Abstract
2-Methylhopanes, molecular fossils of 2-methylbacteriohopanepolyol (2-MeBHP) lipids, have been proposed as biomarkers for cyanobacteria, and by extension, oxygenic photosynthesis. However, the robustness of this interpretation is unclear, as 2-methylhopanoids occur in organisms besides cyanobacteria and their physiological functions are unknown. As a first step towards understanding the role of 2-MeBHP in cyanobacteria, we examined the expression and intercellular localization of hopanoids in the three cell types of Nostoc punctiforme: vegetative cells, akinetes, and heterocysts. Cultures in which N. punctiforme had differentiated into akinetes contained approximately 10-fold higher concentrations of 2-methylhopanoids than did cultures that contained only vegetative cells. In contrast, 2-methylhopanoids were only present at very low concentrations in heterocysts. Hopanoid production initially increased 3-fold in cells starved of nitrogen but returned to levels consistent with vegetative cells within two weeks. Vegetative and akinete cell types were separated into cytoplasmic, thylakoid, and outer membrane fractions; the increase in hopanoid expression observed in akinetes was due to a 34-fold enrichment of hopanoid content in their outer membrane relative to vegetative cells. Akinetes formed in response either to low light or phosphorus limitation, exhibited the same 2-methylhopanoid localization and concentration, demonstrating that 2-methylhopanoids are associated with the akinete cell type per se. Because akinetes are resting cells that are not photosynthetically active, 2-methylhopanoids cannot be functionally linked to oxygenic photosyntheis in N. punctiforme.
INTRODUCTION
Hopanoids are bacterial products that occur pervasively in the geologic record (Ourisson & Albrecht, 1992). One particular structural type, the 2-methylhopane hydrocarbons have been interpreted as indicators of cyanobacteria in paleoenvironments, and by extension, oxygenic photosynthesis (Summons et al., 1999). A recent report that bacteria growing under anaerobic conditions can also produce these hopanoids has called into question the hypothesis that 2-methylhopanoids are biomarkers for the latter (Rashby et al., 2007, Talbot et al., 2007). Nevertheless, since hopanoid hydrocarbons retain significant potential to contribute to our understanding of Earth’s previous environments and the organisms that were present, a much more extensive understanding of their physiological role(s) in modern organisms is needed. Hopanoid lipids have a pentacyclic triterpenoid core that may be modified by the addition of methyl groups or unsaturation, together with a C5 ribose-derived side chain with diverse structural modifications (Fleshe & Rohmer, 1988; Neunlist &Rohmer 1985; Talbot et al., 2008; Rhomer, 1993). The fossilized derivatives comprise suites of C27–C36 hydrocarbons that preserve the methylation patterns of the core but not the structural diversity of the side-chain.
Prior studies have attempted to link hopanoid production to membrane function and the physiological status of bacteria (Kannenberg & Poralla, 1999; Joyeux et al., 2004; Jahnke et al., 1992; Jahnke et al., 1999; Ourisson et al., 1987; Poralla et al., 2000; Simonin et al., 1996). In this report, we describe our studies of the physiological role of hopanoids using the cyanobacterium Nostoc punctiforme as a model organism. N. punctiforme is a filamentous cyanobacterium that has served as a model of bacterial cellular differentiation (Meeks et al., 2002). Under conditions of low nitrogen (N) availability, vegetative cells may undergo differentiation into heterocysts which express nitrogenase that supplies adjacent vegetative cells with fixed N. Alternatively, when cells are exposed to insufficient light to support photoautotrophy or, when phosphorus, necessary for the synthesis of ATP becomes limited, vegetative cells may undergo differentiation into akinetes (Meeks et al., 2002; Argueta & Summers 2005). Akinetes are survival structures that protect the cell from cold and desiccation (Meeks et al., 2002). Cyanobacterial heterocysts and akinetes have been reported, as fossils, in rocks as old as 2100 million years (Tomitani et al., 2006).
Because our preliminary data indicated N. punctiforme produces hopanoid lipids as membrane components, and changes in membrane architecture accompany its cellular differentiation, we focused this study on the subcellular localization of hopanoids in N. punctiforme. Although hopanoid localization has been addressed in a variety of bacteria, including the cyanobacterium Synechocystis PCC6714 (Jürgens et al., 1992), we reasoned that hopanoid localization was worth revisiting in the context of both a 2-methylhopanoid producing organism and cellular differentiation. Knowing where hopanoids localize within the cell provides a foundation upon which to generate hypotheses regarding their biological function.
METHODS
Growth conditions and microscopic analysis of differentiated cells of N. punctiforme
Vegetative cells of N. punctiforme were grown in Allen and Arnon Medium at 25°C under illumination from a cool fluorescent light (7 µM photons × m−2× sec−1), as described previously (Meeks et al., 2002) except cultures were continuously bubbled with air supplied at a rate of 100 cm3 × min−1 rather than shaken. Cultures were harvested by centrifugation (1,000 × g for 20 min) from log phase when chlorophyll a concentration was approximately 1 µM chlorophyll a × ml−1 of culture medium and the presence of vegetative cells was confirmed by light microscopy. For the induction of cell differentiation, vegetative cells were washed three times in buffer consisting of 10 mM NaCl and 5 mM 3-morpholinopropanesulfonic acid (pH 8) and resuspended in fresh medium (vegetative cell control) or medium lacking P or N for the induction of akinetes and heterocysts, respectively. Akinetes differentiation was also initiated by a decrease in the fluorescent light intensity from 7 to 1 µM photons × m−2 × sec−1. Under low light conditions akinetes formed within 5 weeks.
