Abstract
Aims
A balance between apoptosis and proliferation of vascular smooth muscle cells (VSMC) influences the development of intimal hyperplasia. We have previously demonstrated that protein kinase C delta (PKCδ) regulates both apoptosis and proliferation of VSMC in vitro. Here we investigate the role of PKCδ in intimal hyperplasia through gene deletion or overexpression in rodent models of arterial injury.
Methods and results
Arterial injury was induced in mice and rats by means of carotid ligation or balloon angioplasty, respectively. Overexpression of PKCδ was achieved by adenovirus-mediated gene transfer immediately after balloon injury in rat carotid arteries. Levels of PKCδ protein were profoundly increased in the carotid wall 3–7 days after balloon injury, co-localizing to TUNEL-positive medial cells. When subjected to arterial injury, PKCδ gene-deficient mice responded with an enhanced intimal hyperplasia accompanied by an 80% reduction in the number of TUNEL-positive cells detected in the injured arteries as compared with their wild-type littermates. Conversely, arterial gene transfer of PKCδ further increased the arterial expression of PKCδ, which was associated with a marked increase in apoptosis and reduction of intimal hyperplasia. Neither manipulation led to significant alteration in cell proliferation, suggesting that the function of PKCδ after arterial injury is predominantly pro-apoptotic. This notion is further supported by our observation of high PKCδ expression in human restenotic lesions that also co-localized with apoptosis.
Conclusion
The expression of PKCδ is upregulated in the arterial wall in response to injury. This induction appears to be a mechanism of arterial response that negatively influences the degree of intimal hyperplasia by stimulating VSMC apoptosis.
Keywords: Restenosis, Angioplasty, Apoptosis, Protein kinase C
1. Introduction
Intimal hyperplasia is an exaggerated healing response that occurs in the vessel wall after injury. It is a major cause of restenosis, which limits the success of many vascular interventions including bypass grafting, endarterectomy, and balloon angioplasty.1 The development of neointimal hyperplasia is a complex process involving vascular inflammation, re-establishment of the luminal endothelial lining, progenitor cell recruitment, and smooth muscle cell (SMC) proliferation and apoptosis.1,2 It is thought that vascular SMCs undergo a behavioural change in response to injury, transforming from a quiescent and differentiated state to a proliferative and synthetic phenotype.3 Another key characteristic of ‘injured’ SMCs is their resistance to apoptosis. SMCs, isolated from human endarterectomy lesions, were found to be insensitive to apoptotic stimuli.4 As the number of SMCs accumulated in the intimal lesions is determined by the balance between cell proliferation and apoptosis, defining the molecular mechanisms underlying both proliferation and apoptosis is necessary in order to better understand the behavioural changes of SMCs after vascular injury and thus to develop novel therapeutic strategies to inhibit intimal hyperplasia.5
Apoptosis is a multi-step process. In mammalian cells, apoptosis can be initiated through two main pathways. One pathway involves the activation of transmembrane death receptors such as Fas and TNF-α by their specific ligands leading to activation of caspase 8. The other pathway involves mitochondrial depolarization leading to the release of cytochrome c and activation of caspase 9. Both pathways ultimately result in activation of caspase 3, which then leads to apoptotic events including the cleavage of cell proteins, subsequent DNA fragmentation, and cell death.6 Factors that activate death receptors or the mitochondria-mediated apoptotic pathways are found to be abundant at the arterial wall of an injured artery.
Protein kinase C delta (PKCδ) is a novel member of the PKC family, a major group of serine–threonine kinases. All PKC isotypes share a similar structure including an N-terminal regulatory domain and a C-terminal catalytic domain connected via a hinge region. We and others have previously demonstrated in cultured vascular SMCs (VSMCs) that PKCδ is an important mediator of apoptosis in VSMCs.7–9 Inhibition of PKCδ activity or expression diminishes activation of caspase cascade as well as its downstream death events including DNA fragmentation and externalization of phospholipids.7,9 Recently, our group showed that PKCδ undergoes a caspase-3-mediated cleavage in the linker region to produce a catalytic fragment (CF) critical to the execution of cell death.7 Results with PKCδ knockout (KO) mice reveal that mice lacking PKCδ develop normally and are fertile. When subjected to stress or injury, VSMCs of PKCδ null mice showed an anti-apoptotic phenotype.8 In addition, PKCδ null mice are found to exhibit enhanced proliferation of B cells and auto-immunity.10 Using a mouse vein graft bypass model, Leitges and colleagues showed that PKCδ null mice developed an exacerbated graft arteriosclerosis associated with diminished cell apoptosis, suggesting this kinase might be an integral element of vascular injury.8
In cultured VSMCs, PKCδ has been shown to negatively regulate proliferation.11,12 Overexpression of this kinase in VSMC leads to G1 cell cycle arrest12 and impaired activation of mitogen-activated protein kinase extracellular signal-regulated kinase (ERK)1/211. However, inhibition of PKCδ either by gene deletion11 or dominant negative mutant also affects ERK activation.13 The precise relationship between PKCδ and ERK remains to be further investigated.
