Abstract
There has been a fascinating convergence of evidence in recent years implicating the disturbances of neural synchrony in the gamma frequency band (30–100 Hz) as a major pathophysiologic feature of schizophrenia. Evidence suggests that reduced glutamatergic neurotransmission via the N-methyl-D-aspartate (NMDA) receptors that are localized to inhibitory interneurons, perhaps especially the fast-spiking cells that contain the calcium-binding protein parvalbumin (PV), may contribute to gamma band synchrony deficits, which may underlie the failure of the brain to integrate information and hence the manifestations of many symptoms and deficits of schizophrenia. Furthermore, because gamma oscillations are thought to provide the temporal structure that is necessary for synaptic plasticity, gamma deficits may disturb the developmental synaptic reorganization process that is occurring during the period of late adolescence and early adulthood, which may contribute to the onset of schizophrenia and the functional deterioration that is characteristic of the early stage of the illness. Finally, reduced NMDA neurotransmssion on inhibitory interneurons, including the PV-containing cells, may inflict excitotoxic or oxidative injury to downstream pyramidal neurons, leading to further loss of synapses and dendritic branchings. Hence, a key element in the conceptualization of rational early intervention and prevention strategies for schizophrenia may involve correcting the abnormal NMDA neurotransmission on inhibitory interneurons, possibly that on the PV-containing neurons in particular, thus normalizing gamma deficits and attenuating downstream neuronal pathology.
Schizophrenia was classically described as a “splitting of the psychic functions” (Bleuler 1950/1911), in which various aspects of thought and personality were disintegrated. In modern accounts of this illness, it has been proposed that the disorder arises from a failure of the brain to integrate the activity of local and distributed neural circuits (Andreasen 2000; Benes 2000; Friston and Frith 1995). Orchestrated oscillations of neural circuits in the gamma frequency band (30–100 Hz) have been proposed to be the mechanism that supports the integration of brain activities (Engel and Singer 2001; Singer et al 1990; Tallon-Baudry 2004; Uhlhaas and Singer 2006). As a result, there has been increasing interest in recent years in trying to understand how gamma oscillations might play a role in the pathophysiology of schizophrenia. In fact, the role of gamma oscillations in higher brain functions in humans in both normal and disease states, including schizophrenia, has been a major area of research (Engel and Singer 2001; Herrmann and Demiralp 2005; Lee et al 2003a; Lewis et al 2005; Lewis and Moghaddam 2006; Tallon-Baudry 2004; Uhlhaas and Singer 2006). There have also been several excellent reviews that specifically address the roles of gamma deficits in the pathophysiology of schizophrenia (Gonzalez-Burgos and Lewis 2008; Roach and Mathalon 2008; Spencer 2008; Uhlhaas et al 2008). Here, our goal is to critically evaluate the literature in an attempt to integrate the clinical findings of gamma deficits and the well-established observations of inhibitory neuronal dysfunction in schizophrenia. We introduce the hypothesis that the onset and post-onset functional deterioration of schizophrenia may be a manifestation of progressive gamma deficits that result from disturbances of the peri-adolescent maturation of inhibitory neural circuits in the cerebral cortex (Woo and Crowell 2005).
Gamma Band and Higher Cortical Functions
Interest in gamma oscillations and brain functioning can be dated back to the late 80’s when the Eckhorn and Singer groups demonstrated in a series of experiments, first in cats and then in monkeys, that synchronized oscillations of neuronal activity in the gamma frequency band appeared to mediate the integration or binding of visual features that give rise to coherent visual perception (Eckhorn et al 1988; Engel et al 2001; Engel et al 1991; Gray et al 1992; Gray et al 1989; Gray and Singer 1989; Singer et al 1990). The presumed role of gamma in the synchronization of neural circuits for the representation and integration of information is not limited to the sensory/perceptual domain, but it may also mediate a range of other cognitive operations (Salinas and Sejnowski 2001). For example, gamma oscillations may help to mediate selective attention (Fries et al 2001; Salinas and Sejnowski 2001; Tallon-Baudry 2004; Tiitinen et al 1993), working memory (De Pascalis and Ray 1998; Howard et al 2003; Tallon-Baudry et al 1998), long-term memory (Fell et al 2001) and motor control (Schoffelen et al 2005).
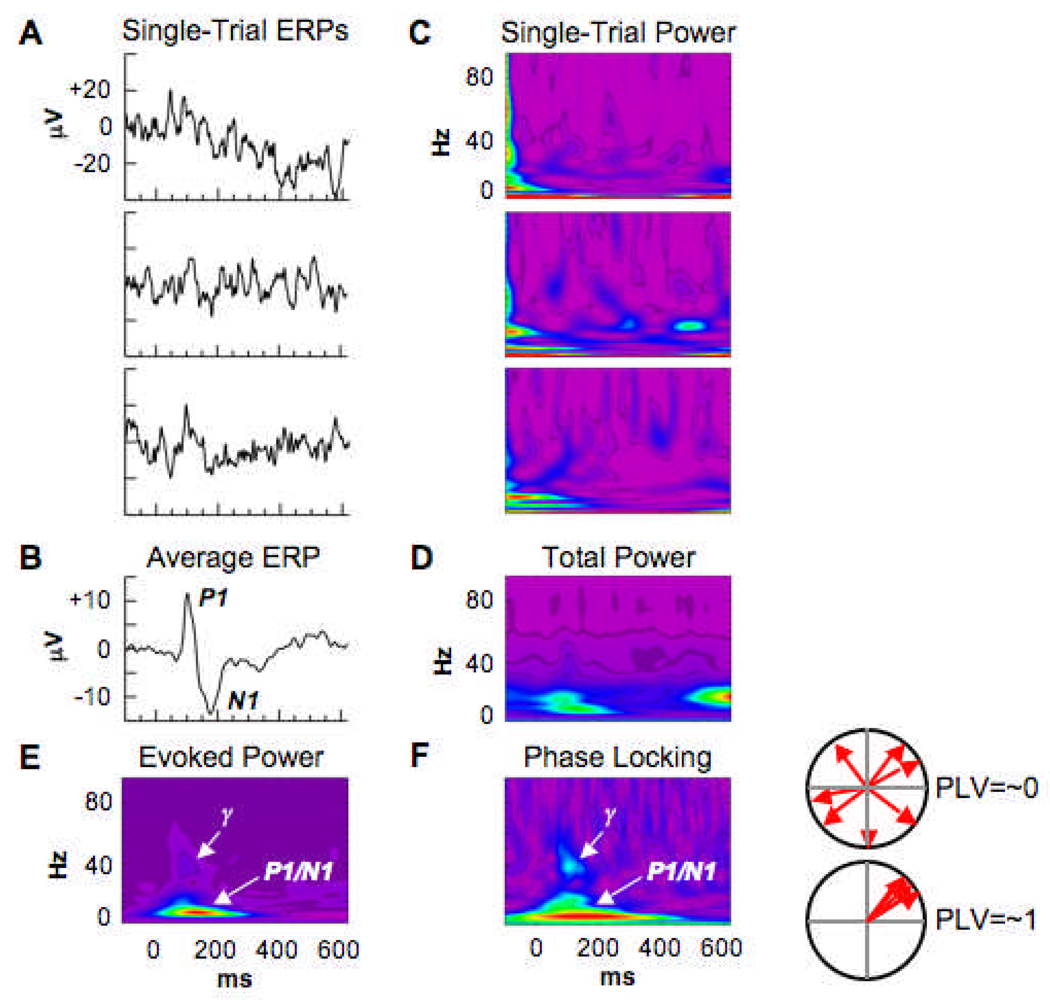
In humans, gamma activity is commonly measured by the electroencephalogram (EEG) during the performance of various tasks. “Evoked” gamma oscillations are phase-locked to a stimulus and therefore show inter-trial phase locking whereas “induced” gamma oscillations are not strictly phase-locked to a stimulus (Tallon-Baudry and Bertrand 1999) (see Fig. 1). Although the precise functional significance of the two forms of gamma activities remains unclear, it has been suggested that early sensory-evoked gamma may reflect sensory or perceptual processing, whereas induced gamma may reflect higher-level cognitive processes (for review see (Engel et al 1992; Pulvermuller et al 1999; Tallon-Baudry and Bertrand 1999)). In addition, in the auditory modality, the repeated presentation of a simple stimulus (such as a click) or the modulation of a sine-wave tone at the frequency of 40 Hz generates a resonant response, the 40 Hz auditory steady-state response (ASSR) (Galambos et al 1981).
