Abstract
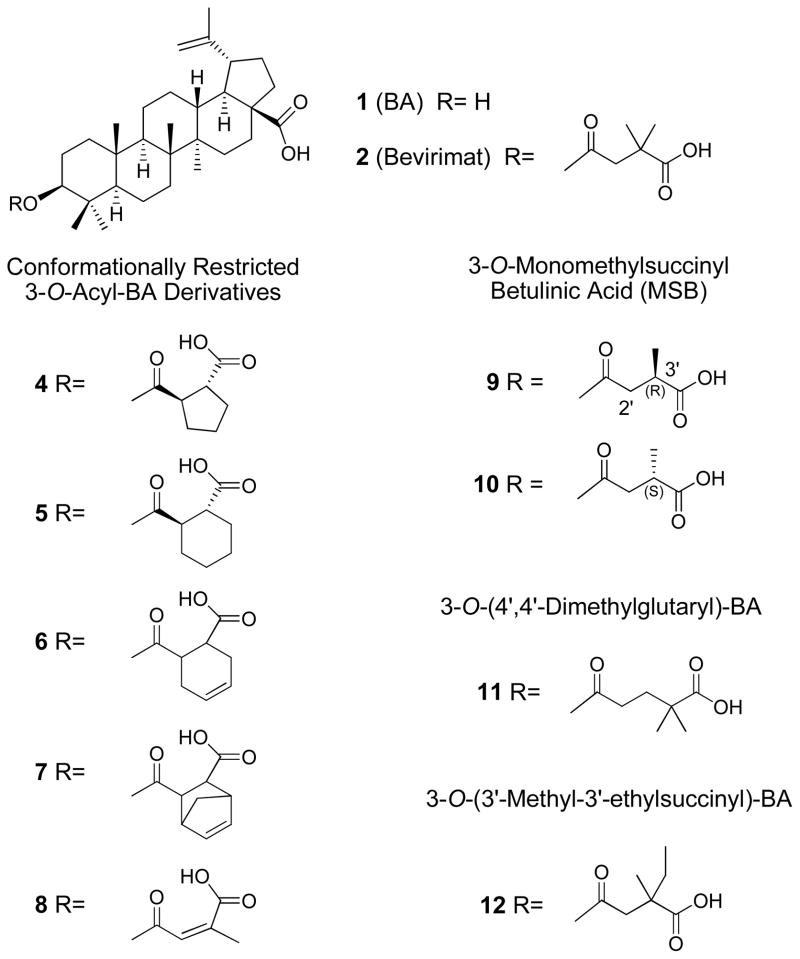
In our continuing study of triterpene derivatives as potent anti-HIV agents, different C-3 conformationally restricted betulinic acid (BA, 1) derivatives were designed and synthesized in order to explore the conformational space of the C-3 pharmacophore. 3-O-Monomethylsuccinyl- betulinic acid (MSB) analogs were also designed to better understand the contribution of the C-3′ dimethyl group of bevirimat (2), the first-in-class HIV maturation inhibitor, which is currently in phase IIb clinical trials. In addition, another triterpene skeleton, moronic acid (MA, 3) was also employed to study the influence of the backbone and the C-3 modification towards the anti-HIV activity of this compound class. This study enabled us to better understand the structure-activity relationships (SAR) of triterpene-derived anti-HIV agents, and led to the design and synthesis of compound 12 (EC50: 0.0006 μM), which displayed slightly better activity than 2 as a HIV-1 maturation inhibitor.
Introduction
It is estimated that at the end of 2008, approximately 33.2 million people were infected worldwide with human immunodeficiency virus (HIV), the etiologic cause of acquired immunodeficiency syndrome (AIDS). Each year, around 2.7 million more people become infected with HIV and 2 million die of AIDS.1 Considering that the development of a safe and effective HIV vaccine is still in the future,2 the current research continues to focus on the disease treatment by chemical anti-HIV agents. Although significant progress has been made since the introduction of highly active antiretroviral therapy (HAART),3, 4 which employs a combinational use of nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and/or protease inhibitors (PIs), it has also led to some serious problems, including increased adverse effects and the emergence of multi-drug-resistant viral strains.5–7 Drug-resistant virus is then involved in HIV transmission, and more than 25% of newly infected individuals harbor HIV-1 isolates that are resistant to at least one ART.8 Therefore, novel potent antiretroviral agents with different targets than currently approved drugs may hold particular promise in addressing issues of current therapies.
Our prior modification study on betulinic acid (BA, 1) resulted in the discovery of bevirimat [3-O-(3′,3′-dimethylsuccinyl)-betulinic acid, 2].9 Bevirimat exhibits extremely potent antiviral activity against HIV-1 primary isolates and several drug-resistant virus, and represents a unique first in a class of anti-HIV compounds termed maturation inhibitors (MIs).10, 11 MIs block the last step of viral gag precursor polyprotein processing from p25 (CA-SP1) to functional p24 (CA), resulting in the production of noninfectious immature HIV-1 particles. Most importantly, MIs retain their high anti-HIV potency against different viral strains that are resistant to current ARTs, including AZT (NRTI), Nevirapine (NNRTI), and Indinavir (PI). Compound 2 has recently succeeded in Phase IIb clinical trials and is in preparation for Phase III clinical trials.12–14
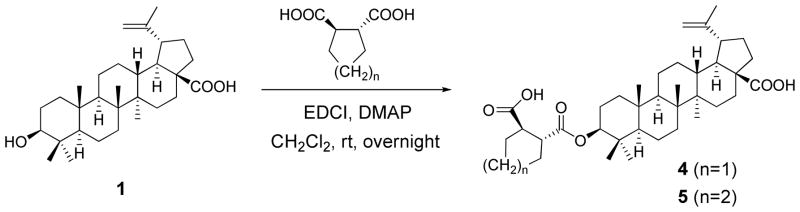
To summarize our prior structure-activity relationship (SAR) study of 2 and other 3- O-acyl-BA derivatives, we know that C-3 ester substitution is important to the enhanced antiviral activity.9, 15 Within the C-3 side chain, the proper length, a terminal carboxylic acid, and C-3′ dimethyl substitution contribute to antiviral potency. However, the C-3 ester groups of prior BA analogs have mainly contained freely rotatable alkyl chains. Therefore, in the present study, five C-3 conformationally restricted BA analogs (4–8) were synthesized in order to explore the conformational space of the C-3 pharmacophore. Two 3-O-monomethylsuccinyl betulinic acid (MSB) derivatives (9–10) and compound 11 were further designed to investigate how the methyl substituents on the C-3 side chain impact the antiviral potency.
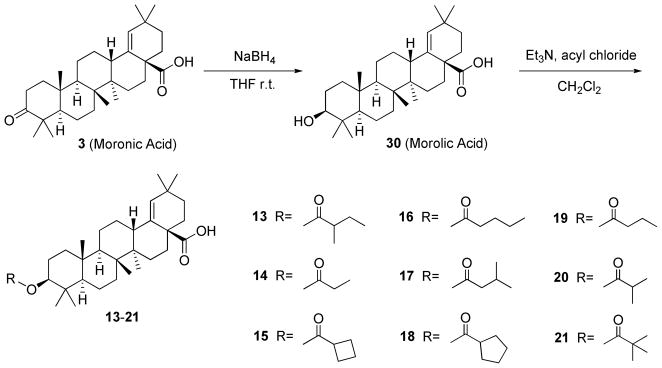
Meanwhile, moronic acid (MA, 3), which was isolated from Brazilian propolis, also exhibited promising anti-HIV activity as a lead compound with an EC50 of 0.1μg/mL.16 However, insertion of the same 3′,3′-dimethylsuccinyl side chain found in 2 at the 3β position of morolic acid (30), the C-3 ketone reduced analog of 3, unexpectedly did not increase the antiviral activity.16 Because 2 and 30 share similar 3D conformational space of their triterpene skeletons, this unexpected result led us to further investigate possible modifications on analogs of 3 and 30.
