Abstract
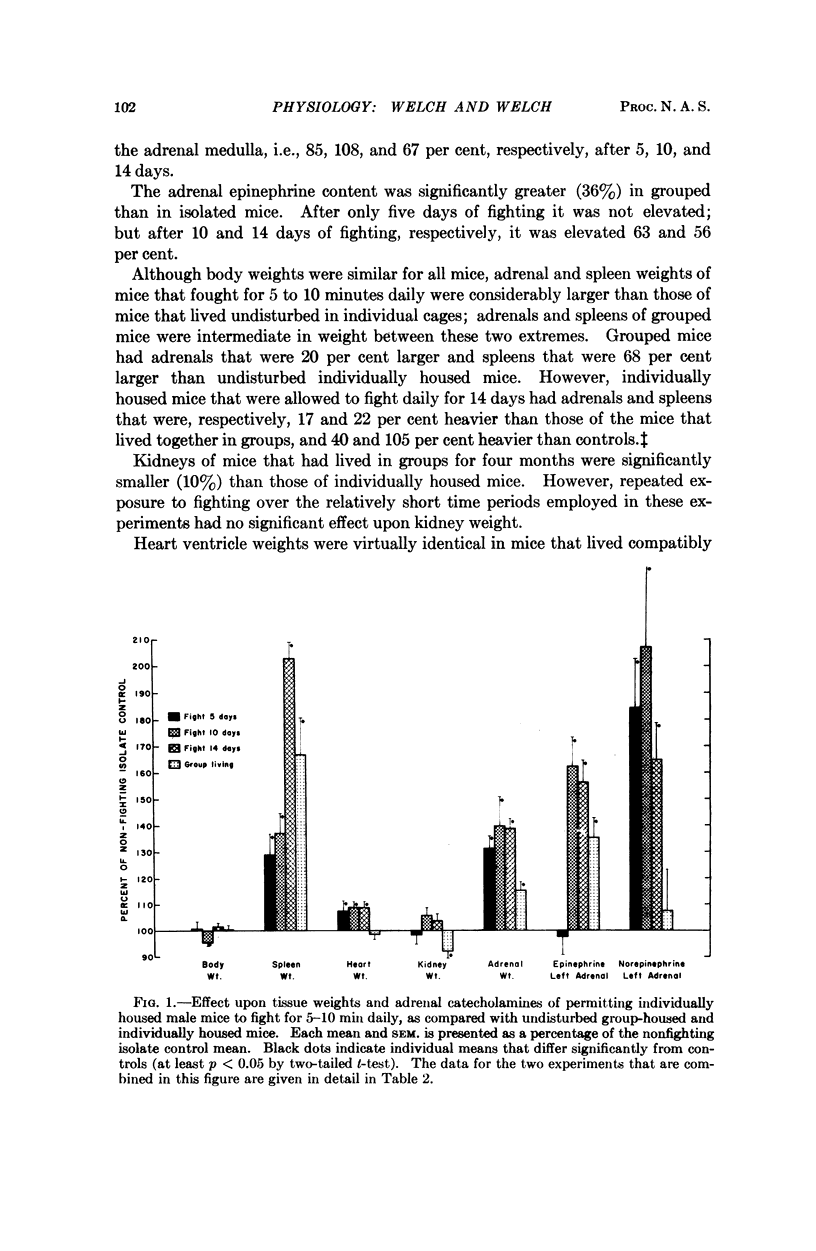
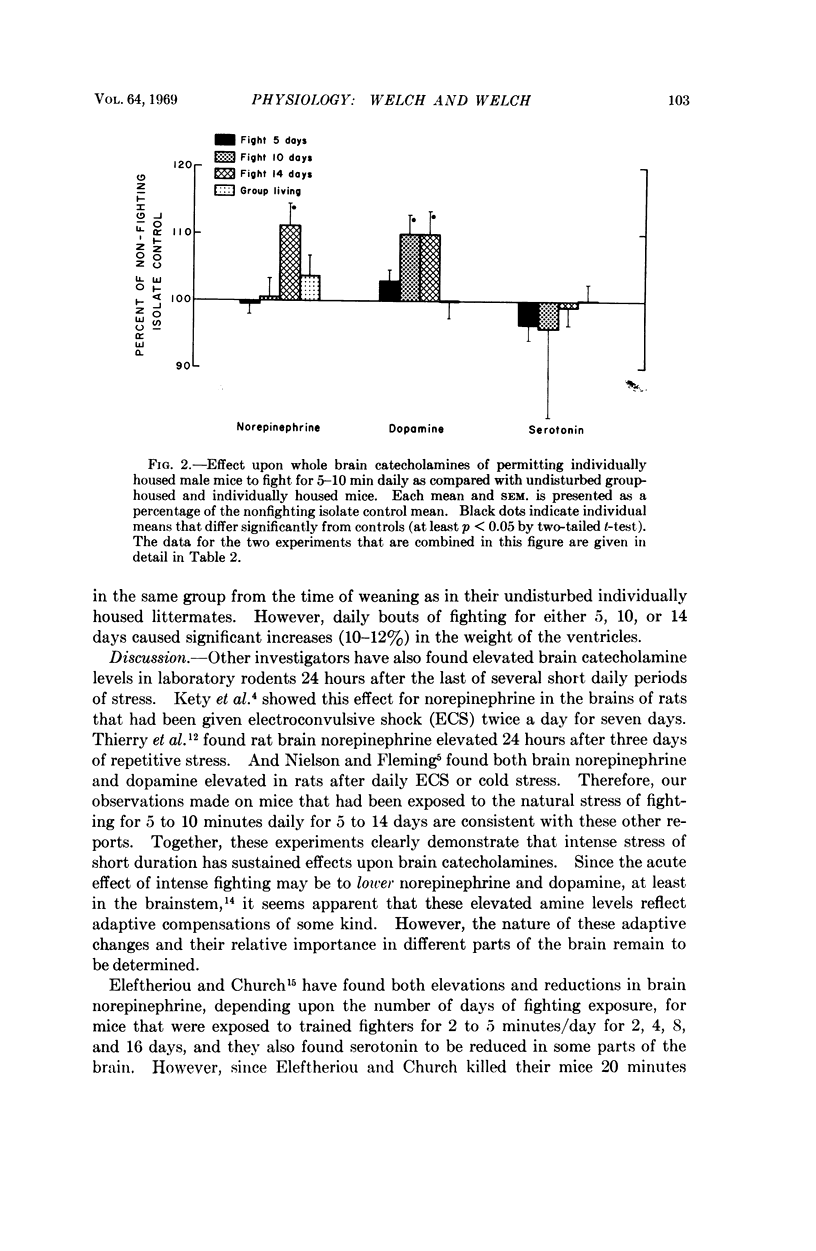
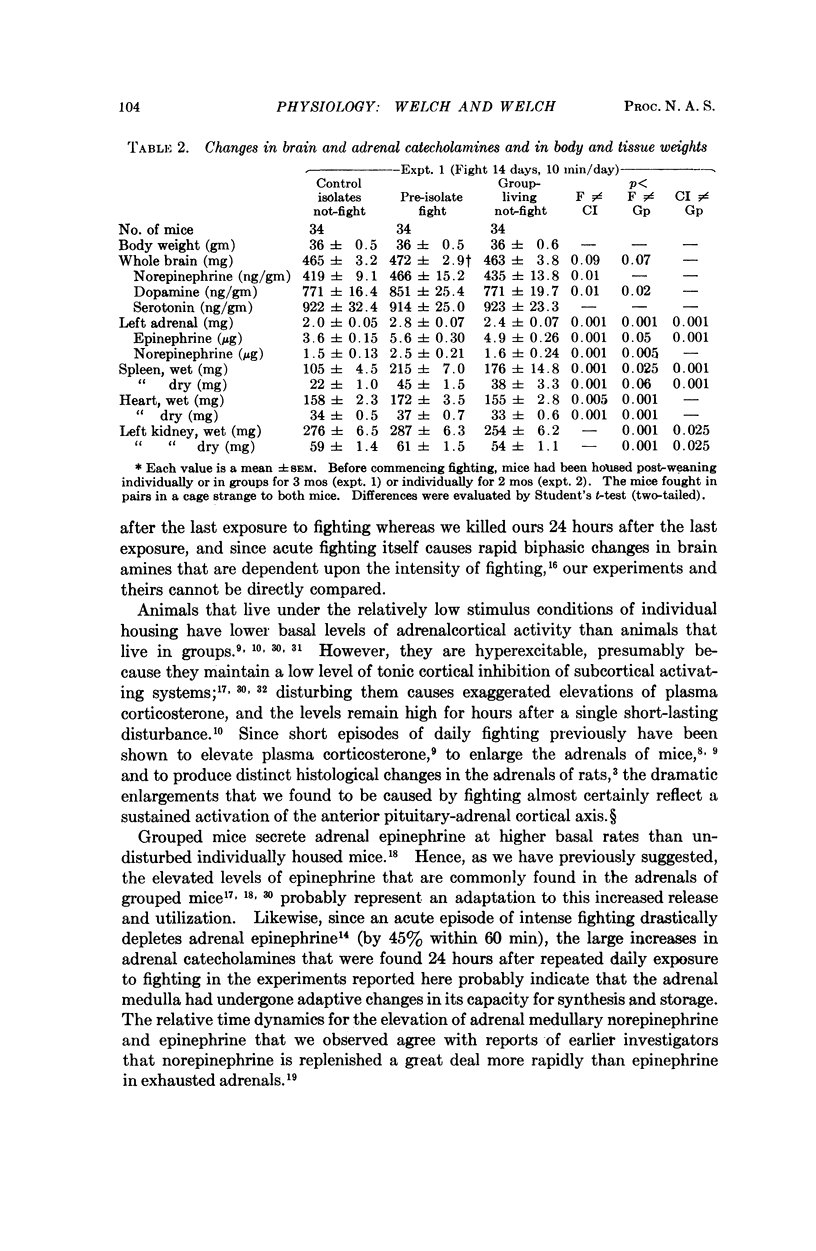
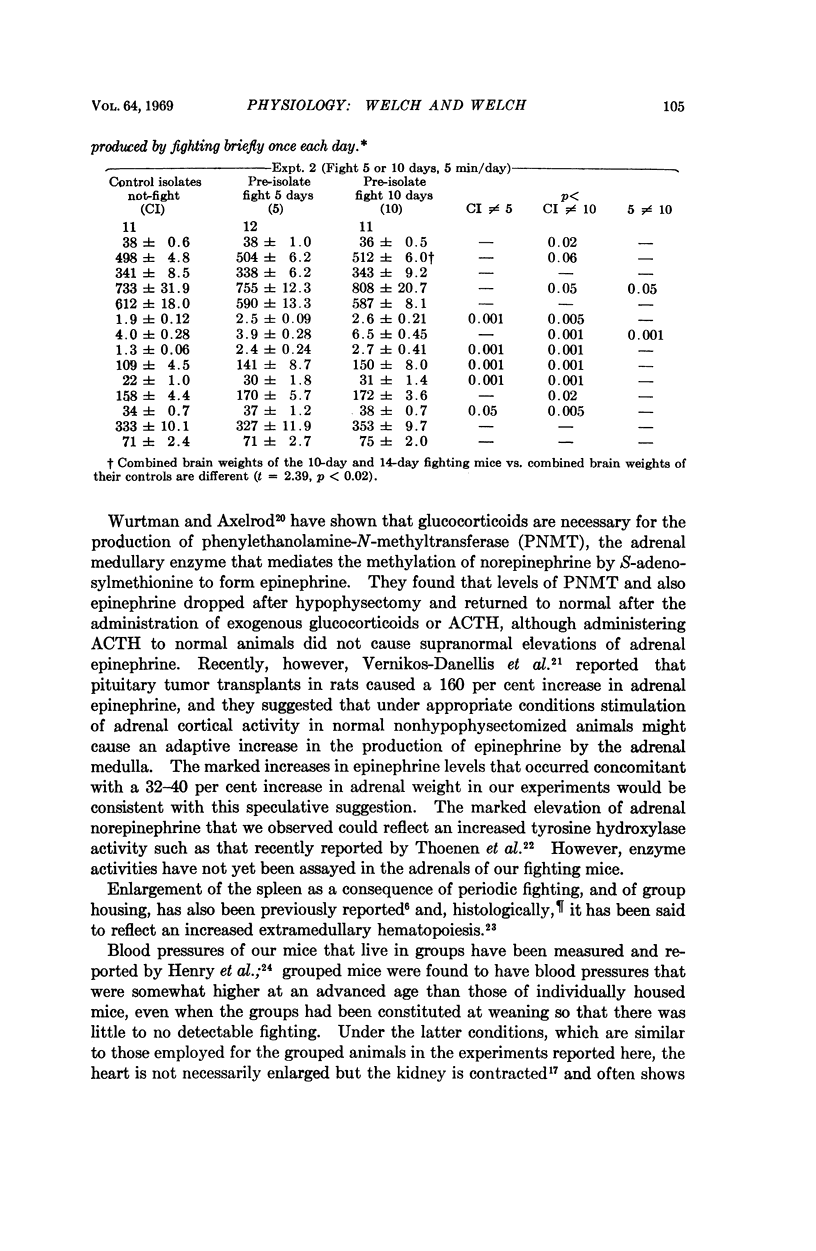
Male white Swiss mice that had previously been made aggressive by several weeks of individual housing were allowed to fight for 5 to 10 minutes each day for 5, 10, or 14 consecutive days; fighting caused a marked enlargement of their adrenals, spleens, and hearts, and a large increase in adrenal catecholamines; brain catecholamines were slightly increased. Long-term group caging, under conditions where the mice did not fight, caused changes that were directionally the same but of smaller magnitude. Similar sociophysiological influences may be important in natural populations. Fighting mice, used under well-defined and closely controlled conditions, may be useful for studying normal mechanisms of neuroendocrine adaptation and control, and, possibly, for studying some forms of hypertension and cardiovascular-renal disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTERWORTH K. R., MANN M. The adrenaline and noradrenaline content of the adrenal gland of the cat following depletion by acetylcholine. Br J Pharmacol Chemother. 1957 Dec;12(4):415–421. doi: 10.1111/j.1476-5381.1957.