One of the distinctive properties of both human atherosclerosis and restenosis is the locality of lesions, which is related to differences in local hemodynamics as well as the regional differences in vessel wall physiology. Different vascular interventions, namely bypass, endarterectomy, angioplasty, and stenting, produce different kinds of injuries to the vessel which could subsequently elicit distinct injury responses. Clinically, the incidence of intimal hyperplasia and restenosis during long-term follow up varies significantly among different vascular interventions.14,15 Therefore, it is important to study PKCδ and its role in VSMC apoptosis and proliferation in multiple experimental models that mimic various vascular interventions.
In this report, we examined PKCδ function in the arterial wall using two rodent arterial injury models. Through genetic or molecular manipulations, we tested the effect of PKCδ inhibition or overexpression on VSMC apoptosis, proliferation, and intimal hyperplasia. These studies provide an explicit link between PKCδ and the pathogenesis of intimal hyperplasia after arterial injury.
2. Methods
2.1. General materials
Dulbecco's modified Eagles medium (DMEM) and cell culture reagents were from Gibco BRL Life Technologies. Chemicals, if not specified, were purchased from Sigma Chemical Co.
2.2. Animal model
Male Sprague-Dawley rats weigh around 300 g underwent angioplasty of the left common carotid artery with or without viral perfusion as described elsewhere.16 Construction of a PKCδ and beta-galactosidase expressing recombinant adenoviral vector (Ad PKCδ and Ad LacZ) and subsequent intraluminal delivery immediately after arterial injury were performed as previously described.16,17
The generation of PKC target deletion was described elsewhere.11 PKCδ KO mouse and their wild-type (WT) littermates, around 12 weeks old, underwent ligation of left carotid artery injury as described previously.18 The arteries were then harvested by perfusion fixation with 4% paraformaldehyde at a physiologic pressure of 100 mmHg.
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, NIH Publication No. 85-23, 1996 revision. Approval from the Institutional Animal Care and Use Committee at University of Wisconsin Madison was granted (#M02285).
2.3. Cell culture
Rat carotid arterial smooth muscle cells (rat VSMCs), and mouse aortic VSMCs, from the thoracic aorta of both PKC KO, and WT mouse, were isolated based on a protocol described by Clowes et al.19 The isolated cells were grown and maintained as recommended at 37°C in 5% CO2 in DMEM modified to contain 4 mM l-glutamine, 1.0 g/L glucose, 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate, supplemented with 10% foetal bovine serum (FBS; Gemini, Woodland, CA, USA), and antibiotics.
2.4. Human atherectomy specimens
Specimens were retrieved from the superficial femoral artery of patients undergoing atherectomy for restenotic lesions after stenting and/or prior angioplasty (n = 3). All specimens were collected under the approval of the Institutional Review Board at Cornell University (# 9704001187). The investigation conforms with the principles outlined in the Declaration of Helsinki.
2.5. Morphometric analysis and immunohistochemistry
Paraffin embedded arteries were cut into 5 µm sections for analysis. Morphometric analysis was carried out on elastin-stained arteries. For rat balloon-injured artery, 10 sections were randomly selected. For mouse ligation injured artery, five transverse sections at 3–4 mm proximal to the ligation site were obtained in each animal as described previously.20 The areas encompassed by the lumen surface (luminal area), internal elastic lamina—lumen surface (intimal area), external elastic lamina (EEL)—internal elastic lamina (medial area), and EEL length were measured. For the evaluation of the degree of intimal hyperplasia, the ratio of intimal area to medial area (I/M ratio) was calculated and compared using digital imaging software (ImageJ 1.36b), as described previously.5,20
Immunohistochemistry using rabbit anti-PKCδ 1:50 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was performed, and PKCδ positive area in the media was calculated as described elsewhere.21,22 TUNEL staining was performed under manufacturer's instruction. Immunofluorescent staining was performed with mouse anti-Ki67 1:100 (AbCam), rabbit anti-PKCδ 1:50 (Santa Cruz Biotechnology), rabbit anti-cleaved caspase-3 antibody (Cell signalling), and donkey anti-mouse Alexa 555 or donkey anti-rabbit Alexa 488 (Molecular Probes). TO-PRO 3 (Molecular Probes) was used to identify nuclei. Staining was visualized with a Nikon Eclipse E800 upright microscope and digital images were acquired using a RetigaEXi CCD digital camera. Fluorescent staining was visualized and digital images were taken on a Zeiss LSM 510 Laser Scanning Confocal imaging system with the appropriate argon beam lasers. For each specimen, cells were counted in four fields at 882× magnification. Digital images were analysed using Zeiss Image Browser software. TUNEL and Ki67 Indices were calculated as (number of positive cells/number of total nuclei) per section by identifying positive cells vs. TO-PRO3 positive cells from at least eight independent fields from five sections in each sample using NIH image software (ImageJ 1.36b).
