Abstract
Type 1 diabetes (T1D) is a chronic autoimmune disorder characterized by destruction of insulin-producing pancreatic β cells. Many broad-based immunosuppressive and antigen-specific immunoregulatory therapies have been and are currently being evaluated for their utility in the prevention and treatment of T1D. Looking forward, this review discusses the potential therapeutic use of antigen-specific tolerance strategies, including tolerance induced by tolerogenic antigen presenting cells pulsed with diabetogenic antigens and transfer of induced or expanded regulatory T cells which have demonstrated efficacy in NOD mice. Depending on the time of therapeutic intervention in the T1D disease process, antigen-specific immunoregulatory strategies may be employed as monotherapies, or in combination with short-term tolerance-promoting immunoregulatory drugs and/or drugs promoting differentiation of insulin-producing β cell from endogenous progenitors.
Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disorder thought to be caused by pro-inflammatory autoreactive T cells which mediate the destruction of insulin-producing pancreatic β cells via both direct and indirect mechanisms leading to lifelong dependence on exogenous insulin (Atkinson and Eisenbarth, 2001). Development of T1D is genetically controlled and thought to be initiated in susceptible individuals by environmental factors such as virus infections, although a viral cause has not been clearly identified (von Herrath, 2009). While both humoral and cell-mediated immune mechanisms are active during diabetes, CD4+ T cells occupy a critical role in T1D pathology (Anderson and Bluestone, 2005) as exemplified by the observation that the majority of the genes associated with elevated disease risk relate to the function of CD4+ Th cells [e.g. a trio of MHC II alleles (Concannon et al., 2009)]. Prior to diagnosis of overt T1D, the pancreatic islets are infiltrated by inflammatory cells including CD4+ T cells (Kent et al., 2005) and antibodies to various β cell antigens are demonstrable in the sera of patients at risk (Achenbach et al., 2005).
Because of the ocular, circulatory, cardiovascular and neurological risks associated with hyperglycemia, treatments which prevent the pathologic autoimmunity from destroying pancreatic tissue is preferable to long-term management of symptoms by insulin replacement therapy since use of exogenous insulin cannot match the precision of endogenous insulin secretion. Much of what is understood about the pathogenesis and regulation of T1D has emerged from the study of spontaneous disease in the non-obese diabetic (NOD) mouse. NOD studies have highlighted the critical role of adaptive immune responses in disease pathogenesis as well as identifying various targets which prevent diabetogenic autoimmune responses as prime therapeutic candidates (Atkinson and Leiter, 1999; Shoda et al., 2005). However, it is critical to understand that there are numerous differences in the pathogenic mechanisms driving the initiation and progression of disease in the NOD mouse vs. human type 1 diabetics, e.g. major differences in the antigens targeted, the composition of inflammatory cell infiltrates in the two species, as well as greatly increased expression of MHC class I in humans (Gianani et al., 2010).
Existing and emerging therapies aimed at regulating the autoimmune response largely involve broad-based immunoregulatory strategies, including the inhibition or deletion of lymphocytes subsets and/or use of agents proposed to induce or re-establish immune tolerance via activation of regulatory T cells (Tregs), e.g. non-mitogenic anti-CD3 or anti-thymocyte globulin (Chatenoud, 2003; Chatenoud et al., 2001; Chung et al., 2007; Kohm et al., 2005). Some of these have shown efficacy in initial clinical trials, but there are risks with any of the broad approaches such as cytokine release and/or reactivation of latent viruses. A highly desired alternative approach is the attempted induction of antigen-specific tolerance to β cell antigens for prevention of disease development in patients at risk or in new onset patients. This review will discuss immunoregulatory strategies employed as monotherapies or in combination, including the use of antigen-specific tolerance strategies, which are under evaluation in clinical trials and/or are being developed based on demonstrated efficacy in preventing or ameliorating disease progression in the NOD mice.
There are numerous pitfalls to the translation of laboratory findings to the clinic. Trials of therapies that alter the natural history of T1D have been hampered by the lack of biomarkers of the immune processes that causes the disease. There are immunologic “readouts” that correlate with the presence of T1D, for instance, the presence of autoantibodies against islet cell antigens including glutamic acid decarboxylase 65 (GAD65), insulin, islet cell antigen 512 (ICA512), and more recently zinc transporter 8 (ZnT8) have supported the autoimmune nature of the disease and have clearly differentiated T1D from Type 2 diabetes where these markers are not found (Seyfert-Margolis et al., 2006). More recently, cellular proliferation assays to islet specific proteins have distinguished responses in patients from normal control subjects (Herold et al., 2009). Other assays have identified antigen-specific cells in the circulation (Pinkse et al., 2005). However, the direct causal relationship between these measures and disease has not yet been established. For instance, in studies in which glycemic control has been modified [e.g. Cyclosporin A (CSA) or anti-CD3 monoclonal antibody (mAb)] there were no identified changes in titers of autoantibodies (Bougneres et al., 1988; Herold et al., 2005; Herold et al., 2002; Keymeulen et al., 2005). Thus, an assay that would reflect tolerance to the immune process in T1D is not currently available, but highly sought after.
Immunologic assays may be used as measures of the effects of immune therapies, but their relationship to the disease process remains speculative. One is left with metabolic parameters as endpoints. Although the relationship of these endpoints to the clinical situation is clearer, it is important to recognize that the most widely employed studies are functional, not anatomic. For example, in murine studies of treatment with CD3 mAb at the diagnosis of T1D in NOD mice, improvement in insulin secretion reflected the recovery of degranulated β cells rather than growth of new cells (Sherry et al., 2006). Even the relationship between improved metabolic function and the sequelae of the disease is controversial, but clinical data have suggested a direct relationship between the two (Palmer et al., 2004).
Chemical and Antibody-Mediated Therapies
Initial clinical studies for treatment of T1D involved small molecule inhibitors with biologics undergoing evaluation in the past decade. These clinical trials have had successes and failures as summarized in Table 1. The following narrative explains the basis for and findings from these trials.
Table 1.
Summary of Successful and Unsuccessful Immunotherapy-Based Approaches in Type 1 Diabetes and Relevant Animal Models.