Fig. 1.
Types of time/frequency domain measures derived from single-trial epochs of EEG. The data were recorded at a parieto-occipital electrode (PO6) from a healthy subject performing a visual discrimination task (44). Stimulus onset is at 0 ms. A) Raw single trial ERPs. B) The average ERP is computed by averaging together the single trials (N=90 here). The P1 N1 components are clearly visible. C) Time frequency maps of spectral power for the corresponding single trials. Time-frequency decomposition was performed using the M orlet wavelet transform. D) Average of the single trial time-frequency power maps. This measure of “total power” represents both “background” (induced, or non-stimulus-locked) and stimulus-locked oscillations. E) Evoked power is the power of stimulus-locked oscillations (i.e., the average ERP). The P1 N1 components occupy the low-frequency rang (<20 Hz). The early evoked visual gamma oscillation (g) is apparent in the time-frequency map. F) phase-locked represents the phase variability across trials and is insensitive to oscillatory power. Low phase locking values (PLV) indicate high phase variability (top circle plot) and high PLV indicate low phase variability (bottom circle plot). (Figure adapted with permission form Javitt et al., 2008, Nat Rev Drug Discovery).
Gamma Band Abnormalities and the Clinical Manifestations of Schizophrenia
Recent evidence suggests that the underlying neural mechanism(s) that generates and/or supports gamma oscillations, including evoked, induced, and steady-state gamma, seems to be impaired in schizophrenia. To date, a number of studies have demonstrated abnormalities in the power and/or phase locking of gamma oscillations in this disorder, suggesting that the neural mechanisms that mediate gamma may be functionally deficient. The most widely studied and replicated effect is the reduction of the 40 Hz ASSR in patients with schizophrenia (Brenner et al 2003; Hong et al 2004a; Kwon et al 1999; Light et al 2006; Spencer et al 2008; Wilson et al 2007a), which is apparent in both the evoked power and phase locking measures. Furthermore, schizophrenia patients demonstrate decreased phase locking of the early visual-evoked gamma oscillation (Spencer et al 2003; Spencer et al 2007), although the early auditory-evoked gamma oscillation does not appear to be affected (Gallinat et al 2004; Spencer et al 2007). Other gamma abnormalities have been reported in association with early auditory processing (Clementz et al 1997; Hong et al 2004b), auditory target detection (Gallinat et al 2004; Haig et al 2000; Symond et al 2005), visual perception (Spencer et al 2004; Wynn et al 2005), and somato-motor processing (Lee et al 2003b).
Given the hypothesis that working memory and prefrontal cortex dysfunction is a core deficit of schizophrenia (e.g. (Goldman-Rakic 1994; Lewis and Gonzalez-Burgos 2007; Park and Holzman 1992; Tan et al 2007)), it is notable that there is evidence that gamma oscillations associated with working memory may be impaired in patients with schizophrenia (Basar-Eroglu et al 2007; Cho et al 2006; Kissler et al 2000). For instance, Basar-Eroglu et al (Basar-Eroglu et al 2007) demonstrated that gamma power fails to increase as working memory load increases, as is seen in healthy control subjects (Gottesman and Gould 2003). Similarly, Cho et al. reported that during the performance of an executive control task containing a working memory component, schizophrenia patients failed to show an increase in gamma activity as cognitive control demands increased, and this deficit was associated with impaired performance of the task (Cho et al 2006).
It is well-established that many of the neurocognitive processes that may be supported by gamma, as described above, are deficient not only in subjects with schizophrenia, but also in their first-degree relatives and in patients with schizophrenia spectrum disorders, such as schizotypal personality disorder (Faraone et al 1999; Gur et al 2007; Seidman et al 2006; Voglmaier et al 1997). Interestingly, gamma band deficits may also occur in first-degree relatives of patients with schizophrenia (Hong et al 2004a). Furthermore, such deficits appear to already exist in first-episode schizophrenia patients (Gallinat et al 2004; Spencer et al 2008; Symond et al 2005). Together these observations raise an interesting possibility that gamma band abnormalities may represent a neurobiologic endophenotype (Gottesman and Gould 2003) and may predate the onset of illness. In this regard, it would be important in future studies to understand whether gamma deficits may occur in individuals at risk for schizophrenia, such as those who are in the prodromal phase of the illness, subjects with schizotypal personality disorder, or children of parents who have schizophrenia, and to possibly link gamma deficits to candidate genes of the illness.
Perhaps one of the most important questions that need to be addressed is whether gamma oscillation disturbances may exhibit any clinical correlates. Indeed, gamma abnormalities have been found to be associated with various symptom domains of schizophrenia, such as hallucinations, thought disorder, disorganization, and psychomotor poverty (Gallinat et al 2004; Lee et al 2003a; Lee et al 2003b; Spencer et al 2004). For example, Spencer et al have shown that in patients with schizophrenia, the strength of the phase locking of visual perception-related oscillation was positively correlated with some of the positive symptoms such as thought disorder, conceptual disorganization and visual hallucinations (Spencer et al 2004). In the domain of motor control, Ford et al. (Ford et al 2007) found that the degree of phase locking of an oscillatory correlate of a corollary discharge mechanism was reduced in schizophrenia patients, and this reduction was related to avolition/apathy symptoms. Also, Gallinat and colleagues have found that there is a positive correlation between the degree of reduction in late gamma in an auditory oddball task and both the positive symptom scale of the PANSS (Positive and Negative Symptom Scale) and the duration of illness (Gallinat et al 2004). Finally, the reduction in gamma power and synchrony deficits have also been shown to be positively correlated with the negative symptomatology of schizophrenia (Lee et al 2003b). In spite of these potentially informative observations, more studies are needed before any conclusions can be drawn about how different kinds of gamma disturbances may be related to the assortment of clinical symptoms of schizophrenia.
Despite the recent excitement surrounding the possible link between the pathophysiology of schizophrenia and gamma band deficits, a number of caveats need to be carefully considered and many questions remain unanswered. First, the strength of the explanatory power of the concept of gamma activity in mediating a variety of brain functions may also represent a major weakness of this concept. For instance, the symptoms and deficits of many neuropsychiatric disorders have all been associated with gamma band abnormalities, such as Alzheimer’s disease (Ribary et al 1991), autism (Welsh et al 2005; Wilson et al 2007b), Tourette’s syndrome (Kalanithi et al 2005; Leckman et al 2006), bipolar disorder (Bhattacharya 2001; O'Donnell et al 2004), attention deficit and hyperactivity disorder (Yordanova et al 2001), or even traumatic brain injury (Slewa-Younan et al 2002). We certainly do not expect that all of these diseases and conditions share identical pathophysiologic mechanisms; thus, gamma band abnormalities may simply be a very sensitive readout or an epiphenomenon of pertubation of cerebral cortical network functions and lack diagnostic specificity. Nevertheless, in this case, gamma band disturbances may still be useful as a clinical outcome measure or as an index of treatment response (Lewis and Moghaddam 2006).
Second, the correlations between gamma abnormalities and behavioral deficits, such as symptoms and cognitive dysfunction, in patients on the whole have not been strong. If gamma synchrony is an essential mechanism for information processing, then it would be expected that gamma abnormalities and behavioral deficits would be linked. However, the evidence for such links may be relatively sparse at this point because many studies do not have a behavioral component (e.g., the ASSR studies), or because tasks were not designed to be sensitive to such relationships (e.g., gamma oscillations elicited in oddball tasks may reflect a variety of processes unrelated to simple target detection). We note that studies in which gamma oscillations do appear to reflect essential mechanisms for task performance in healthy individuals have reported associations between gamma abnormalities and behavioral deficits and/or symptoms in schizophrenia patients (e.g. (Cho et al 2006; Ford et al 2007; Spencer et al 2004)).
Third, the potential effects of antipsychotics on gamma band oscillations are not well understood. The limited data available so far seem to suggest that haloperidol may suppress auditory evoked gamma band activity (Ahveninen et al 2000), raising an important question as to whether previous findings of gamma band deficits in patients with schizophrenia may, at least in part, reflect antipsychotic effects. However, there have also been data suggesting that atypical agents, such as clozapine and olanzapine, may have the opposite effects (Hong et al 2004a; Sperling et al 1999). In addition, abnormal gamma activity has also been observed in unmedicated schizophrenia patients (Gallinat et al 2004), lending support to the notion that gamma abnormality may in fact be intrinsic to the disease process of the illness.