2.6. Immunoblot analysis
Arteries were removed from surrounding tissues and harvested under the surgical microscope. Harvested arteries were rinsed with PBS and homogenized as descried previously.16,23 For cellular studies, VSMCs were made quiescent by incubation in medium containing 0.5% FBS for 48 h. Cells were then treated with H2O2 at various concentrations and times, and then lysed in 50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS and subjected to SDS-PAGE and transferred to a polyvinylidene difluoride (Bio-Rad, Hercules, CA, USA) membrane. Membranes were incubated with rabbit polyclonal antibodies to rabbit anti-PKCδ (Santa Cruz Biotechnology), and mouse anti-β-actin (Sigma). Labelled proteins were visualized with an enhanced chemi-luminescence system (PerkinElmer Life Sciences, Boston, MA).
2.7. Apoptosis assay
DNA fragmentation was determined using the Cell Death Detection ELISA system (Roche Applied Science, Indianapolis, IN, USA), an assay based on a quantitative sandwich-enzyme-immunoassay principle using mouse monoclonal antibodies directed against DNA and histones. Cellular extracts were incubated in 96-well plates coated with anti-histone antibodies. Plates were then incubated with anti-DNA antibodies conjugated to peroxidase for 2 h, and absorbance was measured at 405 nm.
2.8. Statistical analysis
Values were expressed as fold increases (means ± standard error) from at least three independent experiments unless stated otherwise. Differences between two groups were analysed by Student's t test, and one-way analysis of variance followed by Scheffe's test was used for multiple comparisons. Values of P < 0.05 were considered significant.
3. Results
3.1. Temporal expression of PKCδ after balloon angioplasty
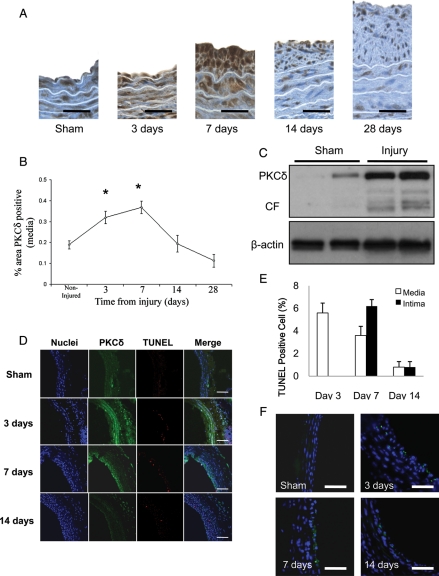
To elucidate the role of PKCδ in vascular injury response, we first evaluated PKCδ expression in rat carotid arteries harvested 3, 7, 14, and 28 days after balloon angioplasty. As compared with non-injured arteries, arteries at day 3 post-injury exhibited significantly higher immunostaining to PKCδ. The PKCδ expression was highest at day 7, and returned to baseline level at day 14 (Figure 1A and B). To better quantify the injury-induced PKCδ expression, we performed western blot analysis. Protein extract isolated from balloon-injured arteries showed a markedly higher level of PKCδ than that from sham-operated arteries (Figure 1C). The CF of PKCδ, known to be associated with PKCδ mediated apoptosis, was abundantly present in injured arteries while it was barely detectable in sham-operated arteries (Figure 1C). To further explore the relationship between high PKCδ expression and apoptosis in injured arteries, we double-stained arterial sections for PKCδ and TUNEL. As shown in Figure 1D, the temporal and spatial patterns of the expression in PKCδ were very similar to that of TUNEL staining (Figure 1D–E). Immunostaining for cleaved caspase-3 also showed similar pattern as TUNEL staining (Figure 1F).
Figure 1.
Expression of PKCδ and apoptosis in balloon-injured arteries. (A) Representative photomicrographs of balloon-injured rat carotid arteries stained for PKCδ (brown) 3, 7, 14, or 28 days post-injury. Sham-operated arteries serve as control (scale bar=50 µm). (B) Percentage of PKCδ positive area in the media by immunostaining (n = 5, *P < 0.05). (C) Balloon-injured rat carotid arteries were harvested 3 days after injury. Extracted proteins were subjected to immunoblotting for PKCδ. Beta-actin serves as a loading control. Each lane represents exatract from a single artery. (D) Representative photomicrographs of immunofluorescence staining for PKCδ (green), TUNEL staining (red), and nuclei (blue). Merged images are shown in the right panels (scale bar=100 µm). (E) TUNEL index were calculated as a ratio of the number of TUNEL-positive cells to total cell number (n = 6). (F) Representative photomicrographs of immunofluorescence staining for cleaved caspase-3 (green) (scale bar=20 um).