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. M., Stockham M. A. The response of the pituitary-adrenal system to a stressful stimulus: the effect of conditioning and pentobarbitone treatment. J Endocrinol. 1965 Aug;33(1):145–152. doi: 10.1677/joe.0.0330145. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. J., LLOYD J. A., DAVIS D. E. THE ROLE OF ENDOCRINES IN THE SELF-REGULATION OF MAMMALIAN POPULATIONS. Recent Prog Horm Res. 1965;21:501–578. [PubMed] [Google Scholar]
- Chipman R. K., Holt J. A., Fox K. A. Pregnancy failure in laboratory mice after multiple short-term exposure to strange males. Nature. 1966 May 7;210(5036):653–653. doi: 10.1038/210653a0. [DOI] [PubMed] [Google Scholar]
- DAVIS D. E., CHRISTIAN J. J. Relation of adrenal weight to social rank of mice. Proc Soc Exp Biol Med. 1957 Apr;94(4):728–731. doi: 10.3181/00379727-94-23067. [DOI] [PubMed] [Google Scholar]
- Geber W. F., Anderson T. A. Cardiac hypertrophy due to chronic audiogenic stress in the rat, Rattus norvegicus albinus, and rabbit, Lepus cuniculus. Comp Biochem Physiol. 1967 May;21(2):273–277. doi: 10.1016/0010-406x(67)90787-6. [DOI] [PubMed] [Google Scholar]
- Giacalone E., Tansella M., Valzelli L., Garattini S. Brain serotonin metabolism in isolated aggressive mice. Biochem Pharmacol. 1968 Jul;17(7):1315–1327. doi: 10.1016/0006-2952(68)90069-5. [DOI] [PubMed] [Google Scholar]