Detailed SAR of triterpene-derived HIV-1 maturation inhibitors has been established from the current study. This analysis led to the design and synthesis of compound 12 with slightly better activity than 2.
Design
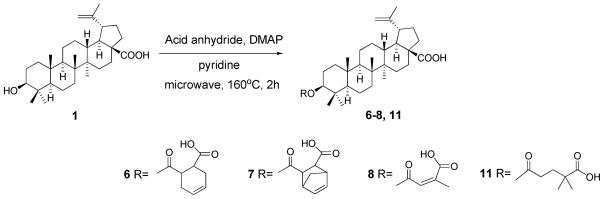
Because the presence of C-3 substitution is critical to the anti-HIV-1 activity of BA derivatives, five conformationally restricted 3-O-acyl-BA analogs (4–8) (Figure 1) were first synthesized and evaluated, in order to further explore the conformational space of this pharmacophore. The C-3′ dimethyl group was also moved towards the 4′-position in a C-3 glutaryl-substituted compound (11) (Figure 1) to study the influence of different positioning of the methyl groups. In addition, although we know that the presence of the C-3′ dimethyl within the C-3 side chain is vital to anti-HIV-1 activity, it is still unclear which, if either, C-3′ methyl group of 2 contributes more toward activity. Therefore, 3-O-monomethylsuccinyl betulinic acid (MSB) derivatives (9–10) (Figure 1) were designed and synthesized.17 Their antiviral activities were then evaluated in vitro against HIV-1IIIB replication in MT-2 cell lines. Diverse substitutions (13–21) (Scheme 4) were also incorporated into 30 to study their influence on the antiviral potency and the impact of the triterpene skeleton itself on the antiviral activity. After reviewing the initial promising bioassay results, compound 12 (Figure 1) was then designed to try to improve the antiviral potency of 2.
Figure 1.
Structures of MSB analogs and other 3-O-acyl BA analogs
Scheme 4.
Synthesis of MA analogs 13–21.
Chemistry
Compounds 4 and 5 were synthesized by reaction of the corresponding cycloalkanedicarboxylic acid with the C-3 β-hydroxyl group of 1 in the presence of 1- ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI) and dimethylaminopyridine (DMAP), resulting in yields of 26% and 35%, respectively (Scheme 1). Compounds 6–8 and 11 were synthesized according to Scheme 2. Reaction of the corresponding acid anhydride with 1 furnished the target compounds in yields of 35–55%.
Scheme 1.
Synthesis of compounds 4 and 5.
Scheme 2.
Syntheses of compounds 6–8 and 11.
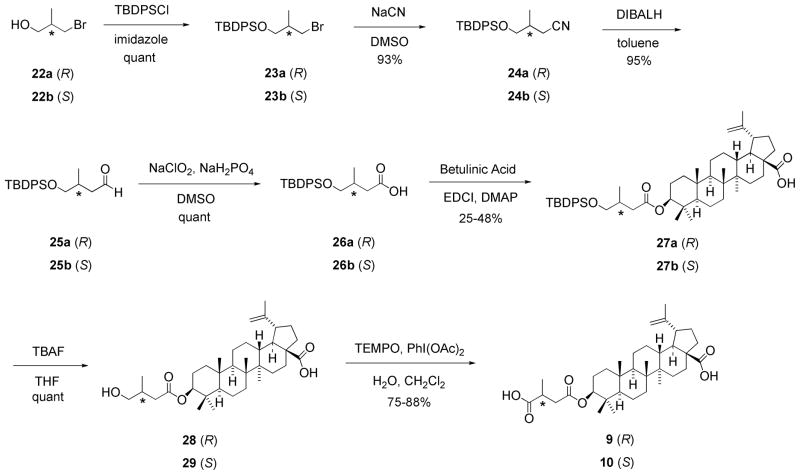
The synthesis of MSB analogs was reported before in Ref 17. As shown in Scheme 3, the hydroxyl of 3R-bromo-2-methylpropanol (22a) was first protected by as a tert-butyldimethylsilyl (TBDPS) moiety to yield 23a quantitatively. The bromide group was then replaced with a cyano moiety (24a), which was reduced to an aldehyde moiety (25a),18 and consequentially oxidized to a carboxylic acid (26a). Reaction of 1 with 26a led to esterification of the 3β-hydroxy group of 1 to provide 27a. After cleavage of the TBDPS group with TBAF in THF, the primary hydroxyl of 28 was oxidized to a carboxylic acid in the presence of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and PhI(OAc)2 to give 3-O-(3′R-methylsuccinyl)-betulinic acid (3′R-MSB, 9).19 The 3′S-MSB isomer (10) was synthesized using the same method starting with 3S-bromo-2- methylpropanol (22b).
Scheme 3.
Total syntheses of compounds 9 and 10.
Scheme 4 depicts the synthesis of MA-analogs. Compound 3 was first treated with NaBH4 to yield 3β-hydroxyl group of 30. Different acyl chloride was then reacted with 30 in the presence of Et3N to furnish 3-O-acyl-MA analogs 13–21.
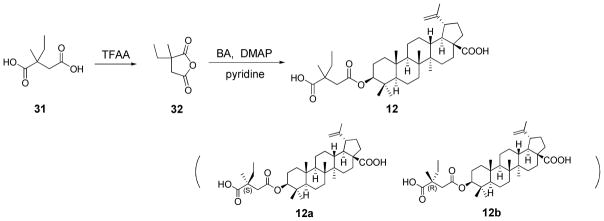
The synthesis of 12 started from the commercially available 2-ethyl-2- methylsuccinic acid (31), which was stirred in trifluoroacetic anhydride (TFAA) to form 2-ethyl-2-methylsuccinic anhydride (32). The reaction of 32 with 1 furnished 3-O-(3′-ethyl-3′-methylsuccinyl) betulinic acid (12) in 55% yield (Scheme 5).
Scheme 5.
Synthesis of compound 12.
Results and Discussion
The newly synthesized conformationally restricted 3-O-acyl-BA analogs (4–8) were first tested in acutely HIV-1IIIB infected MT-2 cell lines, and compared to 2 and AZT. The data are listed in Table 1. Only the 2-methylmaleic acid substituted BA derivative (8) showed moderate anti-HIV-1 activity with a TI of 1.4×102 and an EC50 of 0.18 μM. A pendant cyclopentyl or cyclohexyl ring within the C-3 side chain reduced the antiviral potency of the derivatives (4–7) significantly. One possible reason for the reduced or abolished activity of these compounds is that the pendant ring moieties are locked into a conformation that is not the bioactive one. Alternatively, steric hindrance at the 2′-position [as was seen with 3-O-(2′,2′-dimethylsuccinyl) BA, which had an EC50 of only 2.7 μM]9 may be a contributing factor to the loss in potency. This hindrance may impart an unfavorable interaction with either the binding site, resulting in reduced affinity, or the triterpene template, leading to an unfavorable C-3 side chain conformation.
Table 1.