3.2. The role of PKCδ in VSMC apoptosis in vitro
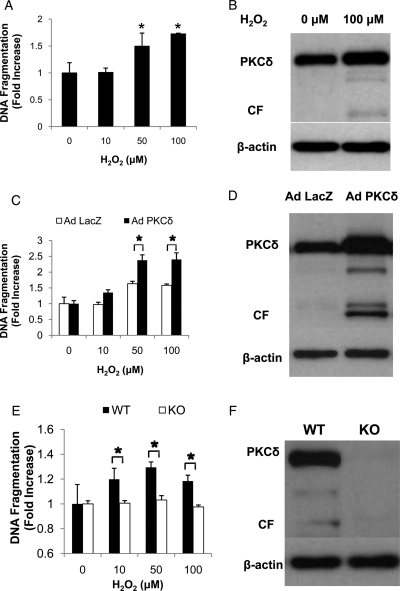
To mimic the high expression we observed in the arterial wall following balloon injury, we forced higher PKCδ expression in normal VSMCs using an adenoviral vector carrying the full-length PKCδ cDNA (Ad PKCδ). As shown in Figure 2A, normal, un-manipulated aortic VSMCs responded to H2O2 with an induction of apoptosis in a dose-dependent manner as measured by ELISA for DNA fragmentation. Western blotting indicated that H2O2 treatment elicited the formation of the pro-apoptotic CF, which was undetectable in control cells (Figure 2B). The level of full-length PKCδ was slightly higher in H2O2-treated cells, however this increase was not statistically significant (fold induction: 1.28 ± 0.12, n = 4, P = 0.14) (Figure 2B). Infection of aortic VSMCs with a control adenovirus (Ad LacZ) did not change the apoptotic responses of these cells to H2O2. In contrast, infection of VSMCs with Ad PKCδ markedly increased their apoptotic responses, including elevated DNA fragmentation and formation of CF (Figure 2C). Conversely, inhibition of PKCδ activity by targeted gene deletion completely eliminated not only the expression of PKCδ but the ability to undergo apoptosis when stimulated with H2O2 (Figure 2E and F).
Figure 2.
Effects of PKCδ on hydrogen peroxide-induced apoptosis of VSMCs. (A–D) Rat VSMCs were treated with 10–100 µM of hydrogen peroxide and harvested after 48 h, with no virus (A and B), with adenovirus encoding PKCδ (Ad PKCδ) or with beta-galactosidase (Ad LacZ) (C and D). In A and C, apoptosis was evaluated by the degree of DNA fragmentation measured with ELISA (*P < 0.05, as compared with untreated control). In B and D, representative immunoblotting for PKCδ are shown. Cell lysates from VSMC that were treated with no virus (B) or with Ad PKCδ or Ad LacZ (D) were harvested and immunoblotted with anti-PKCδ antibody. Beta-actin serves as a protein-loading control. The 79 kDa band labelled as PKCδ represents the full-length version of this kinase and the 40 kDa band labelled as CF represents the catalytic fragment generated by a caspase-mediated cleavage of PKCδ. (E) Mouse VSMCs from PKCδ knock-out (KO) or their wild-type littermates (WT) were treated with 10 –100 µM of hydrogen peroxide and harvested for DNA fragmentation assay after 48 h. (*P < 0.05, KO vs. WT). (F) Cell lysates from both cells were harvested and immunoblotted with anti-PKCδ antibody. Beta-actin serves as a protein-loading control.
3.3. Accelerated intimal hyperplasia in PKCδ KO mouse
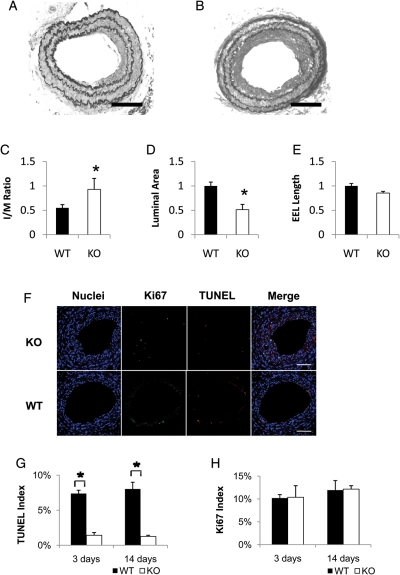
We speculated that the lack of apoptotic response in PKCδ null mice might elicit an accelerated incidence of neointimal formation after arterial injury. To test this hypothesis, we generated arterial injury by ligating the carotid artery of PKCδ WT or KO mouse. Twenty-eight days after ligation, arteries were harvested for morphological analysis. As compared with the WT controls, the injured arteries of PKCδ KO mice showed a significant increase in neointimal formation, reflected by an increase of over 65% in intimal to media ratio (I/M ratio) (Figure 3A–C). Accordingly, the luminal area of the PKCδ null arteries was reduced by ∼50% (Figure 3D). However, there is no significant difference in the length of EEL, a parameter for arterial remodelling (Figure 3E).
Figure 3.