Finally, neurons or neuronal assemblies also oscillate in a variety of other frequency bands (Buzsaki 2006) and the possible pathophysiologic relationship between these oscillation patterns and schizophrenia is largely unexplored. For example, in addition to gamma band disturbances, there is evidence suggesting that synchronized oscillations in the beta frequency band (13–30 Hz), which are thought to link together spatially distributed information across distant regions of the cerebral cortex (Kopell et al 2000), may also be deficient in patients with schizophrenia (Hong et al 2004b; Uhlhaas et al 2006; Yeragani et al 2006). Evidence of oscillation abnormalities in lower frequencies has also been reported, such as the theta band (4–7 Hz) (Koenig et al 2001; Schmiedt et al 2005), and the alpha band (8–13 Hz) (Jin et al 1997).
Disturbances of Parvalbumin-Containing Inhibitory Neurons and Gamma Band Deficits in Schizophrenia
Notwithstanding the caveats raised above, perhaps one of the strongest pieces of evidence in support of the idea of gamma band disturbances playing a role in the pathophysiology of schizophrenia is the increasing understanding that inhibitory interneurons in the cerebral cortex seem to be functionally disturbed in this disorder (Benes and Berretta 2001; Costa et al 2004; Lewis et al 2005), as gamma oscillations are known to emerge from the intricate interplay between inhibitory interneurons and the pyramidal cells they target (e.g.(Whittington et al 2000)). Pyramidal cells drive oscillations among inhibitory interneurons, which in turn phasically modulate the firing rates of pyramidal cells, leading to a synchronized gamma-band oscillation in the whole network. The frequency of the oscillation is determined in part by the decay time constant of inhibitory synaptic conductances via the GABA (γ-aminobutyric acid)A receptor (Brunel and Wang 2001). It should be noted, however, that our current understanding of the neurobiologic mechanisms that mediate gamma oscillations derives virtually exclusively from in vitro experiments using brain slices or in vivo studies in animals. In these studies, gamma oscillations and synchrony are measured based on recordings from individual neurons or small groups of neurons. In humans, on the other hand, gamma oscillations, as measured by EEG, reflect the coordinated activities of neural assemblies that consist of very large numbers of neurons. Therefore, it is unclear whether or if so how mechanisms that support gamma at the level of single neurons as discovered in animals may be applicable to understanding the mechanisms that underlie gamma oscillations within and between very large groups of neurons in humans. Nevertheless, it does appear that the subset of inhibitory neurons that have been found to play a crucial role in sustaining gamma in animals have in fact been found to be altered in postmortem brains from subjects with schizophrenia.
Disturbances of PV-Containing Neurons in Schizophrenia
Multiple lines of evidence strongly suggest that inhibitory interneurons in the cerebral cortex are disturbed in schizophrenia (Benes 2000; Benes and Berretta 2001; Costa et al 2001; Keverne 1999; Lewis et al 2005). For example, the concentrations of GABA and the GABA transporter GAT-1 have been demonstrated to be reduced in the prefrontal cortex (PFC) (Ohnuma et al 1999). In addition, the density of neurons that express the mRNA for GAT-1 and those that express the mRNA for the 67kD isoform of the GABA synthesizing enzyme, glutamic acid decarboxylase (GAD67), have also been found to be decreased in the cerebral cortex in schizophrenia (Akbarian et al 1995; Guidotti et al 2000; Hashimoto et al 2003; Volk et al 2001; Volk et al 2000; Woo et al 2004). In fact, the latter observation is the single most replicated finding in schizophrenia postmortem studies (Akbarian and Huang 2006). Because the amount of expression of GAT-1 or GAD67 mRNA in individual neurons seems to be unaltered and cell loss is not believed to be occurring, at least not in large scale, in the PFC (Selemon and Goldman-Rakic 1999), it appears that the expression of these transcripts in a subset of GABA cells is decreased to a level that is experimentally undetectable. In what may be a compensatory mechanism, postsynaptic GABAA receptor binding has been found to be increased (Benes et al 1996; Benes et al 1992; Newell et al 2006). However, more recent data suggest that, at least at the transcript level, specific subunits of GABAA receptor may be differentially regulated, e.g. the alpha 1 subunit mRNA has been found to be down-regulated (Hashimoto et al 2007; Kim et al 2005) (but see (Ohnuma et al 1999)) whereas the alpha 2 transcript may be up-regulated (Kim et al 2005; Volk et al 2002).
GABA neurons are morphologically, connectionally, chemically, molecularly and biophysically heterogeneous (Gabbott and Bacon 1996; Kawaguchi and Kondo 2002; Kawaguchi and Kubota 1997; Markram et al 2004; Soltesz 2005; Wang et al 2004; Wang et al 2002). Among them, the fast-spiking cells that contain the calcium-binding protein parvalbumin (PV) appear to play a particularly critical role in the generation of synchronized gamma activity (Bartos et al 2007; Cunningham et al 2006; Hajos et al 2004; Klausberger et al 2003; Soltesz and Deschenes 1993). Accumulating evidence suggests that the PV-containing neurons are among the GABA cells that are disturbed in schizophrenia (Lewis et al 2005), thus providing a basis for the pathophysiology of gamma band oscillation abnormalities.
PV-containing neurons consist of two subpopulations of cells, chandelier cells, which synapse onto the axon initial segment of pyramidal neurons (Howard et al 2005), and basket cells, which provide perisomatic inhibitory inputs to pyramidal cells (Freund 2003; Somogyi et al 1983). Lewis and colleagues have shown that the number of cells containing PV mRNA that express a detectable level of GAD67 mRNA is decreased by as much as 45% in the PFC in schizophrenia (Hashimoto et al 2003). Furthermore, in the same study, it was found that the expression of PV mRNA in individual neurons was significantly reduced whereas the expression of the mRNA for calretinin, a calcium-binding protein that is localized to a non-overlapping population of GABA cells, was unaffected (Hashimoto et al 2003). Moreover, the density of the axon terminals of chandelier neurons, which can be immunohistochemically visualized with an antibody against GAT-1 (DeFelipe and Gonzalez-Albo 1998; Inda et al 2006), has been found to be decreased by as much as 40% (Pierri et al 1999; Woo et al 1998). Consistent with this finding, GABAA receptor subunit α2-immunoreactive profiles, which represent the postsynaptic sites of chandelier terminals, and the expression of mRNA for this subunit in pyramidal cells appear to be upregulated in schizophrenia (Kim et al 2005; Volk et al 2002). Together with chandelier cells, the fast-spiking PV-containing basket cells are also crucial in mediating cortical oscillatory dynamics (Csicsvari et al 2003; Freund 2003; Klausberger et al 2003; Mann et al 2005; Tamas et al 2000). However, unlike that of chandelier neurons, axon terminals of basket cells cannot be reliably identified with light microscopy. Therefore, there is currently no definitive evidence directly implicating the involvement of basket cell terminals in the pathophysiology of schizophrenia. However, because basket cells represent the majority, perhaps as many as 80–90%, of all PV-containing neurons in the cortex (Kawaguchi 1995; Krimer et al 2005; Markram et al 2004; Zaitsev et al 2004), it seems almost certain that at least a subset of basket cells must also be involved in order to account of the magnitude of change observed by Hashimoto et al (Hashimoto et al 2003).
