Abstract
The anti-alcoholism drug disulfiram (Antabuse), which is an inhibitor of aldehyde dehydrogenase, induces an aversive reaction to alcohol consumption and thereby helps patients reduce alcohol intake. Recent clinical trials, initiated to investigate whether disulfiram could be used to treat individuals who abuse both alcohol and cocaine, have indicated that disulfiram effectively decreases cocaine consumption. Yet the ability of disulfiram to curb cocaine intake cannot be explained by the disruption of ethanol metabolism. Here, we synthesize clinical and animal data that point to dopamine β-hydroxylase inhibition as a mechanism underlying the efficacy of disulfiram in the treatment of cocaine dependence.
Introduction
Disulfiram (Antabuse) first received pharmacological interest in the 1930s, after workers in a rubber factory, where the compound was used as an antioxidant, became ill. In particular, workers who consumed alcohol after having been exposed to disulfiram experienced flushing of the face, nausea, vertigo, headache, and hypotension. This series of unpleasant symptoms, now known as the “disulfiram-ethanol reaction” (1), eventually led, in 1951, to the approval of disulfiram, under the name Antabuse, by the Food and Drug Administration for the treatment of alcoholism. The drug’s efficacy relies on aversive conditioning; simply put, alcoholics who are prescribed Antabuse learn to avoid alcohol in order to avoid the negative consequences of the disulfiram-alcohol reaction. Today, there is a growing body of evidence to suggest that disulfiram also reduces cocaine use in dependent individuals, regardless of whether they abuse alcohol (2–4).
In this review, we discuss several pharmacological targets of disulfiram, including those involved in cocaine metabolism and catecholamine synthesis, with a focus on dopamine β-hydroxylase (DBH), which catalyzes the conversion of dopamine to norepinephrine and thereby controls the norepinephrine-to-dopamine ratio in central noradrenergic neurons (5). We also review the role of norepinephrine signaling in reward and drug-seeking behavior, and we propose an integrated mechanism to account for disulfiram-induced cocaine abstinence.
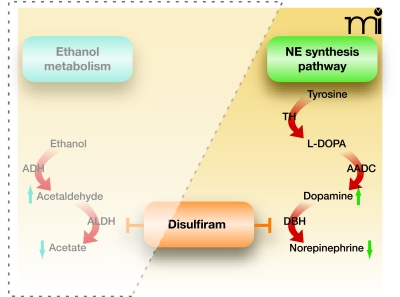
Disulfiram and Ethanol Metabolism
Ethanol is converted to acetaldehyde by the enzyme alcohol dehydrogenase, and acetaldehyde is further metabolized to acetate by aldehyde dehydrogenase (6). Disulfiram is an inhibitor of aldehyde dehydrogenase, which is directly relevant to its role in curbing alcohol consumption (Figure 1). The high levels of acetaldehyde that accumulate following alcohol ingestion in patients taking disulfiram cause the mild to moderate levels of facial flushing, weakness, throbbing headache, nausea, vomiting, sweating, vertigo, hypotension, and other unpleasant symptoms that typify the disulfiram-ethanol reaction (also known as the Antabuse reaction) (7, 8). The direct association of this aversive reaction with alcohol consumption establishes a psychological deterrent in alcoholic patients who abide by the dosing regimen. It is important to note here that, in addition to its effects on aldehyde dehydrogenase, disulfiram has many other targets. Upon absorption, disulfiram is immediately reduced to diethyldithiocarbamate (DDC) when it reacts with thiol groups (9). This metabolite of disulfiram is a potent copper chelator (10), and it can thereby affect the activity of copper-dependent enzymes such as monooxygenases, amine oxidase, cytochrome oxidase, microsomal carboxylesterase, and plasma cholinesterase.
Figure 1.
Disulfiram inhibition of ethanol metabolism. Ethanol is first converted into acetaldehyde by alcohol dehydrogenase (ADH). Acetaldehyde is then transformed into acetate by aldehyde dehydrogenase (ALDH). Disulfiram inhibits ALDH and thereby results in "the disulfiram-ethanol reaction" that promotes abstinence from alcohol. See text for details.