Anti-HIV-1 replication activities for BA and MA derivatives in acutely infected MT-2 cell linesa
| Compd | EC50 (μM) | IC50 (μM) | Therapeutic Index |
|---|---|---|---|
| AZT | 0.034 | 1,870 | 55,000 |
| 2 | 0.0013 | 42.78 | 32,907 |
| 4 | 2.2 | 41.89 | 19 |
| 5 | 17.3 | 40.93 | 2.4 |
| 6 | NS | 41.06 | – |
| 7 | NS | 40.27 | – |
| 8 | 0.18 | 42.51 | 236.2 |
| 9 | 0.12 | 43.83 | 365.3 |
| 10 | 0.0087 | 32.78 | 3,768 |
| 11 | 0.048 | 41.75 | 869.8 |
| 12 | 0.0006 | 36.41 | 60,683 |
| 13 | NS | > 10 | – |
| 14 | NS | > 10 | – |
| 16 | NS | > 10 | – |
| 17 | NS | > 10 | – |
| 18 | NS | > 10 | – |
| 19 | NS | > 10 | – |
| 20 | NS | > 10 | – |
| 21 | NS | > 10 | – |
| 33 (9+10) | 0.016 | 44.93 | 2,808 |
All data presented are averages of at least two separate experiments performed by Panacos Pharmaceutical Inc. EC50: concentration that inhibits HIV-1 replication by 50%. IC50: concentration that inhibits mock-infected cell growth by 50%. TI = IC50/EC50. NS: no suppression at the testing concentration.
In contrast, 3-O-(4′,4′-dimethylglutaryl)-betulinic acid (11) had an antiviral EC50 of 0.048 μM and a TI of 8.7×102. This result confirms that the dimethyl moiety is essential to the anti-HIV-1 potency of 3-O-acyl-BA analogs. Although moving the dimethyl substitution to the 2′-position was highly detrimental, moving the dimethyl group to the 4′-position of the C-3 side chain was well tolerated and significantly increased the antiviral activity, compared with that of the unesterified compound 1. However, the anti- HIV-1 activity of 11 was still 20-fold less than that of 2, indicating that the 3′-position, rather than 4′-position, of the C-3 side chain remains the optimal substitution position. Overall, results of the conformationally restricted compounds (4–8) and 11 stimulated our interest in further study of the importance of the C-3′ dimethyl moiety and the exact contribution of both groups to the anti-HIV-1 potency of 2.
The MSB analogs 9 and 10 were then synthesized and evaluated in parallel with 2 and AZT against viral replication in HIV-1IIIB infected MT-2 cell lines. A 1:1 mixture (33) of 9 and 10 was also tested. The anti-HIV-1 activity data of these derivatives are also listed in Table 1. Among the MSB derivatives, compound 10 with 3′S-methyl showed very potent antiviral activity with a TI of 3.8×103 and EC50 of 0.0087 μM, which is comparable to that of 2 (EC50: 0.0013 μM). Compound 9 with 3′R-methyl exhibited only moderate anti-HIV-1 activity with a TI of 3.7×102 and EC50 of 0.12 μM. The antiviral activity (EC50: 0.016 μM) of the mixture (33) of the two stereoisomers fell in between those of 9 and 10. This result indicates that the two C-3′ methyl groups in the C-3 ester side chain contribute differently to the extremely potent anti-HIV-1 activity of this compound class. We postulate that interaction of the 3′S-methyl group with the viral target might be essential to the anti-HIV-1 activity of 2. The interaction of the 3′R-methyl group within the target is less significant, but still necessary, since 2, with dimethyl substitution at C-3′ position, is slightly more potent than 10.
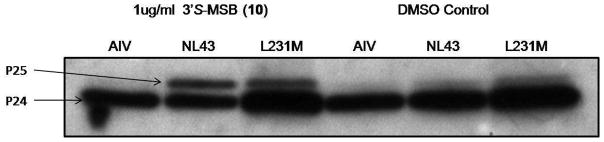
In order to further elucidate the mechanism of action, the production of virus from 10-treated HIV-1 wild-type (NL4-3) or resistant variants transfected Hela cells were subjected to characterization. Radioimmunoprecipitation analyses revealed that, like 2, 10 also functions as a maturation inhibitor. In detail, 10 specifically inhibited the conversion of p25 (CA-SP1) to p24 (CA) in both cell and virion lysates (Figure 3 showing the results from virion lysate), which led to defective Gag processing and production of morphologically abnormal, non-infectious virion particles.
Figure 3. Effect of compound 10 on virus particle production and Gag processing in virion lysate.
Note the accumulation of p25 in the presence of 10. A1V is a CA-SP1 mutant resistant to 2. L231M is a PI-resistant viral mutant.
The anti-HIV replication activity of newly synthesized MA analogs 13–21 were tested against HIV-1IIIB and summarized in Table 1 as well. These compounds have diverse substitutions at the 3β position of analog 30 to explore the conformational space to enhance the antiviral potency. However, none of them showed significant anti-HIV activity. Nevertheless, although the insertion of the 3′,3′-dimethylsuccinyl side chain into the 3β of 30 did not follow the general trend of increasing the activity, the current study indicates that a terminal polar moiety, such as a carboxylic acid group, may still be necessary for the compounds to exert their antiviral replication activity. It also proves that the triterpene skeleton itself plays an important role in the enhanced anti-HIV activity of BA derivatives.
As stated above, our investigation of the C-3′ chiral center of the anti-HIV-1 clinical trials agent 2 showed that the 3′S-methyl group is the major contributor to enhanced anti- HIV-1 activity, while the 3′R-methyl group is less important but still necessary. Accordingly, we postulated that the latter effect may be due to insufficient interaction or a slightly different positioning of the 3′S or 3′R moiety. To confirm our hypothesis, one of the methyl groups was further enlarged to an ethyl group to give the 3′-ethyl-3′-methyl substitution found in 12, which is a mixture of 3′R and 3′S isomers. Compound 12 was evaluated and compared with 2 and AZT in a HIV-1IIIB infected MT-2 cell line. It showed very potent antiviral activity with an EC50 of 0.0006 μM and TI of 6.1×104, which was more active than 33, the mixture of 9 and 10. Indeed, its potency was slightly better than those of 10 and 2.
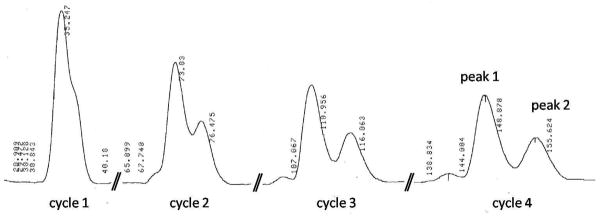
To identify which isomer is the active component, the two C-3′ diastereoisomers (12a and 12b) of 12 were separated by using recycling preparative HPLC (JAI LC-918). After four cycles of separation, the two isomers were obtained successfully (Figure 2). Their purities were ascertained by analytical HPLC. By comparing the 1H-NMR spectra and the optical rotation data of 2, 9, 10, 12a, and 12b, we assigned 12a as the 3′S isomer and 12b as the 3′R isomer. The antiviral activities of 12a and 12b were determined in HIV-1NL4-3 infected MT-4 lymphocytes, where 12a was active and 12b showed no antiviral activity. This result indicates that within the C-3 pharmacophore of BA-derived maturation inhibitors, the 3′S substitution is critical to the anti-HIV activity. Modification on this site may further increase the antiviral potency of the current maturation inhibitors.
Figure 2. Isolation of the two isomers of compound 12 by JAI LC-918 recycling preparative HPLC.
Mobile phase: 85% Acetonitrile in water (0.1% TFA); Flowrate: 4.0 mL/min; Detector: Refractive Index (RI); Column: Alltima 10mm × 250mm C18 5μ. Peak 1 is 12a (3′S isomer); and peak 2 is 12b (3′R isomer).