Altered Glutamatergic Modulation and Disturbances of PV-Containing GABA Neurons
It has long been known that treatment with N-methyl-D-aspartate (NMDA) receptor antagonists produces a syndrome that is highly reminiscent of the clinical picture of schizophrenia, including positive symptoms, negative symptoms and cognitive deficits (Javitt and Zukin 1991; Krystal et al 1994; Newcomer and Krystal 2001), and these data lead to the NMDA receptor hypofunction model of the disorder (Olney and Farber 1995; Olney et al 1999). The paradoxical excitotoxic effects observed by Olney and Farber with NMDA antagonists were explained, at least in part, by blockade of the NMDA receptors that are located on GABA neurons, which have been shown to be some 10-fold more sensitive to NMDA receptor antagonists than the NMDA receptor on pyramidal neurons (Greene et al 2000; Grunze et al 1996; Olney and Farber 1995). Interestingly, it has recently been demonstrated that, in both the anterior cingulate (Woo et al 2004) and prefrontal (Woo et al 2008) cortices, the density of GABA cells, identified with GAD67 labeling, that express the NMDA NR2A subunit is significantly decreased in schizophrenia. These findings are also consistent with the observation that the expression of the mRNA for the vesicular glutamate transporter vGluT1, which is a marker for cortically originated glutamatergic terminals (Fujiyama et al 2001; Kaneko and Fujiyama 2002), appears to be reduced in the PFC in schizophrenia (Eastwood and Harrison 2005). Furthermore, in primary neuronal cultures, Kinney and colleagues have recently found that the amount of NR2A in PV-containing GABA cells seem to be 5-fold greater than that in pyramidal cells at both the transcript and protein levels (Kinney et al 2006). In addition, NR2A, but not NR2B selective antagonist appears to down-regulate GAD67 mRNA and PV expression in PV-containing GABA cells (Kinney et al 2006). Similar reductions of PV expression by NMDA antagonism have been reported in vivo in the prefrontal cortex (Braun et al 2007; Cochran et al 2003; Reynolds et al 2004) and hippocampus (Abdul-Monim et al 2007; Braun et al 2007; Keilhoff et al 2004; Rujescu et al 2006). Also, in a recent study, repeated administration of phencyclidine, an NMDA antagonist, has been shown to reduce the number of PV-expressing axo-axonic cartridges, which presumably represent the axon terminals formed by chandelier cells, in monkeys (Morrow et al 2007). Finally, in line with the idea that PV-containing neurons play an important role in gamma oscillations, NMDA receptor blockade has been found to robustly disrupt gamma rhythms in the entorhinal cortex (Cunningham et al 2006) and it is speculated that this disruption may be mediated by the NMDA receptor on the PV-containing neurons (Cunningham et al 2006). Taken together, reduced glutamatergic inputs onto PV-containing neurons via NMDA receptors, perhaps especially those that contain the NR2A subunit, may mediate, at least in part, the down-regulation of GAD67 and PV and the disruption of gamma band synchrony in schizophrenia. In fact, using double in situ hybridization, we have recently found that the density of PV-containing neurons that express NR2A mRNA seems to be decreased by as much as 50% in subjects with schizophrenia (Bitanihirwe et al 2007). As a result of reduced excitatory drive to PV-containing neurons, cells that are downstream to these neurons may become disinhibited and thus may be rendered more susceptible to excitotoxic or oxidative insults (Lisman et al 2008; Olney and Farber 1995). Short of leading to cell death, cellular injury may be manifested in the form of neuritic and synaptic atrophy (Bernstein and Lichtman 1999; Garden et al 2002; Gilman and Mattson 2002; Glantz et al 2006; Jarskog et al 2005; Mattson et al 1998).
PV Neuronal Disturbances, Gamma Band Synchrony Deficits and the Onset and Progression of Schizophrenia
Synaptic Connectivity Deficits of Schizophrenia
In the past few years, there has been increasing appreciation that deficits in glutamatergic synaptic connectivity may represent a core pathophysiologic feature of schizophrenia (Garey et al 1998; Glantz and Lewis 2000; Kalus et al 2000; McGlashan and Hoffman 2000; Mirnics et al 2001). Of interest, using unbiased counting methods, Selemon and Goldman-Rakic (Selemon and Goldman-Rakic 1999) found that in the PFC it was the volume of neuropil, which contains dendritic and axonal processes and synapses, among other elements, but not cell number that was decreased in schizophrenia. Furthermore, this hypothesis is also consistent with numerous imaging studies demonstrating reduced gray matter volume in patients with this illness (see review in (Shenton et al 2001)), although the possible effects of antipsychotic medications on brain volume remain unclear (Dorph-Petersen et al 2005; Lieberman et al 2005).
One of the strongest pieces of evidence for synaptic deficits in schizophrenia is the observations of reduced expression of synaptic vesicle proteins such as synaptophysin (Eastwood et al 1995; Glantz and Lewis 1997), SNAP-25 (Halim et al 2003; Honer et al 2002), Rab3 (Davidsson et al 1999), complexin (Sawada et al 2002), and VAMP (Halim et al 2003) in the cerebral cortex; the reduction in the expression of some of these proteins may reflect post-translational changes, because in situ hybridization studies have revealed that the mRNA transcripts for these proteins are not affected (Eastwood et al 2000; Glantz et al 2000; Karson et al 1999). In addition, microarray studies have shown that, in the PFC, the expression of many genes encoding proteins that regulate synaptic structure and function are also down-regulated (Hakak et al 2001; Lehrmann et al 2003; Mirnics et al 2000; Pongrac et al 2002; Vawter et al 2001).
In the PFC, the density of spines on the proximal dendrites of layer 3 pyramidal cells, which furnish both feedforward and feedback (recurrent) glutamatergic projections (Pucak et al 1996), both locally and between spatially dispersed cortical regions, is decreased by about 20–50% (Garey et al 1998; Glantz and Lewis 2000). It is also noteworthy that Sweet et al. (Sweet et al 2003) found that pyramidal cell somal area was reduced in layer 3b in Brodmann’s area 42 (auditory association cortex), and noted that this was most compatible with loss of dendritic volume, as that laboratory and others had found in the PFC (Black et al 2004; Kalus et al 2000; Pierri et al 2001). These findings also suggest that, in schizophrenia, both the local cortical excitatory circuitry and the long-range corticocortical connections are disturbed, providing the possible neuroanatomical basis for gamma band synchrony deficits.
Gamma Band Synchrony Deficits May Mediate the Onset and Progression of Schizophrenia by Disturbing Peri-Adolescent Synaptic Pruning
One conundrum in schizophrenia research has been whether the illness represents a purely neurodevelopmental disorder in the sense that deficits that are associated with the pathophysiology of the illness occur early in development or whether there is also a peri-onset process of progression that is neurobiologically distinct (Keshavan 1999; Lewis and Levitt 2002; Lieberman 1999; Weinberger 1987). We think this is an unnecessary dichotomy, that many of the processes that are important in development, such as synaptic and dendritic remodeling and pruning, are also important in the peri-onset changes.
The fast-spiking basket and chandelier cells that contain PV, which exert perisomatic and axo-axonic inhibition (and possibly excitation under some circumstances (Szabadics et al 2006)), respectively, on pyramidal neurons, are known to play a critical role in regulating synchronous neuronal discharges in multiple frequency bands (e.g. theta, gamma, ripple, etc) via both chemical and electrical synapses (Buzsaki et al 2004; Freund 2003; Klausberger et al 2003; Tamas et al 2000; Whittington and Traub 2003). The biophysical characteristics of these neurons that lead to their ability to synchronize neuronal outputs are beyond the scope of this review but have been discussed elsewhere (Buzsaki 2006; Buzsaki and Draguhn 2004; Pike et al 2000; Steriade et al 1998; Traub et al 1998; Whittington and Traub 2003). Here, it is postulated that PV neuronal disturbances may contribute to the onset and progression of schizophrenia via at least two mechanisms: (1) perturbing gamma oscillations and hence activity-dependent synaptic and dendritic remodeling and/or (2) promoting synaptic and dendritic atrophy via an excitotoxic mechanism.
PV-containing basket and chandelier neurons in the cerebral cortex are generated from the same progenitors in the medial ganglionic eminence during development (Wonders and Anderson 2006; Xu et al 2004). These neurons begin to express PV during embryonic stage, but subsequently PV expression dramatically declines until the postnatal period when its expression gradually increases and then stabilizes (Alcantara and Ferrer 1994; de Lecea et al 1995; Gao et al 2000; Patz et al 2004). The postnatal maturation of PV expression in the sensory cortices appears to temporally coincide with the period of experience-dependent refinement of neural circuits (Alcantara and Ferrer 1994; de Lecea et al 1995; Gao et al 2000; Patz et al 2004). The neurobiologic mechanisms that regulate the onset and termination of the synaptic refinement process are just beginning to be unraveled; it appears that the maturation of GABA neural circuits, particularly that of PV neurons, may play a crucial role (Hensch 2005; Jiang et al 2005). For example, Tropea et al have found that dark rearing, which is known to prolong the duration of critical period and the maturation of the visual cortex, is associated with a significant down-regulation of the transcript for PV (Tropea et al 2006). Interestingly, it has been found that the effects of dark rearing can be rescued by overexpression of the neurotrophin brain-derived neurotrophic factor (BDNF) (Gianfranceschi et al 2003). Furthermore, in transgenic mice in which the BDNF is developmentally over-expressed, the maturation of PV neuronal circuits in the visual cortex is accelerated and, at the same time, the critical period for developmental synaptic plasticity is also precociously terminated (Hanover et al 1999; Huang et al 1999). The fact that PV-containing neurons appear to highly express trkB (Cellerino et al 1996; Gorba and Wahle 1999), the high affinity receptor tyrosine kinase for BDNF, is also consistent with the idea that BDNF/trkB signaling may be particularly important in mediating the maturation of these neurons and hence determining the time course of developmental synaptic plasticity.