Effects of PKCδ gene deficiency on intimal hyperplasia after carotid ligation. (A and B) Representative photomicrographs of the carotid arteries of PKCδ knock-out mice (KO) and their wild-type littermates (WT) 28 days after ligation. Sections were stained with Elastic-van Gieson staining (scale bar=50 µm). (C–E) Quantitative morphological analyses of the carotid artery of WT and KO 28 days after injury. The intimal area to medial area ratio (I/M ratio) (C), luminal area (D), and length of external elastic lamina (EEL) (E) were measured as described in the Methods (n = 6, *P < 0.05 as compared with WT mouse). (F) Representative micrographs of immunofluorescence staining of the carotid arteries of PKCδ knock-out mouse (KO) and their wild-type littermates (WT) 14 days after ligation. The cross sections were stained for nuclei (blue), Ki67 (green), and TUNEL (red). Merged images are shown on the right panels (scale bar=50 µm). TUNEL index (G) and Ki67 index (H) were calculated as a percentage of the number of positive cells to the number of total cells (n = 6, *P < 0.05 as compared with WT).
3.4. Cell apoptosis in PKCδ KO mouse after carotid ligation
Next we compared the degree of apoptosis between PKCδ KO and WT littermates after ligation injury. The carotid arteries of PKCδ KO and WT were subjected to carotid ligation, and were harvested after 3 or 14 days. As shown in Figure 3F and G, apoptosis was attenuated in PKCδ KO arteries at both 3 and 14 days. In contrast, PKCδ gene deletion did not lead to any significant alteration in arterial cell proliferation as measured by immunostaining for the proliferation marker Ki67 (Figure 3F and H).
3.5. PKCδ gene transfer following arterial injury
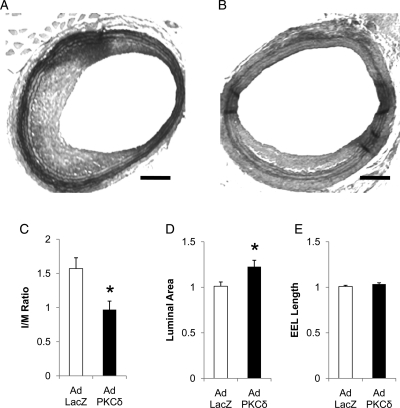
Next, we extended our in vitro PKCδ gene transfer study to an arterial injury model. Here, we used a rat carotid balloon injury because luminal gene transfer cannot be flawlessly accomplished in mice due to their small arterial size. Immediately following balloon angioplasty, the injured arteries were infected with Ad PKCδ or Ad LacZ. The arteries were harvested after 14 days for morphological analyses. As shown in Figure 4, Ad PKCδ infection significantly reduced the neointimal formation, reflected by a 30% reduction in I/M ratio in comparison with Ad LacZ infected arteries. In addition, Ad PKCδ increased luminal area by ∼30% without significantly changing EEL length (Figure 4D).
Figure 4.
Effects of PKCδ overexpression on intimal hyperplasia after balloon injury. (A and B) Representative photomicrographs of rat carotid arteries 14 days after balloon injury, infected with an adenovirus encoding PKCδ (Ad PKCδ) (A) or beta-galactosidase (Ad LacZ) (B). Sections were stained with Elastic-van Gieson staining (scale bar =100 µm). (C–E) Quantitative morphological analyses of the carotid arteries 14 days after balloon injury. The intimal area to medial area ratio (I/M ratio) (C), luminal area (D), and length of external elastic lamina (EEL) (E) were measured as described in Methods (n = 6, *P < 0.05 as compared with Ad LacZ).
3.6. Apoptosis after balloon injury in the presence of PKCδ overexpression
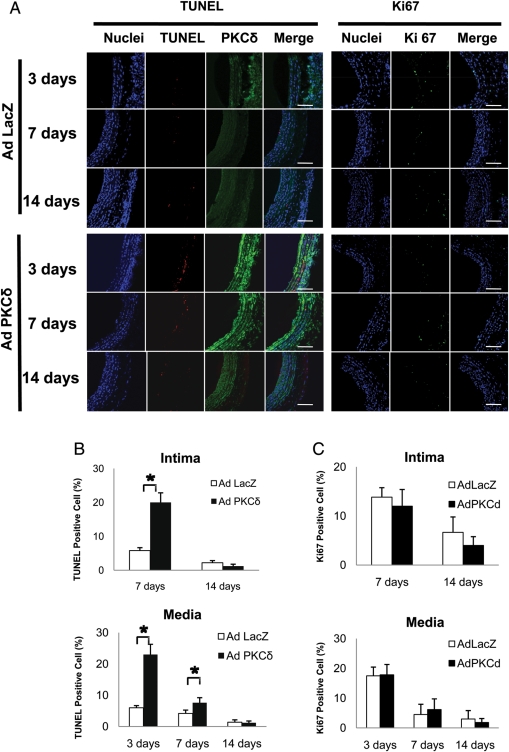
To test whether Ad PKCδ inhibits neointimal formation by stimulating apoptosis of arterial VSMC, we evaluated PKCδ expression, cell apoptosis, and proliferation in the carotid arteries after balloon injury/gene transferring. Immunostaining for PKCδ confirmed the success of gene transfer, indicated by the elevated PKCδ at both days 3 and 7 as compared with Ad LacZ infected arteries (Figure 5A). TUNEL analysis showed extensive upregulation of apoptosis in Ad PKCδ-infected arteries (Figure 5A and B). At day 3, the percentage of TUNEL-positive cells in the tonic media was 21% in Ad PKCδ infected arteries as compared with 6% of Ad LacZ-infected arteries. The upregulation of apoptosis persisted to day 7 in both media and intima, then subsided at 14 days (Figure 5A and B). In contrast, immunostaining for Ki67 showed no significant difference in proliferation between Ad PKCδ and Ad LacZ-infected arteries at all time points (Figure 5C). Since the intima at day 3 was undetectable, we have presented both TUNEL and Ki67 index in the intima only at days 7 and 14.