To date, there have been eight supervised clinical trials, ranging from 56 to 270 days in duration, that assess oral disulfiram for the treatment of alcoholics [see (11)]. The percentage of disulfiram-treated patients who completed these trials, ranging from eighteen to eighty-five percent, was higher for those populations in which drug administration was supervised by clinic staff or a family member. Only one study (comprising four disulfiram-treated patients and one placebo control) reported adverse side effects as a factor in patient drop-out (12). Four trials had a completely randomized design; three of these compared supervised versus unsupervised disulfiram administration (13–15). All groups reported better abstinence from drinking after supervised disulfiram administration compared with unsupervised administration or placebo. The studies of Robichaud et al. (16) and Sereny et al. (17), both using a within-subject design, found that disulfiram treatment led to alcohol abstinence in ninety-nine percent of patients during a 14-day treatment period, with fifty-eight percent of patients reaching “significant periods of sobriety.” In a six-month study (18), patterns of compliance within a supervised treatment program revealed that forty-five percent of subjects who took disulfiram regularly remained abstinent for the duration, whereas twenty-four percent of treated subjects relapsed. A recent investigation of 180 alcoholic patients randomly assigned to placebo or administration of the glutamatergic modulator acamprosate included a subset of subjects who volunteered to receive disulfiram (19). The simultaneous administration of acamprosate and disulfiram doubled the “mean cumulative abstinence” score in patients compared with the placebo-alone group.
Adherence to treatment must be recognized as a confounding factor in interpreting the effectiveness of disulfiram from clinical trial data. Problems with adherence pose the main clinical challenge in using disulfiram to treat alcoholism. Other limitations of its use are side effects (20–22) and hepatic toxicity in alcoholics with compromised liver function (10, 23), both of which are a result of disulfiram’s multiple enzymatic targets and lead to the underuse of the drug as a pharmacotherapy for alcoholism. Disulfiram can be an effective treatment for alcoholism, especially when patient compliance is supervised or supported within the context of stable social circles and community ties. It is against this backdrop that the idea of treating cocaine dependence with disulfiram emerged in the early 1990s.
Disulfiram and Cocaine Addiction
Cocaine and Alcohol Co-abuse
According to clinical and epidemiological studies, fifty to ninety percent of patients who abuse cocaine also abuse alcohol (24–27), and several factors could account for this comorbidity. Not only is there evidence that ethanol inhibits cocaine metabolism, but in addition, the simultaneous administration of the two drugs results in the formation of the active metabolite cocaethylene (CE) (28–32). CE manifests a pharmacological profile similar to that of cocaine in a number of respects: it has a high affinity for the dopamine transporter; it increases extracellular dopamine concentrations (33–35); it decreases the firing rate of dopamine neurons in the ventral tegmental area (VTA); and it potentiates ethanol-induced excitation of VTA neurons (36). In contrast to cocaine, however, CE is less potent in elevating heart rate and has a longer half-life (37). In the rat, CE is more reinforcing and less anxiogenic than cocaine (38), and CE overdose is more lethal than an overdose of either cocaine or ethanol alone (39). Taken together, these observations may explain the frequent anecdotal accounts from cocaine users who report that alcohol use prolongs their cocaine-induced euphoria (“high”) and reduces their paranoia during a cocaine binge. Similarly, alcohol reportedly helps “take the edge off” of cocaine-induced anxiety, hyperactivity, and insomnia. And finally, alcohol intoxication can impair judgment and inhibition, which may lead to further risky behavior, including illicit drug use.
Disulfiram Treatment for Dual Cocaine and Alcohol Dependence: Clinical Trials
On the hypothesis that withholding alcohol from cocaine-and-alcohol–dependent individuals might lead to a decrease in cocaine use, two research groups examined the use of disulfiram in this patient population in 1993. In the first study, although disulfiram treatment reduced both alcohol and cocaine use, the effect on cocaine use was attributed to a course of behavioral therapy that had been implemented in the patient population (40). The second study, a randomized twelve-week pilot trial, compared the effects of disulfiram administration to the effects of naltrexone, an opioid antagonist that may prevent drug craving; both treatments were accompanied by cognitive behavioral therapy. Disulfiram proved more effective than naltrexone at lowering the frequency of cocaine use (41), and a larger clinical trial ensued, in which disulfiram treatment improved abstinence from cocaine as compared to no treatment; disulfiram treatment in this larger trial appeared more effective for those outpatients who also received cognitive behavioral therapy (CBT) (42). The beneficial effect of disulfiram was still evident a year later in a follow-up study (43), and the efficacy of disulfiram therapy in diminishing cocaine use as observed earlier in comorbid addicts (41) was corroborated.