| A. Successful Clinical Trials | ||||
|---|---|---|---|---|
| Therapy | Efficacy in animal model | Comments | Principle adverse events | References |
| Cyclosporine A | + NOD mouse, BB/W rat | Continued use of the drug was needed. | Renal toxicity | (Assan et al., 1994; Bougneres et al., 1988; Bougneres et al., 1990; Feutren et al., 1986; Laupacis et al., 1983; Mori et al., 1986; Stiller etal., 1984) |
| Antithymocyte globulin (alone or with prednisone) | + NOD mouse with Exendin-4, not as single agent | Also part of a hematopoietic stem cell transplant protocol | Thrombocytopenia, serum sickness | (Eisenbarth et al., 1985; Ogawa et al., 2004; Saudek et al., 2004; Simon et al., 2008; Voltarelli et al., 2007) |
| Anti-CD3 mAb | + NOD mouse | Late timing was an issue in the first report but not in a second report. | Mild transient cytokine release, Transient EBV reactivation. | (Chatenoud et al., 1997; Chatenoud et al., 1994; Herold et al., 1992; Herold et al., 2005; Herold et al., 2002; Keymeulen et al., 2005) |
| Rituximab | + NOD mouse | Grade 1 or 2 infusion related reactions | (Hu et al., 2007; Pescovitz et al., 2009) | |
| Etanercept | +NOD mouse but depending on timing: - for older mice | Pilot human trial | No significant drug related adverse events | (Jacob et al., 1990; Mastrandrea et al., 2009) |
| GAD65 | +NOD mouse | Only in those with diabetes < 6mos duration | Mild site irritation, no significant drug related adverse events | (Agardh et al., 2005; Ludvigsson et al., 2008; Tian et al., 1996; Tisch et al., 1993) |
| Oral insulin (Prevention) | + NOD mouse | Only a subset of pre-diabetic subjects with high IAA titer | No significant drug related adverse events | (Skyler et al., 2005; Zhang et al., 1991) |
| Closed loop insulin | A “biostator” (closed loopsystem) was used and suppressed endogenous insulin production. No immune therapy was given. | Hypoglycemia | (Shah et al., 1989) | |
| B. Unsuccessful Clinical Trials | ||||
| Nicotinamide | + NOD mouse | No significant drug related adverse events | (Gale et al., 2004; Kolb and Burkart, 1999; O’Brien et al., 2000; Yamada et al., 1982) | |
| Intranasal insulin | +NOD mouse | Nasal irritation and discharge, cough, fever, GI symptoms | (Bonifacio et al., 2008; Every etal., 2006; Harrison et al., 2004; Nanto- alonen et al., 2008) | |
| Parenteral insulin | + NOD mouse | A pilot clinical trial showed efficacy | Chemical hypoglycemia | (Atkinson et al., 1990; Diabetes Prevention Trial –Type 1 Diabetes Study Group, 2002) |
| Oral insulin | + NOD mouse | See above. A change in the IAA titer for inclusion appeared to result in dilution of the drug effect. | No significant drug related adverse events | (Skyler et al., 2005; Zhang et al., 1991) |
| Insulin in incomplete Freund s adjuvant | + NOD mouse | Small pilot trial, generated high titers of anti-insulin antibodies | No significant drug related adverse events | (Orban et al., 2009; Skyler etal., 2005; Zhang et al., 1991) |
Cyclosporin A
CSA was employed in the first trials showing effects of immune therapies on T1D. Continuous CSA treatment initiated soon after diagnosis eliminated the need for exogenous insulin (Bougneres et al., 1988; Stiller et al., 1984). However, the lack of lasting effects and renal toxicity of the drug diminished enthusiasm for this approach and other broad spectrum immune modulating agents such as Azathioprine and prednisone (Bougneres et al., 1990; Silverstein et al., 1988).
CD3 monoclonal antibody
CD3 mAb without Fc receptor (FcR) binding was developed with the goal of reducing T cell activation, but maintaining immunoregulatory capacity in vivo via suboptimal TCR signals and/or induction of Tregs. Preclinical studies indicated, however, that not only was in vivo activation quantitatively reduced, but the signal delivered by the modified Ab was qualitatively different from FcR binding mAb (Belghith et al., 2003; Smith et al., 1998; Smith et al., 1997). These studies indicated a selective inhibitory effect on differentiated Th1 cells, which had been thought to be involved in β cell destruction. Rather than a direct inhibitory effect of the drug which would require the continued presence of the agent, tolerance was achieved likely via by induction of Tregs. Disease did not recur over time following short-term treatment of newly hyperglycemic mice, and if treated mice did not completely reverse hyperglycemia following drug treatment, they did not destroy syngeneic transplants after anti-CD3 mAb treatment (Chatenoud et al., 1997; Chatenoud et al., 1994).
FcR non-binding anti-CD3 mAbs carrying mutations of the IgG1 Fc chain or with elimination of glycosylation sites [hOKT3γ1(Ala-Ala) and aglycosyl anti-CD3] were found to be less activating than OKT3 (Bisikirska et al., 2005; Herold et al., 2003; Xu et al., 2000). In two trials, brief treatment of new-onset T1D patients was shown to attenuate loss of β cell function for ≥2 years (Herold et al., 2005; Herold et al., 2002; Keymeulen et al., 2005). Clinical parameters including hemoglobin A1c and insulin usage improved. Importantly, there was no evidence for long-term immune suppression. The number of circulating T cells recovered to pretreatment levels by one month after treatment and the drug was well tolerated – the cytokine storm had largely been eliminated, although about 10% of subjects discontinued drug because of adverse events attributed to cytokine release. In the European trial in which the number of circulating T cells was less than in the North American trial, EBV reactivation was seen, but in all cases, the infection resolved, and the reduced numbers of circulating lymphocytes were transient.
Children who are relatives of patients with T1D and have islet cell autoantibodies are at extraordinary high risk for progression to diabetes. About 90% of subjects who meet these criteria, identified in the Diabetes Prevention Trial-1 will have clinical disease within 7 years and the median time to disease onset is 3.31 years (Sherr et al., 2008). The progression of β cell destruction in these individuals, therefore, resembles those with disease and therefore because of the near certainty that disease will progress, interventions that have shown efficacy in subjects with diabetes could be considered in this group. Accordingly, TrialNet has initiated a trial of anti-CD3 mAb treatment in individuals at high risk of diabetes. Based on information from clinical trials in patients with the disease, the suggested outcome is maintenance of insulin secretion and prevention of disease onset.
The mechanism of drug action in patents is not resolved, but may differ from that described in NOD mice. In this regard, Herold, et al. isolated IL-10 producing CD4+ cells from the circulation of drug treated patients, and there was an increase in the relative ratio of production of IL-10:IFN-γ in patient cells activated ex vivo (Herold et al., 2003). An increase in adaptive CD4+CD25+Foxp3+ cells that inhibit immune responses through a TGF-β-dependent mechanism has been found in the pancreatic draining lymph nodes of anti-CD3 treated mice, even in the absence of naturally occurring Tregs (i.e. in CD28−/− mice) (Belghith et al., 2003; Bisikirska et al., 2005). It is not clear whether the same cells can be found in the circulation of patients. It has also been suggested that the mAb induces adaptive CD8+ Tregs whose mechanism of inhibition is not clear and may be similar to CD8+ suppressor cells described in other clinical settings. The absence of a tolerance biomarker or even a functional assay that correlates with the pathogenic process has made it difficult to answer whether the drug induces tolerance in patients.
Anti-Thymocyte Globulin
Anti-Thymocyte Globulin (ATG) with prednisone had been shown to reduce insulin requirements in a pilot trial involving new-onset patients, but was discontinued because of thrombocytopenia (Eisenbarth et al., 1985). In a more recent study, ATG (Fresinius) retarded the loss of C-peptide (which correlates with loss of pancreatic β cell function) in new-onset patients without the need for continuous drug administration (Saudek et al., 2004). The importance of the multiple specificities of ATG compared to anti-CD3 or other anti-T cell mAbs is not known – CD3 is an important target of ATG, but ATG causes a more prolonged peripheral T cell depletion. Thus the effects of these two biologics on the T cell repertoire may be different.
Anti-CD20 (Rituximab)
Anti-CD20 (Rituximab) was recently employed in a T1D trial. B lymphocytes were first thought to be important in the initiation of insulitis because the islets were clear of inflammatory lesions in B cell-deficient NOD mice (Serreze et al., 1996). Previous evidence however had questioned B cell directed therapeutic approaches in established disease because it was possible to adoptively transfer disease with diabetogenic splenic T cells into NOD.SCID recipients, lacking B cells and antibodies (Miller et al., 1988). Hu, et al. and Xiu, et al recently showed that diabetes was prevented in NOD mice by depleting B cells with CD20 mAb before and at the time of onset of hyperglycemia (9–12 week old mice) and even reversed disease in about 30% of animals at the appearance of hyperglycemia (Hu et al., 2007; Xiu et al., 2008). Interestingly, co-transfer of B cells from the successfully treated mice diminished the rate of adoptive transfer of disease suggesting a possible role for activation of “regulatory” B cells. Others have since shown that IL-10 producing B cells can be induced in mice depleted of CD20+ B cells (Yanaba et al., 2008).
A recent randomized placebo-controlled trial of CD20 mAb (Rituximab) showed modest (23%) but significant improvement in β cell function 3 months after diagnosis and overall at 1 year, in drug-treated compared to placebo-treated subjects (Pescovitz et al., 2009). There were also significant improvements in clinical parameters including Hemoglobin A1c and insulin use. Following 3 months, however, there was a parallel decline in β cell function in the drug- and placebo-treated subjects. Subtle but significant differences in the depletion of CD19+CD27+IgD+ cells differentiated clinical responders from non-responders. However, there was little evidence that the drug induced immunologic tolerance. The CD19+ cells, which reached a nadir level at study month 1 had not recovered to control levels after 12 months, and the levels of IgM were still significantly depressed. Once again, maintenance of clinical efficacy will require either a combination of drugs or repeated treatment, but the chronicity of immune suppression is of concern.