Figure 5.
Effects of PKCδ overexpression on proliferation and apoptosis in vivo. (A) Representative micrographs of immunofluorescence staining of the rat carotid arteries infected with adenovirus encoding PKCδ (Ad PKCδ) or beta-galactosidase (Ad LacZ). Arteries were harvested and analysed at the indicated time points post-injury. For double staining of PKCδ and TUNEL (left panels), the cross-sections were stained for nuclei (blue), TUNEL (red), and PKCδ (green). For Ki67 staining (right panels), the sections were stained for nuclei (blue) and Ki67 (green). Merged images are shown on the right panels (scale bar=100 µm). TUNEL index (B) and Ki67 index (C) were calculated as a percentage of the number of positive cells to the number of total cells in the balloon-injured arteries infected with Ad PKCδ or Ad LacZ (n = 6, *P < 0.05 as compared with Ad LacZ-infected arteries).
3.7. PKCδ expression and apoptosis in human restenotic artery
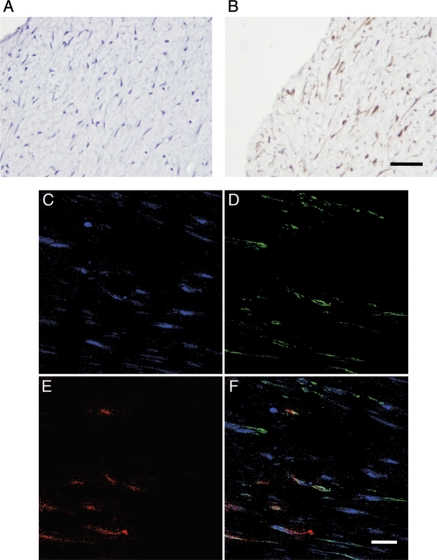
To determine whether PKCδ expression is altered in restenotic arteries, samples were collected from the superficial femoral artery of patients who underwent atherectomy for restenotic lesions. Immunohistochemical analysis for PKCδ showed robust expression of PKCδ in restenotic lesions (Figure 6). The double staining for PKCδ and TUNEL revealed co-localization of PKCδ and TUNEL-positive cells (Figure 6C–F).
Figure 6.
PKCδ expression and apoptosis in human restenostic lesions. Representative micrographs of negative control (A) and immunostaining for PKCδ (B) of atherectomy specimens of human restenotic lesions (scale bar=50 µm). (C–E) Representative photomicrographs of double immunofluorescence staining for PKCδ (D: green), TUNEL staining (E: red), nuclei (C: blue), and merged image (F) (scale bar=20 µm).
4. Discussion
Arterial responses to injury involve many molecular changes, including the upregulation of cytokines, growth factors, and certain matrix proteins.24–26 Here, we have reported a novel molecular alteration following arterial injury. The injury-induced elevation in PKCδ protein expression was profound and acute. By day 3 following balloon angioplasty of a rat carotid artery, the protein level of PKCδ was increased significantly evidenced by both immunohistological and biochemical analyses. The induction of the CF of PKCδ was even more dramatic. Of note, the tumour suppressor p53, a pro-apoptotic factor that we have previously shown to be regulated by PKCδ, was previously reported to be upregulated 7 days after injury.27
The co-localization of PKCδ with apoptotic cells extended to human restenotic lesions (as seen in this study) as well as aortic specimens of aneurysm patients,28 strongly supporting a link between PKCδ and apoptosis of VSMCs. This link is further demonstrated by our in vivo studies using targeted gene deletion and adenovirus-mediated gene transfer. Inhibition of PKCδ expression via gene deletion led to a marked decrease in the number of apoptotic cells following carotid ligation. Conversely, further elevation of PKCδ expression via adenovirus-mediated gene transfer resulted in increased cell apoptosis. Our findings are consistent with Leitges's report using the mouse vein graft model.8 Since these experimental models, i.e. carotid ligation, balloon angioplasty, and vein grafting, cause injuries to the arterial wall through different mechanisms, the combined findings between Leitges's and ours indicate that PKCδ is a common mechanism underlying many types of vascular injury and therefore may serve as a useful target in anti-intimal hyperplasia therapies.