To conclude our SAR investigation of the C-3 modification on triterpene-derived anti-HIV agents, we found that no conformationally restricted 3-O-acyl BA analog (4–8) showed significant antiviral activity, indicating that C-3′ dimethyl substitution of the succinyl side chain is crucial to the high potency of 2. A C-3 terminal polar moiety, such as carboxylic acid, is also very important to the antiviral activity, as was proven by MA analogs 13–21. Further SAR study of the C-3′ chiral center of the newly designed MSB analogs (9–10) revealed that the 3′S-methyl group of 2 is the major contributor to the compound’s enhanced anti-HIV-1 activity. This result led us to design and synthesize compound 12, which has an enlarged C-3′ substituent (methylethyl instead of dimethyl). As we anticipated, 12 showed extremely potent antiviral activity, and was slightly better than 2. Further investigation confirmed that 12a, the 3′S isomer, is the active compound. This result provides us with better SAR information to further design the next generation of potent BA derived HIV-1 maturation inhibitors.
Experimental Section
Chemistry
The melting points were measured with a Fisher Johns melting apparatus without correction. 1H NMR spectra were measured on a 300MHz Varian Gemini 2000 spectrometer or 500MHz Inova spectrometer using Me4Si (TMS) as internal standard. The solvent used was CDCl3 unless otherwise indicated. Mass spectra were measured on Shimadzu LCMS-2010 and LCMS-IT/TOF (ESI-MS). HPLC for purity determinations were conducted using Shimadzu LCMS-2010 with a Grace Alltima 2.1mm × 150mm HP C18 5μ column or a 2.1mm × 100mm HP C18 3μ column, and a Shimadzu SPD-M20A detector at 205 nm wavelength. Two different solvent systems for HPLC purity analyses were as follows: 1) acetonitrile:water = 80:20, 2) MeOH:water = 90:10. The isocratic HPLC mode was used and the flow rate was 0.3 mL/min. All target compounds were at least 95% pure, as determined by HPLC-UV-MS. Optical rotations were measured with a Jasco Dip-2000 digital polarimeter at 20 °C at the sodium D line. Thin-layer chromatography (TLC) was performed on Merck precoated silica gel 60 F-254 plates. Flash+™ and CombiFlash systems were used as medium pressure column chromatography. All other chemicals were obtained from Aldrich, Inc.
Synthesis of BA derivatives 4 and 5
A solution of 1 (1 eq), EDCI (8 eq), DMAP (2 eq) and the proper cycloalkanedicarboxylic acid (5 eq) in anhydrous CH2Cl2 (8 mL) was stirred at rt overnight until the starting material was not observed by TLC. The solution was diluted with CH2Cl2 (20 mL) and washed three times with brine and distilled water. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
3β-O-[(2′R, 3′R)-3′-Carboxycyclopentanecarbonyl]-betulinic acid (4)
26% yield (starting with 150 mg of 1); white amorphous powder. Mp 228–230 °C. MS (ESI−) m/z: 595.4 (M− − H) for C37H56O6. 1H NMR (300 MHz, CDCl3): δ 4.70, 4.57 (1H each, s, H-29), 4.45 (1H, dd, J = 7.8, 5.7 Hz, H-3), 3.20 (1H, m, H-19), 3.01, 2.88 (1H each, m, H-3′, H-2′), 1.85–2.06 (6H, m, 3 × CH2, H-4′, 5′, 6′), 1.65 (3H, s, H-30), 0.94 (6H, s, 2 × CH3), 0.85, 0.81, 0.75 (3H each, s, 3 × CH3). [α]20D −15.29 ° (c = 0.17, MeOH).
3β-O-[(2′R, 3′R)-3′-Carboxycyclohexanecarbonyl]-betulinic acid (5)
35% yield (starting with 150 mg of 1); white amorphous powder. Mp 233–235 °C. MS (ESI−) m/z: 609.4 (M− − H) for C38H58O6. 1H NMR (300 MHz, CDCl3): δ 4.71, 4.59 (1H each, s, H-29), 4.46 (1H, dd, J = 10.2, 5.1 Hz, H-3), 2.98 (1H, m, H-19), 2.89, 2.71 (1H each, m, H-3′, H-2′), 1.84–2.16 (8H, m, 4 × CH2, H-4′, 5′, 6′, 7′), 1.66 (3H, s, H-30), 0.94 (9H, s, 3 × CH3), 0.81, 0.79 (3H each, s, 2 × CH3). [α]20D −27.00 ° (c = 0.20, MeOH).
Synthesis of BA derivatives 6–8 and 11
A solution of 1 (1 eq), DMAP (2 eq) and the proper acid anhydride (5 eq) in anhydrous pyridine (1.5 mL) was stirred at 160 °C for 2 h using microwave. The reaction mixture was diluted with EtOAc (20 mL) and washed three times with 20% HCl solution and distilled water. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
3β-O-(3′-Carboxycyclohex-5′-enecarbonyl)-betulinic acid (6)
40% yield (starting with 100 mg of 1); white amorphous powder. Mp 178–180 °C. MS (ESI−) m/z: 607.4 (M− − H) for C38H56O6. 1H NMR (300 MHz, CDCl3): δ 5.70 (2H, m, H-5′, 6′), 4.72, 4.60 (1H each, s, H-29), 4.48 (1H, m, H-3), 3.02 (1H, m, H-19), 2.83, 2.72 (1H each, m, H-3′, H-2′), 2.32–2.27 (4H, m, 2 × CH2, H-4′, 7′), 1.68 (3H, s, H-30), 1.01, 0.97 (3H each, s, 2 × CH3), 0.89, 0.83, 0.81 (3H each, s, 3 × CH3). [α]20D +14.17 ° (c = 0.18, MeOH).
3β-O-[3′-Carboxybicyclo[2.2.1]hept-5′-enecarbonyl]-betulinic acid (7)
35% yield (starting with 100 mg of 1); white amorphous powder. Mp 165–167 °C. MS (ESI−) m/z: 619.4 (M− − H) for C39H56O6. 1H NMR (300 MHz, CDCl3): δ 5.73 (2H, m, H-5′, 6′) 4.71, 4.59 (1H each, s, H-29), 4.45 (1H, m, H-3), 3.01 (1H, m, H-19), 2.95, 2.76 (1H each, m, H-3′, H-2′), 2.52-2.39 (4H, m, 2 × CH2, H-4′, 7′), 1.67 (3H, s, H-30), 0.96 (6H, s, 2 × CH3), 0.91, 0.85, 0.82 (3H each, s, 3 × CH3). [α]20D +24.00 ° (c = 0.10, MeOH).
3β-O-[(Z)-3′-Carboxybut-2-enoyl]-betulinic acid (8)
55% yield (starting with 150 mg of 1); red amorphous powder. Mp 169–171 °C. MS (ESI−) m/z: 567.4 (M− − H) for C35H52O6. 1H NMR (300 MHz, CDCl3): δ 5.89 (1H, s, H-2′), 4.70, 4.58 (1H each, s, H-29), 4.46 (1H, m, H-3), 2.96 (1H, m, H-19), 1.93 (3H, s, CH3-3′), 1.66 (3H, s, H-30), 0.94, 0.93, 0.90 (3H each, s, 3 × CH3), 0.80, 0.79 (3H each, s, 2 × CH3). [α]20D +9.41 ° (c = 0.17, MeOH).
3β-O-(4′,4′-Dimethylglutaryl)-betulinic acid (11)
53% yield (starting with 100 mg of 1); white amorphous powder. Mp 228–230 °C. MS (ESI−) m/z: 597.4 (M− − H) for C37H58O6. 1H NMR (300 MHz, CDCl3): δ 4.73, 4.61 (1H each, s, H-29), 4.45 (1H, dd, J = 9.0, 5.2 Hz, H-3), 3.00 (1H, m, H-19), 2.32 (2H, dd, J = 6.6, 5.4 Hz, H-2′), 2.16 (1H, m, H-13), 1.69 (3H, s, H-30), 1.22, 1.21 (3H each, d, J = 6 Hz, 2 × CH3-4′), 0.97, 0.91 (3H each, s, CH3-23, 24), 0.86, 0.85, 0.83 (3H each, s, CH3-25, 26, 27). [α]20D −0.77 ° (c = 0.13, MeOH).