The notion that the mechanism of disulfiram-induced cocaine abstinence might not be related to the disulfiram-alcohol reaction emerged from the results of two studies published in 2000. In these trials, the effects of disulfiram on cocaine use were assessed in patients who were addicted to both cocaine and opiates, and who were maintained on methadone (3) or buprenorphine (2). In agreement with previous studies, the addicts treated with disulfiram were better able, relative to those addicts not receiving disulfiram, to reduce their intake of alcohol, cocaine, and opiates (3). In addition, disulfiram shortened the time necessary for patients to reach continuous cocaine, but not heroin, abstinence (2). Throughout these trials, alcohol consumption was minimal for all subjects, regardless of medication group, and baseline alcohol use did not predict responses to disulfiram. It was therefore something of a conceptual breakthrough when, in 2004, a randomized, placebo-controlled trial not only confirmed the effectiveness of disulfiram in treating cocaine dependence, but moreover revealed that the drug’s effectiveness in this regard could be differentiated from its role in curbing alcohol abuse (4). Specifically, the groundbreaking trial addressed cocaine use both with and without comorbidity for alcohol abuse, showing that the benefits of disulfiram therapy were most pronounced in patients who either were not alcohol dependent at baseline or who fully abstained from alcohol during treatment. These observations directly suggest that disulfiram undermines cocaine addiction in a manner independent of its action in inhibiting alcohol intake. Several other double-blind, randomized, placebo-controlled trials have confirmed the efficacy of disulfiram on cocaine intake (44, 45), with a potentially greater effect in males (46), but none have been designed to investigate the mechanisms of disulfiram-induced cocaine abstinence. Nevertheless, some clues can be gleaned from human laboratory and animal studies assessing the interactions between disulfiram and the physiological, behavioral, and subjective effects of cocaine.
Disulfiram and Cocaine: Human Laboratory and Animal Studies
To understand how disulfiram treatment affects an individual’s response to cocaine, and to shed light on the mechanism driving the drop in cocaine intake, a series of human laboratory studies looked at whether disulfiram influences the self-reported subjective effects of cocaine. The results have been conflicting. Two groups reported no difference in the subjective effects of cocaine, such as cravings or high, using a “Yes/No” scale (3) or a visual analog scale (47). Another group reported a modest, non-significant increase in “high” and “anxiety” (48), whereas others found increases in nervousness, paranoia (49, 50), or psychosis (51, 52). Two other studies report decreased “rush,” “high,” or “craving” (44, 53). Disulfiram is also reported to enhance some subjective effects of the psychostimulant dextroamphetamine, including “high,” anxiety, “bad drug effects,” “craving,” and “drug liking” (54). The modulation of subjective effects by disulfiram may in fact vary, increasing or decreasing the rewarding effects of cocaine; the most consistent finding is a worsening of the aversive effects of cocaine, such as anxiety and paranoia. Other side effects of disulfiram clinical trials on cocaine dependence are headaches, fatigue, and paranoia (55). Thus, there may be a “disulfiram-cocaine” reaction that is similar to but distinct from the “disulfiram-alcohol” reaction and that promotes cocaine abstinence.
There are a few published preclinical studies in rodents that address how disulfiram pretreatment affects behavioral responses to cocaine. Early studies showed that disulfiram pretreatment suppresses amphetamine-induced (56) and cocaine-induced (57) locomotor activity in mice and rats. More recent studies indicate that disulfiram has minimal effects on baseline activity levels, but repeated administration prior to cocaine facilitates the development of behavioral sensitization to cocaine in rats (58) and mice (our unpublished data). Disulfiram pretreatment also enhances cocaine-induced seizures in mice (59).
Molecular Mechanisms of Disulfiram Action
Dopamine Metabolism and Release
Because dopamine mediates many of the addictive properties of cocaine and other psychostimulants, the effect of disulfiram on dopamine neurotransmission is a logical place to look for clues to the drug’s clinical efficacy. Although clinical trials have shown that the disulfiram-ethanol reaction cannot account for effects on cocaine dependence, aldehyde dehydrogenase in fact plays two important roles in dopamine metabolism. Dopamine is metabolized by monoamine oxidase into the intermediate metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is then converted by aldehyde dehydrogenase into 3,4-dihydroxypenylacetic acid (DOPAC) (60). DOPAL is a reactive electrophile and is toxic to dopaminergic neurons (61). In a separate metabolic pathway, dopamine is converted by catechol-O-methyl-transferase and monoamine oxidase to produce 3-methoxy-4-hydroxyphenylac-etaldehyde (MHPA), which is a substrate of aldehyde dehydrogenase. In this way, disulfiram might be expected to increase levels of both DOPAL and MHPA (Figure 2). Because the accumulation of DOPAL decreases dopamine uptake into synaptic vesicles and is toxic to dopamine neurons, disulfiram might be predicted to undermine dopamine transmission and dampen the euphoric and stimulant effects of cocaine. However, patients in some laboratory studies report just the opposite, namely, augmentation of the “high” elicited from cocaine (48) and dextroamphetamine (54); these results are inconsistent with the inhibition of aldehyde dehydrogenase as a basis for disulfiram-induced cocaine abstinence.
