Abstract
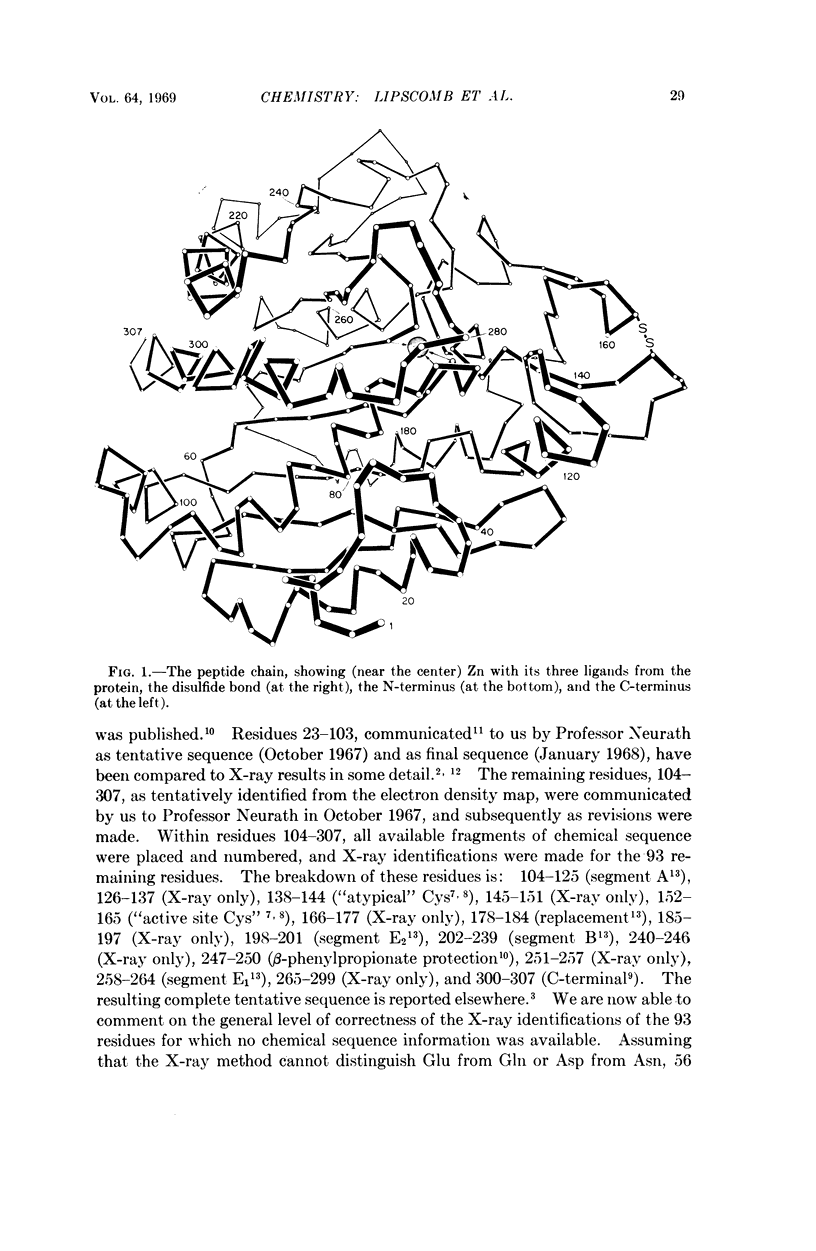
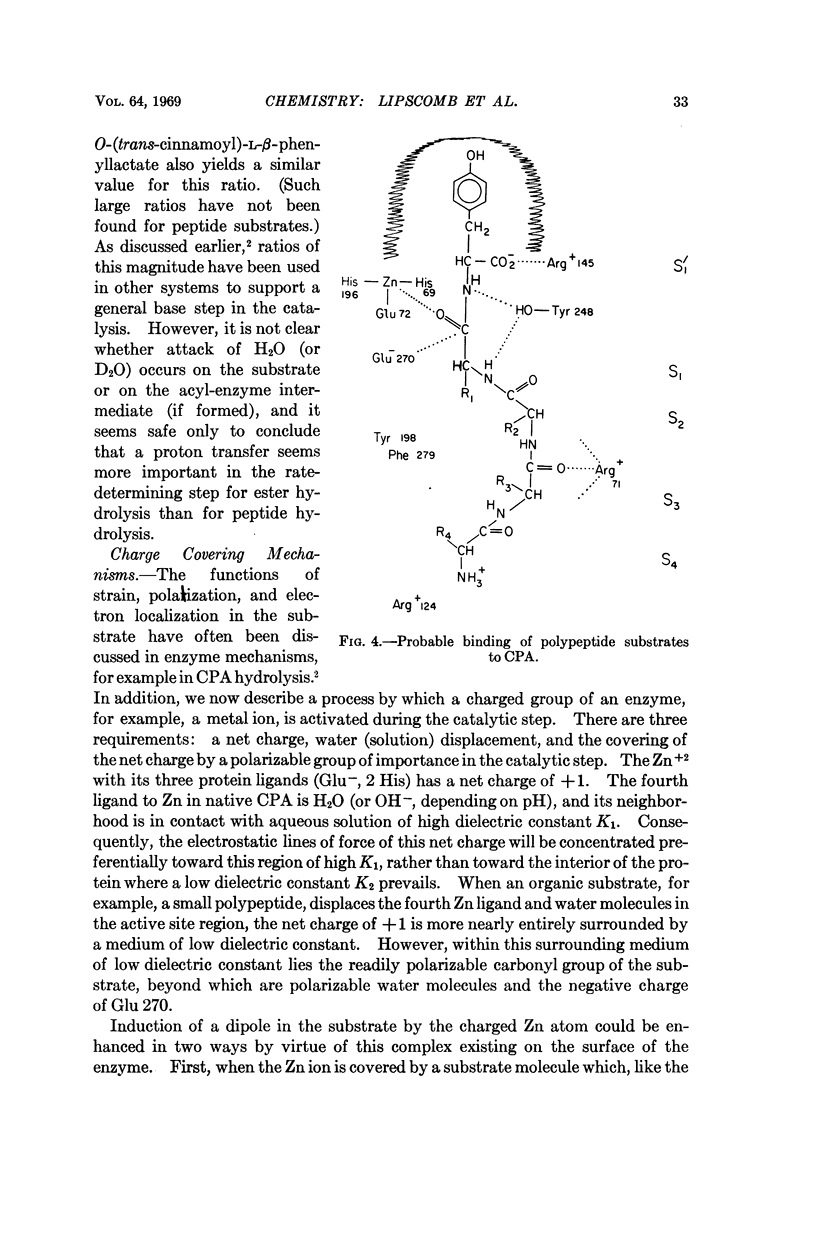
Several features of carboxypeptidase A (CPA) which were previously established by the X-ray diffraction structure studies have now been confirmed by the chemical sequence analysis. These results include the number (307) of amino acid residues in CPAα, the identities of the residues (Arg 145, Glu 270, and Tyr 248) shown by the X-ray study to be involved in substrate binding and catalysis, and the existence of a disulfide bond.