- Henry J. P., Meehan J. P., Stephens P. M. The use of psychosocial stimuli to induce prolonged systolic hypertension in mice. Psychosom Med. 1967 Sep-Oct;29(5):408–432. doi: 10.1097/00006842-196709000-00002. [DOI] [PubMed] [Google Scholar]
- Kety S. S., Javoy F., Thierry A. M., Julou L., Glowinski J. A sustained effect of electroconvulsive shock on the turnover of norepinephrine in the central nervous system of the rat. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1249–1254. doi: 10.1073/pnas.58.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson H. C., Fleming R. M. Effects of electroconvulsive shock and prior stress on brain amine levels. Exp Neurol. 1968 Jan;20(1):21–30. doi: 10.1016/0014-4886(68)90121-0. [DOI] [PubMed] [Google Scholar]
- RAPP J. P., CHRISTIAN J. J. SPLENIC EXTRAMEDULLARY HEMATOPOIESIS IN GROUPED MALE MICE. Proc Soc Exp Biol Med. 1963 Oct;114:26–28. doi: 10.3181/00379727-114-28576. [DOI] [PubMed] [Google Scholar]
- Raab W. Emotional and sensory stress factors in myocardial pathology. Neurogenic and hormonal mechanisms in pathogenesis, therapy, and prevention. Am Heart J. 1966 Oct;72(4):538–564. doi: 10.1016/0002-8703(66)90112-8. [DOI] [PubMed] [Google Scholar]