Lipid extractions and analysis
Cultures were harvested by centrifugation at 1,000 × g for 20 min and the cell pellets were freeze dried for later analysis. Total membrane lipids were extracted by the Bligh-Dyer solution as described previously (Bligh & Dyer, 1959; Rashby et al., 2007) with two exceptions. First, all extractions were performed for 24 h rather than 45 min, so as to maximize hopanoid recovery; additional extractions did not yield measurable increases in hopanoid content. Second, dichloromethane and aqueous phase separation of the mixture was facilitated by the addition of 15 ml of a 0.1 M solution of NaCl in water as opposed to water alone. Bacteriohopanepolyols were peracetylated by treatment with a mixture (1:1) of pyridine and acetic anhydride incubated at 65°C for 30 min. Total lipid extracts were dried and resuspended in dichloromethane to a concentration of 10 µg total lipid extract/ml. Cholestanol (100 ng) was added to the freeze dried material prior to lipid extraction to serve as a recovery standard and epiadrosterone (43.6 ng × µl−1) was added to the samples immediately prior to derivatization to serve as an internal standard. Hopanoids were detected and quantified by GC-MS using a HP 890 GC attached to an Agilent 5973 mass selective detector equipped with a Gerstel PTV injector according to the protocol of Welander et al. (2009). Hopanoids were quantified using epiadrosterone as a standard and should be regarded as pseudoquantitative. Several of the hopanoids detected were tentatively identified as desaturated hydrocarbons based upon previously published specra (Summons & Jahnke, 1992) and elution from silica gel columns in the hexane fraction. Because hopanoid hydrocarbons were only present at low concentrations and they are proposed intermediates in the synthesis of hopane polyols (Fliesch & Rohmer, 1988), we choose to focus the remainder of the study on bacteriohopanepolyols (Figure 1). Based upon previously published spectra and elution times, the hopanoid compounds bacteriohopanetetrol (1) and 2-methylbacteriohopanetetrol (2) were identified (Welander et al., 2009). 2-methylbacteriohopanepentol was detected and its structure analyzed by treatment of total lipid extracts with periodic acid as described by (Rashby et al., 2007). The periodic acid cleavage product 2-methyl-31-baceriohopanol was detected confirming the presence of 2-methyl-31,32,33,34,35 bacteriohopanepentol (3). We did not observe the 30, 32 diol predicted to be produced from periodic acid treatment 2-methyl-30,32,33,34,35-bacteriohopanepentol (Zhao et al., 1996). Further analysis of total lipid extract by LC/MS indicated the presence of bacteriohopane cyclitol ether, however, the focus of our study was on 2-methylhopanoids and this hopanoids was not methylated.
Figure 1.
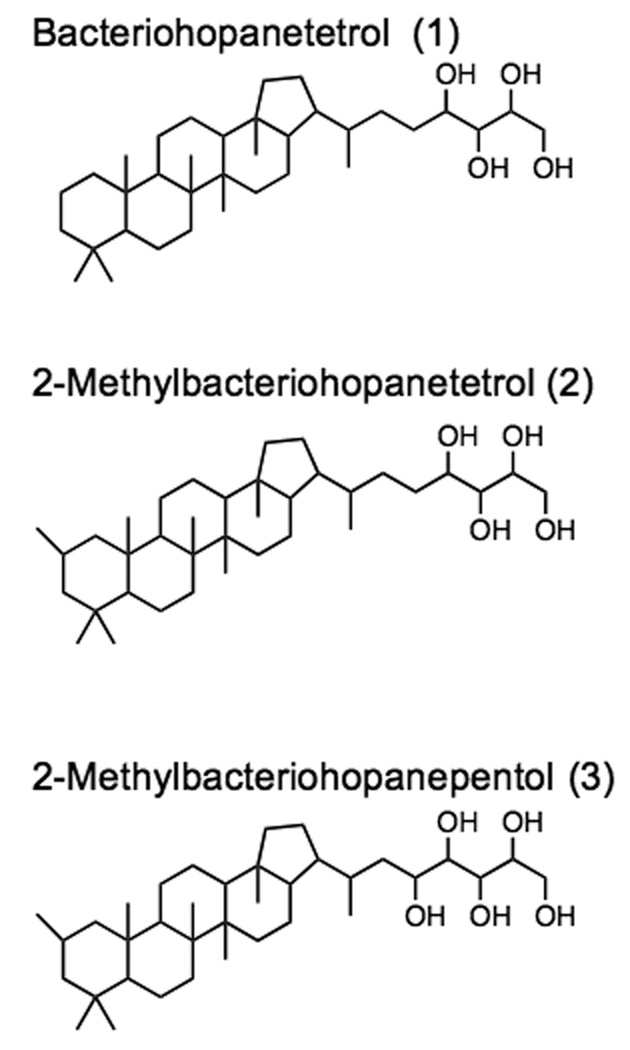
Scheme showing the structures of bacteriohopanetetrol (1), 2-methylbacteriohopaneterol (2) and 2-methylbacteriohopanepentol (3) found in N. punctiforme
Light, fluorescence and electron microscopy of differentiated cell types of N. punctiforme
The progress of cell differentiation was followed with a fluorescence microscope, as described previously (Meeks et al., 2002). For transmission electron micrographs, cultures containing vegetative cells, vegetative cells and heterocysts, or akinetes were harvested by centrifugation at 1000 × g for 20 min. Harvested cells were enrobed in 2% (wt/vol) noble agar and placed in 2% glutaraldehyde for 2 h. Agar blocks were then washed twice in N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES) buffer and fixed in 2% OsO4 for 2 h, followed by 2% (wt/vol) uranyl acetate (UA) staining for 2 h. Blocks were then dehydrated through a graded ethanol series (25%, 50%, 75%, 95%, and 3 × 100%) for 15 min in each solution. Blocks were suspended in a 50/50 ethanol/LR White resin solution for 30 min, followed by 100% LR White for 1 h. Samples were then embedded in gelation capsules filled with fresh LR White resin and were allowed to polymerized at 60°C for 1 h. Capsules were thin-sectioned on a Reichert-Jung Ultracut E ultramicrotome and ultra-thin sections were mounted on Formvar carbon-coated copper grids. To improve contrast, grids containing thin sections were post- stained in 2% (wt/vol) UA. Electron microscopy was performed on a JEOL JEM-1200EXII transmission electron microscope.
Preparation of purified cytoplasmic, thylakoid, and outer membrane fractions from differentiated cell types of N. punctiforme
Cultures of N. punctiforme were harvested by centrifugation at 1,000 × g for 20 min, washed three times in ice-cold 5 mM sodium potassium phosphate buffer pH 7.8 (consisting of 0.44g × l−1 K2PO4 and 0.36g × l−1 NaHPO4), and then re-suspended in the same buffer used to wash the cells. A pre-cooled French press and pressure cell were used in a refrigerated room to ensure cells and membranes remained at 4°C. Differential lysis of vegetative and heterocyst cell types was accomplished using the procedure described by Fay & Lang (1971). Membrane fractions were obtained by discontinuous sucrose gradient centrifugation using the protocol described by Moslavac et al. (2005) except the digitonin, added to facilitate the separation of TK and CM membrane fractions, was replaced by 0.1 % Tween-20.
