Abstract
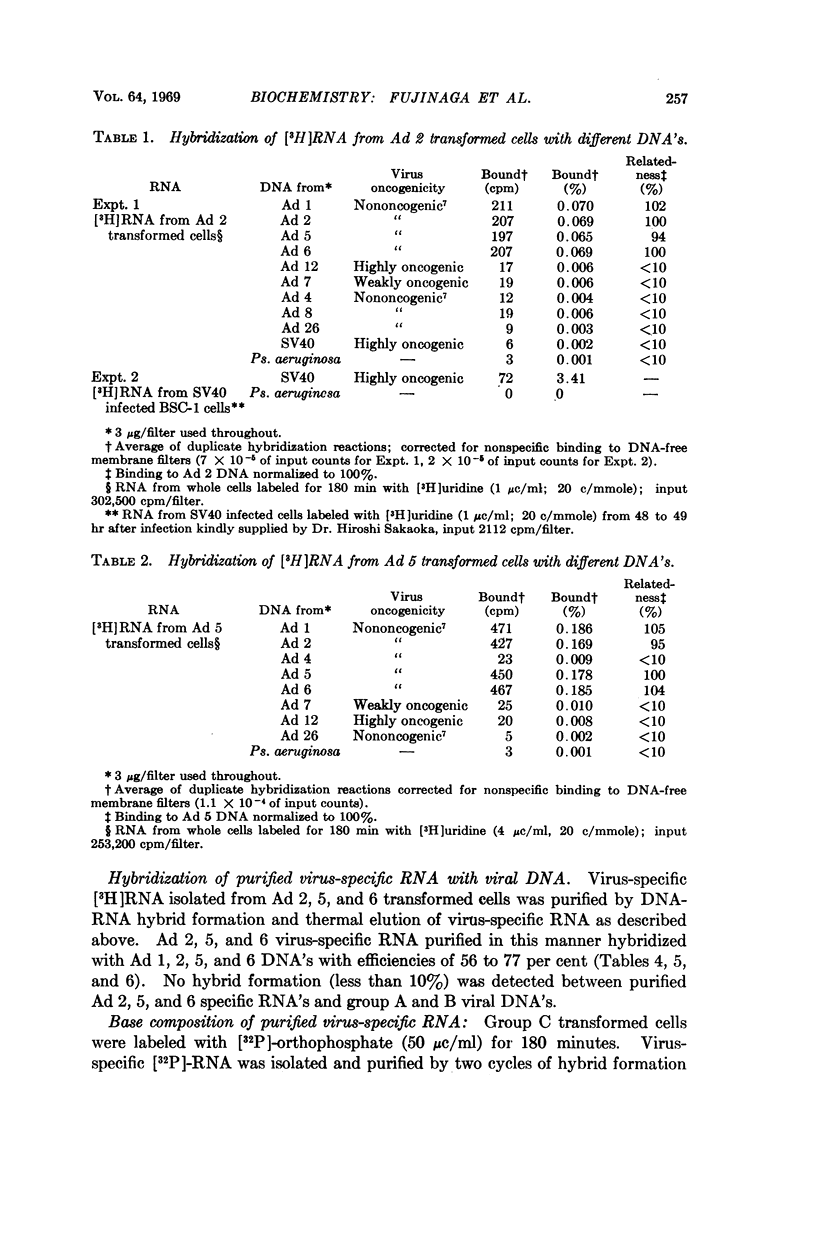
A new class of virus-specific RNA molecules was found in cells transformed by group C human adenovirus types 2, 5, and 6. RNA isolated from virus-free rat embryo cells transformed by adenovirus 2, 5, and 6 hybridized with all group C adenovirus DNA's (adenovirus 1, 2, 5, and 6) equally well, but not appreciably with group A and B adenovirus DNA's. Most likely no viral genes common to group A, B, and C adenoviruses are transcribed in adenovirus-transformed cells.