The functional maturation of the PFC is quite protracted; the underlying process of refinement of glutamatergic synapses is not completed until late adolescence and early adulthood (Bourgeois et al 1994; Huttenlocher 1979; Woo et al 1997), which coincides with the period of time when schizophrenia symptomatology typically begins to emerge. Interestingly, it is also during this period of development when PV neuronal circuits appear to gradually achieve maturation, as reflected in their attaining the adult pattern of PV protein expression (Anderson et al 1995; Erickson and Lewis 2002). At the same time, as these neurons apparently are becoming functionally mature, gamma power also progressively increases (Poulsen et al 2007; Rojas et al 2006). Furthermore, available evidence indicates that BDNF and trkB also achieves the adult patterns of expression during the peri-adolescent period (Hayashi et al 2000; Ohira et al 1999; Webster et al 2002). Taken together, these observations raise a tantalizing possibility that in schizophrenia disturbed BDNF/trkB signaling during the peri-adolescent period may, at least in part, lead to disturbances in the maturation of PV-containing neurons. Consistent with this idea, postmortem studies have in fact shown that the expression of BDNF and trkB mRNAs in the PFC is decreased in schizophrenia (Hashimoto et al 2004; Weickert et al 2003). Finally, most, but not all (Shimizu et al 2003) studies have found that serum BDNF level appears to be reduced in patients with schizophrenia (Grillo et al 2007; Pirildar et al 2004; Tan et al 2005; Toyooka et al 2002), including first-episode patients (Buckley et al 2007).
Synaptic refinement is an activity-dependent process that is governed by the Hebbian principle of coincidence detection (Hebb 1949; Katz and Shatz 1996). In the simplest term, when the pre-and post-synaptic elements of a synapse are coincidentally (within a narrow time window) active, the synapse is strengthened; otherwise, the synapse is either not strengthened, weakened or eliminated. Interestingly, the duration of the time window that is required for activity-dependent strengthening of synapses via coincidence detection closely matches the time scale of gamma oscillations (Bi and Poo 1998; Buzsaki and Draguhn 2004; Engel et al 1992; Harris 2005; Harris et al 2003; Konig et al 1996; Magee and Johnston 1997) and some evidence actually points to a direct relationship (Wespatat et al 2004). In other words, gamma oscillations may provide a temporal structure for activity-dependent synaptic refinement to take place. Thus, functional disturbances of PV neurons, perhaps mediated by disturbances of BDNF/trkB signaling, may lead to aberrant pruning of synapses (Feinberg 1982; Keshavan et al 1994; McGlashan and Hoffman 2000) by disturbing gamma oscillations. Furthermore, BDNF/trkB signaling appears to play an important role in regulating the expression of NMDA receptor, perhaps especially the NR2A subunit (Caldeira et al 2007; Glazner and Mattson 2000; Margottil and Domenici 2003; Small et al 1998). Hence, disturbed BDNF/trkB signaling could potentially also explain the finding of reduced NR2A expression in PV-containing neurons (Bitanihirwe et al 2007). Reduced glutamatergic activity on PV neurons may inflict excitotoxic damage to pyramidal cells via disinhibition, which may then lead to additional loss of dendrites and synapses (Bernstein and Lichtman 1999; Garden et al 2002; Gilman and Mattson 2002; Glantz et al 2006; Jarskog et al 2005; Mattson et al 1998). This idea appears to be supported by the results of a recent study in which Moghaddam and colleagues found that NMDA receptor blockade reduced inhibition furnished by GABA neurons but increased, at a delayed rate, the firing of pyramidal cells (Homayoun and Moghaddam 2007). Together, these mechanisms may play a role in triggering disease onset and leading to progressive functional deterioration during the early phase of the illness. The observations of progressive reduction in gray matter volume in both clinically high risk individuals (Job et al 2005; Job et al 2006; Pantelis et al 2003) and during the early post-onset course of the illness (Cahn et al 2002; DeLisi et al 1997; Ho et al 2003; Lieberman et al 2005; Salisbury et al 2007) are consistent with this idea. So far, the bulk of the evidence for gamma deficits in schizophrenia has come from studies in patients who are in the chronic phase of the illness. In future studies, it will be important to map the course of emergence and progression of gamma abnormalities in prodromal and first-episode schizophrenia patients to see if it correlates with the clinical course of disease onset and progression to chronicity and MRI gray matter measures. In addition, the pathophysiologic model proposed here can be tested in animals by experimentally perturbing the BDNF/trkB signaling cascade during adolescence to see if the neurobiologic and clinical phenotypes of schizophrenia, such as PV neuronal disturbances, glutamatergic synaptic deficits and gamma impairment can be reproduced in the experimental conditions.
Implications for Treatment, Early Intervention and Prevention
In recent years, there has been increasing momentum towards realizing the concept of early intervention and ultimately perhaps even prevention of the onset of schizophrenia. Despite the excitement and hope that have been generated, at present, it is unclear what specific early intervention and prevention strategies may be effective. The interesting convergence of recent preclinical and clinical findings implicates gamma oscillatory synchrony deficits mediated by disturbances of the PV-containing class of GABA cells as a key feature in the pathophysiology of schizophrenia. It is postulated that abnormal gamma synchrony may disturb the peri-adolescent synaptic and dendritic remodeling process, which may play a role in triggering the onset and functional deterioration of schizophrenia. An important goal in future studies would be to further define the cellular and molecular mechanisms that mediate gamma band synchrony (via animal studies) and how these mechanisms may be disturbed in schizophrenia (via postmortem human brain research), and then to translate this understanding into meaningful intervention strategies. As the tradition of the development of schizophrenia therapeutics has been one of serendipity, it is exciting that we can now begin to think about rational strategies that aim at correcting a possible pathophysiologic pathway that may mediate the onset of schizophrenia.
The idea that drugs or compounds that modulate glutamate neurotransmission via the NMDA receptor may be effective in the treatment of schizophrenia is based on strong theoretical rationale and has been advocated by many investigators (e.g. (Goff and Coyle 2001; Goff et al 2001; Javitt 2004; Krystal et al 2003; Moghaddam 2004; Tsai et al 2004)). However, direct stimulation of NMDA receptors may carry serious risks such as seizures or stroke. Thus, drugs that indirectly enhance NMDA neurotransmission, such as the metabotropic glutamate receptor agonists (Lewis and Moghaddam 2006; Moghaddam 2004), have been a focus of interest. In fact, the preliminary results of a phase II clinical trial testing the possible efficacy of a novel class II metabotropic receptor mGlu2/3 agonist appear to be very encouraging (Patil et al 2007). The data reviewed in this article extend the concept of glutamatergic enhancement to suggest that selective strengthening of the glutamatergic inputs to the NMDA receptors on PV-containing neurons may be particularly important. In fact, metabotropic glutamate receptors, such as the class I subunits mGluR1 and mGluR5, are expressed by PV-containing GABA neurons (Muly et al 2003), although the expression of mGluR2/3, to our knowledge, has not been studied. Furthermore, stimulation of mGluR5 subunit powerfully elicits long-term potentiation at glutamatergic synapses on PV-containing neurons (Tennigkeit et al 2006). In addition, activation of mGluR1 and 5 receptors induces gamma and theta oscillations (Gillies et al 2002; Martin 2001; Whittington et al 1995). However, because mGluR1 and mGluR5 receptors are not selectively expressed on PV-containing GABA neurons, but are also localized to other neuronal types, including pyramidal cells (Muly et al 2003), it is potentially difficult to predict the functional outcomes of enhancement of these receptors. Given the reported efficacy of the mGluR2/3 agonist, it would be interesting to find out if this subunit may be selectively, or at least preferentially localized to PV-containing neurons. Before we can develop strategies that can enhance NMDA transmission on PV-containing neurons, it will be necessary to better define the pharmacologic and/or downstream signaling mechanisms that are specific for PV-containing neurons. However, it should be mentioned that in addition to the gamma rhythm, other rhythms, such as the higher beta band, are also known to be disrupted in schizophrenia. In contrast to gamma oscillation, the generation of beta band rhythm does not appear to require GABA inhibition but can be partly replicated by acute NMDA receptor blockade. Hence, dysfunction of NMDA receptors on neural elements other than PV-containing neurons must also contribute to disrupted brain dynamics in schizophrenia (Roopun et al 2008).