Immunodetection of specific antigens in the cytoplasmic, thylakoid, and outer membrane fractions of N. punctiforme
Comparative analysis of the complete genome of N. punctiforme to sequences of commercially available antibodies specific to membrane antigens indicated that N. punctifrome has an outer membrane porin with 42% amino acid (AA) similarity to Toc75 of Pisum sativum, and a PSII component PsbD with 90% AA similarity to the PsbD from Arabidopsis thaliana. Rabbit anti-Toc75 and PSII polyclonal antibodies were purchased from Agrisera. Goat anti-rabbit IgG conjugated to horse radish peroxidase served as the secondary antibody and was purchased from Abcam. All antibiodies cross reacted as expected with proteins from N. punctiforme. Western blots were performed according to standard procedures. The CDP-star chemiluminesent detection kit (New England Biolabs Inc. USA) was used for the detection and the radiograms were quantified with Quantity One image analyzer (Biorad).
Effect of akinete germination on hopanoid localization in N. punctiforme
Akinetes formed in response to P-limitation were maintained for 6 months under the conditions used to form the akinetes. Akinetes were harvested by centrifugation 1000 × g for 20 min and re-suspended in sterile water. To remove any remaining vegetative cells, the re-suspended akinete culture was placed in a sonicating water bath for 20 min. Following sonication, cells were washed three more times in sterile water to remove cell debris and finally re-suspended in complete medium and separated into 5 ml aliquots. At time intervals of 0, 5, 24, 48 h three replicate aliquots were visualized by light microscopy and then harvested by centrifugation (1000 × g for 5 min). Cells were washed three times in complete medium to ensure that any loose akinete envelopes were separated from the cells. To collect akinete envelopes all supernatants were collected and subjected to ultracerifugation (50,000 × g for 12 h). Pelleted akinete envelopes were re-suspended in water and extracted by the same method used to isolate hopanoids from intact cells, as described above.
RESULTS
Expression of bacteriohopanepolyols
Bisseret et al. (1985) and Zhao et al. (1996) described a variety of hopanoids in cyanobacteria including 1, 2, and 3 in Nostoc species. Our work shows that the expression of hopanoids is positively correlated to the depletion of P or N from the medium, but that N and P deprivation affect hopanoid expression to different extents (Figure 2A–C). The expression of 3 was significantly higher following one week of P or N deprivation, but in N- deprived cultures, 3 decreased to values that were not significantly different than the complete medium controls following four weeks of incubation (Figure 2C). In contrast, 3 expression continued to increase in cultures deprived of P. Furthermore, the expression of 1 and 2 only increased in response to P starvation and this effect occurred only after three weeks of P-starvation (Figure 2 A & B). The 2-methylhopanoid index (2-methylhopanoid/ desmethylhopanoid +2-methylhopanoid) varied from an initial value of 2.1 in the inoculum to 1 to 6.5 during the course of the experiment and the highest value for the 2-methylhopanoid index was recorded in cultures containing heterocysts in which total hopanoid concentrations had decreased from 2 ng/µg−1 TLE on week 1 to 1 ng/µg−1 total lipid extract (TLE) on week 5 (Figure 3).
Figure 2.
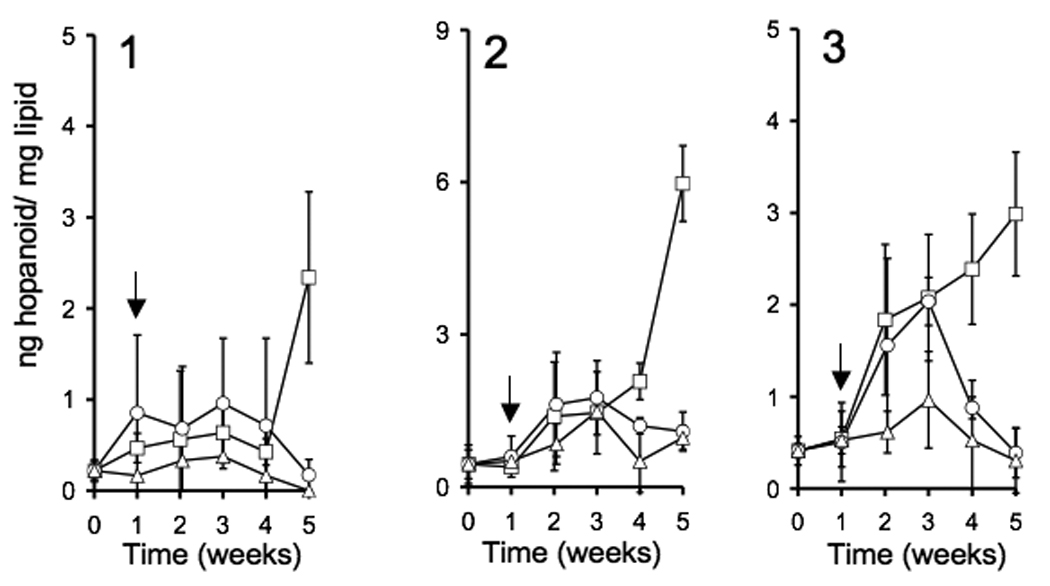
Time course of the effects of N or P limitation on the expression of 1, 2, and 3 in N. punctiforme. At time zero vegetative cells of N. punctiforme were inoculated into fresh medium. Following 7 days of growth, at the time indicated by the arrow, cells were harvested, washed and resuspened in either complete medium (△) or medium lacking either P (□) or N (○). Data points are the means of three replicates and error bars represent the standard deviation of the means.
Figure 3.