Cytokine and cytokine receptor directed therapies
Cytokine and cytokine receptor directed therapies are also in development for treatment of T1D. Human insulitis shows a considerably greater infiltration of innate immune cells such as macrophages and NK T cells compared to NOD insulitis (Dotta et al., 2007; Itoh et al., 1993). Moreover, innate mediators, TNFα, IL-1 and Type 1 interferons, were among the first molecules shown to have direct cytotoxic effects on β cells and were postulated to be the direct cause of β cell killing (Rabinovitch et al., 1990). Possibly because of its innate role in activating adaptive immune responses, it was not surprising that IL-1 receptor-deficient NOD mice had reduced development of diabetes (Thomas et al., 2004). Treatment with the IL-1 receptor antagonist, Anakinra, was shown to improve glucose control in patients with Type 2 diabetes, which is not thought to be mediated by adaptive immune responses but has a significant inflammatory component (Donath and Mandrup-Poulsen, 2008). Interestingly, the drug mechanism appeared to involve a beneficial effect on β cells, reflected by an increase in the insulin:proinsulin ratio, rather than effect on reduced insulin sensitivity that had been thought to be the result of the inflammatory cytokine. β cells may be a source of IL-1, particularly in response to glucose, suggesting a destructive cycle in which hyperglycemia induces expression of the inflammatory mediator resulting in immune activation and further β cell destruction. Initial preclinical data do not suggest that IL-1 blockade alone will prevent or reverse type 1 diabetes, but this axis may be an important target of a combination strategy. Studies to evaluate the effects of IL-1 blockade in disease progression are in progress.
TNFα and IFNγ are directly cytotoxic to β cells suggesting these cytokines as rational targets for immune therapy. However, TNFα has a more complicated role in diabetes progression. Jacob et al. reported that TNFα prevented development of insulitis and diabetes and even the adoptive transfer of diabetes by lymphocytes into young NOD mice (Jacob et al., 1990). Moreover, neutralization of TNFα accelerated diabetes in older mice but at a younger age, prevented disease. These paradoxical effects may have led to reluctance for clinical translation but a recent report by Mastrandrea et al. found that the soluble TNF receptor, Etanercept, reduced loss of C-peptide responses in a small pilot trial (Mastrandrea et al., 2009).
Small molecule protease inhibitors therapy
Small molecule protease inhibitors are also under development for the treatment of T1D. The role of innate immune responses in T1D pathogenesis is further supported by the studies by Koulamanda, et al. in which infusions of alpha-1 antitrypsin (AAT), a serine protease inhibitor which protects tissues from enzymes produced from inflammatory cells, were found to reverse new onset diabetes in NOD mice (Koulmanda et al., 2008). Multiple effects were noted in the NOD studies including reduced insulitis, enhanced β cell regeneration, and improvement in peripheral insulin sensitivity. This non-conventional approach is now in clinical testing.
The small molecule tyrosine kinase inhibitor, Gleevec, used widely for treatment of leukemia, was shown to prevent and reverse diabetes in NOD mice (Louvet et al., 2008). The effects appeared to be linked to inhibition of platelet-derived growth factor receptor (PDGFR) since targeting c-Abl kinase with sunitinib or c-Kit kinase and c-Fms kinase with another tyrosine kinase inhibitor showed marginal efficacy whereas soluble PDGFR reversed diabetes.
Aggressive insulin therapy
Lastly, aggressive insulin therapy has been tested for therapeutic efficacy in T1D. Shah, et al. showed that use of a closed loop system, in which patients with new onset T1D were administered insulin to suppress endogenous insulin production, resulted in improved metabolic function, similar to more recent trials of immune modulators (Shah et al., 1989). It was possible that the intensive insulin therapy had an immune modulatory effect, but this early observation also raises the question as to whether reducing metabolic demand on the targets themselves might alter the immune response to islets.
Antigen-specific Tolerance Approaches to T1D Therapy
The gold standard therapy for the treatment of autoimmune diseases, including T1D, would be the development treatment strategies in which only the pathogenic autoreactive T cells are inactivated safely and in an autoantigen-specific manner while leaving the remainder of immune system unperturbed, i.e. the induction antigen-specific immunologic tolerance. There are multiple strategies under development and/or currently being evaluated in T1D trials that are proposed to target multiple diabetogenic antigens and have been demonstrated to operative via a number of cell intrinsic (anergy) and/or cell extrinsic (Tregs) mechanisms.
Insulin therapy
Insulin therapy has been widely studied in both animal models of T1D as well as in human prevention and new-onset trials. In several autoimmune disease models, mucosal exposure to auto-antigens induce tolerance largely via induction of a variety of Treg cells (Faria and Weiner, 2005). Insulin and proinsulin molecules have been identified to play a prime role in the initiation of the autoimmune process that ultimately leads to destruction of β cells and onset of clinical diabetes. Since early 1990s, mucosal exposure of insulin and many of its immunogenic epitopes has been used for diabetes prevention in animal models. Oral insulin at a dose of 1mg twice a week for 5 weeks followed by weekly treatment was able to delay diabetes onset and reduce diabetes incidence in NOD mice (Zhang et al., 1991). Adjuvants such as cholera toxin B subunit could significantly reduce the amount of antigen (insulin) needed to microgram amounts (Bergerot et al., 1997). Similarly to oral treatment, intranasal aerosol insulin treatment of pre-diabetic NOD mice also significantly delayed diabetes incidence in NOD mice (Aspord and Thivolet, 2002; Harrison et al., 1996). In addition to whole insulin, insulin-derived peptides, such as B9–23, mutated proinsulin peptide B24-C33, and proinsulin II, have also been shown to be efficacious in pre-diabetic NOD mice (Chen et al., 2001; Daniel and Wegmann, 1996; Martinez et al., 2003).
Despite the persistence of even “clinically significant” levels of residual insulin and the potential for recovery of dysfunctional β cells with immune therapy at the time of diagnosis, prevention of T1D will have a greater impact than treatment approaches. Autoimmunity to islets can be identified ≥ three years before presentation with hyperglycemia in many individuals. Interventions that are effective at onset would be postulated to be effective in the pre-diabetic period. In addition, by intervening at an early stage, antigen-specific approaches might be more effective since the repertoire is more restricted and the number of different effectors that are involved is more restricted. Based on the success in animal models, clinical trials of oral or nasal insulin have been conduced in humans. These trials can be divided into prevention trials in pre-diabetics and therapeutic trials in recent-onset diabetics.
Human prevention trials have included a double-blind crossover safety study conducted in 38 individuals with antibodies to one or more islet antigens showed that intranasal insulin was safe in that it did not accelerate loss of β-cell function in individuals at risk for type 1 diabetes, but instead induced an increase in antibody and a decrease in T cell responses to insulin consistent with mucosal tolerance (Harrison et al., 2004). The subsequent Diabetes Prevention Trial-1 (DPT-1) tested the efficacy of oral insulin in 388 pre-diabetic patients who were first- and second-degree relatives of T1D patients and were also classified as at increased risk for developing T1D by genetic, immunological, and metabolic staging (Skyler et al., 2005; Sosenko et al., 2006). Oral insulin therapy did not delay or prevent type 1 diabetes. However, in subgroup analysis, it appeared that there might be a potential benefit in diabetes prevention in those subjects with higher autoantibody levels. A more recent prevention trial using intranasal insulin conducted in 224 Finnish children with genetic and immunological risks for developing T1D, showed that nasal insulin administration at 1 unit/kg/day initiated soon after detection of autoantibodies had no beneficial effect on diabetes prevention (Nanto-Salonen et al., 2008). Furthermore, children positive for three of four autoantibodies before initiation of treatment appeared to be at possibly increased risk for accelerated onset of diabetes. This is a classic example of where pre-clinical studies were not predictive of the outcome of a human trial.