In a recently published proof-of-principle study, Lewis and colleagues observed that working memory deficits in schizophrenia patients appeared to respond to treatment with a partial agonist of the GABAA alpha 2 subunit, which is preferentially localized to synapses formed by chandelier neurons (Lewis et al 2008). In addition, they found that such treatment resulted in improvement in gamma band power in the PFC in these patients. It would be interesting to see if this compound might be efficacious in correcting or reversing the hypothesized deficits that occur during the peri-onset phase of schizophrenia.
So far, we have emphasized the role of PV-containing cells in the generation of gamma oscillations. However, in addition to these neurons, another class of basket cells that do not contain PV but contain the neuropeptide cholecystokinin (CCK) appears to play a crucial role not necessarily in the generation but in the fine-tuning of network oscillatory activities (Freund 2003; Freund and Katona 2007). In fact, gene expression studies have revealed that the expression of the mRNA for CCK may be decreased in the frontal (Hashimoto et al 2007; Virgo et al 1995) and temporal (Virgo et al 1995) cortices in subjects with schizophrenia. An interesting aspect of these cells is that their axon terminals selectively express the cannabinoid CB1 receptor (Katona et al 2001; Katona et al 1999). Thus, these presynaptic CB1 receptors may be a critical element in the modulation of gamma oscillation by controlling the release of GABA by CCK-containing cells (Beinfeld and Connolly 2001; Hajos et al 2000). In addition, the relative selectivity in the localization of the CB1 receptor, as has already been noted by other investigators (Lewis and Gonzalez-Burgos 2007), may further increase its attractiveness as a potential drug target in any attempt to modulate gamma band oscillation. In addition, the possible role of cannabis in contributing to the development of schizophrenia in vulnerable individuals (Broome et al 2005; Moore et al 2007; Semple et al 2005) might well be mediated by its effects on cortical oscillatory synchrony (Hajos et al 2007; Hajos et al 2000; Skosnik et al 2006). Finally, CCK-containing basket cells also appear to preferentially express the nicotinic α7 receptor subunit (Frazier et al 1998; Freedman et al 1993), raising the interesting possibility that the potential cognitive enhancing effects of nicotinic α7 receptor agonists (Martin et al 2004; Olincy et al 2006) may be mediated, at least in part, by modulating gamma band oscillations via regulating the activities of CCK-containing basket cells. In light of this, it has recently been demonstrated that nicotine seems to enhance gamma-band power in the context of auditory sensory gating (Crawford et al 2002). Together these findings may also provide a physiological basis for the exceedingly high prevalence of cigarette smoking in patients with schizophrenia (Brown et al 1999; Dalack and Meador-Woodruff 1996).
In summary, there has been compelling evidence linking PV neuronal dysfunction to the pathophysiology of gamma deficits in schizophrenia. Furthermore, disturbances in the functional maturation of PV neurons during the peri-adolescent period may lead to gamma deficits and hence aberrant synaptic pruning, triggering the onset of schizophrenia. Thus, in future studies, it would be important to characterize the genetic and molecular cascades that mediate the functional disturbances of PV-containing neurons, as such knowledge will inspire the conceptualization and development of rational, neurobiology-based treatment, early intervention and prevention strategies.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- Ahveninen J, Kahkonen S, Tiitinen H, et al. Suppression of transient 40-Hz auditory response by haloperidol suggests modulation of human selective attention by dopamine D2 receptors. Neurosci Lett. 2000;292:29–32. doi: 10.1016/s0304-3940(00)01429-4. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Brain Res Rev. 2006 doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ferrer I. Postnatal development of parvalbumin immunoreactivity in the cerebral cortex of the cat. Journal of Comparative Neurology. 1994;348:133–149. doi: 10.1002/cne.903480108. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Brain Res Rev. 2000;31:106–112. doi: 10.1016/s0165-0173(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Connolly K. Activation of CB1 cannabinoid receptors in rat hippocampal slices inhibits potassium-evoked cholecystokinin release, a possible mechanism contributing to the spatial memory defects produced by cannabinoids. Neurosci Lett. 2001;301:69–71. doi: 10.1016/s0304-3940(01)01591-9. [DOI] [PubMed] [Google Scholar]
- Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Research - Brain Research Reviews. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Khan Y, Vincent SL, Wickramasinghe R. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996;22:338–349. doi: 10.1002/(SICI)1098-2396(199604)22:4<338::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. Journal of Neuroscience. 1992;12:924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M, Lichtman JW. Axonal atrophy: the retraction reaction. Curr Opin Neurobiol. 1999;9:364–370. doi: 10.1016/s0959-4388(99)80053-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J. Reduced degree of long-range phase synchrony in pathological human brain. Acta Neurobiol Exp (Wars) 2001;61:309–318. doi: 10.55782/ane-2001-1406. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe B, Lim M, kim A, Viscidi E, Woo T-UW. Expression of N-methy-D-aspartate receptors in parvalbumin-containing neurons in the prefrontal cortex in schizophrenia. 2007 In preparation. [Google Scholar]
- Black JE, Kodish IM, Grossman AW, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox or the group of schizophrenias. J. Zinkin (trans.) 1950/1911 [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Braun I, Genius J, Grunze H, Bender A, Moller HJ, Rujescu D. Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophr Res. 2007 doi: 10.1016/j.schres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Sporns O, Lysaker PH, O'Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- Broome MR, Woolley JB, Tabraham P, et al. What causes the onset of psychosis? Schizophr Res. 2005;79:23–34. doi: 10.1016/j.schres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29:697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang XJ. Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. J Comput Neurosci. 2001;11:63–85. doi: 10.1023/a:1011204814320. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 2007;91:1–5. doi: 10.1016/j.schres.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Geisler C, Henze DA, Wang XJ. Interneuron Diversity series: Circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27:186–193. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–219. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Maffei L, Domenici L. The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci. 1996;8:1190–1197. doi: 10.1111/j.1460-9568.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical {gamma} synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiology of Disease. 2001;8:723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis JM, Dong E, et al. A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol. 2004;16:1–23. doi: 10.1615/critrevneurobiol.v16.i12.10. [DOI] [PubMed] [Google Scholar]
- Crawford HJ, McClain-Furmanski D, Castagnoli N, Jr, Castagnoli K. Enhancement of auditory sensory gating and stimulus-bound gamma band (40 Hz) oscillations in heavy tobacco smokers. Neurosci Lett. 2002;317:151–155. doi: 10.1016/s0304-3940(01)02454-5. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Hunt J, Middleton S, et al. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalack GW, Meador-Woodruff JH. Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr Res. 1996;22:133–141. doi: 10.1016/s0920-9964(96)80441-5. [DOI] [PubMed] [Google Scholar]
- Davidsson P, Gottfries J, Bogdanovic N, et al. The synaptic-vesicle-specific proteins rab3a and synaptophysin are reduced in thalamus and related cortical brain regions in schizophrenic brains. Schizophrenia Research. 1999;40:23–29. doi: 10.1016/s0920-9964(99)00037-7. [DOI] [PubMed] [Google Scholar]
- de Lecea L, del Rio JA, Soriano E. Developmental expression of parvalbumin mRNA in the cerebral cortex and hippocampus of the rat. Brain Res Mol Brain Res. 1995;32:1–13. doi: 10.1016/0169-328x(95)00056-x. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Ray WJ. Effects of memory load on event-related patterns of 40-Hz EEG during cognitive and motor tasks. Int J Psychophysiol. 1998;28:301–315. doi: 10.1016/s0167-8760(97)00083-4. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Gonzalez-Albo MC. Chandelier cell axons are immunoreactive for GAT-1 in the human neocortex. Neuroreport. 1998;9:467–470. doi: 10.1097/00001756-199802160-00020. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PW, Harrison PJ. Altered synaptophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience. 1995;66:309–319. doi: 10.1016/0306-4522(94)00586-t. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Cairns NJ, Harrison PJ. Synaptophysin gene expression in schizophrenia. Investigation of synaptic pathology in the cerebral cortex. British Journal of Psychiatry. 2000;176:236–242. doi: 10.1192/bjp.176.3.236. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res. 2005;73:159–172. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, et al. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Engel AK, Konig P, Kreiter AK, Schillen TB, Singer W. Temporal coding in the visual cortex: new vistas on integration in the nervous system. Trends Neurosci. 1992;15:218–226. doi: 10.1016/0166-2236(92)90039-b. [DOI] [PubMed] [Google Scholar]