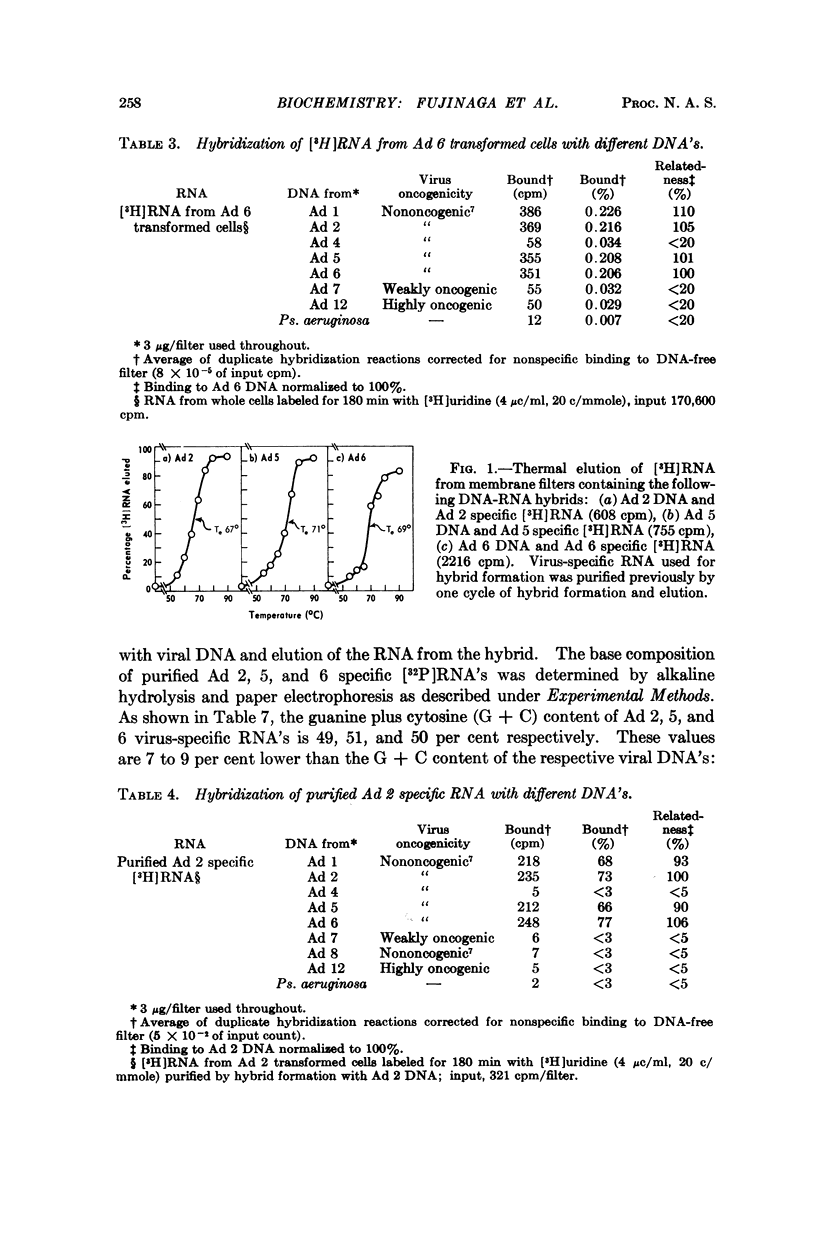
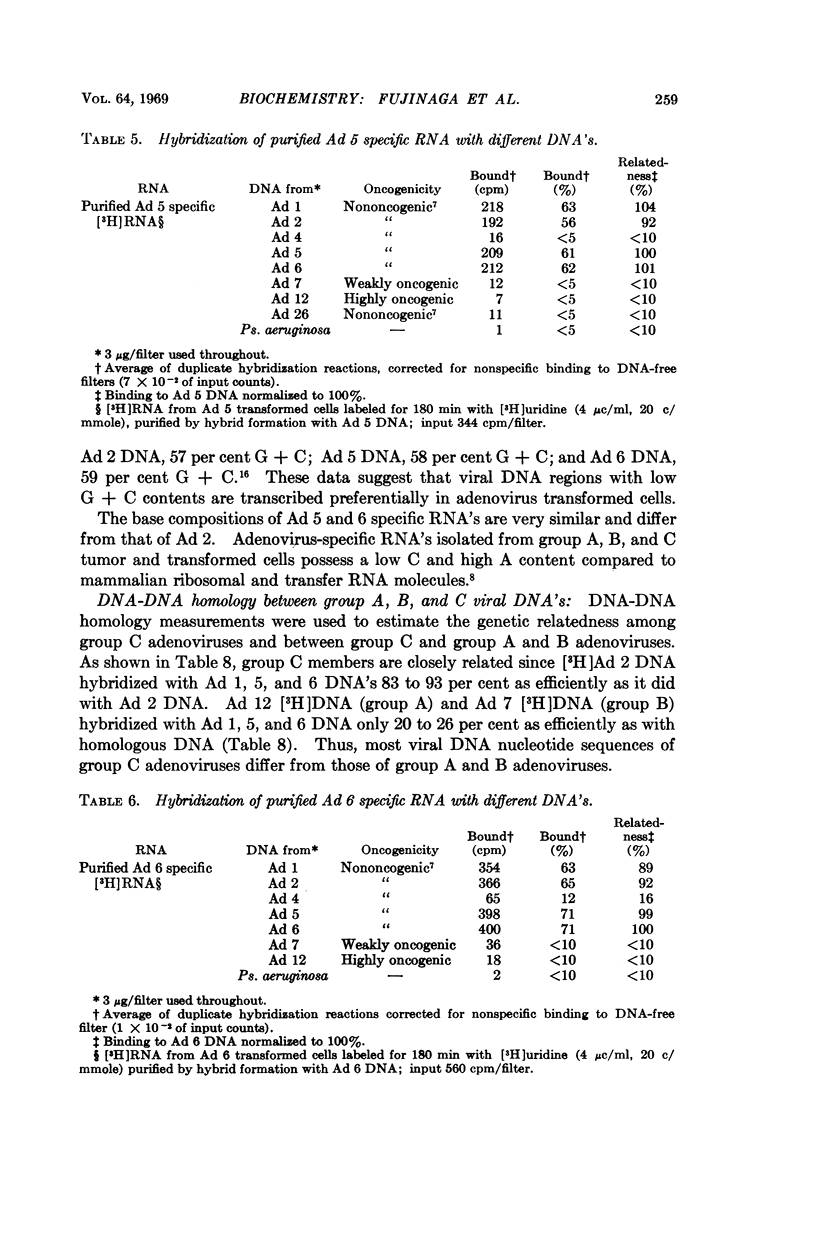
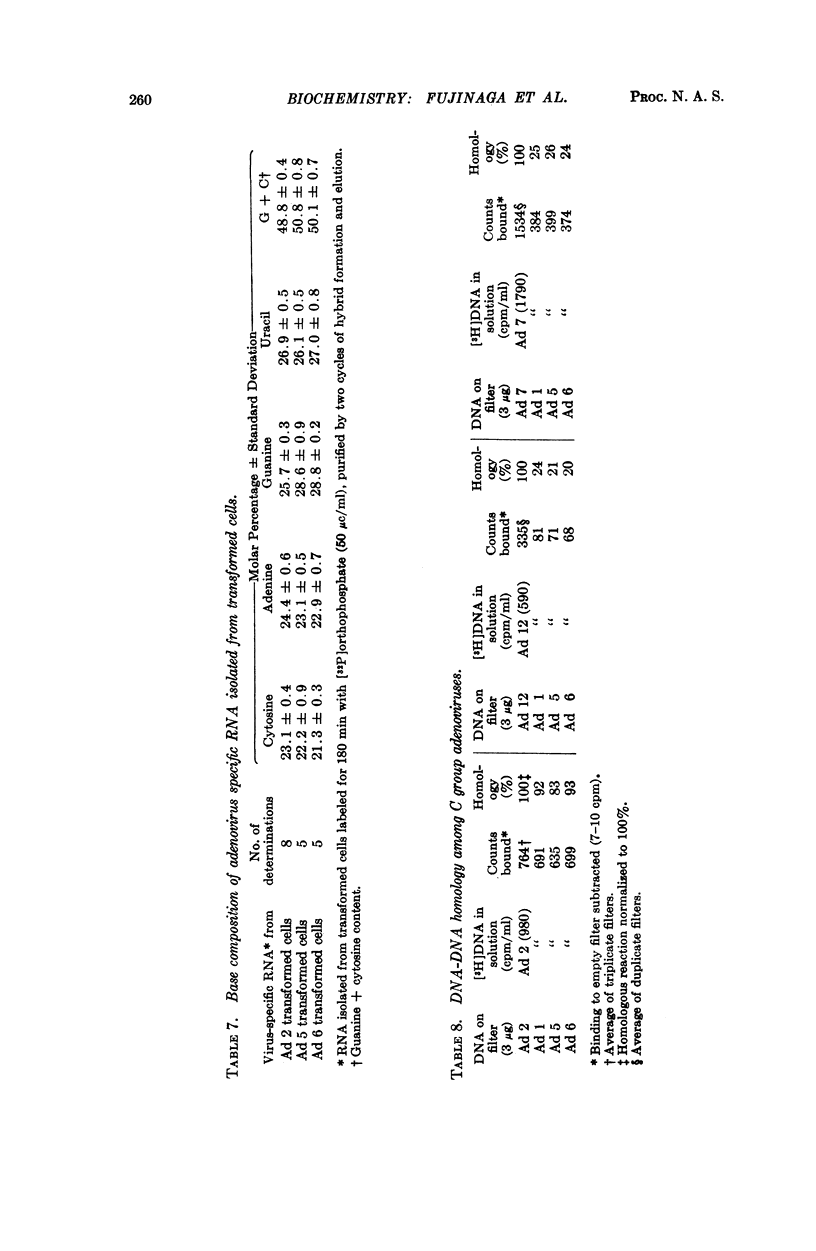
Group C adenoviruses are closely related since they share 83 to 93 per cent of their base sequences as shown by DNA-DNA homology measurements. Group C DNA's share only 10 to 26 per cent of their base sequences with group A and B DNA's. Moreover, the shared sequences are not transcribed detectably in adenovirus transformed cells.