Synthesis of 23a and 23b
A solution of 22a or 22b (1 eq), imidazole (1.1 eq) and TBDPSCl (1.1 eq) in dry DMF was stirred at rt, until the starting material was not observed by TLC. The reaction mixture was diluted with EtOAc and washed with 20% HCl solution and distilled water. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
(3R-Bromo-2-methylpropoxy)(tert-butyl)diphenylsilane (23a)
2.03 g (100%) yielded from 22a; colorless oil. MS (ESI+) m/z: 391.2 (M+ + H), 413.2 (M+ + Na) for C20H27BrOSi.
(3S-Bromo-2-methylpropoxy)(tert-butyl)diphenylsilane (23b)
1.44 g (100%) yielded from 22b; colorless oil. MS (ESI+) m/z: 391.1 (M+ + H), 413.1 (M+ + Na) for C20H27BrOSi.
Synthesis of 24a and 24b
To a solution of 23a or 23b (1 eq) in dry DMSO (10 mL) was added sodium cyanide (3 eq). The mixture was stirred at 120 °C for 2 h until the starting material was not observed. After cooling to rt, the reaction was diluted with diethyl ether (30 mL) and washed with distilled water. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
4-(tert-Butyldiphenylsilyloxy)-3R-methylbutanenitrile (24a)
1.70g (93%) yield starting with 23a; colorless oil. MS (ESI+) m/z: 338.2 (M+ + H) for C21H27NOSi.
4-(tert-Butyldiphenylsilyloxy)-3S-methylbutanenitrile (24b)
1.163 g (93%) yield starting with 23b; colorless oil. MS (ESI+) m/z: 338.2 (M+ + H) for C21H27NOSi.
Synthesis of 25a and 25b
To a solution of 24a or 24b (1 eq) in anhydrous toluene (10 mL) was added diisobutylaluminium hydride (DIBALH, 1.1 eq) dropwise at 0 °C under argon. After stirring for 30 min, ice water was added slowly followed by 10% HCl and aqueous saturated potassium tartrate. Stirring was continued for 15 min at 0 °C and then the aqueous layer was extracted three times with CH2Cl2. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
4-(tert-Butyldiphenylsilyloxy)-3R-methylbutanal (25a)
1.02 g (95%) yield starting with 24a; colorless oil. MS (ESI+) m/z: 341.2 (M+ + H) for C21H28O2Si.
4-(tert-Butyldiphenylsilyloxy)-3S-methylbutanal (25b)
1.047 g (90%) yield starting with 24a; colorless oil. MS (ESI+) m/z: 341.2 (M+ + H) for C21H28O2Si.
Synthesis of 26a and 26b
To a solution of 25a or 25b (1 eq) in dry DMSO (1.0 mL) was added NaH2PO4·H2O (0.8 eq in 2.0 mL H2O) and 80% NaClO2 (1.5 eq in 2.0 mL H2O) dropwise over 5 min. The mixture was stirred overnight. The reaction was quenched with saturated NH4Cl solution and extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
4-(tert-Butyldiphenylsilyloxy)-3R-methylbutanoic acid (26a)
700 mg (100%) yield starting with 25a; colorless oil. MS (ESI−) m/z: 355.2 (M− − H) for C21H28O3Si. 1H NMR (300 MHz, CDCl3): δ 7.68-7.62 (4H, m, H ar-2′), 7.45-7.33 (6H, m, H ar-3′, 4′), 3.59, 3.43 (2H, dd, J = 7.6, 1.5 Hz, H-4), 2.61-2.30 (3H, m, H-2, H-3), 1.05 (9H, s, SiC(CH3)3), 0.95 (3H, d, J = 10 Hz, H-5).
4-(tert-Butyldiphenylsilyloxy)-3S-methylbutanoic acid (26b)
830 mg (76%) yield starting with 25b; colorless oil. MS (ESI−) m/z: 355.2 (M− − H) for C21H28O3Si. 1H NMR (300 MHz, CDCl3): δ 7.68-7.62 (4H, m, H ar-2′), 7.45-7.33 (6H, m, H ar-3′, 4′), 3.58, 3.42 (2H, dd, J = 7.6, 1.5 Hz, H-4), 2.60-2.30 (3H, m, H-2, H-3), 1.05 (9H, s, SiC(CH3)3), 0.95 (3H, d, J = 10 Hz, H-5).
Synthesis of 27a and 27b
A solution of 1 (1 eq), EDCI (8 eq), DMAP (2 eq) and 26a or 26b (5 eq) in anhydrous CH2Cl2 (5 mL) was stirred at rt overnight, until the starting material was not observed by TLC. The solution was diluted with CH2Cl2 (15 mL) and washed three times with brine and distilled water. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
3β-O-[4′-(tert-Butyldiphenylsilyloxy)-3′R-methylbutanoyl]-betulinic acid (27a)
32% yield starting with 340 mg BA, white powder. Mp 179–181 °C. MS (ESI−) m/z: 793.5 (M− − H) for C51H74O5Si. 1H NMR (300 MHz, CDCl3): δ 7.68-7.62 (4H, m, H ar-2′), 7.45-7.33 (6H, m, H ar-3′, 4′), 4.72, 4.60 (1H each, s, H-29), 4.45 (1H, m, H-3), 3.58, 3.42 (2H, m, H-4′), 2.87–2.95 (2H, m, H-19, H-3′), 2.64-2.42 (2H, m, H-2′), 1.69 (3H, s, H-30), 1.28, 1.26 (3H, d, J = 6 Hz, CH3-3′), 1.01 (9H, s, SiC(CH3)3), 0.96 (6H, s, 2 × CH3), 0.89, 0.85, 0.82 (3H each, s, 3 × CH3).
3β-O-[4′-(tert-Butyldiphenylsilyloxy)-3′S-methylbutanoyl]-betulinic acid (27b)
48% yield starting with 170 mg BA, white powder. Mp 186-187 °C. MS (ESI−) m/z: 793.5 (M− − H) for C51H74O5Si. 1H NMR (300 MHz, CDCl3): δ 7.68-7.62 (4H, m, H ar-2′), 7.45-7.33 (6H, m, H ar-3′, 4′), 4.73, 4.60 (1H each, s, H-29), 4.51 (1H, dd, J = 11.1, 4.8 Hz, H-3), 3.60-3.42 (2H, m, H-4′), 2.96–3.03 (1H, m, H-19), 2.86–2.93 (1H, m, H-3′), 2.73, 2.69 (1H each, dd, J = 11.2, 5.7, 4.8 Hz, H-2′), 1.69 (3H, s, H-30), 1.27, 1.25 (3H, d, J = 6 Hz, CH3-3′), 1.02 (9H, s, SiC(CH3)3), 0.97, 0.94, 0.86, 0.85, 0.81 (3H each, s, CH3- 23, 24, 25, 26, 27).
Synthesis of 28 and 29
A solution of 27a or 27b (1 eq) and TBAF (1.0 M in THF, 3 eq) in anhydrous THF was stirred at 0 °C for 1.5 h. The mixture was then allowed to warm to rt and stirred until there was no starting material detected by TLC. The reaction was diluted with 15 mL of CH2Cl2 and washed with saturated NH4Cl solution and brine. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
3β-O-(4′-Hydroxy-3′R-methylbutanoyl)-betulinic acid (28)
100% yield from 27a, white powder. Mp 201–203 °C. MS (ESI−) m/z: 555.4 (M− − H) for C35H56O5. 1H NMR (300 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.54 (1H, dd, J = 9.9, 5.7 Hz, H-3), 3.55, 3.42 (2H, m, H-4′), 3.01 (1H, m, H-19), 2.87–2.95 (1H, m, H-3′), 2.70-2.42 (2H, m, H-2′), 1.68 (3H, s, H-30), 1.27, 1.25 (3H, d, J = 6 Hz, CH3-3′), 0.93 (6H, s, 2 × CH3), 0.85, 0.84, 0.81 (3H each, s, 3 × CH3). [α]20D +15.50 ° (c = 0.12, MeOH).
