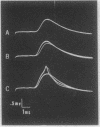
Abstract
Experiments were undertaken to determine if the effects of epinephrine in promoting neuromuscular transmission were mediated by adenosine 3′:5′-cyclic phosphate (cyclic AMP). Dibutyryl cyclic AMP and the methyl xanthines, theophylline and caffeine (which inhibit cyclic AMP hydrolysis), were found to increase the amplitude of the end plate potential in the isolated rat diaphragm. Like epinephrine (which is known to promote cyclic AMP synthesis), these agents appear to facilitate the release of acetylcholine from the motor neuron. This interpretation is supported by the observation that theophylline and dibutyryl cyclic AMP markedly increased the frequency but not the amplitude of the spontaneous miniature end plate potentials. In addition, these drugs increased the number of transmitter packets released in response to nerve stimulation. These results are consistent with the view that cyclic AMP plays a role in the release of acetylcholine and in the “defatiguing effect” of epinephrine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Babad H., Ben-Zvi R., Bdolah A., Schramm M. The mechanism of enzyme secretion by the cell. 4. Effects of inducers, substrates and inhibitors on amylase secretion by rat parotid slices. Eur J Biochem. 1967 Mar;1(1):96–101. doi: 10.1111/j.1432-1033.1967.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Breckenridge B. M., Burn J. H., Matschinsky F. M. Theophylline, epinephrine, and neostigmine facilitation of neuromuscular transmission. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1893–1897. doi: 10.1073/pnas.57.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Inhibition of cyclic nucleotide phosphodiesterase by adenosine 5'triphosphate and inorganic pyrophosphate. Biochem Biophys Res Commun. 1966 Apr 19;23(2):214–219. doi: 10.1016/0006-291x(66)90530-4. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., LOEWENSTEIN W. R. Nature of neuromuscular facilitation by sympathetic stimulation in the frog. J Physiol. 1955 Dec 29;130(3):559–571. doi: 10.1113/jphysiol.1955.sp005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H., Stamenović B. A., Whitaker B. D. The effect of noradrenaline on the end-plate potential in twitch fibres of the frog. J Physiol. 1968 Apr;195(3):743–754. doi: 10.1113/jphysiol.1968.sp008486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Some effects produced by adrenaline upon neuromuscular propagation in rats. J Physiol. 1958 Apr 30;141(2):291–304. doi: 10.1113/jphysiol.1958.sp005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka R. G., Sternlicht E. Enzyme secretion in mouse pancreas mediated by adenosine-3'5'-cyclic phosphate and inhibited by adenosine-3'-phosphate. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1123–1128. doi: 10.1073/pnas.61.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Tenenhouse A. Cyclic adenosine monophosphate, CA++, and membranes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1364–1370. doi: 10.1073/pnas.59.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The metabolic requirements from catecholamine release from the adrenal medulla. J Physiol. 1969 May;202(1):197–209. doi: 10.1113/jphysiol.1969.sp008804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Turtle J. R., Littleton G. K., Kipnis D. M. Stimulation of insulin secretion by theophylline. Nature. 1967 Feb 18;213(5077):727–728. doi: 10.1038/213727a0. [DOI] [PubMed] [Google Scholar]