Figure 2.
Dopamine metabolism. Dopamine (DA) is metabolized intracellularly and extracellularly by the same group of enzymes, but in different orders. Inside dopaminergic cells, monoamine oxidase (MAO) converts dopamine into 3,4-dihydrophenylacetaldeyde (DOPAL), a substrate of aldehyde dehydrogenase (ALDH). ALDH then converts DOPAL into 3,4-dihydroxyphenylacetic acid (DOPAC). After DOPAC diffuses out of the cell, catechol-O-methyltransferase (COMT) converts it into homovanillic acid (HVA). Extracellularly, dopamine metabolism begins by transformation into 3-methoxytyramine (3-MT) by COMT. 3-MT is then oxidized into 3-methoxy-4-hydroxyphenylacetaldehyde (MHPA) by MAO, and finally transformed into HVA by ALDH.
There has been one microdialysis investigation into the effects of disulfiram upon the storage and release of striatal dopamine and DOPAC in rats (62). In this study, disulfiram was reported to increase dopamine and DOPAC release in the striatum; however, the acute systemic dose (500 mg/kg) was notably higher than behaviorally relevant doses administered in previous studies (56–58, 63–66). The observed increase in dopamine release (145% above baseline level), moreover, peaked at forty minutes after treatment and returned to baseline after eighty minutes, whereas lower (behaviorally relevant) disulfiram dosing has been observed by ourselves (unpublished data) and others (65, 66) to elevate tissue dopamine concentrations for hours following administration. In vitro indications that disulfiram inhibits uptake and increases efflux of dopamine in bovine striatal synaptic vesicles (62) are similarly problematic, because the concentration of disulfiram used (1.7 mM) was well beyond physiologically relevant levels. Nevertheless, the data indicate that disulfiram can directly disrupt dopamine metabolism and homeostasis. There is not sufficient evidence, however, to support the conclusion that such disruption can account for the drug’s efficacy in promoting cocaine abstinence. It is, rather, an indirect effect upon dopamine release that likely accounts for the therapeutic effect of disulfiram (see below).
Glutamate Neurotransmission
Glutamate is the primary excitatory neurotransmitter in the mammalian central nervous system. Brain regions that mediate the addictive properties of cocaine, such as the mesolimbic dopamine system (VTA and nucleus accumbens), amygdala, and frontal cortex, all receive extensive glutamatergic projections [see (67) and (68)]. Given this anatomical correlation, as well as the functional correlation between dopamine and glutamate in learning and memory, much recent research has focused on dopamine-glutamate interactions in the modulation of psychostimulant-induced synaptic plasticity and addiction (69–71). Pharmacological manipulation of glutamate receptors can have profound effects on behavioral responses to psychostimulants in animal models of addiction. For example, ionotropic and metabotropic glutamate receptor antagonism impairs the acquisition and expression of cocaine-conditioned place preference (72, 73) and attenuates cue-induced cocaine seeking in rats (74–79).
The effects of disulfiram on glutamatergic neurotransmission are not well characterized. One study showed that S-methyl-N,N-diethylthiocarbamate sulfoxide (DETC-MeSO), a metabolite of disulfiram, blocked glutamate binding to receptors in mouse brains (80). The blockade was dose-and time-dependent partially (up to 50%) irreversible, and affected more than one receptor subtype. Another group reported that disulfiram, but not its primary metabolite DDC, attenuated synaptic glutamate uptake in rat brain and in cultured neurons, resulting in higher extracellular levels of glutamate (81). Still another study showed that both disulfiram and DDC increased extracellular glutamate levels in the rat striatum (82); here, DCC (290 mg/kg) elicited a brief two-fold increase in glutamate, whereas disulfiram (500 mg/kg) was less potent but longer-lasting. The interpretation of this microdialysis study is limited by the high doses of drug used. Furthermore, extracellular levels of glutamate during cocaine withdrawal differ among brain areas, and there are disulfiram interactions with cocaine in this regard. For example, during cocaine withdrawal, glutamate is decreased in the nucleus accumbens (83–85) but increased in the prefrontal cortex (86). Although normalizing glutamatergic transmission in the nucleus accumbens might therefore seem like a promising therapeutic strategy for treating psychostimulant addiction (87), there is currently insufficient evidence to support a critical role for altered glutamatergic transmission in the therapeutic efficacy of disulfiram.