Several explanations have been offered for the failure of these trials including insufficient dosing as well as the fact that by the time an individual is identified with autoantibodies, the disease process is well-established. Therefore, the opportunity to intervene before the autoreactive repertoire is expanded via epitope spreading (Miller et al., 2007), i.e. before the appearance of multiple autoantibodies, using tolerance strategies with or without broader immunosuppressive agents, should be further explored. In addition, this may also reflect the complexity of mucosal immunology. Depending on pre-existing milieu, both tolerance and immunity are potential outcomes after mucosal antigen exposures. This could explain why possible disease acceleration has been observed with mucosal insulin therapy in certain sub-populations. Again, understanding individual immune responses elicited by mucosal insulin therapy based on the dose, route, frequency, duration, and stage of disease at which therapy is instituted will likely significantly enhance our ability to design individualized mucosal insulin therapy that will be safe and efficacious.
There have been a number of human new onset trials using insulin therapy. Two published trials examined the effect of oral insulin therapy on residual β cell function in recent-onset T1D patients. In the IMDIAB trial, a total of 82 patients with clinical type 1 diabetes were randomized to receive oral insulin at 5 mg/day or placebo (Pozzilli et al., 2000). At 1 year follow-up, there was no difference between the insulin treated and the placebo treated groups with respect to mean C-peptide secretion, requirement for insulin therapy, or IgG insulin antibodies. Furthermore, in patients younger than 15 years a tendency for low C-peptide at 9 and 12 months was observed in the oral insulin group, suggesting an acceleration in the decline of β cell function. In the ORALE trial, 131 new onset T1D patients were randomized to a low dose (2.5mg/day) or a high dose (7.5mg/day) oral insulin versus placebo for 1 year, and again no benefit was observed in preventing deterioration of β cell function (Chaillous et al., 2000). These results are consistent with those seen in murine models where oral insulin was shown not to reverse new onset diabetes (Fousteri et al., 2007). Interestingly, if nasal insulin therapy is used in combination with anti-CD3 therapy, a significant benefit in reversing recent-onset diabetes is then achieved in two animal models of autoimmune diabetes (Bresson et al., 2006). Expansion of insulin-specific Tregs producing IL-10, TGF-β, and IL-4, and possibly their modulation of antigen-presenting cells in local draining lymph nodes were proposed as likely mechanisms. These findings should provide the basis for using combinatorial therapies in future trials for humans with recent-onset diabetes as discussed below.
Interestingly, a more recent phase I study using a single intramuscular injection of human insulin B-chain in incomplete Freund s adjuvant in 12 subjects with recent-onset diabetes showed that this therapy led to the development of lasting (at 2 year follow-up) insulin B-chain-specific CD4+ Tregs (Orban et al., 2009). This study provides the basis for testing this modality of insulin B-chain therapy in a larger T1D trial. Another ongoing phase I-II clinical trial of subcutaneous BHT-3021, a plasmid encoding proinsulin, is testing the safety, dose and preliminary efficacy of this therapeutic modality in recent-onset T1D patients with promising early results (http://www.bayhilltx.com/JDRF-Bayhill_Joint_Press_Release_11_08.pdf).
Glutamate decarboxylase 65
Immune therapies targeting glutamate decarboxylase 65 (GAD65), an early target of autoantibodies during the initiation of T1D (Kaufman et al., 1993; Tisch et al., 1993), have also been tested in both animal models and human T1D. Interestingly, the initial antigenic region is confined to a few epitopes near the C-terminus of the GAD protein, but later spreads intramolecularly to other GAD determinants, followed by further intermolecular spreading to other β cell antigens. Consequently, tolerance with intravenous or intrathymic injections of GAD in female NOD mice at 3 weeks of age eliminates the anti-GAD T cell responses, as well as subsequent spreading of the cascade of T cell responses to other β cell antigens and from development of insulitis or clinical diabetes (Tisch et al., 1993). Intravenous injections of GAD during the later stages of disease still effectively blocked disease progression in pre-diabetic mice and protect syngeneic islet graft survival in diabetic NOD mice (Tian et al., 1996). The identification of CD4+ Tregs in GAD-treated mice suggests a major role for bystander suppression in the induction of tolerance by treatment with this autoantigen which raises questions if GAD is targeted early in T1D (Tisch et al., 1998).
Detection of anti-GAD65 antibodies in the sera of pre-diabetic individuals is a reliable predictive marker for the progression to overt diabetes (Leslie et al., 1999). Promising preclinical data in the NOD model prompted two clinical trials using alum-formulated human recombinant GAD65. A phase II safety and dose finding trial conducted in patients with Latent Autoimmune Diabetes in Adults (LADA) (Agardh et al., 2005) showed the drug to be safe, and administration of two 20 μg subcutaneous doses one month apart led to an increase of fasting and stimulated C-peptide at 24 weeks compared to baseline, a benefit that was associated with an increase in CD4+CD25+ Tregs. The second trial used the 20 μg dosing regimen in recent-onset T1D children between 10 and 18 years of age (Ludvigsson et al., 2008). A slower decline of fasting and stimulated C-peptide was observed in the GAD-alum group compared to the placebo. More importantly, the protective effect of GAD-alum was preferentially exhibited in those who received treatment within 6 months of diagnosis suggesting that the autoimmune process is more susceptible to GAD-based modulatory therapy if initiated at an earlier stage.
Heat Shock Protein
Therapies targeting heat shock protein (hsp) have also been tested in animal models and human trials. Early controversies existed as to whether heat shock proteins (hsp) were true autoantigens implicated in the pathogenesis of T1D (Atkinson et al., 1991). However, extensive pre-clinical studies using the hsp60 peptide p277, demonstrated efficacy of peptide vaccination in halting disease progression in the NOD mice (Elias and Cohen, 1995; Elias et al., 1991). p277 treatment appeared to promote Th2 type response with up-regulation of IL-10 and IL-13, and down-regulation of IFN-γ (Elias et al., 1997; Jin et al., 2008). p277 also exerts inhibitory effects on the innate immune system via signaling through TLR-2, leading to inhibition of inflammatory lymphocyte chemotaxis (Nussbaum et al., 2006).
The equivalent of human hsp60 p277 is a 24-amino acid synthetic peptide derived from the C-terminus of the human hsp60, termed DiaPep277. Several phase I and II clinical trials in human T1D patients have been completed in Europe, while phase III trials are currently underway. A phase II trial was conducted in patients with established T1D, but with residual β cell function (Huurman et al., 2007) using a dose range of subcutaneously administered DiaPep277. Results showed a trend of dose-dependent preservation of stimulated C-peptide secretion. Three additional trials were performed in new-onset T1D patients (Lazar et al., 2007; Raz et al., 2001; Schloot et al., 2007). Two of these trials enrolled adult TID patients, while the third enrolled pediatric T1D patients. The adult trials showed significantly better preservation of insulin synthesis as measured by C-peptide production in the treated groups compared with placebo, but this effect was not seen in the pediatric trial. Similar results were observed in one other trial performed in pediatric patients (Schloot et al., 2007), although in children with less aggressive disease progression based on genetic background, there appeared to be a trend to better preserved C-peptide at the end of the study period. In summary, phase II trials with DiaPep277 have shown some promise in preserving residual β cell function, which appears to be less effective in patients with more aggressive disease. A phase III trial is currently underway with results expected in 2011.
