Abstract
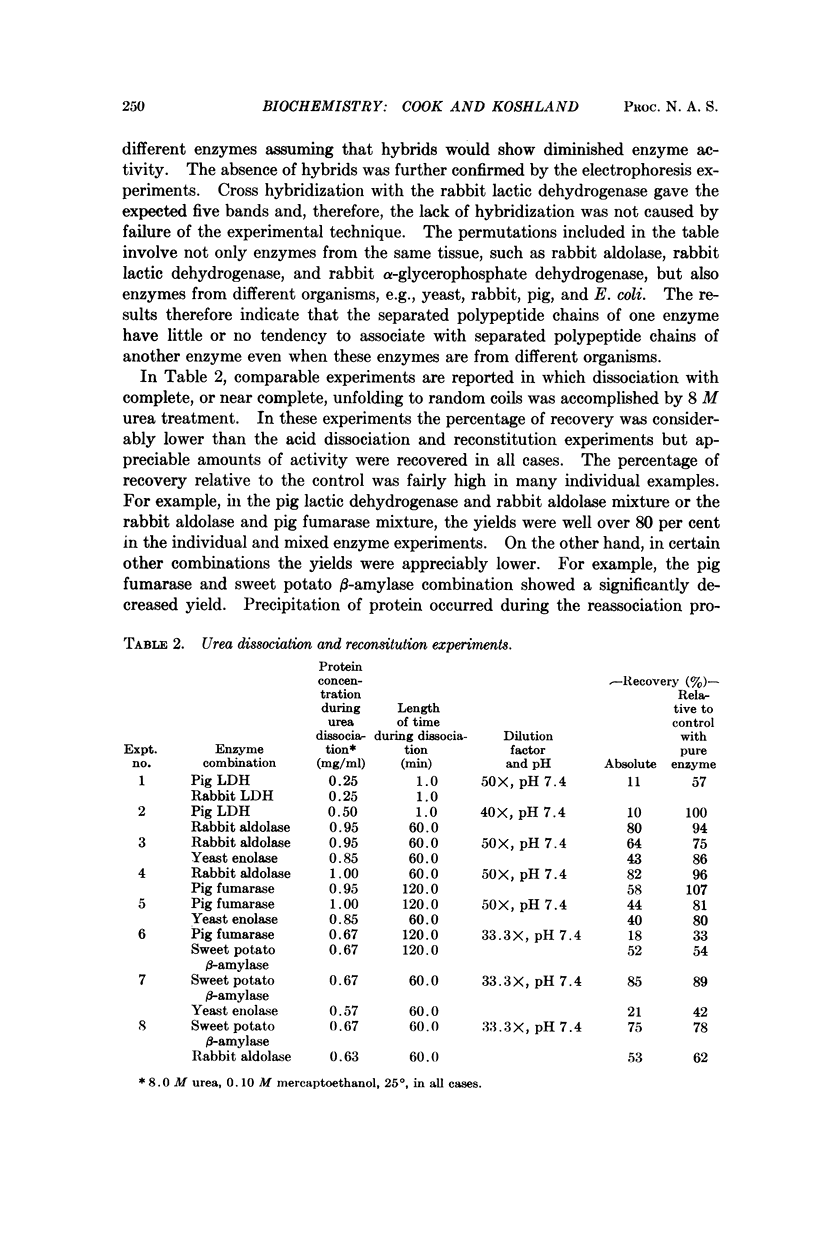
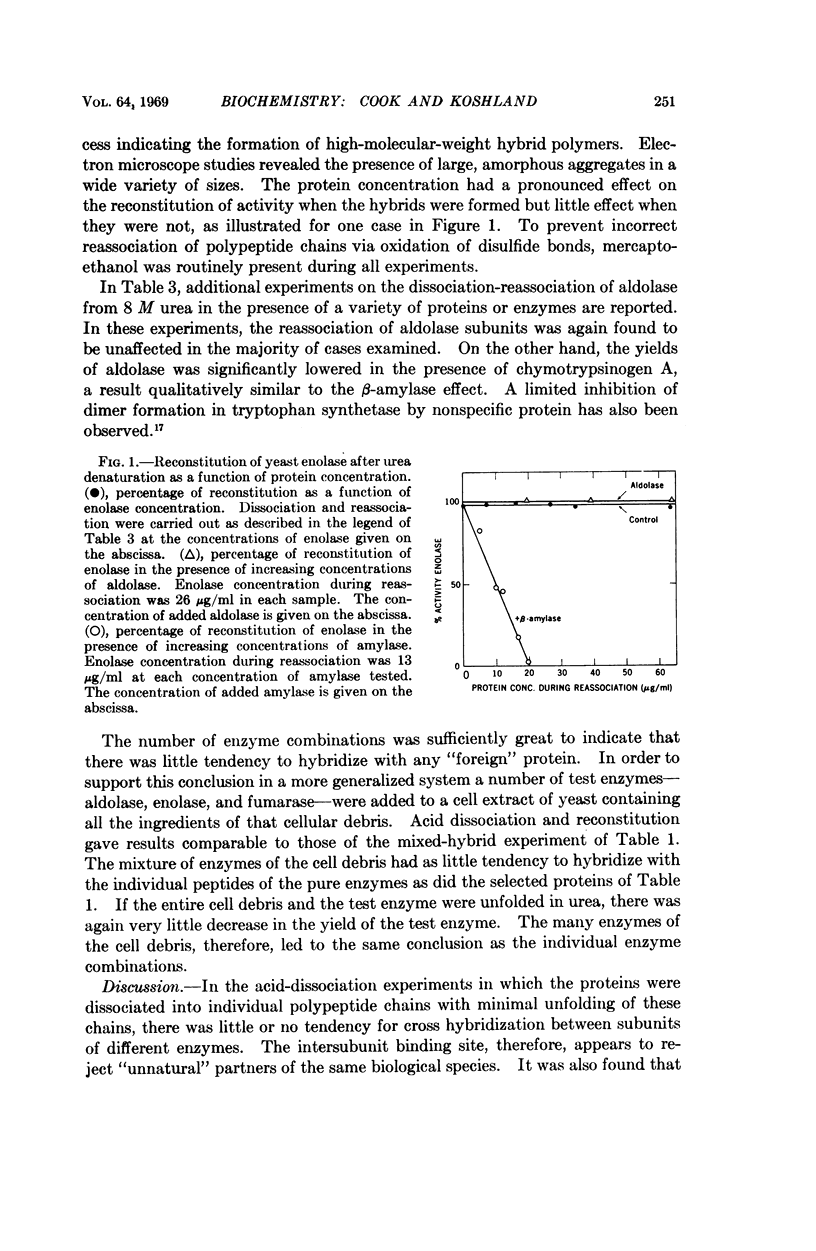
The recoveries of activity in the presence of mixtures of several enzymes and a cellular debris were compared with the recoveries of the pure enzymes. In the acid-dissociation experiments, no interference from foreign proteins was observed nor were any cross hybrids between subunits of different enzymes found. This suggests that the intersubunit binding sites are highly specific and have been selected over evolutionary time for correct assembly. In the urea experiments, cross hybridization and decreased yields were observed in a few cases but in most cases the “foreign” unfolded chains did not influence the recovery of the test enzyme. The results suggest that compartmentalization, temporal or spatial, will not be required as far as assembly of subunits of cytoplasmic enzymes is concerned. The results suggest some cross reaction might occur if all peptide chains were unfolding together. If folding occurs during or immediately after translation, this difficulty would be avoided.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Weber G. The reversible acid dissociation and hybridization of lactic dehydrogenase. Arch Biochem Biophys. 1966 Sep 26;116(1):207–223. doi: 10.1016/0003-9861(66)90028-2. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkare U. W., Colowick S. P. Reversible inactivation and dissociation of yeast hexokinase. J Biol Chem. 1965 Dec;240(12):4570–4584. [PubMed] [Google Scholar]
- REITHEL F. J. THE DISSOCIATION AND ASSOCIATION OF PROTEIN STRUCTURES. Adv Protein Chem. 1963;18:123–226. doi: 10.1016/s0065-3233(08)60269-7. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Conformational states of the subunit of Escherichia coli alkaline phosphatase. Biochemistry. 1967 Nov;6(11):3552–3559. doi: 10.1021/bi00863a029. [DOI] [PubMed] [Google Scholar]
- STELLWAGEN E., SCHACHMAN H. K. The dissociation and reconstitution of aldolase. Biochemistry. 1962 Nov;1:1056–1069. doi: 10.1021/bi00912a016. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., MacLennan D. H., McConnell D. G., Green D. E. Studies on the electron transfer system. 68. Formation of membranes as the basis of the reconstitution of the mitochondrial electron transfer system. J Biol Chem. 1967 May 10;242(9):2051–2061. [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S., King J., Lielausis I., Henninger M. Bacteriophage assembly. Fed Proc. 1968 Sep-Oct;27(5):1160–1166. [PubMed] [Google Scholar]
- Woodward D. O. Functional and organizational properties of Neurospora mitochondrial structural protein. Fed Proc. 1968 Sep-Oct;27(5):1167–1173. [PubMed] [Google Scholar]