3β-O-(4′-Hydroxy-3′S-methylbutanoyl)-betulinic acid (29)
100% yield from 27b, white powder. Mp 229–231 °C. MS (ESI−) m/z: 555.4 (M− − H) for C35H56O5. 1H NMR (300 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.51 (1H, dd, J = 12.5, 5.6 Hz, H-3), 3.58, 3.43 (2H, m, H-4′), 2.97–3.01 (1H, m, H-19), 2.83–2.92 (1H, m, H-3′), 2.70, 2.62 (1H each, dd, J = 16.2, 6.3, 4.8 Hz, H-2′), 1.69 (3H, s, H-30), 1.27, 1.25 (3H, d, J = 6 Hz, CH3-3′), 1.01, 0.96, 0.86, 0.85, 0.81 (3H each, s, CH3-23, 24, 25, 26, 27). [α]20D −16.10 ° (c = 0.11, MeOH).
Synthesis of 9 and 10
To a solution of 28 or 29 (1 eq) in CH2Cl2 (5 mL) was added TEMPO (0.1 eq), and PhI(OAc)2 (1.5 eq). The mixture was stirred at rt until the starting material was not observed by TLC. The reaction was filtered through thin silica gel pad and eluted with CH2Cl2 and washed with saturated NH4Cl solution and brine. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
3β-O-(3′R-Methylsuccinyl)-betulinic acid (9)
75% yield from 28, white amorphous powder. Mp 268–271 °C. MS (ESI−) m/z: 569.38 (M− − H) for C35H54O6. 1H NMR (300 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.54 (1H, dd, J = 9.9, 5.7 Hz, H-3), 3.01 (1H, m, H-19), 2.87-2.95 (1H, m, H-3′), 2.84, 2.42 (1H each, dd, J = 15.9, 8.3, 5.6, H-2′), 2.10–2.20 (1H, m, H-13), 1.69 (3H, s, H-30), 1.28, 1.26 (3H, d, J = 6 Hz, CH3- 3′), 0.97 (6H, s, 2 × CH3), 0.86, 0.83, 0.80 (3H each, s, 3 × CH3). [α]20D +13.00 ° (c = 0.05, MeOH).
3β-O-(3′S-Methylsuccinyl)-betulinic acid (10)
88% yield from 29, white amorphous powder. Mp 279–281 °C. MS (ESI−) m/z: 569.38 (M− − H) for C35H54O6. 1H NMR (300 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.51 (1H, dd, J = 11.1, 4.8 Hz, H-3), 2.96–3.03 (1H, m, H-19), 2.86–2.93 (1H, m, H-3′), 2.70, 2.61 (1H each, dd, J = 16.2, 6.3, 4.8 Hz, H-2′), 2.06–2.16 (1H, m, H-13), 1.69 (3H, s, H-30), 1.27, 1.25 (3H, d, J = 6 Hz, CH3-3′), 0.97, 0.94, 0.86, 0.85, 0.81 (3H each, s, CH3-23, 24, 25, 26, 27). [α]20D − 12.88 ° (c = 0.08, MeOH).
Synthesis of 12
2-Ethyl-2-methylsuccinic acid (50 mg, 4 eq) was stirred in TFAA at rt for 3 h until the reaction mixture become homogenous. The solution was then concentrated to dryness under reduced pressure to yield 2-ethyl-2-methylsuccinic anhydride (32). Compound 32 was reacted without further purification with 1 (36 mg, 1 eq) and DMAP (19 mg, 2 eq) in anhydrous pyridine (1.5 mL), stirring at 160 °C for 2 h in microwave. The reaction mixture was diluted with EtOAc (10 mL) and washed three times with 20% HCl solution and distilled water. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield 30 mg (55%) of 12; white amorphous powder.
3β-O-(3′S-Ethylmethylsuccinyl)-betulinic acid (12a)
Mp 186–187 °C. MS (ESI−) m/z: 597.4 (M− − H) for C37H58O6. 1H NMR (500 MHz, CDCl3): δ 4.71, 4.59 (1H each, s, H-29), 4.48 (1H, m, H-3), 3.00 (1H, m, H-19), 2.75, 2.52 (1H each, d, J = 15.5, H-2′), 1.66 (3H, s, H-30), 1.34-1.32 (2H, m, CH2-3′), 1.23 (3H, s, CH3-3′), 1.18 (2H, m, CH3-3″), 0.94 (6H, s, 2 × CH3), 0.85, 0.84, 0.78 (3H each, s, 3 × CH3). [α]20D −9.00 ° (c = 0.10, MeOH).
3β-O-(3′R-Ethylmethylsuccinyl)-betulinic acid (12b)
Mp 179–181 °C. MS (ESI−) m/z: 597.4 (M− − H) for C37H58O6. 1H NMR (500 MHz, CDCl3): δ 4.71, 4.58 (1H each, s, H-29), 4.51 (1H, dd, J = 10.2, 6.4 Hz, H-3), 2.99 (1H, m, H-19), 2.98, 2.26 (1H each, d, J = 15.5, H-2′), 1.67 (3H, s, H-30), 1.35 (2H, m, CH2-3′), 1.25 (3H, s, CH3-3′), 1.19 (3H, m, CH3-3″), 0.98, 0.95 (3H each, s, 2 × CH3), 0.86, 0.82, 0.79 (3H each, s, 3 × CH3). [α]20D +9.57 ° (c = 0.07, MeOH).
Synthesis of 30
To a solution of 3 (1 g, 1eq) in anhydrous THF was added sodium borohydride (209 mg, 2.5 eq). The reaction was neutralized with 10% HCl after stirring in rt for 4 h, and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield 905 mg (90%) of 30; white amorphous powder. Mp 197–199 °C. MS (ESI−) m/z: 455.5 (M− − H) for C30H47O3. 1H NMR (300 MHz, CDCl3): δ 5.15 (1H, s, H-19), 3.19 (1H, dd, J = 10.8, 5.6 Hz, H-3), 0.97, 0.96 (3H each, s, H-29, H-30), 0.95 (6H, s, H-24, H-26), 0.84 (3H, s, H-25), 0.75, 0.74 (3H each, s, H-23, H-27).
Synthesis of MA derivatives 13–21
To a solution of 30 in dry CH2Cl2 were added triethylamine (50 μL) and corresponding acyl chloride (50 μL). The reaction was stirred at rt for 30 min. The mixture was diluted with CH2Cl2 and washed with 10% HCl. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds.
3β-O-(2′-Methylbutyryl)-moronic acid (13)
73% yield (starting with 20 mg of 1); white amorphous powder. Mp 237–239 °C. MS (ESI−) m/z: 539.4 (M− − H) for C35H56O4. 1H NMR (300 MHz, CDCl3): δ 5.17 (1H, s, H-19), 4.47 (1H, dd, J = 10.0, 5.6 Hz, H-3), 2.35 (1H, dd, J = 10.0, 6.0 Hz, H-2′), 1.96 (2H, m, H-3′), 1.17, 1.15 (3H, d, J = 6 Hz, CH3-2′), 0.98, 0.97 (3H each, s, H-29, H-30), 0.91 (3H, m, H-4′), 0.88, 0.84 (6H), 0.82 (3H each, s, H-26, H-25, H-24, H-23), 0.77 (3H, s, H-27).