Cocaine Metabolism
Disulfiram is an inhibitor of plasma cholinesterase and microsomal carboxylesterase activities (88), both of which are essential for cocaine metabolism (89, 90). This inhibitory activity likely explains the reported three-to sixfold increase in plasma cocaine levels following intranasal administration of disulfiram (47–49). In one study (48), serum cholinesterase activity was surprisingly not affected by disulfiram treatment; however, the authors acknowledged the limitations of the assay used and their sample preparation, so that cholinesterase inhibition may have been present but undetected. We observed no effect of disulfiram on peak serum cocaine levels in wild-type mice despite potent behavioral effects (59). In theory, increases in plasma cocaine levels in disulfiram-treated addicts could enhance both rewarding and aversive responses to cocaine. Nonetheless, addicts are quite adept at titrating their doses to maximize euphoric and minimize unpleasant effects and could thereby circumvent the abstinence-promoting effects of treatment.
Dopamine β-Hydroxylase Inhibition
Although any of the possible mechanisms described above could contribute to disulfiram-induced cocaine abstinence, none of them are especially compelling, and some are difficult to test. In contrast, data from animal and human laboratory studies strongly support an important role for DBH inhibition in the ability of disulfiram to reduce cocaine use. The following section will review and integrate the available data and suggest further testable hypotheses concerning DBH inhibition and the blockade of norepinephrine synthesis as primary mechanisms underlying disulfiram-induced cocaine abstinence.
Cocaine inhibits the reuptake of dopamine, serotonin, and norepinephrine by transporters at the plasma membrane of mono-aminergic neurons and thus elevates extracellular concentrations of these neurotransmitters (91–93). The psychostimulant effects of cocaine are mediated primarily by enhancement of dopaminergic transmission in the mesolimbic system in the brain, while serotonin and norepinephrine play a modulatory role. Based on how these neurotransmitters mediate drug responses and reward, most pharmacotherapeutic strategies to combat cocaine addiction have focused on the modulation of dopamine signaling.
DBH converts dopamine to norepinephrine, thereby playing a direct role in determining the ratio of dopamine to norepinephrine in noradrenergic neurons (64, 94). The enzyme is copper-dependent (95), and because the primary metabolite of disulfiram, DDC, is a copper chelator, DBH activity is inhibited by disulfiram (65, 96). Disulfiram administration decreases central conversion of dopamine into norepinephrine and significantly lowers the ratio of norepinephrine to dopamine in the brains of mice and rats (57, 64, 66, 97). (See Figure 3.)
Figure 3.
Disulfiram inhibition of the norepinephrine (NE) biosynthetic pathway. In the catecholamine synthesis pathway, tyrosine is converted into 3,4-dihydroxy-L-phenylalanine (L-DOPA) by tyrosine hydroxylase (TH), which is then transformed into dopamine by aromatic amino acid decarboxylase (AADC), whereupon dopamine b-hydroxylase (DBH) converts dopamine into norepinephrine. Disulfiram inhibits DBH, reducing the production of norepinephrine and increasing the pool of dopamine.
DBH Inhibition in Cocaine Reward, Aversion, and Relapse
We begin this section with three ways in which DBH inhibition has been hypothesized to decrease cocaine use: (1) by acting as a “dopamine replacement therapy;” (2) by decreasing the rewarding effects of cocaine; and (3) by increasing the aversive effects of cocaine. Ultimately, however, we will argue that relapse prevention mediated by decreased levels of norepinephrine is likely the most important mechanism underlying disulfiram-induced cocaine abstinence.
The “Dopamine Agonist” Hypothesis of DBH Inhibition
Chronic cocaine users are hypothesized to be hypodopaminergic, and a popular treatment strategy has been to “normalize” dopaminergic tone [e.g., dopamine agonist therapy; reviewed by Grabowski et al. (98)]. Indeed, disulfiram has sometimes been classified as a “dopamine agonist,” as its beneficial effect on cocaine intake has been attributed to increased dopamine concentration following DBH inhibition (99). However, we will argue that, because of the functional interaction between the noradrenergic and dopaminergic systems, this hypothesis is unlikely to be correct.