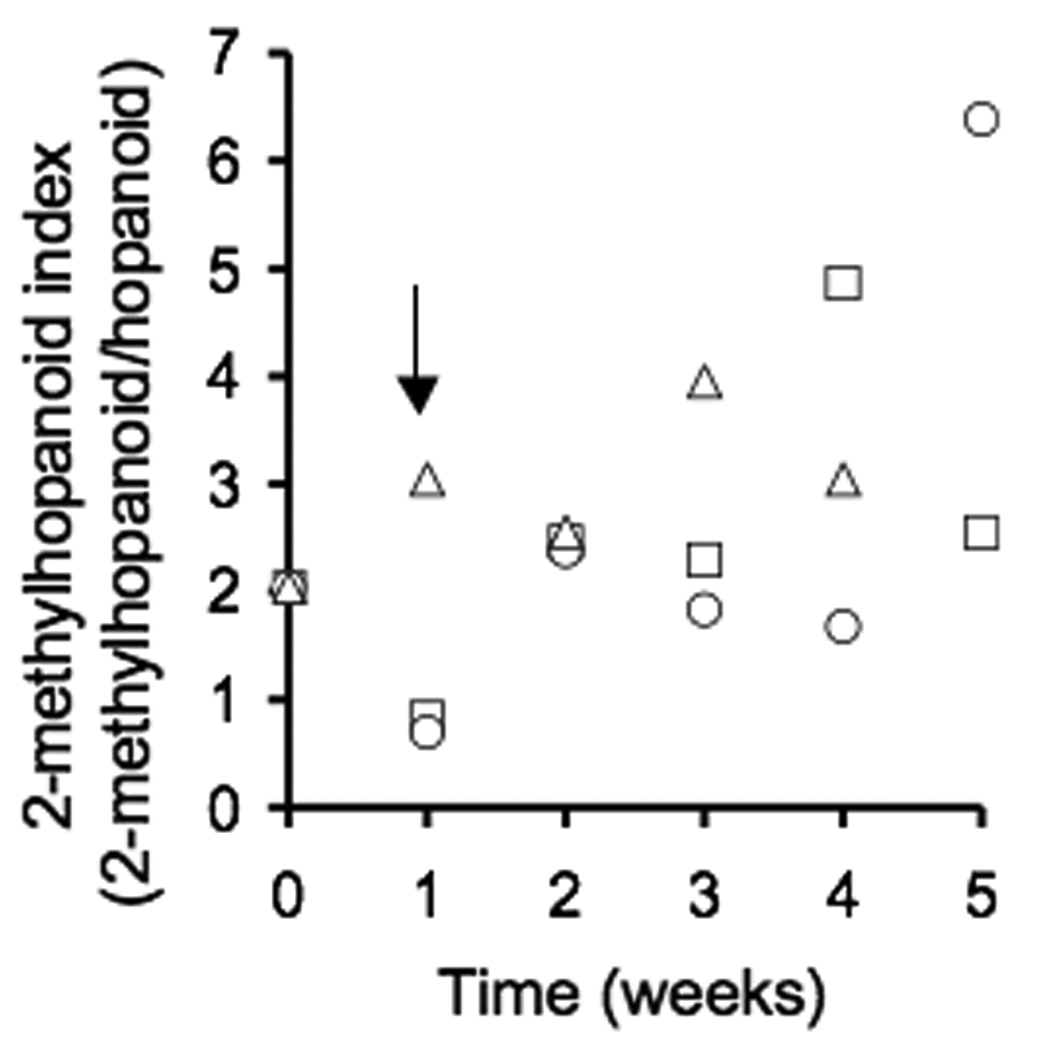
Time course of N or P limitation on the ratio of 2-methylhopanoid to desmethylhopanoids (i.e. 2-methylhopanoid index). At time zero, vegetative cells of N. punctiforme were inoculated into fresh medium. Following one week (arrow), cells were harvested, washed and resuspened in either complete medium (△) or medium lacking either P (□) or N (○).
Differentiation of N. punctiforme cells into heterocysts and akinetes
Cultures of N. punctiforme that had been grown in complete medium or medium lacking P or N were examined by fluorescence and transmission electron microscopy. Because heterocysts do not express phycobilisomes, which fluoresce red under illumination with a Cy3 fluorescence filter, heterocyst frequency in filaments was detected by an absence of fluorescence. Following one week of N starvation, approximately 10% of cells in N. punctiforme filaments differentiated into heterocysts (Arrows on Figure 4 A & B). Filaments consisting of akinetes, formed following the re-suspension of vegetative cells into medium lacking P, were larger than vegetative filaments and divisions between the cells of the filament were more pronounced, as described by Meeks et al. (2002) (Figure 4 C). Electron micrographs of differentiated cells revealed structural changes in the membranes of heterocysts and akinetes relative to vegetative cells. For example, a decrease in intercellular thylakoid membrane in akinetes (Figure 4 E), as compared to vegetative cells (Figure 4 D). Because cellular differentiation from vegetative cells into either heterocysts or akinetes in N. punctiforme is accompanied by visible changes in membrane architecture and hopanoids are components of lipid membranes, we sought to quantify the intracellular localization of hopanoids in the differentiated cell types.
Figure 4.