3β-O-Propionyl-moronic acid (14)
57% yield (starting with 11.2 mg of 1); white amorphous powder. Mp 210–211 °C. MS (ESI−) m/z: 511.5 (M− − H) for C33H52O4. 1H NMR (300 MHz, CDCl3): δ 5.175 (1H, s, H-19), 4.48 (1H, dd, J = 9.9, 6.6 Hz, H-3), 2.32 (2H, q, J = 7.2 Hz, H-2′), 1.14 (3H, t, J = 7.8 Hz, H-3′), 0.99, 0.98 (3H each, s, H-29, H-30), 0.88 (6H), 0.84 (6H), 0.77, (3H each, s, H-26, H-25, H-24, H-23, H-27).
3β-O-Cyclobutancarbnyl-moronic acid (15)
58% yield (starting with 12.2 mg of 1); white amorphous powder. Mp 231–233 °C. MS (ESI−) m/z: 537.4 (M− − H) for C35H54O4. 1H NMR (300 MHz, CDCl3): δ 5.17 (1H, s, H-19), 4.48 (1H, dd, J = 11.7, 4.8 Hz, H-3), 3.12 (1H, quintet, J = 8.4 Hz, H-2′), 2.34-2.13, 2.04-1.86 (4H, m, H-3′, H-5′),1.62-1.27 (2H, m, H-4′), 0.99, 0.98, 0.97, 0.88, 0.84 (6H), 0.77 (3H each, s, H-29, H-30, H-25, H-26, H24, H- 23, H-27).
3β-O-Valeroryl-moronic acid (16)
84% yield (starting with 13.5 mg of 1); white amorphous powder. Mp 197–199 °C. MS (ESI−) m/z: 539.4 (M− − H) for C35H56O4. 1H NMR (300 MHz, CDCl3): δ 5.17 (1H, s, H-19), 4.48 (1H, dd, J = 10.6, 6.0 Hz, H-3), 2.30 (2H, t, J = 6.0 Hz, H-2′), 1.76 (2H, m, H-3′), 0.99, 0.98, 0.97, 0.88, 0.84 (6H), 0.77, (3H each, s, H-29, H-30, H-26, H-25, H-24, H-23, H-27).
3β-O-Isovaleroryl-moronic acid (17)
54% yield (starting with 12.2 mg of 1); white amorphous powder. Mp 134–136 °C. MS (ESI−) m/z: 539.4 (M− − H) for C35H56O4. 1H NMR (300 MHz, CDCl3): δ 5.17 (1H, s, H-19), 4.48 (1H, dd, J = 11.6, 5.6 Hz, H-3), 2.17 (2H, d, J = 10.0, H-2′), 1.63 (1H, m, H-3′), 0.99 (6H), 0.97 (6H), 0.95, 0.88, 0.84, 0.835, 0.77, (3H each, s, 2 × H-4′, H-29, H-30, H-26, H-25, H-24, H-23, H-27).
3β-O-Cyclopetanecarbonyl-moronic acid (18)
75% yield (starting with 20 mg of 1); white amorphous powder. Mp 209–210 °C. MS (ESI−) m/z: 551.4 (M− − H) for C36H56O4. 1H NMR (300 MHz, CDCl3): δ 5.17 (1H, s, H-19), 4.48 (1H, dd, J = 10.6, 5.6 Hz, H-3), 3.13 (1H, quintet, J = 6.0 Hz, H-2′), 2.36-2.13, 2.06-1.89 (4H, m, H-3′, H-6′),1.78-1.19 (4H, m, H-4′, H-5′), 1.00, 0.98, 0.97, 0.88, 0.84 (6H), 0.77 (3H each, s, H-29, H-30, H-25, H-26, H24, H-23, H-27).
3β-O-Butyryl-moronic acid (19)
71% yield (starting with 20 mg of 1); white amorphous powder. Mp 242–244 °C. MS (ESI−) m/z: 525.5 (M− − H) for C34H54O4. 1H NMR (300 MHz, CDCl3): δ 5.18 (1H, s, H-19), 4.48 (1H, dd, J = 10.0, 6.0 Hz, H-3), 2.28 (2H, t, J = 6.0 Hz, H-2′), 1.76 (2H, m, H-3′), 1.00, 0.98 (3H each, s, H-29, H-30), 0.97 (3H, t, J = 6.0 Hz, H-4′), 0.92, 0.88, 0.84 (6H), 0.77, (3H each, s, H-26, H-25, H-24, H-23, H-27).
3β-O-Isobutyryl-moronic acid (20)
66% yield (starting with 20 mg of 1); white amorphous powder. Mp 206–207 °C. MS (ESI−) m/z: 525.4 (M− − H) for C34H54O4. 1H NMR (300 MHz, CDCl3): δ 5.18 (1H, s, H-19), 4.46 (1H, dd, J = 10.0, 6.0 Hz, H-3), 2.54 (1H, septet, J = 6.0 Hz, H-2′), 1.19, 1.17, 1.16, 1.15 (6H, d, J = 3.0 Hz, H-3′, CH3-2′), 0.98 (9H), 0.88, 0.85, 0.84, 0.77 (3H each, s, H-29, H-30, H-25, H-26, H24, H-23, H-27).
3β-O-Trimethylacetyl-moronic acid (21)
34% yield (starting with 25 mg of 1); white amorphous powder. Mp 103–105 °C. MS (ESI−) m/z: 539.5 (M− − H) for C35H56O4. 1H NMR (300 MHz, CDCl3): δ 5.17 (1H, s, H-19), 4.46 (1H, dd, J = 10.0, 5.4 Hz, H-3), 1.15 (9H, s, 3 × CH3-2′), 0.98, 0.97, 0.96, 0.88, 0.84 (6H), 0.77 (3H each, s, H-29, H-30, H-25, H-26, H24, H-23, H-27).
HIV-1IIIB Replication Inhibition Assay in MT-2 Cell Lines.17, 20, 21
The evaluation of HIV-1 inhibition was carried out as follows using MT-2 lymphocytes. Test samples were first dissolved in dimethyl sulfoxide (DMSO). The following drug concentrations were routinely used for screening: 100, 20, 4 and 0.8 μg/mL. For agents found to be active, additional dilutions were prepared for subsequent testing so that an accurate EC50 value could be determined. Test samples were prepared, and to each sample well, was added 90 μl of media containing MT-2 cells at 3×105 cells/mL and 45 μL of virus inoculum (HIV-1 IIIB isolate) containing 125 TCID50. Control wells containing virus and cells only (no drug) and cells only (no virus or drug) were also prepared. A second identical set of samples were added to cells under the same conditions without virus (mock infection) for toxicity determinations (IC50 defined below). In addition, AZT and 2 were also assayed during each experiment as positive drug controls. On day 1 PI (post-infection), 140 μL fresh cell specific media was added to each cell. On day 4 PI, the assay was terminated and culture supernatants were harvested for p24 antigen ELISA analysis. The compound toxicity was determined by XTT using the mock-infected sample wells. If a test sample inhibited virus replication and was not toxic, its effects were reported in the following terms: EC50, the concentration of the test sample that was able to suppress HIV replication by 50%; IC50, the concentration of test sample that was toxic to 50% of the mock-infected cells; and therapeutic index (TI), the ratio of the IC50 to EC50.
HIV-1 Maturation Inhibition Assay.10
Hela cells were transfected with pNL4-3 or mutated HIV-1 virus (A1V or L231M) and cultured in the absence or presence of 10. Two days post-transfection, cells were metabolically labeled for 2h with 35S-Met/Cys. Cell lysates were prepared, and virions were pelleted by ultracentrifugation. Cell and viral lysates were immunoprecipitated with HIV-Ig. Western blotting was then performed,22, 23 and p25 and p24 are indicated.