Anatomical and functional connections between the noradrenergic and dopaminergic systems affect the responsiveness of the mesolimbic reward system to psychostimulants. The locus coeruleus, the primary noradrenergic nucleus in the brain, directly innervates the VTA and the prefrontal cortex. The A1 and A2 noradrenergic cell groups in the brain stem also project to the VTA and the nucleus accumbens. In general, norepinephrine (i.e., the product of DBH activity) provides excitatory drive onto midbrain dopamine neurons, both directly (through the VTA) and indirectly (via glutamatergic projections from the prefrontal cortex to the VTA) [reviewed by Weinshenker and Schroeder (100) and Mejías-Aponte et al. (101)]. In this way, DBH inhibition would decrease excitatory drive onto midbrain dopamine neurons, and thus, despite increased tissue levels of dopamine, the firing of dopaminergic neurons (particularly burst firing) would be attenuated, and extracellular DA levels would actually fall. For example, pharmacological blockade of norepinephrine signaling inhibits basal and psychostimulant-induced burst firing in midbrain dopaminergic neurons (102, 103), and lesions of the locus coeruleus, along with norepinephrine depletion, reduce dopamine release in terminal regions (104). Also, norepinephrine depletion in the prefrontal cortex attenuates psychostimulant-induced dopamine release in the nucleus accumbens (105, 106). Finally, although DBH knockout (Dbh−/−) mice completely lack norepinephrine and have high tissue dopamine levels, the animals manifest low basal and psychostimulant-evoked extracellular dopamine levels in the dorsal and ventral striatum (107). The specific effects of disulfiram on extracellular catecholamines have yet to be tested, but treatment of mice with another copper chelator (fusaric acid) also acts to inhibit DBH and diminishes basal and methamphetamine-provoked dopamine release in the striatum (108). These results suggest that extracellular dopamine levels are likely reduced, not raised, by disulfiram, effectively refuting the idea that disulfiram treatment promotes cocaine abstinence by “normalizing” dopamine levels in addicts (see Figure 4).
Figure 4.
Effect of chronic DBH inhibition on dopamine transmission. Genetic DBH inhibition, and presumably pharmacological DBH inhibition by disulfiram, leads to decreased norepinephrine synthesis in the locus coeruleus and brainstem and norepinephrine release in the midbrain. Because midbrain dopaminergic neurons require noradrenergic drive for normal burst firing and neurotransmitter release, dopamine release is decreased and a compensatory upregulation of high-affinity state dopamine receptors ensues, resulting in behavioral hypersensitivity to psychostimulants.
Reduction in Cocaine-Induced Reward Effects by DBH Inhibition
Because noradrenergic facilitation of dopamine transmission is crucial for the rewarding effects of psychostimulants, disulfiram might be reasoned to reduce cocaine use by inhibiting the noradrenergic-mediated high. Indeed, norepinephrine depletion or adrenergic receptor blockade attenuates the stimulant and rewarding effects of amphetamine and cocaine in rodents [reviewed in (100)]. For example, norepinephrine promotes the locomotor-activating effects of psychostimulants and also facilitates dopamine release in the nucleus accumbens and conditioned place preference (105, 106, 109–112). Consistent with this hypothesis of DBH inhibition, one human laboratory study found that disulfiram decreased cocaine “high” and “rush” (53). On the other hand, several studies show no effect or even higher levels of psycho-stimulant reward following disulfiram administration (3, 47–49, 54). In any event, therapeutic strategies to blunt the rewarding effects of psychostimulants are unlikely to be successful, because drug addicts can easily defeat such measures by adjusting their drug intake. For instance, dopamine receptor antagonists actually tend to increase rather than decrease cocaine self-administration in rats (113–116). It is thus not reasonable to expect partial reduction of cocaine reward to significantly modify the behavior of cocaine-dependent individuals.