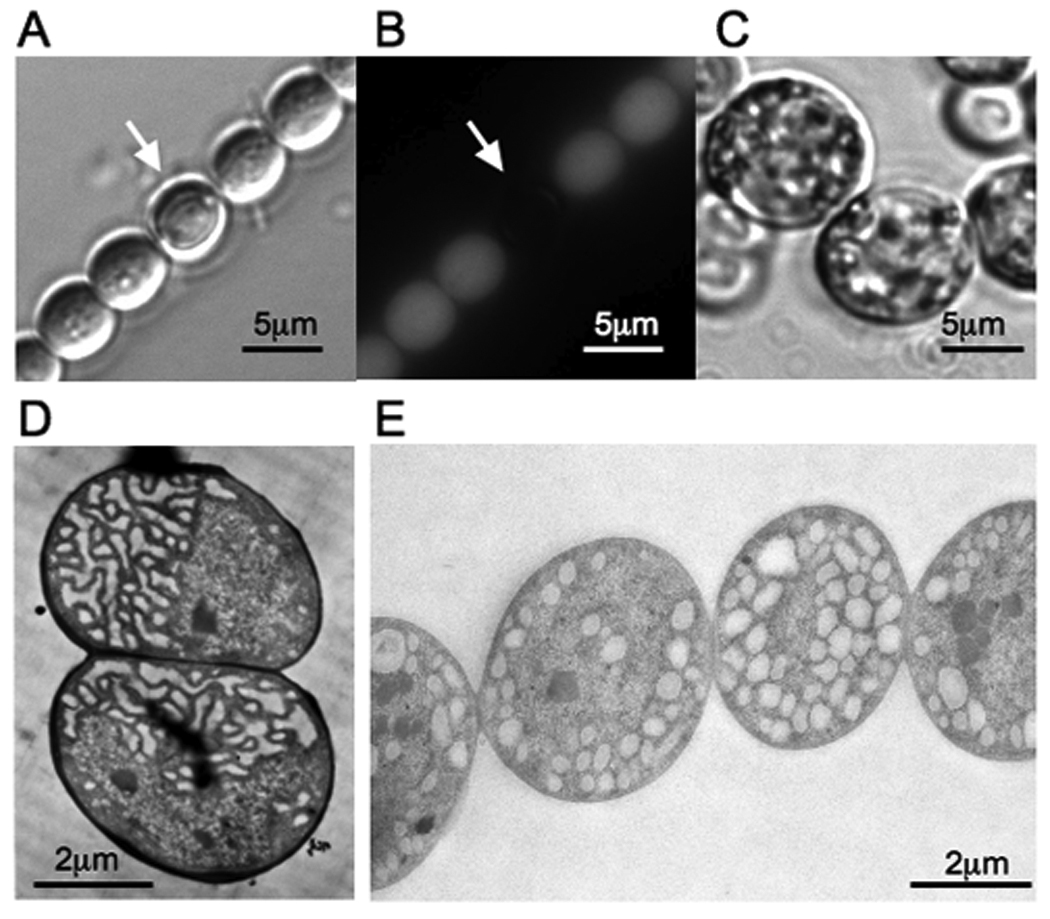
Images of differentiated cell types of N. punctiforme. Vegetative filaments containing heterocysts are shown under light (A) and fluorescence (B) microscopy. The white arrow indicates the position of heterocyst. Akinete cell morphology is shown under light microscopy (C). Electron micrographs of vegetative and akinete cell types are shown in panels D and E, respectively.
Membrane fractionation and immunological analysis of membrane fractions
Antibodies directed against PsbD and Toc75 served as markers for the thylakoid and outer membrane, respectively. These membrane markers allowed for the development of a method for the discrete separation of cytoplasmic, thylakoid and outer membranes. Furthermore, the anti-Toc75 antibody specifically labeled the outer membrane fraction and anti-PsbD specifically labeled the thylakoid membrane fraction, providing evidence that the outer membrane and the thylakoid membrane were not cross contaminated (data not shown). These data also indicated that the cytoplasmic membrane was not contaminated with the outer or thylakoid membranes.
Intercellular localization of hopanoids in differentiated cell types of N. punctiforme
In vegetative cells the cytoplasmic, thylakoid and outer membrane consisted of 0, 0.44, and 0.12 % hopanoid by weight, respectively (lower limit of detection approximately 0.1%). Vegetative cells from N-fixing cultures contained higher concentrations of hopanoids than did cells grown in complete medium and the overall hopanoid concentrations of the cytoplasmic, thylakoid and outer membranes were 0, 0.24, and 0.57 % hopanoid by weight, respectively (Table 2). This increase in hopanoid content was most significant for 3 in the outer membrane of vegetative cells. Hopanoids were not produced in sufficient amounts for quantitative measurement in heterocysts (lower limit of detection 0.1% by weight). However, by far the greatest concentration of hopanoids was found in the outer membranes of akinetes which consisted of nearly 4% hopanoid by weight. Thylakoid and cytoplasmic membrane fractions did not show significant changes in hopanoid content in akinetes.
Table 2.
Hopanoid composition of membrane fractions. Numbers represent the mean value of three replicates (µg/mg of total lipid extract). Typical standard deviation of the means were 25%, and the lower limit of detection was approximately 1 µg/mg.
| Cell Type | Membrane Fraction |
Hopanoid Structure | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Vegetative | ||||
| Cytoplasmic | 0 | 0 | 0 | |
| Thylakoid | 1.7 | 1.7 | 1 | |
| Outer | 1.2 | 0 | 0 | |
| Vegetative from N-fixing culture |
||||
| Cytoplasmic | 0 | 0 | 0 | |
| Thylakoid | 0 | 0 | 2.4 | |
| Outer | 1.2 | 0 | 4.5 | |
| Heterocyst | ||||
| Cytoplasmic | 0 | 0 | 0 | |
| Thylakoid | 0 | 0 | 0 | |
| Outer | 0 | 0 | 2 | |
| Akinete | ||||
| Cytoplasmic | 0 | 0 | 0 | |
| Thylakoid | 2 | 2.1 | 0 | |
| Outer | 10.4 | 0 | 29.9 | |
The effect of light on hopanoid production during vegetative cell differentiation into akinetes
Given that we had stimulated akinete formation by starving vegatative cells for P, our data could not distinguish whether the increase in hopanoids associated with akinetes was a specific response to decreased P availability or associated with the akinete cell type per se. We thus explored hopanoid expression and localization in akinetes formed by a P-independent mechanism: light deprivation. When visible light was supplied to vegetative cultures below 1 µmole×(m2×min)−1 vegetative cells differentiated into akinetes. The outer membrane of light-deprived akinetes contained 29-fold more hopanoids than the outer membrane fraction of vegetative cells. This suggests that the increased expression of hopanoids in the outer membrane of akinetes is specific to the akinete cell type and not simply a response to P-limitation.
Germination of N. punctiforme akinetes
We explored the effect of akinete germination on hopanoid localization. Mature akinetes were harvested following six months of P-starvation, and germination was initiated by re-suspending cells in complete medium. Hopanoid content was measured along the germination time course. Several physiological changes were noted within 48 h (Figure 5A): First, average cell size decreased from 7.5 +/−2.1 to 4.2 +/−0.9 µm within 48 h of re-suspension in complete medium. Second, storage granules in akinetes decreased in size and number. Third, cells appeared to break away from the akinete envelope eventually leaving jettisoned envelopes in the medium (Figure 5A, arrow). Following 48 h of incubation in complete medium, cells were harvested and separated from jettisoned envelopes. Analysis of total lipid extracts indicated that germinated cells were strongly depleted in hopanoids relative to the initial concentrations in the akinete (Figure 5B). In contrast, the jettisoned akinete envelopes contained abundant BHP. Interestingly, the hopanoid content of the jettisoned envelopes was only about 10% of the hopanoid associated with akinetes suggesting that hopanoids were degraded or modified during the germination process. At this time, pathways for hopanoid biosynthesis or degradation are unknown and the identification of hopanoids with modified or novel structures remains to be explored in N. punctiforme.