HIV-1NL4-3 Replication Inhibition Assay in MT-4 Lymphocytes.21
A previously described HIV-1 infectivity assay was used. A 96-well microtiter plate was used to set up the HIV-1NL4-3 replication screening assay. NL4-3 variants at a multiplicity of infection (MOI) of 0.01 were used to infect MT4 cells. Culture supernatants were collected on day 4 PI for the p24 antigen capture using an ELISA kit from ZeptoMetrix Corporation (Buffalo, New York).
Supplementary Material
Acknowledgments
We thank Panacos Pharmaceutical Inc. for the anti-HIV replication assay in MT-2 lymphocytes. This investigation was supported by Grant AI-077417 from the National Institute of Allergy and Infectious Diseases (NIAID) awarded to K.H.L.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- HIV-1
human immunodeficiency virus type 1
- BA
betulinic acid
- MA
moronic acid
- HAART
highly active antiretroviral therapy
- NRTI
nucleoside/nucleotide reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- MI
maturation inhibitor
- P24 (CA)
capsid
- P25 (CA-SP1)
capsid precursor
- Bevirimat
3-O-(3′,3′-dimethylsuccinyl)-betulinic acid
- MSB
3-O-monomethylsuccinyl betulinic acid
- EDCI
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride
- DMAP
4-(dimethylamino)pyridine
- TBDPS
tert-butyldimethylsilyl
- TBAF
tetra-n-butylammonium fluoride
- THF
tetrahydrofuran
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxy
- PhI(OAc)2
(diacetoxyiodo) benzene
- NaBH4
Sodium borohydride
- Et3N
triethylamine
- TFAA
trifluoroacetic anhydride
- AZT
zidovudine
- PI
post-infection
Footnotes
For part 80, see the following. Lee, K. H. “Discovery and Development of Natural Product-derived Chemotherapeutic Agents Based on a Medicinal Chemistry Approach”, J. Nat. Prod., in press.
Supporting Information Available: Additional information on compound purity, high-resolution mass spectral data, and HPLC analysis results of the target compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.UNAIDS. AIDS epidemic update. Nov, 2009. [Google Scholar]
- 2.Kawalekar OU, Shedlock DJ, Weiner DB. Current strategies and limitations of HIV vaccines. Curr Opin Investig Drugs. 2010;11:192–202. [PubMed] [Google Scholar]
- 3.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 4.Hammer SM, Katzenstein DA, Hughes MD, Gundacker H, Schooley RT, Haubrich RH, Henry WK, Lederman MM, Phair JP, Niu M, Hirsch MS, Merigan TC. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N Engl J Med. 1996;335:1081–1090. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 5.Piscitelli SC, Flexner C, Minor JR, Polis MA, Masur H. Drug interactions in patients infected with human immunodeficiency virus. Clin Infect Dis. 1996;23:685–693. doi: 10.1093/clinids/23.4.685. [DOI] [PubMed] [Google Scholar]
- 6.Boden D, Hurley A, Zhang L, Cao Y, Guo Y, Jones E, Tsay J, Ip J, Farthing C, Limoli K, Parkin N, Markowitz M. HIV-1 drug resistance in newly infected individuals. Jama. 1999;282:1135–1141. doi: 10.1001/jama.282.12.1135. [DOI] [PubMed] [Google Scholar]
- 7.Pereira CF, Paridaen JT. Anti-HIV drug development--an overview. Curr Pharm Des. 2004;10:4005–4037. doi: 10.2174/1381612043382459. [DOI] [PubMed] [Google Scholar]
- 8.Wegner SA, Brodine SK, Mascola JR, Tasker SA, Shaffer RA, Starkey MJ, Barile A, Martin GJ, Aronson N, Emmons WW, Stephan K, Bloor S, Vingerhoets J, Hertogs K, Larder B. Prevalence of genotypic and phenotypic resistance to anti-retroviral drugs in a cohort of therapy-naive HIV-1 infected US military personnel. AIDS. 2000;14:1009–1015. doi: 10.1097/00002030-200005260-00013. [DOI] [PubMed] [Google Scholar]
- 9.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem. 1996;39:1016–1017. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Goila-Gaur R, Salzwedel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Martin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc Natl Acad Sci U S A. 2003;100:13555–13560. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamoto T, Kashiwada Y, Kanbara K, Gotoh K, Yoshimori M, Goto T, Sano K, Nakashima H. Anti-human immunodeficiency virus activity of YK-FH312 (a betulinic acid derivative), a novel compound blocking viral maturation. Antimicrob Agents Chemother. 2001;45:1225–1230. doi: 10.1128/AAC.45.4.1225-1230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith PF, Ogundele A, Forrest A, Wilton J, Salzwedel K, Doto J, Allaway GP, Martin DE. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-o-(3′,3′-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob Agents Chemother. 2007;51:3574–3581. doi: 10.1128/AAC.00152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin DE, Blum R, Doto J, Galbraith H, Ballow C. Multiple-dose pharmacokinetics and safety of bevirimat, a novel inhibitor of HIV maturation, in healthy volunteers. Clin Pharmacokinet. 2007;46:589–598. doi: 10.2165/00003088-200746070-00004. [DOI] [PubMed] [Google Scholar]
- 14.Martin DE, Blum R, Wilton J, Doto J, Galbraith H, Burgess GL, Smith PC, Ballow C. Safety and pharmacokinetics of Bevirimat (PA-457), a novel inhibitor of human immunodeficiency virus maturation, in healthy volunteers. Antimicrob Agents Chemother. 2007;51:3063–3066. doi: 10.1128/AAC.01391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu D, Wild CT, Martin DE, Morris-Natschke SL, Chen CH, Allaway GP, Lee KH. The discovery of a class of novel HIV-1 maturation inhibitors and their potential in the therapy of HIV. Expert Opin Investig Drugs. 2005;14:681–693. doi: 10.1517/13543784.14.6.681. [DOI] [PubMed] [Google Scholar]
- 16.Ito J, Chang FR, Wang HK, Park YK, Ikegaki M, Kilgore N, Lee KH. Anti-AIDS agents 48. Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J Nat Prod. 2001;64:1278–1281. doi: 10.1021/np010211x. [DOI] [PubMed] [Google Scholar]
- 17.Qian K, Nakagawa-Goto K, Yu D, Morris-Natschke SL, Nitz TJ, Kilgore N, Allaway GP, Lee KH. Anti-AIDS agents 73: structure-activity relationship study and asymmetric synthesis of 3-O-monomethylsuccinyl-betulinic acid derivatives. Bioorg Med Chem Lett. 2007;17:6553–6557. doi: 10.1016/j.bmcl.2007.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keyling-Bilger F, Schmitt G, Beck A, Luu B. Synthesis of optically active diastereomers of a nonproteic neurotrophic mimetic. Tetrahedron. 1996;52:14891–14904. [Google Scholar]
- 19.Kawasaki M, Shinada T, Hamada M, Ohfune Y. Total synthesis of (−)- kaitocephalin. Org Lett FIELD Full Journal Title:Organic letters. 2005;7:4165–4167. doi: 10.1021/ol0515154. [DOI] [PubMed] [Google Scholar]
- 20.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 21.Qian K, Yu D, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ, Salzwedel K, Reddick M, Allaway GP, Lee KH. Anti-AIDS agents. 78. Design, synthesis, metabolic stability assessment, and antiviral evaluation of novel betulinic acid derivatives as potent anti-human immunodeficiency virus (HIV) agents. J Med Chem. 2009;52:3248–3258. doi: 10.1021/jm900136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demirov DG, Ono A, Orenstein JM, Freed EO. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc Natl Acad Sci U S A. 2002;99:955–960. doi: 10.1073/pnas.032511899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiernan RE, Ono A, Englund G, Freed EO. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J Virol. 1998;72:4116–4126. doi: 10.1128/jvi.72.5.4116-4126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.