Cocaine Aversion via DBH Inhibition
The mesolimbic system and dopamine release are important not only for reward, but also for responses to aversive stimuli (117–119). Because norepinephrine depletion attenuates drug-induced dopamine release, as described above, DBH inhibition might also be expected to blunt the aversive effects of cocaine. Although this may be true for acute DBH inhibition, chronic DBH inhibition (as might be observed following days or weeks of disulfiram treatment) leads to compensatory changes in the dopaminergic system that result, paradoxically perhaps, in a hypersensitivity to the aversive effects of cocaine. A striking example of this phenomenon occurs in Dbh−/− mice, which essentially have total and lifelong DBH inhibition (120, 121). Given their low basal levels of dopamine release, which remains low in spite of stimulant administration, these mice manifest a compensatory increase in striatal high-affinity state dopamine receptors (107). Consequently, Dbh−/− mice have a paradoxical hypersensitivity to psychostimulants, manifested in heightened locomotor activity and related stereotypical behavior (107, 122). Even more relevant to this discussion is the observation that the same cocaine doses that evoke conditioned place preference in wild-type mice result in conditioned place aversion in Dbh−/− mice. The Dbh−/− phenotype thus suggests that chronic norepinephrine deficiency augments the aversive effects of cocaine.
The augmentation of aversive behavior in Dbh−/− mice may offer an explanation for similar behavior seen in people. Plasma DBH activity is highly variable in humans, and a significant proportion of this variability is genetic (123). A common polymorphism, a C-to-T change at nucleotide position -1021 of the DBH gene (allele frequency ~0.2) accounts for most of the genetic variance in DBH activity; individuals heterozygous for the low-activity T allele (i.e., individuals who are “CT”) have approximately 50% lower DBH activity than CC homozygotes; DBH activity in TT homozygotes is typically reduced by greater than 90% relative to that of CC individuals (124). Strikingly, cocaine addicts with genetically low DBH activity report increased cocaine-induced paranoia (125, 126), an effect that is phenocopied by disulfiram treatment prior to cocaine administration [R. Malison, personal communication; see also (49, 50, 127)].
Could an increase in the aversive effects of cocaine, such as paranoia, underlie disulfiram-induced cocaine abstinence? The success of disulfiram in the treatment of alcoholism depends on just such a negative response to the addictive drug. In the case of ethanol, inhibition of aldehyde dehydrogenase produces an aversive reaction mediated by acetaldehyde. Perhaps in the case of cocaine, chronic inhibition of DBH produces an aversive reaction mediated by the induced expression of high-affinity dopamine receptors and excessive dopamine signaling. Appealing as this hypothesis may be, however, increased cocaine aversion is probably not a major contributor to the clinical efficacy of disulfiram. As discussed above, cocaine addicts habitually titrate their cocaine intake to achieve the desired subjective effects. If high doses of cocaine were producing a negative experience, they would probably lower their intake, not abstain. In fact, at low doses of cocaine, Dbh−/− mice actually seem to experience increased reward instead of aversion (107). Furthermore, neither adrenergic receptor antagonists nor disulfiram affect operant cocaine self-administration in rats (128–130). Finally, if cocaine aversion induced by chronic DBH inhibition discouraged cocaine use, one would predict that low-activity DBH alleles would be underrepresented in addicts, a prediction that is refuted by current data (131).
In summary, DBH inhibition is by far the most likely mechanism underlying disulfiram-induced cocaine abstinence for several reasons: i) the inhibition of DBH alters neurotransmitter levels known to be critical for the rewarding, aversive, and addictive properties of cocaine; ii) both mice and humans with low DBH activity have altered responses to cocaine; and iii) the altered responses to cocaine in DBH-deficient animals can be mimicked by disulfiram treatment. Interestingly, a pharmacogenetic interaction between disulfiram and DBH may exist; disulfiram appears to lower cocaine use primarily in individuals carrying at least one low-activity DBH allele (132, 133). This intriguing finding reinforces the idea that DBH inhibition contributes to the therapeutic effects of disulfiram in the treatment of cocaine dependence. However, all the potential hypotheses to account for why DBH inhibition is effective (e.g., dopamine receptor agonism, decreased cocaine reward, and increased cocaine aversion) fail upon a reasoned consideration of current data. In the final section, we will argue that relapse prevention, mediated by DBH inhibition and a decrease in norepinephrine, provides the most likely mechanistic explanation.