Figure 5.
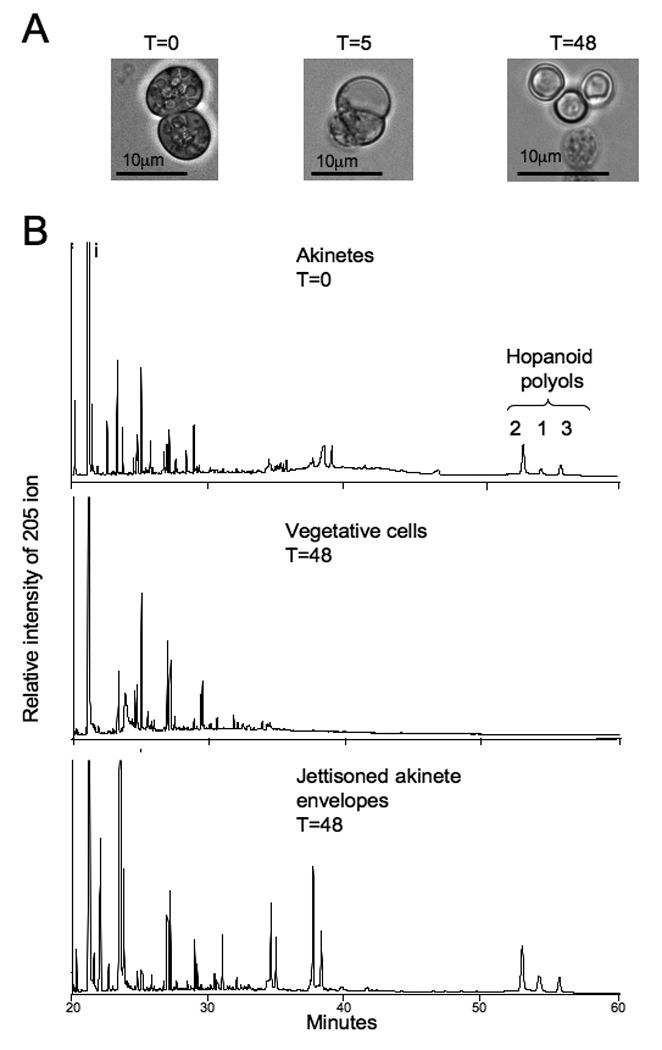
The effect of Nostoc punctiforme akinete germination on hopanoid localization. A. Physiological changes in membrane architecture that accompany the germination process. The white arrow indicates cell envelopes that have been jettisoned from cells. B. Chromatograms of the 205 Da ion from the total lipid extracts of 6-month-old akinetes, cells collected 48 hours after germination was initiated, and jettisoned akintete envelope as indicated. Lipid analysis was performed on a DB-XLB column as indicated in the methods and hopanoid structures are identified in the figure by their respective numbers (see Figure 1).
DISCUSSION
Previous membrane localization studies have indicated that hopanoids are components of the outer and intercytoplasmic membranes in a variety of Gram negative and methanotrophic bacteria (Jahnke et al., 1992; Jurgens et al., 1992; Simonin et al., 1996).
Here we extend these observations to show that 2-methylhopanoids localize to the outer membrane of N. punctiforme, a cyanobacterium capable of cellular differentiation. Based on our survey of differentiated cell types, several patterns emerge regarding hopanoid methylation. First, 2-methylhopanoids are only minimally produced by either vegetative or heterocyst cell-types, implying that 2-methylhopanoids are neither functionally linked to oxygenic photosynthesis nor nitrogen fixation. Second, 2-methylhopanoids are maximally expressed in the akinete outer membrane, which suggests a protective role for 2-methylhopanoids, possibly by maintaining membrane fluidity during periods of cold or desiccation. We discuss these findings in the context of 2-methylhopanoids as biomarkers for cyanobacteria and oxygenic photosynthesis.
We found that both 2-methylhopanoids and their desmethyl equivalents were most abundant in the outer membranes of akinetes, an environmentally recalcitrant structure in which oxygenic photosynthesis has been down regulated (Argueta & Summers, 2005). Such localization is thus inconsistent with a role for hopanoids in oxygenic photosynthesis in N. punctiforme. More generally, it is important to consider that cyanobacteria are capable of other types of metabolism besides oxygenic photosynthesis, such as fermentation, and some strains, such as Oscillatoria limnetica, have been shown to engage in anoxygenic photosynthesis (Cohen et al., 1975). Thus, even if 2-methylhopanoids prove to be reliable biomarkers for cyanobacteria, without a functional link between oxygenic photosynthesis and 2-methylhopanoids, the use of 2-methylhopanes as a proxy to date the evolutionary origin of oxygenic photosynthesis can only be indirect.
The fact that N. punctiforme heterocysts do not contain hopanoids is noteworthy in light of previously published work in which N-fixing Frankia were reported to contain hopanoids (Berry et al., 1993). The authors of this other study proposed that hopanoids limit the diffusion of oxygen into the cell, thereby protecting the oxygen sensitive enzyme nitrogenase, providing a biological rationale for the hypothesis that 2-methyl hopanes may be a proxy for N-limitation in paleoenvironments (Kuypers et al., 2004). However, hopanoids have also been proposed to be a response to extremes in pH, dessication and alcohols (Moreau et al., 1997; Porella et al., 2000; Welander et al., 2009), in all cases by limiting the diffusion of substances into or out of the cell. Although the absence of hopanoids in heterocysts suggests there is no functional link between N-fixation and hopanoids, hopanoids may well serve a general function to stabilize membranes and limit diffusion through membranes.