Insulin-coupled, ECDI-fixed Antigen Presenting Cells
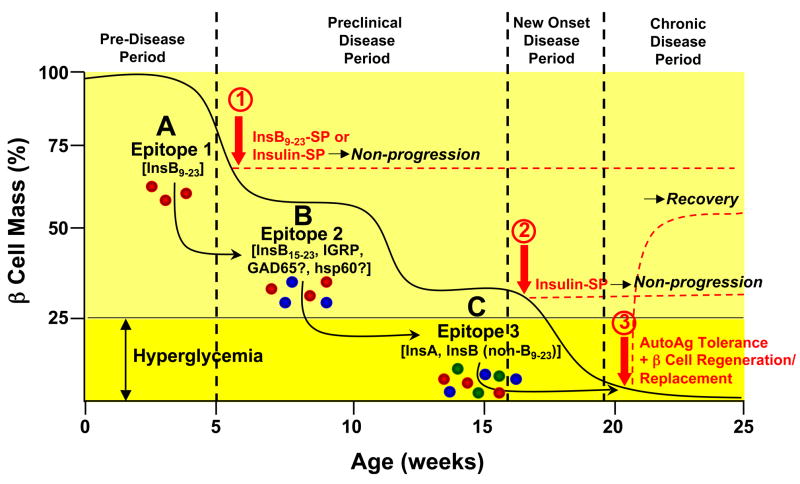
An alternative technique for effective tolerance induction for treatment of autoimmune diseases is the administration of autoantigenic peptides covalently cross-linked to cellular vehicles using ethylene carbodiimide [reviewed in (Miller et al., 2007)]. In preclinical models of various autoimmune diseases, this approach involves chemically cross-linking autoantigenic proteins or peptides to syngeneic splenic leukocytes using ethylene-carbodiimide (ECDI) (Miller et al., 1979). It has been demonstrated that intravenous injection of these antigen-coupled splenocytes (Ag-SP) is a highly efficacious method for the induction of tolerance for both the prevention and treatment of a variety of immune-mediated disorders in animal models, including the EAE model of MS (Kennedy et al., 1990; Miller et al., 2007; Tan et al., 1992), T1D in the NOD mouse (Fife et al., 2006) (Miller, et al. unpublished), and islet transplant rejection (Luo et al., 2008). Ag-SP tolerance induced by this method is indirect in that the input Ag-SP, which are induced to undergo rapid apoptotic cell death following ECDI fixation (Turley and Miller, 2007), are uptaken in the host spleen which is critical for tolerance induction as splenectomy abrogates tolerance induction to both autoantigens and alloantigens (unpublished). ECDI-fixed cells accumulate in the splenic marginal zone and induce splenic antigen presenting cells (APCs) to upregulate inhibitory costimulatory molecules (i.e. PD-L1) and to secrete regulatory cytokines (i.e. IL-10 and TGF-β) leading to unresponsiveness via two independent, but synergistic mechanisms – T cell-intrinsic PD-L1-PD-1-mediated anergy and activation of iTregs as demonstrated for regulation of EAE (Miller et al., 2007), T1D (Fife et al., 2006; Fife et al., 2009) and allogeneic islet cell transplantation (Luo et al., 2008). Reprocessing and representation of antigens coupled to apoptotic Ag-SP debris by host splenic DCs gives this strategy the advantage that tolerance to autoantigenic epitopes can be induced by cellular carriers fixed with intact proteins or even crude homogenates of the disease target organ (Kennedy et al., 1990). The mechanisms of Ag-APC tolerance are fundamentally different from tolerance strategies using mucosal antigen administration or alum injections in that unresponsiveness is exquisitely antigen-specific and does not appear to involve bystander suppression (Vanderlugt et al., 2000). This tolerance induction method is currently being tested in a recently initiated magnetic resonance imaging (MRI)-controlled Phase I-IIa clinical trial in new-onset relapsing-remitting multiple sclerosis (MS) patients at the Center for Multiple Sclerosis, Univ. of Hamburg, Germany. The trial is examining the effects of tolerance induction using peptide-coupled, ECDI-fixed autologous peripheral blood leukocytes (Ag-PBL) coupled with a cocktail of seven myelin peptides (encompassing immunodominant MS-associated CD4 T cell epitopes on three separate myelin proteins) in an attempt to inhibit the potential of epitope spreading to multiple endogenous myelin epitopes. A second clinical trial using insulin-coupled PBLs for prevention of T1D is currently under development by the Immune Tolerance Network. The ability to simultaneously target multiple myelin epitopes has been demonstrated in several mouse EAE models employing Ag-SP tolerance (Smith and Miller, 2006) and is likely to be important in T1D as epitope spreading is an important component of disease pathogenesis in the NOD mice (Figure 1). Disease appears to be initiated by T cell responses to the immunodominant InsB9–23 epitope and then spread to other insulin epitopes as illustrated by the finding that tolerance induced in young NODs by splenocytes coupled with either intact insulin or InsB9–23 inhibits development of T1D, but prevention of new onset disease (18–20 weeks in our colony) can only be induced by tolerance to intact insulin (unpublished). This suggests that InsB9–23 is an initiating diabetogenic epitope in NOD mice, as supported by a recently reported genetic approach (Nakayama et al., 2005), and that the response evolves to target other insulin epitopes outside of this region as mice transition to overt hyperglycemia. A similar scenario of epitope spreading is postulated to occur in human T1D which will influence the antigenic specificities needed to be targeted in tolerance-based immunoregulatory strategies.
Figure 1. Model of Epitope Spreading and Tolerance Therapy in the Pathogenesis of Type 1 Diabetes in the NOD Mouse.
Progression of T1D in the NOD mouse involves the sequential activation of autoreactive T cells to multiple diabetogenic epitopes via epitope spreading which accumulate until clinical diagnosis when sufficient autoreactive effector cells are present to cause destruction of the majority of the β cell mass. The insulin B chain epitope 9–23 (InsB9–23) (A - red effector cells) appears to be the initiating or very early pathogenic diabetogenic epitope in the NOD mouse based on the ability of tolerance induced by ECDI-fixed splenocytes coupled with either intact insulin or InsB9–23 in 4–6 week old mice to inhibit development of clinical diabetes (1). As β cell destruction continues responses to additional islet antigens, e.g. InsB15–23 and/or IRGP (B - blue effector cells) and eventually epitopes on the insulin A or B chains (C - green effector cells) are activated. Epitopes on the InsA or Ins B chain (outside of B9–23) epitopes on chain appear to be dominant at the stage of transition to overt disease (loss of approximately 75% of islet mass) based on the ability tolerance induced by insulin-coupled, but not InsB9–23-coupled, splenocytes to ameliorate disease progression in 18–20 week old NOD mice (2). Recovery from (i.e., reversal) chronic T1D when all of the β cells have been destroyed would be expected to require a combination of tolerance to the autoantigens which were responsible for initial β cell destruction and a β cell regeneration and/or replacement strategy which may require allo- or xenoantigen tolerance in therapies involving islet cell transplantation (3). A similar pattern of epitope spreading is postulated to occur in human T1D.
Combination therapies
The lack of permanent remission of T1D with any single agent suggests that combination therapies may be required for treating T1D. A combination of approaches may be needed for effective prevention of disease or reversal of new-onset T1D. Various broader spectrum immunoregulatory or suppressive agents used in combination or together with antigen-specific tolerance strategies have been tested in animal models of T1D and in a limited number of clinical trials.
As effector T cell responses are highly influenced by the cytokines in the environment, combination of an agent that can create a tolerogenic environment with a diabetogenic antigen would be predicted to better modulate antigen response. Synergy has been observed in reversal of diabetes in the NOD and lymphocytic chroriomeningitis virus (LCMV) models of the disease when insulin peptide was administered intranasally together with anti-CD3 mAb (Bresson et al., 2006). Insulin peptide-specific T cells isolated from these mice exhibited regulatory function and produced IL-10 and TGFβ in response to antigen. This synergy likely involved both the reduction of the ongoing response by the anti-CD3 mAb in combination with the induction of antigen-specific Tregs since neither treatment alone was able to induce the antigen-specific regulatory cells. Other drug combinations have shown synergistic effects in the NOD T1D model, e.g. synergy between IL-1 blockade with anti-CD3 mAb therapy (unpublished). Interestingly, despite the complementary effects on effector cells while promoting expansion of Tregs, rapamycin negated the effects of anti-CD3 mAb on diabetes in NOD mice without altering the frequency or phenotype of T cells. Even mice that had been rendered normoglycemic with anti-CD3 mAb had their tolerance broken by treatment with rapamycin. Other studies have combined immunologic approaches with approaches aimed at restoring β cell function to achieve glycemic control. For example, the combination of a glucagon-like peptide 1 (GLP-1) receptor agonist (Exendin-4) was found to augment β cell function in diabetic mice treated with anti-CD3 mAb or ATG (Ogawa et al., 2004; Sherry et al., 2007). There was little evidence for immune effects but the insulin content of pancreatic β cells was increased, possibly by enhancing recovery of degranulated β cells that can be identified in islets at the time of diagnosis.