- Shapiro A. P., Scheib E. T., Croker B. Change in blood pressure due to crowding of mice and rats. Psychosom Med. 1968 May-Jun;30(3):347–348. doi: 10.1097/00006842-196805000-00010. [DOI] [PubMed] [Google Scholar]
- Thierry A. M., Javoy F., Glowinski J., Kety S. S. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat. I. Modifications of norepinephrine turnover. J Pharmacol Exp Ther. 1968 Sep;163(1):163–171. [PubMed] [Google Scholar]
- Thoenen H., Mueller R. A., Axelrod J. Increased tyrosine hydroxylase activity after drug-induced alteration of sympathetic transmission. Nature. 1969 Mar 29;221(5187):1264–1264. doi: 10.1038/2211264a0. [DOI] [PubMed] [Google Scholar]
- Tislow R. Delayed psychosomatic responses. Life Sci. 1965 Nov;4(21):2047–2056. doi: 10.1016/0024-3205(65)90321-8. [DOI] [PubMed] [Google Scholar]
- VAN LIERE E. J., NORTHUP D. W. Cardiac hypertrophy produced by exercise in albino and in hooded rats. J Appl Physiol. 1957 Jul;11(1):91–92. doi: 10.1152/jappl.1957.11.1.91. [DOI] [PubMed] [Google Scholar]
- Vernikos-Danellis J., Ciaranello R., Barchas J. Adrenal epinephrine and phenylethanolamine N-methyl transferase (PNMT) activity in the rat bearing a transplantable pituitary tumor. Endocrinology. 1968 Dec;83(6):1357–1358. doi: 10.1210/endo-83-6-1357. [DOI] [PubMed] [Google Scholar]