Using isolated VSMCs, we and others have reported that PKCδ also plays a key role in regulation of proliferation.11–13,29,30 However, neither PKCδ gene deficiency nor adenovirus-mediated gene transfer led to significant alterations in the number of proliferating cells after arterial injury in both ligation and angioplasty models. Accordingly, no significant change in cell proliferation was also reported in PKCδ KO mice when subjected to vein bypass.8 The lack of effect on vascular proliferation by PKCδ suggests that the dominant role of this kinase in an injured artery is to regulate cell apoptosis rather than proliferation.
PKCδ can be activated by many extracellular factors including mechanical stress, pro-inflammatory cytokines, and oxidative stress, all of which are known to be associated with arterial injury.7,26,30 Mechanisms regarding how PKCδ is activated by various stimuli include binding of second messenger diacylglycerol, phosphorylation, membrane translocation, and proteolysis. Recently, we used a rat aortic VSMC line to show that the caspase-3-mediated cleavage of PKCδ is a necessary step in the apoptotic pathway leading to execution of death events elicited by H2O2.7 In the present study, we detected the CF, the product of PKCδ cleavage, in the tissue extract of injury carotid arteries but not in uninjured arteries. This finding indicates that a similar activation mechanism of PKCδ exists in the arterial wall, perhaps responding to increased oxidative stress associated with arterial injury.
We are tempted to postulate that alterations in intimal hyperplasia produced by PKCδ KO or gene expression are primarily mediated by the pro-apoptotic function of this kinase. However, our study does not rule out potential effects of PKCδ on other players of intimal hyperplasia. Compared with the extensive degree of apoptosis induced by PKCδ overexpression, the suppressive effect of PKCδ overexpression on intimal hyperplasia was not as profound as we had anticipated. We performed a preliminary evaluation of inflammation in the overexpression model revealing no significant presence of CD69+ macrophages in injured carotid wall in either the presence or absence of Ad PKCδ. In order to completely rule out any potential effect of PKCδ on inflammatory response after arterial injury, markers for other inflammatory cells need to be evaluated. Alternatively, PKCδ could affect the recruitment of bone marrow derived progenitor cells to the injured artery. In a separate study, we found that VSMCs can attract progenitor cells by secreting monocyte chemoattracting protein-1 (MCP-1).16 The vital role of MCP-1 in the pathogenesis of arterial remodelling has been previously reported.31 In vitro, we have found that PKCδ is a major mediator of MCP-1 expression by VSMCs.28 If this stimulatory function of PKCδ regarding to MCP-1 expression is proven to exist during arterial injury, the beneficial effect of PKCδ in the promotion of VSMC apoptosis may be offset by its coincident stimulation of MCP-1, thus recruitment of progenitor cells. A reliable bone marrow transplant model is necessary in order to test this hypothesis following rat carotid balloon injury.
In summary, we showed upregulation of PKCδ accompanied by apoptosis of VSMC in the arterial wall in response to injury. Through alterations of PKCδ expression in rodent artery injury models, we demonstrated the suppressive effect of PKCδ on the development of intimal hyperplasia after arterial injury, primarily through the stimulation of VSMC apoptosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by a Public Health Service Grant R01 HL-81424 (K.C.K. and B.L.) from the National Heart Lung, Blood Institute and an American Heart Association grant-in-aid 0455859T (B.L.) and Uehara Memorial Foundation Research Fellowship Award (D.Y.).
Supplementary Material
Acknowledgements
The authors like to thank Dr K. Craig Kent for scientific discussions and Karla Esbona, Stephanie Morgan, Justin Lengfeld, Rachel Edlin, and Chunjie Wang for technical assistance and Dr Nakayama of Kyusyu University for PKCδ knockout mice.
Conflict of interest: none declared.