- Engel AK, Konig P, Singer W. Direct physiological evidence for scene segmentation by temporal coding. Proc Natl Acad Sci U S A. 1991;88:9136–9140. doi: 10.1073/pnas.88.20.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a 4-year follow-up study. J Abnorm Psychol. 1999;108:176–181. doi: 10.1037//0021-843x.108.1.176. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of Psychiatric Research. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH. Out-of-Synch and Out-of-Sorts: Dysfunction of Motor-Sensory Communication in Schizophrenia. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L. Alpha-bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clinical Neuroscience. 1995;3:89–97. [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. Journal of Comparative Neurology. 1996;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Galambos R, Makeig v, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981;78:2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Wormington AB, Newman DE, Pallas SL. Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: immunocytochemical localization of calbindin- and parvalbumincontaining neurons. J Comp Neurol. 2000;422:140–157. doi: 10.1002/(sici)1096-9861(20000619)422:1<140::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of Neurology, Neurosurgery & Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, et al. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MJ, Traub RD, LeBeau FE. A model of atropine-resistant theta oscillations in rat hippocampal area CA1. J Physiol. 2002;543:779–793. doi: 10.1113/jphysiol.2002.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman CP, Mattson MP. Do apoptotic mechanisms regulate synaptic plasticity and growth-cone motility? Neuromolecular Med. 2002;2:197–214. doi: 10.1385/NMM:2:2:197. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Austin MC, Lewis DA. Normal cellular levels of synaptophysin mRNA expression in the prefrontal cortex of subjects with schizophrenia. Biological Psychiatry. 2000;48:389–397. doi: 10.1016/s0006-3223(00)00923-9. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Archives of General Psychiatry. 1997;54:660–669. doi: 10.1001/archpsyc.1997.01830190088009. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mattson MP. Differential effects of BDNF, ADNF9, and TNFalpha on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp Neurol. 2000;161:442–452. doi: 10.1006/exnr.1999.7242. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. American Journal of Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Goff DC, Leahy L, Berman I, et al. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J Clin Psychopharmacol. 2001;21:484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. Journal of Neuropsychiatry & Clinical Neurosciences. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gray CM, Engel AK, Konig P, Singer W. Synchronization of oscillatory neuronal responses in cat striate cortex: temporal properties. Vis Neurosci. 1992;8:337–347. doi: 10.1017/s0952523800005071. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R, Bergeron R, McCarley R, Coyle JT, Grunze H. Short-term and long-term effects of N-methyl-D-aspartate receptor hypofunction. Arch Gen Psychiatry. 2000;57:1180–1181. doi: 10.1001/archpsyc.57.12.1180. author reply 1182–1183. [DOI] [PubMed] [Google Scholar]
- Grillo RW, Ottoni GL, Leke R, Souza DO, Portela LV, Lara DR. Reduced serum BDNF levels in schizophrenic patients on clozapine or typical antipsychotics. J Psychiatr Res. 2007;41:31–35. doi: 10.1016/j.jpsychires.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of GeneralPsychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, et al. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig AR, Gordon E, De Pascalis V, Meares RA, Bahramali H, Harris A. Gamma activity in schizophrenia: evidence of impaired network binding? Clin Neurophysiol. 2000;111:1461–1468. doi: 10.1016/s1388-2457(00)00347-3. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biological Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.12.005. In press. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hajos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, et al. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8:797–810. doi: 10.1038/sj.mp.4001319. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. Journal of Neuroscience. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD. Neural signatures of cell assembly organization. Nat Rev Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. Journal of Neuroscience. 2004;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Mitsunaga F, Itoh M, Shimizu K, Yamashita A. Development of full-length Trk B-immunoreactive structures in the prefrontal and visual cortices of the macaque monkey. Anatomy & Embryology. 2000;201:139–147. doi: 10.1007/pl00008234. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: John Wiley; 1949. [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Bayer TA, et al. Abnormalities of SNARE mechanism proteins in anterior frontal cortex in severe mental illness. Cereb Cortex. 2002;12:349–356. doi: 10.1093/cercor/12.4.349. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004a;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW. Gamma/beta oscillation and sensory gating deficit in schizophrenia. Neuroreport. 2004b;15:155–159. doi: 10.1097/00001756-200401190-00030. [DOI] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Inda MC, Defelipe J, Munoz A. The Distribution of Chandelier Cell Axon Terminals that Express the GABA Plasma Membrane Transporter GAT-1 in the Human Neocortex. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl114. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Glantz LA, Gilmore JH, Lieberman JA. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:846–858. doi: 10.1016/j.pnpbp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev. 2005;50:126–133. doi: 10.1016/j.brainresrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG, Sandman CA, Bunney WE., Jr Electroencephalographic photic driving in patients with schizophrenia and depression. Biol Psychiatry. 1997;41:496–499. doi: 10.1016/S0006-3223(96)00473-8. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Med. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus P, Muller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11:3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for 'hypofrontality'. Molecular Psychiatry. 1999;4:39–45. doi: 10.1038/sj.mp.4000459. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res. 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of Psychiatric Research. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Keverne EB. GABA-ergic neurons and the neurobiology of schizophrenia and other psychoses. Brain Research Bulletin. 1999;48:467–473. doi: 10.1016/s0361-9230(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Kim A, Matzilevich D, Walsh JP, Benes FM, Woo TUW. Parvalbumin-containing neurons and disturbances of prefrontal cortical circuitry in schizophrenia. Society for Neuroscience Abstract. 2005 [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler J, Muller MM, Fehr T, Rockstroh B, Elbert T. MEG gamma band activity in schizophrenia patients and healthy subjects in a mental arithmetic task and at rest. Clin Neurophysiol. 2000;111:2079–2087. doi: 10.1016/s1388-2457(00)00425-9. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Koenig T, Lehmann D, Saito N, Kuginuki T, Kinoshita T, Koukkou M. Decreased functional connectivity of EEG theta-frequency activity in first-episode, neuroleptic-naive patients with schizophrenia: preliminary results. Schizophr Res. 2001;50:55–60. doi: 10.1016/s0920-9964(00)00154-7. [DOI] [PubMed] [Google Scholar]
- Konig P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, et al. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol. 2005;94:3009–3022. doi: 10.1152/jn.00156.2005. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Archives of General Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Vaccarino FM, Kalanithi PS, Rothenberger A. Annotation: Tourette syndrome: a relentless drumbeat--driven by misguided brain oscillations. J Child Psychol Psychiatry. 2006;47:537–550. doi: 10.1111/j.1469-7610.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003a;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Haig A, Gordon E. "Gamma (40 Hz) phase synchronicity" and symptom dimensions in schizophrenia. Cognit Neuropsychiatry. 2003b;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Hyde TM, Vawter MP, Becker KG, Kleinman JE, Freed WJ. The use of microarrays to characterize neuropsychiatric disorders: postmortem studies of substance abuse and schizophrenia. Curr Mol Med. 2003;3:437–446. doi: 10.2174/1566524033479690. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of Neocortical Circuits in Schizophrenia. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biological Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, et al. Gamma Band Oscillations Reveal Neural Network Cortical Coherence Dysfunction in Schizophrenia Patients. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Margottil E, Domenici L. NR2A but not NR2B N-methyl-D-aspartate receptor subunit is altered in the visual cortex of BDNF-knock-out mice. Cell Mol Neurobiol. 2003;23:165–174. doi: 10.1023/A:1022945821455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Martin SJ. Activation of metabotropic glutamate receptors induces gamma frequency oscillations in the rat dentate gyrus in vivo. Neuropharmacology. 2001;40:634–637. doi: 10.1016/s0028-3908(00)00190-8. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Exp Neurol. 1998;153:35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Archives of General Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends in Neurosciences. 2001;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0708-0. [DOI] [PubMed] [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Jew SK, Huang XF. Alterations of muscarinic and GABA receptor binding in the posterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006 doi: 10.1016/j.pnpbp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport. 2004;15:1369–1372. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- Ohira K, Shimizu K, Hayashi M. Change of expression of full-length and truncated TrkBs in the developing monkey central nervous system. Brain Res Dev Brain Res. 1999;112:21–29. doi: 10.1016/s0165-3806(98)00151-5. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. Journal of Psychiatric Research. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007 doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Patz S, Grabert J, Gorba T, Wirth MJ, Wahle P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an Early period of postnatal development. Cereb Cortex. 2004;14:342–351. doi: 10.1093/cercor/bhg132. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. American Journal of Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Archives of General Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol. 2000;529(Pt 1):205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirildar S, Gonul AS, Taneli F, Akdeniz F. Low serum levels of brain-derived neurotrophic factor in patients with schizophrenia do not elevate after antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:709–713. doi: 10.1016/j.pnpbp.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Pongrac J, Middleton FA, Lewis DA, Levitt P, Mirnics K. Gene expression profiling with DNA microarrays: advancing our understanding of psychiatric disorders. Neurochem Res. 2002;27:1049–1063. doi: 10.1023/a:1020904821237. [DOI] [PubMed] [Google Scholar]
