464161. [DOI] [PubMed] [Google Scholar]
- 19.van't Veer MB, Brooijmans AM, Langerak AW, Verhaaf B, Goudswaard CS, Graveland WJ, van Lom K, Valk PJ. The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica. 2006;91:56–63. [PubMed] [Google Scholar]
- 20.Nikitin EA, Malakho SG, Biderman BV, Baranova AV, Lorie YY, Shevelev AY, Peklo MM, Vlasik TN, Moskalev EA, Zingerman BV, Vorob'ev IA, Poltaraus AB, Sudarikov AB, Vorobjev AI. Expression level of lipoprotein lipase and dystrophin genes predicts survival in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:912–922. doi: 10.1080/10428190701245112. [DOI] [PubMed] [Google Scholar]
- 21.Heintel D, Kienle D, Shehata M, Kröber A, Kroemer E, Schwarzinger I, Mitteregger D, Le T, Gleiss A, Mannhalter C, Chott A, Schwarzmeier J, Fonatsch C, Gaiger A, Döhner H, Stilgenbauer S, Jäger U, CLL Study Group High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1216–1223. doi: 10.1038/sj.leu.2403748. [DOI] [PubMed] [Google Scholar]
- 22.Bilban M, Heintel D, Scharl T, Woelfel T, Auer MM, Porpaczy E, Kainz B, Kröber A, Carey VJ, Shehata M, Zielinski C, Pickl W, Stilgenbauer S, Gaiger A, Wagner O, Jäger U, German CLL Study Group Deregulated expression of fat and muscle genes in B-cell chronic lymphocytic leukemia with high lipoprotein lipase expression. Leukemia. 2006;20:1080–1088. doi: 10.1038/sj.leu.2404220. [DOI] [PubMed] [Google Scholar]
- 23.Buhl AM, Jurlander J, Geisler CH, Pedersen LB, Andersen MK, Josefsson P, Petersen JH, Leffers H. CLLU1 expression levels predict time to initiation of therapy and overall survival in chronic lymphocytic leukemia. Eur J Haematol. 2006;76:455–464. doi: 10.1111/j.0902-4441.2005.t01-1-EJH2530.x. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy H, Wierda WG, Barron LL, Cromwell CC, Wang J, Coombes KR, Rangel R, Elenitoba-Johnson KS, Keating MJ, Abruzzo LV. High expression of activation-induced cytidine deaminase (AID) and splice variants is a distinctive feature of poor-prognosis chronic lymphocytic leukemia. Blood. 2003;101:4903–4908. doi: 10.1182/blood-2002-09-2906. [DOI] [PubMed] [Google Scholar]
- 25.Ghia P, Stamatopoulos K, Belessi C, Moreno C, Stilgenbauer S, Stevenson F, Davi F, Rosenquist R, European Research Initiative on CLL ERIC recommendations on IGHV gene mutational status analysis in chronic lymphocytic leukemia. Leukemia. 2007;21:1–3. doi: 10.1038/sj.leu.2404457. [DOI] [PubMed] [Google Scholar]
- 26.Dobbin KK, Simon RM. Comparison of microarray designs for class comparison and class discovery. Bioinformatics. 2002;18:1438–1445. doi: 10.1093/bioinformatics/18.11.1438. [DOI] [PubMed] [Google Scholar]
- 27.Dobbin KK, Shih JH, Simon RM. Comment on ‘Evaluation of the gene-specific dye bias in cDNA microarray experiments’. Bioinformatics. 2005;21:2803–2804. doi: 10.1093/bioinformatics/bti428. [DOI] [PubMed] [Google Scholar]
- 28.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 30.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruniquel D, Borie N, Hannier S, Triebel F. Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics. 1998;48:116–124. doi: 10.1007/s002510050411. [DOI] [PubMed] [Google Scholar]
- 33.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein CD223 (LAG-3) Eur J Immunol. 2002;32:2255–2263. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 34.Foa R, Fierro MT, Giovarelli M, Lusso P, Benetton G, Bonferroni M, Forni G. Immunoregulatory T-cell defects in B-cell chronic lymphocytic leukemia: cause or consequence of the disease? The contributory role of decreased availability of interleukin 2 (IL-2) Blood Cells. 1987;12:399–412. [PubMed] [Google Scholar]
- 35.Huard B, Prigent P, Pages F, Bruniquel D, Triebel F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur J Immunol. 1996;26:1180–1186. doi: 10.1002/eji.1830260533. [DOI] [PubMed] [Google Scholar]
- 36.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3(CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 37.Foa R, Giovarelli M, Jemma C, Fierro MT, Lusso P, Ferrando ML, Lauria F, Forni G. Interleukin 2 (IL 2) and interferon-gamma production by T lymphocytes from patients with B-chronic lymphocytic leukemia: evidence that normally released IL 2 is absorbed by the neoplastic B cell population. Blood. 1985;66:614–619. [PubMed] [Google Scholar]
- 38.Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005;35:2081–2088. doi: 10.1002/eji.200526090. [DOI] [PubMed] [Google Scholar]
- 39.Fink K, Manjarrez-Orduño N, Schildknecht A, Weber J, Senn BM, Zinkernagel RM, Hengartner H. B cell activation state-governed formation of germinal centers following viral infection. J Immunol. 2007;179:5877–5885. doi: 10.4049/jimmunol.179.9.5877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A-H. Relative expression of tested genes in groups with mutated and unmutated IGHV detected using qRT-PCR.
List of used TaqMan® Gene Expression Assays.