There are few completed human trials with combinations of immune modulators, in part because of the regulatory issues involved with testing unapproved drugs. Published studies have been limited to agents that have previously been approved for use in other illnesses. A combination trial of IL-2 with rapamycin is currently underway supported by the Immune Tolerance Network (http://www.immuntolerance.org). This approach is based on the complementary actions of the two agents to cause activation induced cell death with sparing and perhaps expansion of Tregs. An older study involved the combination of Azathioprine and Prednisone, which showed efficacy comparable to other agents such as CSA (Silverstein et al., 1988). The most notable combination has been the use of autologous non-myeloablative hematopoietic stem cell transplantation in subjects with new onset T1D. Subjects received pretreatment with cyclophosphamide and granulocyte-colony stimulating factor (G-CSF) to expand CD34+ cells that were harvested and re-infused after treatment of subjects with ATG and cyclophosphamide. Unlike the experience in other immune modulation trials, 14 of 15 patients were rendered insulin free for an average of 16 months (Couri et al., 2009; Voltarelli et al., 2007). Toxicity was a clear problem – oligospermia was seen in 10 of 22 subjects and 1 case of pneumonia was reported. Nonetheless, the extent and duration of insulin recovery was unequaled by other approaches.
Forward Thinking
Antigen-induced and/or antigen-specific Treg-mediated tolerance-based strategy targeting only autoreactive T cells in the absence of long-term application of broad- based immunoregulatory or suppressive drugs or antibodies is the targeted immunotherapy for prevention or early reversal of T1D. Ideally the tolerance therapy would specifically target β cell antigens involved in initiation of disease pathogenesis as well as identified endogenous islet autoantigens which may be recruited to become targets of the ongoing autoimmune disease process via epitope spreading. Antigen- or Treg-induced tolerance therapies must also be durable, i.e. have the ability to regulate the autoimmune response permanently or at least for many years following induction perhaps acting in part via the activity of renewable populations of autoantigen-specific Tregs. Depending on the status of the autoimmune repertoire at the time therapy is initiated, tolerance induction may also have to be combined with or induced shortly following a tolerable immunoregulatory treatment (small molecule or antibody-based) which can function to reduce the autoantigen-specific T cell frequency to a level which can be effectively and durably suppressed. In addition, additional drugs may be required in combination to promote β cell regeneration. Regardless of the tolerance method employed for therapy, early intervention in T1D patients is critical to prevent ongoing islet destruction and to establish a microenvironment conducive to allow for the recovery of a normal β cell mass from endogenous progenitor cells. The chances for disease prevention will be improved by the identification of biomarkers identifying patients at risk as early in the disease process as possible.
Cellular adoptive transfer-based approaches have shown significant promises in pre-clinical NOD models, both in pre-diabetic and post-diabetic stages. Specifically, both ex vivo expanded nTregs or induced CD4+CD25+Foxp3+ Tregs (iTregs) have been shown to control ongoing autoimmunity and either prevent progression to overt diabetes or protect syngeneic islet grafts and/or allow unperturbed β cell recovery thereby inducing diabetes remission in NOD mice (Godebu et al., 2008; Luo et al., 2007; Tang et al., 2004; Weber et al., 2006). It is unclear if antigen specificity is critically important in this approach as both non-specifically expanded or induced Tregs and islet antigen-specific Tregs have shown efficacy in controlling the disease. Additionally, it also appears that Tregs of one antigen specificity may be sufficient in controlling ongoing autoimmunity that is likely caused by auto-aggressive T cells of multiple islet antigen specificities (Luo et al., 2007; Tarbell et al., 2004). Clearly delineating these characteristics of Treg adoptive transfer therapy will have significant impact on the design of future clinical trials using this modality.
Another strategy for enhancing Treg numbers in vivo is by dendritic cell-based therapy. It has been shown that direct injection of either dendritic cells from pancreatic draining lymph nodes or β cell antigen-pulsed immature dendritic cells protect prediabetic NOD mice from developing overt diabetes, possibly through the in vivo induction of Treg cells (Clare-Salzler et al., 1992; Lo et al., 2006). However, direct ex vivo dendritic cell therapy carries the potential risk of their acquiring an activated phenotype activation upon adoptive transfer, leading to ultimate immunity rather than tolerance. An alternative approach for targeting dendritic cells for tolerance induction is by the in vivo delivery of cognate antigens to steady-state dendritic cells through the endocytic receptor DEC 205 (Bonifaz et al., 2002). It has been recently shown that delivery of β cell antigens in such a fashion leads to deletion of diabetogenic CD8+ T cells in the context of ongoing autoimmunity (Mukhopadhaya et al., 2008). Ultimately, adoptive cell therapies that target both the CD4 and the CD8 compartments (Han et al., 2005; Santamaria, 2008) may provide synergy for protection against ongoing autoimmunity.
The question then becomes what is the ideal therapy to treat patients with long-standing T1D who have presumably destroyed all or the majority of their β cell mass perhaps including renewable β cell progenitor cells? Again tolerance-based therapies would be ideal in early onset, but intervention late in disease would still require that pancreatic autoantigen-specific processes be targeted prior to the transplant of stem cells capable of differentiation into insulin-producing β cells or by transplantation of allogeneic (islets harvested from cadaver donors) or xenogeneic (e.g., porcine islet) islet cells. The critical requirement for autoantigen tolerance in advanced disease is amply illustrated by the fact that healthy islets from young NODs transplanted into long-term diabetic NOD recipients are vigorously rejected due to the residual autoimmune responses (Tian et al., 1996) and by anecdotal human data where pancreas transplants from identical twins are rejected (Sibley et al., 1985). Assuming that the immunosuppressive drugs required for the conditioning and/or maintenance of allo- or xenografts may not be compatible with induction or maintenance autoantigen-specific tolerance, future therapies attempting reversal of overt diabetes in long-standing T1D patients secondary to islet transplantation will likely require tolerance to diabetogenic autoantigens combined with tolerance to the alloantigens or xenoantigens on the donor islets, an approach currently under test in the NOD model using ECDI-fixed cells (Luo et al., 2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach P, Bonifacio E, Ziegler AG. Predicting type 1 diabetes. Curr Diab Rep. 2005;5:98–103. doi: 10.1007/s11892-005-0035-y. [DOI] [PubMed] [Google Scholar]
- Agardh CD, Cilio CM, Lethagen A, Lynch K, Leslie RD, Palmer M, Harris RA, Robertson JA, Lernmark A. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005;19:238–246. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Aspord C, Thivolet C. Nasal administration of CTB-insulin induces active tolerance against autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol. 2002;130:204–211. doi: 10.1046/j.1365-2249.2002.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assan R, Timsit J, Feutren G, Bougneres P, Czernichow P, Hannedouche T, Boitard C, Noel LH, Mihatsch MJ, Bach JF. The kidney in cyclosporin A-treated diabetic patients: a long-term clinicopathological study. Clin Nephrol. 1994;41:41–49. [PubMed] [Google Scholar]
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Holmes LA, Scharp DW, Lacy PE, Maclaren NK. No evidence for serological autoimmunity to islet cell heat shock proteins in insulin dependent diabetes. J Clin Invest. 1991;87:721–724. doi: 10.1172/JCI115051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Maclaren NK, Luchetta R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes. 1990;39:933–937. doi: 10.2337/diab.39.8.933. [DOI] [PubMed] [Google Scholar]
- Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:4610–4614. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacio E, Ziegler A, Achenbach P, Barker J, Eisenbarth G. Translating mucosal antigen based prevention of autoimmune diabetes to human. Novartis Found Symp. 2008;292:187–199. doi: 10.1002/9780470697405.ch17. discussion 199–201, 202–183. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougneres PF, Carel JC, Castano L, Boitard C, Gardin JP, Landais P, Hors J, Mihatsch MJ, Paillard M, Chaussain JL, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med. 1988;318:663–670. doi: 10.1056/NEJM198803173181103. [DOI] [PubMed] [Google Scholar]
- Bougneres PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, Chaussain JL, Bach JF. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39:1264–1272. doi: 10.2337/diab.39.10.1264. [DOI] [PubMed] [Google Scholar]
- Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet. 2000;356:545–549. doi: 10.1016/s0140-6736(00)02579-4. [DOI] [PubMed] [Google Scholar]
- Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol. 2003;3:123–132. doi: 10.1038/nri1000. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- Chatenoud L, Salomon B, Bluestone JA. Suppressor T cells--they’re back and critical for regulation of autoimmunity! Immunol. Rev. 2001;182:149–163. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Bergerot I, Elliott JF, Harrison LC, Abiru N, Eisenbarth GS, Delovitch TL. Evidence that a peptide spanning the B-C junction of proinsulin is an early Autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol. 2001;167:4926–4935. doi: 10.4049/jimmunol.167.9.4926. [DOI] [PubMed] [Google Scholar]
- Chung DT, Korn T, Richard J, Ruzek M, Kohm AP, Miller S, Nahill S, Oukka M. Anti-thymocyte globulin (ATG) prevents autoimmune encephalomyelitis by expanding myelin antigen-specific Foxp3+ regulatory T cells. Int Immunol. 2007;19:1003–1010. doi: 10.1093/intimm/dxm078. [DOI] [PubMed] [Google Scholar]
- Clare-Salzler MJ, Brooks J, Chai A, Van Herle K, Anderson C. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest. 1992;90:741–748. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simoes BP, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- Daniel D, Wegmann DR. Intranasal administration of insulin peptide B: 9–23 protects NOD mice from diabetes. Ann N Y Acad Sci. 1996;778:371–372. doi: 10.1111/j.1749-6632.1996.tb21146.x. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Trial– Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- Donath MY, Mandrup-Poulsen T. The use of interleukin-1-receptor antagonists in the treatment of diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2008;4:240–241. doi: 10.1038/ncpendmet0783. [DOI] [PubMed] [Google Scholar]
- Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A. 2007;104:5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth GS, Srikanta S, Jackson R, Rabinowe S, Dolinar R, Aoki T, Morris MA. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res. 1985;2:271–276. [PubMed] [Google Scholar]
- Elias D, Cohen IR. Treatment of autoimmune diabetes and insulitis in NOD mice with heat shock protein 60 peptide p277. Diabetes. 1995;44:1132–1138. doi: 10.2337/diab.44.9.1132. [DOI] [PubMed] [Google Scholar]
- Elias D, Meilin A, Ablamunits V, Birk OS, Carmi P, Konen-Waisman S, Cohen IR. Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and downregulates autoimmunity to various beta-cell antigens. Diabetes. 1997;46:758–764. doi: 10.2337/diab.46.5.758. [DOI] [PubMed] [Google Scholar]
- Elias D, Reshef T, Birk OS, van der Zee R, Walker MD, Cohen IR. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991;88:3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Every AL, Kramer DR, Mannering SI, Lew AM, Harrison LC. Intranasal vaccination with proinsulin DNA induces regulatory CD4+ T cells that prevent experimental autoimmune diabetes. J Immunol. 2006;176:4608–4615. doi: 10.4049/jimmunol.176.8.4608. [DOI] [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, Du Rostu H, Rodier M, Sirmai J, Lallemand A, et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2:119–124. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009 doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fousteri G, von Herrath M, Bresson D. Mucosal exposure to antigen: cause or cure of type 1 diabetes? Curr Diab Rep. 2007;7:91–98. doi: 10.1007/s11892-007-0017-3. [DOI] [PubMed] [Google Scholar]
- Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, Kent SC, Hering BJ, West E, Steck A, et al. Dimorphic histopathology of longstanding childhood-onset diabetes. Diabetologia. 2010;53:690–698. doi: 10.1007/s00125-009-1642-y. [DOI] [PubMed] [Google Scholar]
- Godebu E, Summers-Torres D, Lin MM, Baaten BJ, Bradley LM. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J Immunol. 2008;181:1798–1805. doi: 10.4049/jimmunol.181.3.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, Santamaria P. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11:645–652. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J Exp Med. 1996;184:2167–2174. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, Bonifacio E, Couper JJ, Colman PG. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27:2348–2355. doi: 10.2337/diacare.27.10.2348. [DOI] [PubMed] [Google Scholar]
- Herold KC, Bluestone JA, Montag AG, Parihar A, Wiegner A, Gress RE, Hirsch R. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes. 1992;41:385–391. doi: 10.2337/diab.41.3.385. [DOI] [PubMed] [Google Scholar]
- Herold KC, Brooks-Worrell B, Palmer J, Dosch HM, Peakman M, Gottlieb P, Reijonen H, Arif S, Spain LM, Thompson C, Lachin JM. Validity and reproducibility of measurement of islet autoreactivity by T-cell assays in subjects with early type 1 diabetes. Diabetes. 2009;58:2588–2595. doi: 10.2337/db09-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala) J Clin Invest. 2003;111:409–418. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huurman VA, Decochez K, Mathieu C, Cohen IR, Roep BO. Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab Res Rev. 2007;23:269–275. doi: 10.1002/dmrr.691. [DOI] [PubMed] [Google Scholar]
- Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, Waguri M, Imagawa A, Tamura S, Inada M, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92:2313–2322. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob CO, Aiso S, Michie SA, McDevitt HO, Acha-Orbea H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci U S A. 1990;87:968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Zhu A, Wang Y, Chen Q, Xiong Q, Li J, Sun Y, Li T, Cao R, Wu J, Liu J. A Th1-recognized peptide P277, when tandemly repeated, enhances a Th2 immune response toward effective vaccines against autoimmune diabetes in nonobese diabetic mice. J Immunol. 2008;180:58–63. doi: 10.4049/jimmunol.180.1.58. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MK, Tan LJ, Dal Canto MC, Tuohy VK, Lu ZJ, Trotter JL, Miller SD. Inhibition of murine relapsing experimental autoimmune encephalomyelitis by immune tolerance to proteolipid protein and its encephalitogenic peptides. J Immunol. 1990;144:909–915. [PubMed] [Google Scholar]
- Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, Hering BJ, Hafler DA. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, Bluestone JA, Miller SD. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- Kolb H, Burkart V. Nicotinamide in type 1 diabetes. Mechanism of action revisited. Diabetes Care. 1999;22(Suppl 2):B16–20. [PubMed] [Google Scholar]
- Koulmanda M, Bhasin M, Hoffman L, Fan Z, Qipo A, Shi H, Bonner-Weir S, Putheti P, Degauque N, Libermann TA, et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci U S A. 2008;105:16242–16247. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupacis A, Stiller CR, Gardell C, Keown P, Dupre J, Wallace AC, Thibert P. Cyclosporin prevents diabetes in BB Wistar rats. Lancet. 1983;1:10–12. doi: 10.1016/s0140-6736(83)91558-1. [DOI] [PubMed] [Google Scholar]
- Lazar L, Ofan R, Weintrob N, Avron A, Tamir M, Elias D, Phillip M, Josefsberg Z. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabetes Metab Res Rev. 2007;23:286–291. doi: 10.1002/dmrr.711. [DOI] [PubMed] [Google Scholar]
- Leslie RD, Atkinson MA, Notkins AL. Autoantigens IA-2 and GAD in Type I (insulin-dependent) diabetes. Diabetologia. 1999;42:3–14. doi: 10.1007/s001250051105. [DOI] [PubMed] [Google Scholar]