References
- 1.Kent KC, Liu B. Intimal hyperplasia—still here after all these years! Ann Vasc Surg. 2004;18:135–137. doi: 10.1007/s10016-004-0019-4. [DOI] [PubMed] [Google Scholar]
- 2.Tsai S, Butler J, Rafii S, Liu B, Kent KC. The role of progenitor cells in the development of intimal hyperplasia. J Vasc Surg. 2009;49:502–510. doi: 10.1016/j.jvs.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.McCaffrey TA, Consigli S, Du B, Falcone DJ, Sanborn TA, Spokojny AM, et al. Decreased type II/type I TGF-beta receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-beta1. J Clin Invest. 1995;96:2667–2675. doi: 10.1172/JCI118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamanouchi D, Banno H, Nakayama M, Sugimoto M, Fujita H, Kobayashi M, et al. Hydrophilic statin suppresses vein graft intimal hyperplasia via endothelial cell-tropic Rho-kinase inhibition. J Vasc Surg. 2005;42:757–764. doi: 10.1016/j.jvs.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Stennicke HR, Salvesen GS. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 7.Kato K, Yamanouchi D, Kamiya K, Zhang F, Kent KC, Liu B. Caspase-mediated protein kinase C-delta cleavage is necessary for apoptosis of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00274.2009. doi:10.1152/ajpheart.00274.2009, published online ahead of print October 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, et al. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JL, Lin HH, Kim KJ, Lin A, Ou JH, Ann DK. PKC delta signaling: a dual role in regulating hypoxic stress-induced autophagy and apoptosis. Autophagy. 2009;5:244–246. doi: 10.4161/auto.5.2.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Ryer EJ, Kundi R, Kamiya K, Itoh H, Faries PL, et al. Protein kinase C-delta regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2. J Vasc Surg. 2007;45:160–168. doi: 10.1016/j.jvs.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumoto S, Nishizawa Y, Hosoi M, Koyama H, Yamakawa K, Ohno S, et al. Protein kinase C delta inhibits the proliferation of vascular smooth muscle cells by suppressing G1 cyclin expression. J Biol Chem. 1997;272:13816–13822. doi: 10.1074/jbc.272.21.13816. [DOI] [PubMed] [Google Scholar]
- 13.Ginnan R, Singer HA. PKC-delta-dependent pathways contribute to PDGF-stimulated ERK1/2 activation in vascular smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1193–C1201. doi: 10.1152/ajpcell.00499.2004. [DOI] [PubMed] [Google Scholar]
- 14.Lee LK, Kent KC. Infrainguinal occlusive disease: endovascular intervention is the first line therapy. Adv Surg. 2008;42:193–204. doi: 10.1016/j.yasu.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Mwipatayi BP, Hockings A, Hofmann M, Garbowski M, Sieunarine K. Balloon angioplasty compared with stenting for treatment of femoropopliteal occlusive disease: a meta-analysis. J Vasc Surg. 2008;47:461–469. doi: 10.1016/j.jvs.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Tsai S, Kato K, Yamanouchi D, Wang C, Rafii S, et al. Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells. J Biol Chem. 2009;284:17564–17574. doi: 10.1074/jbc.M109.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryer EJ, Hom RP, Sakakibara K, Nakayama KI, Nakayama K, Faries PL, et al. PKCdelta is necessary for Smad3 expression and transforming growth factor beta-induced fibronectin synthesis in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006;26:780–786. doi: 10.1161/01.ATV.0000209517.00220.cd. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Takeshita K, Liu PY, Satoh M, Oyama N, Mukai Y, et al. Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation. 2009;119:2686–2692. doi: 10.1161/CIRCULATIONAHA.108.790485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 20.Prus K, Edstrom A, Wallin M. Actin-induced stimulation of microtubule-associated ATPase activity. FEBS Lett. 1981;125:49–52. doi: 10.1016/0014-5793(81)80993-3. [DOI] [PubMed] [Google Scholar]
- 21.Hollenbeck ST, Sakakibara K, Faries PL, Workhu B, Liu B, Kent KC. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004;120:288–294. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Edlin RS, Tsai S, Yamanouchi D, Wang C, Liu B, Kent KC. Characterization of primary and restenotic atherosclerotic plaque from the superficial femoral artery: Potential role of Smad3 in regulation of SMC proliferation. J Vasc Surg. 2009;49:1289–1295. doi: 10.1016/j.jvs.2008.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banno H, Takei Y, Muramatsu T, Komori K, Kadomatsu K. Controlled release of small interfering RNA targeting midkine attenuates intimal hyperplasia in vein grafts. J Vasc Surg. 2006;44:633–641. doi: 10.1016/j.jvs.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007;97:730–737. [PubMed] [Google Scholar]
- 25.Simons M. VEGF and restenosis: the rest of the story. Arterioscler Thromb Vasc Biol. 2009;29:439–440. doi: 10.1161/ATVBAHA.109.183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zampetaki A, Zhang Z, Hu Y, Xu Q. Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1-p38 MAPK-NF-kappaB signaling pathways. Am J Physiol Heart Circ Physiol. 2005;288:H2946–H2954. doi: 10.1152/ajpheart.00919.2004. [DOI] [PubMed] [Google Scholar]
- 27.Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL, et al. Protein kinase C delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J Biol Chem. 2005;280:35310–35317. doi: 10.1074/jbc.M507187200. [DOI] [PubMed] [Google Scholar]
- 28.Schubl S, Tsai S, Ryer EJ, Wang C, Hu J, Kent KC, et al. Upregulation of protein kinase cdelta in vascular smooth muscle cells promotes inflammation in abdominal aortic aneurysm. J Surg Res. 2009;153:181–187. doi: 10.1016/j.jss.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh H, Yamamura S, Ware JA, Zhuang S, Mii S, Liu B, et al. Differential effects of protein kinase C on human vascular smooth muscle cell proliferation and migration. Am J Physiol Heart Circ Physiol. 2001;281:H359–H370. doi: 10.1152/ajpheart.2001.281.1.H359. [DOI] [PubMed] [Google Scholar]
- 30.Skaletz-Rorowski A, Eschert H, Leng J, Stallmeyer B, Sindermann JR, Pulawski E, et al. PKC delta-induced activation of MAPK pathway is required for bFGF-stimulated proliferation of coronary smooth muscle cells. Cardiovasc Res. 2005;67:142–150. doi: 10.1016/j.cardiores.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, et al. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–314. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.