Virus-specific RNA isolated from group C transformed cells contains 49 to 51 per cent G + C, but viral DNA's possess a 7 to 9 per cent higher G + C content. These differences suggest that only a portion of the viral genome with an average G + C content of 49 to 51 per cent is transcribed in group C adenovirus transformed cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freeman A. E., Black P. H., Vanderpool E. A., Henry P. H., Austin J. B., Huebner R. J. Transformation of primary rat embryo cells by adenovirus type 2. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1205–1212. doi: 10.1073/pnas.58.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA Mammalian viruses, ii. Viral-specific RNA in tumor cells induced by "weakly" oncogenic human adenoviruses. Proc Natl Acad Sci U S A. 1967 Mar;57(3):806–812. doi: 10.1073/pnas.57.3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. V. Properties of purified viral-specific RNA from human adenovirus-induced tumor cells. J Mol Biol. 1968 Jan 14;31(1):63–73. doi: 10.1016/0022-2836(68)90054-5. [DOI] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by deoxyribonucleic acid mammalian viruses. IV. Related virus-specific ribonucleic acids in tumor cells induced by "highly" oncogenic adenovirus types 12, 18, and 31. J Virol. 1967 Jun;1(3):576–582. doi: 10.1128/jvi.1.3.576-582.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. The mechanism of viral carcinogenesis by DNA mammalian viruses: viral-specific RNA in polyribosomes of adenovirus tumor and transformed cells. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1567–1574. doi: 10.1073/pnas.55.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Mak S., Green M. A method for determining the fraction of the viral genome transcribed during infection and its application to adenovirus-infected cells. Proc Natl Acad Sci U S A. 1968 Jul;60(3):959–966. doi: 10.1073/pnas.60.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Kern J., Freeman A. E., Martin C. E., McAllister R. C., Turner H. C., Huebner R. J. T and tumour antigens of adenovirus group C-infected and transformed cells. Nature. 1968 Aug 3;219(5153):517–518. doi: 10.1038/219517a0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., TURNER H. C., LANE W. T. SPECIFIC ADENOVIRUS COMPLEMENT-FIXING ANTIGENS IN VIRUS-FREE HAMSTER AND RAT TUMORS. Proc Natl Acad Sci U S A. 1963 Aug;50:379–389. doi: 10.1073/pnas.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACY S., GREEN M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION. VII. HOMOLOGY BETWEEN DNA'S OF TUMORIGENIC AND NONTUMORIGENIC HUMAN ADENOVIRUSES. Proc Natl Acad Sci U S A. 1964 Oct;52:1053–1059. doi: 10.1073/pnas.52.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La S., Green M. The mechanism of viral carcinogenesis by DNA mammalian viruses: DNA-DNA homology relationships among the "weakly" oncogenic human adenoviruses. J Gen Virol. 1967 Oct;1(4):413–418. doi: 10.1099/0022-1317-1-4-413. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M. O., Lewis A. M., Jr, Macpherson I., Huebner R. J. Transformation of rat embryo cells by adenovirus type 1. J Gen Virol. 1969 Jan;4(1):29–36. doi: 10.1099/0022-1317-4-1-29. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. IX. Chemical and base composition analysis of 28 human adenoviruses. Proc Natl Acad Sci U S A. 1965 Aug;54(2):547–551. doi: 10.1073/pnas.54.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEBRING E. D., SALZMAN N. P. AN IMPROVED PROCEDURE FOR MEASURING THE DISTRIBUTION OF P32O4--AMONG THE NUCLEOTIDES OF RIBONUCLEIC ACID. Anal Biochem. 1964 May;8:126–129. doi: 10.1016/0003-2697(64)90177-0. [DOI] [PubMed] [Google Scholar]
- Warmaar S. O., Cohen J. A. A quantitative assay for DNA-DNA hybrids using membrane filters. Biochem Biophys Res Commun. 1966 Aug 23;24(4):554–558. doi: 10.1016/0006-291x(66)90356-1. [DOI] [PubMed] [Google Scholar]