Alternatively, because hopanoids are produced by akinetes formed in response to different environmental stresses, our data indicate that 2-methyl hopanoids could be a developmental marker for the spore-like akinete cell type per se. Fossilized akinetes have been dated back to 2.1 Ga in marine sediments and molecular analyses of modern cyanobacterial genes suggest that the capacity for cellular differentiation into the heterocyst and akinete cell types arose once between 2.1 and 2.45 Ga when heterocysts evolved to cope with increasing oxygen concentrations, with akinetes later evolving from heterocysts (Knoll et al., 2007; Tomitani et al., 2006). Because the akinetes of N. punctiforme are abundant sources of 2-methylhopanoids, it seems reasonable that at least one important source of 2-methylhopanes in the rock record may have been cyanobacteria capable of cellular differentiation. We note that other 2-methylhopanoid-producing cyanobacteria, such as Phormidium luridum (a member of the Oscillatoriales), do not make akinetes; moreover, other 2-methylhopanoid producers such as Rhodopseudomonas palustris (a member of the Rhizobacteriales) do not make akinetes either (Rashby et al., 2007). Accordingly, while it is interesting to speculate on the contribution of akinetes to the 2-methylhopane fossil record, we are unable to constrain how significant this contribution might have been without additional information that would speak to the probable ecological distribution of these different sources.
Vegetative cell differentiation into akinetes is accompanied by the development of a thickened cell envelope that protects cells from cold or desiccation (Argueta & Summers, 2005); the presence of 2-methylhopanoids in the akinete envelope could therefore indicate a protective role for 2-methylhopanoids. A similar role for unmethylated hopanoids was proposed in the Gram positive species Streptomyces coelicolor in which sporulation and the production of aerial hyphae corresponded to the production of hopanoids (Poralla et al., 2000). While our focus has been on the biological function of 2-methylhopanoids because of their geological significance, hopanoid diversity is primarily defined by different polar headgroups. Much remains to be learned about the specific function of any given type of hopanoid, and whether methylation makes a difference. Previous researchers have suggested that hopanoids are bacterial equivalents of eukaryotic sterols that contribute to membrane stability, and that the ratio of 2-methylhopanoids to desmethylhopanoids could regulate membrane fluidity (Bisseret et al., 1985). Hopanoid methylation would likely destabilize the chair conformation of the A-ring of the hopanoid through steric interactions between the methyl groups at C2, C4, and C10, resulting in the A-ring adopting a boat confirmation (Bisseret et al., 1985). Could a conformational change in the A-ring affect hydrophobic interactions between hopanoids and other membrane components? As we gain greater insight into the functional distinction between 2-methylhopanoids and their unmethylated counterparts and their associations with other membrane constituents, we will be able to better interpret the 2-methylhopanoid index from paleo-environments.
In summary, we explored the physiological role of 2-methylhopanoids in the cyanobacterium N. punctiforme. While it will be important to confirm these findings in other cyanobacteria to determine how general they are, our results suggest that 2-methylhopanoids are components of resting cell types. Given the pattern of distribution we observed in N. punctiforme, the fact that not all cyanobacteria produce 2-methylhopanoids, and their presence in the Rhizobiales, it seems clear that they do not have a direct role in the machinery of oxygenic photosynthesis. We have also demonstrated that 2-methylhopanoids may be shed with the envelope during the germination of akinetes, providing a mechanism for the entry of hopanoids into the sediment. The total hopanoid content of akinetes could not be accounted for following germination, suggesting that N. punctiforme has a previously unrecognized ability to degrade hopanoids. Because hopanoid biosynthesis and degradation appear to be up-regulated by the process of akinete formation and germination, we can now move forward with molecular analyses to gain a better understanding of the metabolic pathways involved in hopanoid processing.
Supplementary Material
Table 1.
Summary of hopanoids detected by high-temperature GC-MS and their identifying fragment ions.
| Hopanoid | Molecular ion |
Side-chain Loss |
A+B ring fragment ion |
D+E ring + side chain |
|---|---|---|---|---|
| acetate of 1 | 714 | 369 | 191 | 493 |
| acetate of 2 | 728 | 383 | 205 | 493 |
| acetate of 3 | 786 | 383 | 205 | 551 |
REFERENCES
- Argueta C, Summers ML. Characterization of a model system for the study of Nostoc punctiforme akinetes. Archives of Microbiology. 2005;183:338–346. doi: 10.1007/s00203-005-0778-5. [DOI] [PubMed] [Google Scholar]
- Berry AM, Harriott OT, Moreau RA, Osman SF, Benson DR, Jones AD. Hopanoids compose the Frankia vesicle envelope, presumptive barrier of oxygen diffusion to nitrogenase. Proceedings of the National Acadamy of Science USA. 1993;90:6091–6094. doi: 10.1073/pnas.90.13.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseret P, Zundel M, Rohmer M. Prokaryotic triterpenoids. 2. 2 beta-methylhopanoids from Methylobacterium organophilum and Nostoc muscorum, a new series of prokaryotic triterpenoids. European Journal of Biochemistry. 1985;150:29–34. doi: 10.1111/j.1432-1033.1985.tb08982.x. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Padan E, Shilo M. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. Journal of Bacteriology. 1975;123:855–861. doi: 10.1128/jb.123.3.855-861.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P, Lang NJ. The heterocysts of bluegreen algae: I. Ultrastructural integrity after isolation. Proceedings of the Royal Society London. 1971;178:185–192. [Google Scholar]