- Lo J, Peng RH, Barker T, Xia CQ, Clare-Salzler MJ. Peptide-pulsed immature dendritic cells reduce response to beta cell target antigens and protect NOD recipients from type i diabetes. Ann N Y Acad Sci. 2006;1079:153–156. doi: 10.1196/annals.1375.023. [DOI] [PubMed] [Google Scholar]
- Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105:18895–18900. doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, Miller SD. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding R, Steinman RM, Suthanthiran M. Dendritic cells with TGF-{beta}1 differentiate naive CD4+CD25− T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez NR, Augstein P, Moustakas AK, Papadopoulos GK, Gregori S, Adorini L, Jackson DC, Harrison LC. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest. 2003;111:1365–1371. doi: 10.1172/JCI17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–1249. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Appel MC, O’Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988;140:52–58. [PubMed] [Google Scholar]
- Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Suko M, Okudaira H, Matsuba I, Tsuruoka A, Sasaki A, Yokoyama H, Tanase T, Shida T, Nishimura M, et al. Preventive effects of cyclosporin on diabetes in NOD mice. Diabetologia. 1986;29:244–247. doi: 10.1007/BF00454884. [DOI] [PubMed] [Google Scholar]
- Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, Steinman RM, Tarbell KV, DiLorenzo TP. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci U S A. 2008;105:6374–6379. doi: 10.1073/pnas.0802644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanto-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipila JI, Haavisto L, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- Nussbaum G, Zanin-Zhorov A, Quintana F, Lider O, Cohen IR. Peptide p277 of HSP60 signals T cells: inhibition of inflammatory chemotaxis. Int Immunol. 2006;18:1413–1419. doi: 10.1093/intimm/dxl074. [DOI] [PubMed] [Google Scholar]
- O’Brien BA, Harmon BV, Cameron DP, Allan DJ. Nicotinamide prevents the development of diabetes in the cyclophosphamide-induced NOD mouse model by reducing beta-cell apoptosis. J Pathol. 2000;191:86–92. doi: 10.1002/(SICI)1096-9896(200005)191:1<86::AID-PATH573>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes. 2004;53:1700–1705. doi: 10.2337/diabetes.53.7.1700. [DOI] [PubMed] [Google Scholar]
- Orban T, Farkas K, Jalahej H, Kis J, Treszl A, Falk B, Reijonen H, Wolfsdorf J, Ricker A, Matthews JB, et al. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J Autoimmun. 2009 doi: 10.1016/j.jaut.2009.1010.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, Spera S, Suraci C, Multari G, Cervoni M, et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia. 2000;43:1000–1004. doi: 10.1007/s001250051482. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990;71:152–156. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- Santamaria P. Genetic and therapeutic control of diabetogenic CD8+ T cells. Novartis Found Symp. 2008;292:130–136. doi: 10.1002/9780470697405.ch12. discussion 136–145, 202–133. [DOI] [PubMed] [Google Scholar]
- Saudek F, Havrdova T, Boucek P, Karasova L, Novota P, Skibova J. Polyclonal anti-T-cell therapy for type 1 diabetes mellitus of recent onset. Rev Diabet Stud. 2004;1:80–88. doi: 10.1900/RDS.2004.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloot NC, Meierhoff G, Lengyel C, Vandorfi G, Takacs J, Panczel P, Barkai L, Madacsy L, Oroszlan T, Kovacs P, et al. Effect of heat shock protein peptide DiaPep277 on beta-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev. 2007;23:276–285. doi: 10.1002/dmrr.707. [DOI] [PubMed] [Google Scholar]
- Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD. Ig mu null mice J Exp Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfert-Margolis V, Gisler TD, Asare AL, Wang RS, Dosch HM, Brooks-Worrell B, Eisenbarth GS, Palmer JP, Greenbaum CJ, Gitelman SE, et al. Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes. 2006;55:2588–2594. doi: 10.2337/db05-1378. [DOI] [PubMed] [Google Scholar]
- Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989;320:550–554. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- Sherr J, Sosenko J, Skyler JS, Herold KC. Prevention of type 1 diabetes: the time has come. Nat Clin Pract Endocrinol Metab. 2008;4:334–343. doi: 10.1038/ncpendmet0832. [DOI] [PubMed] [Google Scholar]
- Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology. 2007;148:5136–5144. doi: 10.1210/en.2007-0358. [DOI] [PubMed] [Google Scholar]
- Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–3245. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Sibley RK, Sutherland DE, Goetz F, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985;53:132–144. [PubMed] [Google Scholar]
- Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988;319:599–604. doi: 10.1056/NEJM198809083191002. [DOI] [PubMed] [Google Scholar]
- Simon G, Parker M, Ramiya V, Wasserfall C, Huang Y, Bresson D, Schwartz RF, Campbell-Thompson M, Tenace L, Brusko T, et al. Murine antithymocyte globulin therapy alters disease progression in NOD mice by a time-dependent induction of immunoregulation. Diabetes. 2008;57:405–414. doi: 10.2337/db06-1384. [DOI] [PubMed] [Google Scholar]
- Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- Smith CE, Miller SD. Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities. J Autoimmunity. 2006;27:218–231. doi: 10.1016/j.jaut.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Tang Q, Bluestone JA. Partial TCR signals delivered by FcR-nonbinding anti-CD3 monoclonal antibodies differentially regulate individual Th subsets. J Immunol. 1998;160:4841–4849. [PubMed] [Google Scholar]
- Smith JA, Tso JY, Clark MR, Cole MS, Bluestone JA. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J Exp Med. 1997;185:1413–1422. doi: 10.1084/jem.185.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, et al. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2006;29:643–649. doi: 10.2337/diacare.29.03.06.dc05-1006. [DOI] [PubMed] [Google Scholar]
- Stiller CR, Dupre J, Gent M, Jenner MR, Keown PA, Laupacis A, Martell R, Rodger NW, von Graffenried B, Wolfe BM. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984;223:1362–1367. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- Tan LJ, Kennedy MK, Miller SD. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. II. Fine specificity of effector T cell inhibition. J Immunol. 1992;148:2748–2755. [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas HE, Irawaty W, Darwiche R, Brodnicki TC, Santamaria P, Allison J, Kay TW. IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes. 2004;53:113–121. doi: 10.2337/diabetes.53.1.113. [DOI] [PubMed] [Google Scholar]
- Tian J, Clare-Salzler M, Herschenfeld A, Middleton B, Newman D, Mueller R, Arita S, Evans C, Atkinson MA, Mullen Y, et al. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat Med. 1996;2:1348–1353. doi: 10.1038/nm1296-1348. [DOI] [PubMed] [Google Scholar]
- Tisch R, Liblau RS, Yang XD, Liblau P, McDevitt HO. Induction of GAD65-specific regulatory T-cells inhibits ongoing autoimmune diabetes in nonobese diabetic mice. Diabetes. 1998;47:894–899. doi: 10.2337/diabetes.47.6.894. [DOI] [PubMed] [Google Scholar]
- Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- Turley DM, Miller SD. Peripheral tolerance Induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Eagar TN, Neville KL, Nikcevich KM, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J Immunol. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simoes BP, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- von Herrath M. Diabetes: A virus-gene collaboration. Nature. 2009;459:518–519. doi: 10.1038/459518a. [DOI] [PubMed] [Google Scholar]
- Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, Ziegler SF, Bradley LM. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B Lymphocyte Depletion by CD20 Monoclonal Antibody Prevents Diabetes in Nonobese Diabetic Mice despite Isotype-Specific Differences in Fc{gamma}R Effector Functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PW, Bluestone JA, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nonaka K, Hanafusa T, Miyazaki A, Toyoshima H, Tarui S. Preventive and therapeutic effects of large-dose nicotinamide injections on diabetes associated with insulitis. An observation in nonobese diabetic (NOD) mice. Diabetes. 1982;31:749–753. doi: 10.2337/diab.31.9.749. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci USA. 1991;88:10252–10256. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]