- Poulsen C, Picton TW, Paus T. Age-related changes in transient and oscillatory brain responses to auditory stimulation in healthy adults 19–45 years old. Cereb Cortex. 2007;17:1454–1467. doi: 10.1093/cercor/bhl056. [DOI] [PubMed] [Google Scholar]
- Pucak ML, Levitt JB, Lund JS, Lewis DA. Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. Journal of Comparative Neurology. 1996;376:614–630. doi: 10.1002/(SICI)1096-9861(19961223)376:4<614::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Lutzenberger W, Preissl H. Nouns and verbs in the intact brain: evidence from event-related potentials and high-frequency cortical responses. Cereb Cortex. 1999;9:497–506. doi: 10.1093/cercor/9.5.497. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia--postmortem studies and animal models. Neurotox Res. 2004;6:57–61. doi: 10.1007/BF03033297. [DOI] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991;88:11037–1041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale PD, et al. Development of the 40Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin Neurophysiol. 2006;117:110–117. doi: 10.1016/j.clinph.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-Specific Changes in Gamma and Beta2 Rhythms in NMDA Receptor Dysfunction Models of Schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, et al. A pharmacological model for psychosis based on N-methyl-Daspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Young CE, Barr AM, et al. Altered immunoreactivity of complexin protein in prefrontal cortex in severe mental illness. Mol Psychiatry. 2002;7:484–492. doi: 10.1038/sj.mp.4000978. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Smith CW, et al. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr Bull. 2006;32:507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biological Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Watanabe H, et al. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neurosci Lett. 2003;351:111–114. doi: 10.1016/j.neulet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray C, Engel A, Konig P, Artola A, Brocher S. Formation of cortical cell assemblies. Cold Spring Harb Symp Quant Biol. 1990;55:939–952. doi: 10.1101/sqb.1990.055.01.088. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Aydt EE, Kuhlenshmidt HA, O'Donnell BF. Psychophysiological evidence of altered neural synchronization in cannabis use: relationship to schizotypy. Am J Psychiatry. 2006;163:1798–1805. doi: 10.1176/ajp.2006.163.10.1798. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Green AM, Baguley IJ, Felmingham KL, Haig AR, Gordon E. Is 'gamma' (40 Hz) synchronous activity disturbed in patients with traumatic brain injury? Clin Neurophysiol. 2002;113:1640–1646. doi: 10.1016/s1388-2457(02)00239-0. [DOI] [PubMed] [Google Scholar]
- Small DL, Murray CL, Mealing GA, Poulter MO, Buchan AM, Morley P. Brain derived neurotrophic factor induction of N-methyl-D-aspartate receptor subunit NR2A expression in cultured rat cortical neurons. Neurosci Lett. 1998;252:211–214. doi: 10.1016/s0304-3940(98)00587-4. [DOI] [PubMed] [Google Scholar]
- Soltesz I. Diversity in the Neuronal Machine. New York: Oxford University Press; 2005. [Google Scholar]
- Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Kisvarday ZF, Martin KA, Whitteridge D. Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience. 1983;10:261–294. doi: 10.1016/0306-4522(83)90133-1. [DOI] [PubMed] [Google Scholar]
- Spencer KM. Visual gamma oscillations in schizophrenia: implications for understanding neural circuitry abnormalities. Clin EEG Neurosci. 2008;39:65–68. doi: 10.1177/155005940803900208. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-Evoked Gamma Oscillations in Chronic Schizophrenia. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.02.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W, Vieth J, Martus M, Demling J, Barocka A. Spontaneous slow and fast MEG activity in male schizophrenics treated with clozapine. Psychopharmacology (Berl) 1999;142:375–382. doi: 10.1007/s002130050902. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Durmuller N, Grenier F. Dynamic properties of corticothalamic neurons and local cortical interneurons generating fast rhythmic (30–40 Hz) spike bursts. J Neurophysiol. 1998;79:483–490. doi: 10.1152/jn.1998.79.1.483. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology. 2003;28:599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- Symond MB, Harris AW, Gordon E, Williams LM. "Gamma synchrony" in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C. Attention and awareness in synchrony. Trends Cogn Sci. 2004;8:523–525. doi: 10.1016/j.tics.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nature Neuroscience. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17 Suppl 1:i171–i181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Tan YL, Zhou DF, Cao LY, Zou YZ, Zhang XY. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27–32. doi: 10.1016/j.neulet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- Tennigkeit F, Fukuda T, Geiger JR. Long-lasting plasticity emerges in adult neocortical fast-spiking interneurons. Society for Neuroscience Abstract. 2006 [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Toyooka K, Asama K, Watanabe Y, et al. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249–257. doi: 10.1016/s0165-1781(02)00127-0. [DOI] [PubMed] [Google Scholar]
- Traub RD, Spruston N, Soltesz I, Konnerth A, Whittington MA, Jefferys GR. Gamma-frequency oscillations: a neuronal population phenomenon, regulated by synaptic and intrinsic cellular processes, and inducing synaptic plasticity. Prog Neurobiol. 1998;55:563–575. doi: 10.1016/s0301-0082(98)00020-3. [DOI] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, et al. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Tsai G, Lane HY, Yang P, Chong MY, Lange N. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 2004;55:452–456. doi: 10.1016/j.biopsych.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Barrett T, Cheadle C, et al. Application of cDNA microarrays to examine gene expression differences in schizophrenia. Brain Research Bulletin. 2001;55:641–650. doi: 10.1016/s0361-9230(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Virgo L, Humphries C, Mortimer A, Barnes T, Hirsch S, de Belleroche J. Cholecystokinin messenger RNA deficit in frontal and temporal cerebral cortex in schizophrenia. Biol Psychiatry. 1995;37:694–701. doi: 10.1016/0006-3223(94)00206-I. [DOI] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW. Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry. 1997;41:530–540. doi: 10.1016/s0006-3223(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Archives of General Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101:1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gupta v, Toledo-Rodriguez M, Wu CZ, Markram H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 2002;12:395–410. doi: 10.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res Dev Brain Res. 2002;139:139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Ahn ES, Placantonakis DG. Is autism due to brain desynchronization? Int J Dev Neurosci. 2005;23:253–263. doi: 10.1016/j.ijdevneu.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Wespatat V, Tennigkeit F, Singer W. Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J Neurosci. 2004;24:9067–9075. doi: 10.1523/JNEUROSCI.2221-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical Gamma Generators Suggest Abnormal Auditory Circuitry in Early-Onset Psychosis. Cereb Cortex. 2007a doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol Psychiatry. 2007b;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Crowell AL. Targeting synapses and myelin in the prevention of schizophrenia. Schizophr Res. 2005;73:193–207. doi: 10.1016/j.schres.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218:267–277. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TUW, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Walsh JP, Benes FM. Density of Glutamic Acid Decarboxylase 67 Messenger RNA-Containing Neurons That Express the N-Methyl-D-Aspartate Receptor Subunit NR2A in the Anterior Cingulate Cortex in Schizophrenia and Bipolar Disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeragani VK, Cashmere D, Miewald J, Tancer M, Keshavan MS. Decreased coherence in higher frequency ranges (beta and gamma) between central and frontal EEG in patients with schizophrenia: A preliminary report. Psychiatry Res. 2006;141:53–60. doi: 10.1016/j.psychres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Banaschewski T, Kolev V, Woerner W, Rothenberger A. Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder--evidence from event-related gamma oscillations. Clin Neurophysiol. 2001;112:1096–1108. doi: 10.1016/s1388-2457(01)00524-7. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of Calcium-binding Proteins in Physiologically and Morphologically Characterized Interneurons of Monkey Dorsolateral Prefrontal Cortex. Cereb Cortex. 2004 doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]