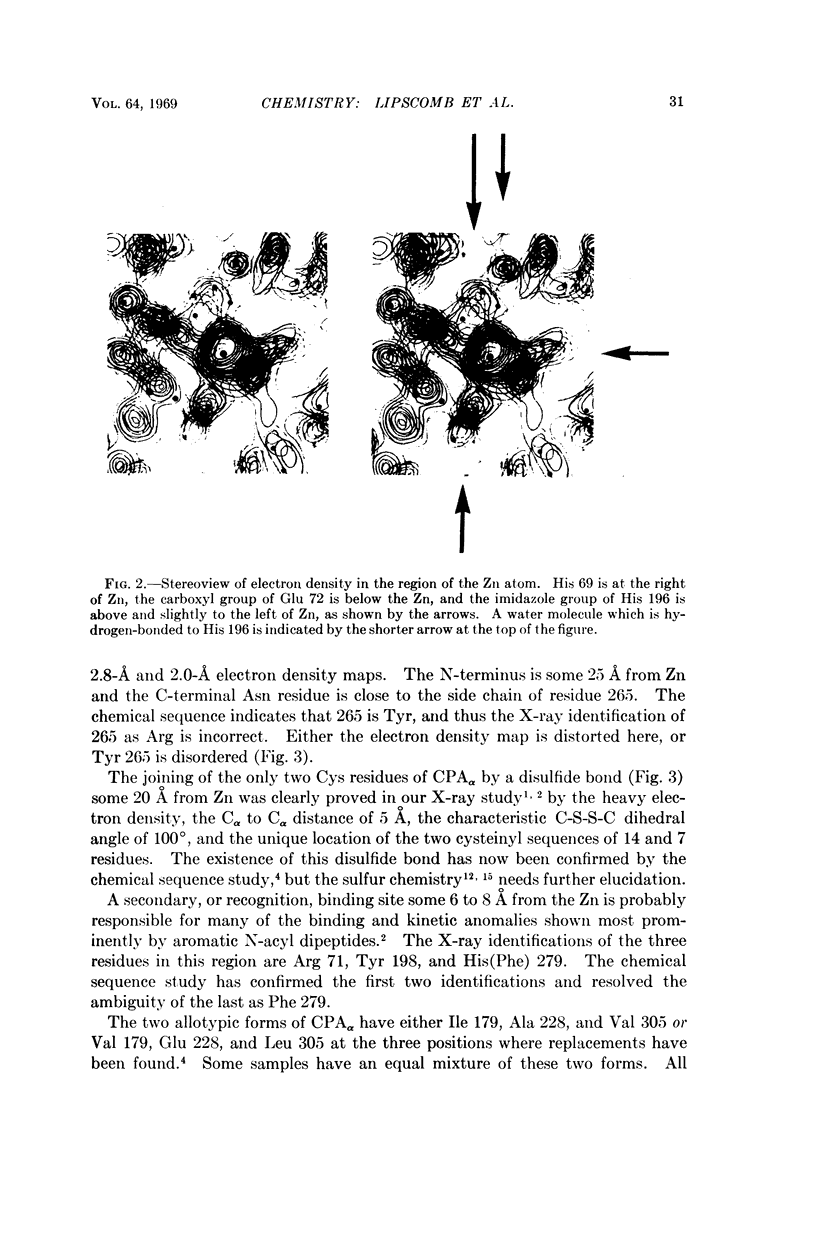
The Zn ligands, shown by the X-ray study to be residues 69, 72, and 196 and identified as His, Glx, and either Glx or Lys, are proved by the chemical sequence to be His, Glu, and His, respectively.
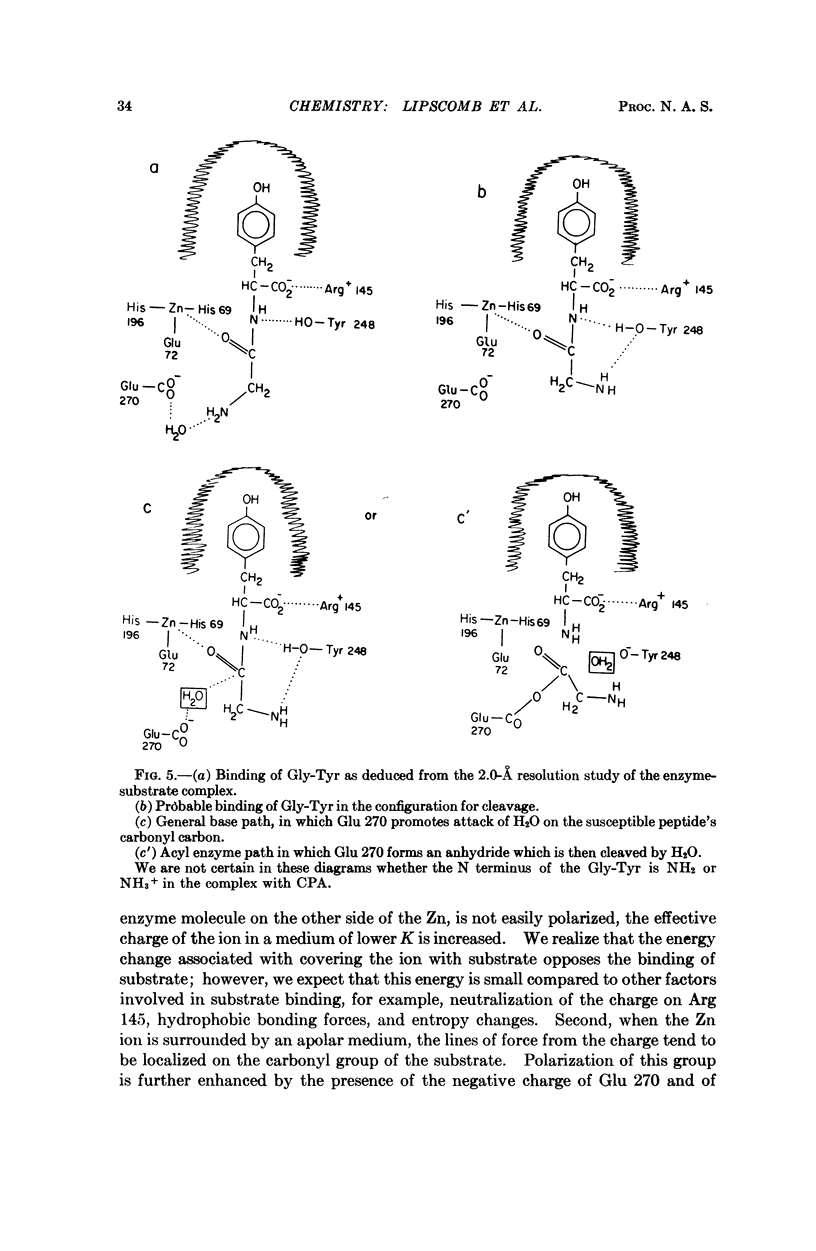
No change is required in our previous mechanistic deductions, which are here extended to include a specific mechanism of activation of the substrate by a net charge on the metal ion, which suffers a change in local dielectric constant when it is covered by a substrate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARGETZI J. P., THOMPSON E. O., SAMPATHKUMAR K. S., WALSH K. A., NEURATH H. THE AMINO- AND CARBOXYL-TERMINAL RESIDUES AND THE SELF-DIGESTION OF BOVINE PANCREATIC CARBOXYPEPTIDASE A. J Biol Chem. 1964 Nov;239:3767–3774. [PubMed] [Google Scholar]
- Bradshaw R. A., Ericsson L. H., Walsh K. A., Neurath H. The amino acid sequence of bovine carboxypeptidase A. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1389–1394. doi: 10.1073/pnas.63.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR K. S., CLEGG J. B., WALSH K. A. THE N-TERMINAL SEQUENCE OF BOVINE CARBOXYPEPTIDASE A AND ITS RELATION OF ZYMOGEN ACTIVATION. Biochemistry. 1964 Nov;3:1728–1732. doi: 10.1021/bi00899a024. [DOI] [PubMed] [Google Scholar]
- Kaiser B. L., Kaiser E. T. Effect of D2O on the carboxypeptidase-catalyzed hydrolysis of O-(trans-cinnamoyl)-L-beta-phenyllactate and N-(N-benzoylglycyl)-L-phenylalanine. Proc Natl Acad Sci U S A. 1969 Sep;64(1):36–41. doi: 10.1073/pnas.64.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEURATH H. MECHANISM OF ZYMOGEN ACTIVATION. Fed Proc. 1964 Jan-Feb;23:1–7. [PubMed] [Google Scholar]
- Reeke G. N., Hartsuck J. A., Ludwig M. L., Quiocho F. A., Steitz T. A., Lipscomb W. N. The structure of carboxypeptidase a, vi. Some results at 2.0-a resolution, and the complex with glycyl-tyrosine at 2.8-a resolution. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2220–2226. doi: 10.1073/pnas.58.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roholt O. A., Pressman D. The sequence around the active-center tyrosyl residue of bovine pancreatic carboxypeptidase A. Proc Natl Acad Sci U S A. 1967 Jul;58(1):280–285. doi: 10.1073/pnas.58.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]