- Welch A. S., Welch B. L. Reduction of norepinephrine in the lower brainstem by psychological stimulus. Proc Natl Acad Sci U S A. 1968 Jun;60(2):478–481. doi: 10.1073/pnas.60.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch A. S., Welch B. L. Solvent extraction method for simultaneous determination of norepinephrine, dopamine, serotonin, and 5-hydroxyindoleacetic acid in a single mouse brain. Anal Biochem. 1969 Aug;30(2):161–179. doi: 10.1016/0003-2697(69)90387-x. [DOI] [PubMed] [Google Scholar]
- Welch B. L., Welch A. S. Graded effect of social stimulation upon d-amphetamine toxicity, aggressiveness and heart and adrenal weight. J Pharmacol Exp Ther. 1966 Mar;151(3):331–338. [PubMed] [Google Scholar]
- Welch B. L., Welch A. S. Greater lowering of brain and adrenal catecholamines in group-housed than in individually-housed mice administered DL-alpha-methyltyrosine. J Pharm Pharmacol. 1968 Mar;20(3):244–246. doi: 10.1111/j.2042-7158.1968.tb09734.x. [DOI] [PubMed] [Google Scholar]
- Weltman A. S., Sachler A. M., Sparber S. B. Endocrine, metabolic and behavioral aspects of isolation stress on female albino mice. Aerosp Med. 1966 Aug;37(8):804–810. [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem. 1966 May 25;241(10):2301–2305. [PubMed] [Google Scholar]