- Flesch G, Rohmer M. Prokaryotic hopanoids: the biosynthesis of the bacteriohopane skeleton: formation of isoprenic units from two distinct acetate pools and a novel type of carbon/carbon linkage between a triterpene and D-ribose. European Journal of Biochemistry. 1988;175:405–411. doi: 10.1111/j.1432-1033.1988.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Jahnke LL, Stan-Lotter H, Kato K, Hochstein LI. Presence of methylsterol and bacteriohopanepolyol in an outer membrane preparation from Methylococcus capsulatus (Bath) Journal of General Microbiology. 1992;138:1759–1766. doi: 10.1099/00221287-138-8-1759. [DOI] [PubMed] [Google Scholar]
- Jahnke LL, Summons RE, Hope JM, Des Marais DJ. Carbon isotopic fractionation in lipids from methanotrophic bacteria II: The effects of physiology and environmental parameters on the biosynthesis and isotopic signatures of biomarkers. Geochimica et Cosmochimica Acta. 1999;63:79–93. doi: 10.1016/s0016-7037(98)00270-1. [DOI] [PubMed] [Google Scholar]
- Joyeux C, Fouchard S, Llopiz P, Neunlist S. Influence of the temperature and the growth phase on the hopanoids and fatty acids content of Frateuria aurantia (DSMZ 6220) FEMS Microbiology Ecology. 2004;47:371–379. doi: 10.1016/S0168-6496(03)00302-7. [DOI] [PubMed] [Google Scholar]
- Jürgens UJ, Simonin P, Rohmer M. Localization and distribution of hopanoids in membrane systems of the cyanobacterium Synechocystis PCC 6714. FEMS Microbiology Letters. 1992;71:285–288. doi: 10.1016/0378-1097(92)90723-2. [DOI] [PubMed] [Google Scholar]
- Kannenberg EL, Poralla K. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften. 1999;86:168–176. [Google Scholar]
- Knoll AH, Summons RE, Waldbauer JR, Zumberge JE. The geological succession of primary producers in the oceans. In: Falkowski P, Knoll AH, editors. The Evolution of Primary Producers in the Sea. Boston: Academic Press; 2007. pp. 133–163. [Google Scholar]
- Kuypers MMM, van Breugel Y, Schouten S, Erba E, Sinninghe Damsté JS. N2-fixing bacteria supplied nutrient N for Cretaceous oceanic anoxic events. Geology. 2004;32:853–856. [Google Scholar]
- Meeks JC, Campbell EL, Summers ML, Wong FC. Cellular differentiation in the cyanobacterium Nostoc punctiforme. Archives of Microbiology. 2002;178:395–403. doi: 10.1007/s00203-002-0476-5. [DOI] [PubMed] [Google Scholar]
- Moreau RA, Powell MJ, Fett WF, Whitaker BD. The effect of ethanol and oxygen on the growth of Zymomonas mobilis and the levels of hopanoids and other membrane lipids. Current Microbiology. 1997;35:124–128. doi: 10.1007/s002849900224. [DOI] [PubMed] [Google Scholar]
- Moslavac S, Bredemeier R, Mirus O, Granvogl B, Eichacker LA, Schleiff E. Proteomic analysis of the outer membrane of Anabaena sp. Strain PCC 7120. Journal of Proteome Research. 2005;4:1330–1338. doi: 10.1021/pr050044c. [DOI] [PubMed] [Google Scholar]
- Neunlist S, Rohmer M. A novel hopanoid, 30-(5'-adenosyl)hopane, from the purple non-sulphur bacterium Rhodopseudomonas acidophila, with possible DNA interactions. Biochemistry Journal. 1985;228:769–771. doi: 10.1042/bj2280769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourisson G, Albrecht P. Geohopanoids: The most abundant natural products on earth? Accounts of Chemical Research. 1992;25:298–402. [Google Scholar]
- Ourisson G, Rohmer M, Poralla K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annual Review of Microbiology. 1987;41:301–333. doi: 10.1146/annurev.mi.41.100187.001505. [DOI] [PubMed] [Google Scholar]
- Poralla K, Muth G, Härtner T. Hopanoids are formed during transition from substrate to aerial hyphae in Streptomyces coelicolor A3(2) FEMS Microbiology Letters. 2000;189:93–95. doi: 10.1111/j.1574-6968.2000.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Rashby SE, Sessions AL, Summons RE, Newman DK. Biosynthesis of 2-methylbacteriohopanepolyols by an anoxygenic phototroph. Proceedings of the National Academy of Science USA. 2007;104:15099–15104. doi: 10.1073/pnas.0704912104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer M. The biosynthesis of the hopane series in eubacteria: A mine of new enzyme reactions. Pure and Applied Chemistry. 1993;65:1293–1298. [Google Scholar]
- Simonin P, Jürgens UJ, Rohmer M. Bacterial triterpenoids of the hopane series from the prochlorophyte Prochlorothrix hollandica and their intercellular localization. European Journal of Biochemistry. 1996;241:865–871. doi: 10.1111/j.1432-1033.1996.00865.x. [DOI] [PubMed] [Google Scholar]
- Summons RE, Jahnke LL. Hopenes and hopanes methylated in ring-A: correlation of the hopanoids of extant methylotrophic bacteria with their fossil analogues. In: Moldowan JM, Albrecht P, Philp RP, editors. Biomarkers in Sediments and Petroleum. New Jersey: Prentice Hall; 1992. pp. 182–200. [Google Scholar]
- Summons RE, Jahnke LL, Hope JM, Logan GA. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature. 1999;400:554–557. doi: 10.1038/23005. [DOI] [PubMed] [Google Scholar]
- Talbot HM, Rohmer M, Farrimond P. Rapid structural elucidation of composite bacterial hopanoids by atmospheric pressure chemical ionisation liquid chromatography/ion trap mass spectrometry. Rapid Communications in Mass Spectrometry. 2007;21:880–892. doi: 10.1002/rcm.2911. [DOI] [PubMed] [Google Scholar]
- Talbot HM, Summons RE, Jahnke LJ, Cockell CS, Rohmer M, Farrimond P. Cyanobacterial bacteriohopanepolyol signatures from cultures and natural environmental settings. Organic Geochemistry. 2008;39:232–263. [Google Scholar]
- Tomitani A, Knoll AH, Cavanaugh CM, Ohno T. The evolutionary diversification of cyanobacteria: Molecular-phylogenetic and paleontological perspectives. Proceedings of the National Academy of Sciences USA. 2006;103:5442–5447. doi: 10.1073/pnas.0600999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welander PV, Hunter RC, Zhang L, Sessions AL, Summons RE, Newman DK. Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1. Journal of Bacteriology. 2009 doi: 10.1128/JB.00460-09. doi:10.1128/JB.00460-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Berova N, Nakanishi K, Rohmer M, Mougenot P, Jürgens UJ. Structures of two bacteriohopanoids with acyclic pentol side-chains from the cyanobacterium Nostoc PCC 6720. Tetrahedron. 1996;8:2777–2788. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