Norepinephrine and Reinstatement
Drug addiction is a chronic relapsing disorder (134, 135), as patients in treatment often slip back into drug-taking behaviors after periods of sobriety. Several types of stimuli can trigger drug craving and lead to relapse. Environmental and sensory cues associated with past drug use can prompt strong urges to renew drug use, an occurrence known as cue-induced relapse (136–138). Re-exposure to illicit drugs can also lead to reinstatement of drug abuse, which is called drug-primed relapse. Stress is the classic trigger for drug use, in the initiation of drug use, and the maintenance of addictive behavior (137, 139). Anecdotally, stress is cited as the most common cause of relapse. The organism’s natural reward system is neurologically usurped in addiction (140, 141), and so it is crucial that pharmacotherapies for cocaine addiction be selective in repressing the drive to seek drugs without altering the healthy impulse to seek out natural rewards. Prevention of relapse is thus considered one of the most promising strategies for current pharmacotherapies targeted at cocaine dependence; such strategies are based on efforts to dissociate drug-related cues and the effects of stress from drug use.
Although pharmacological manipulation of the noradrenergic system does not affect cocaine self-administration in rats or non-human primates, it profoundly affects the reinstatement of cocaine seeking after a period of abstinence or extinction of the behavior, and the effect on reinstatement in experimental mammals has become a commonly used model of human relapse. In such experiments, animals are first conditioned to self-administer a drug by pressing a lever that results in drug infusion, and then they are exposed to an “extinction” phase, during which responses on the active lever result only in saline infusions. During extinction, cues previously associated with drug delivery (i.e., tone and illumination of cue light) are absent. Following the extinction phase, “reinstatement” tests begin, during which rats are exposed to a relapse trigger, such as an injection of drug, acute stress (e.g., foot shock), or environmental cues associated with drug delivery (i.e., tone and light). These tests are referred to as drug-primed, stress-induced, and cue-induced reinstatement, respectively. Reinstatement of drug seeking is operationally defined as the number of responses on the active lever that previously resulted in cocaine infusion, even though the animals are under extinction conditions (i.e., still receiving saline infusions). The validity of reinstatement tests to model human addiction is high, as determined in studies of both humans and laboratory animals (142).
Studies have shown that reduction of norepinephrine signaling attenuates reinstatement of amphetamine- (143) and cocaine-seeking, although it appears that this attenuation can be mediated by different receptors; blockade of β-adrenergic receptors prevents stress-induced reinstatement, whereas blockade of α1-adrenergic receptors abrogates drug-primed reinstatement (144, 145). Preliminary results indicate that the blockade of cocaine-primed reinstatement by α1-adrenergic receptor antagonists can be mimicked by disulfiram (our unpublished data). Conversely, the facilitation of norepinephrine transmission (e.g., induces reinstatement in rats and non-human primates (146–148).
The contribution of norepinephrine to stress-induced reinstatement is particularly intriguing: emotional stress has been associated with increases in drug craving (149, 150); high stress is a good predictor of continued drug use in addicts (149); and individuals exposed to stress are more likely to relapse into drug use (150, 152). There is an overlap among the neurocircuits implicated in drug abuse and stress [see (153–155)]. The extended amygdala [i.e., bed nucleus of the stria terminalis, central amygdala, and the shell of the nucleus accumbens (156)], receives corticotropin-releasing factor and noradrenergic innervation, and this concerted innervation underlies the role of the extended amygdala in sensory information integration, emotion, and appetitive learning. Thus, it functions as a mediator of both stress and drug responses and integrates the function of norepinephrine in influencing stress-induced reinstatement [see (157)]. For example, local infusions of β-adrenergic receptor antagonists within the extended amygdala block stress-induced reinstatement of cocaine self-administration in rats (144). Finally, we and others have shown that β-adrenergic receptor antagonists attenuate cocaine-induced and cocaine withdrawal-induced anxiety (158, 159). Together, these results indicate that norepinephrine mediates stress responses that contribute to relapse. We propose that by decreasing noradrenergic transmission, disulfiram can dampen the stress response and lower the likelihood of relapse in the face of environmental influences and stressors.
Currently, there is little direct evidence supporting an effect of disulfiram on relapse prevention, as most clinical trials have focused on its effect on current cocaine use. To date, there has been only one study that measured outcomes in a group of patients for one year following monitored disulfiram treatment (43). Recorded outcomes in this follow-up study were complete abstinence and number of days of cocaine use (during the previous four weeks) self-reported at one, three, six, and twelve months after treatment completion. Frequency of drug use is a point-prevalence variable that gives a snapshot of a disease at a specific point in time. This measure is touted as a good reflection of the nature of drug use because it takes into account the fact that patients often relapse briefly into drug use and also allows for validation of self-reports with biochemical measures. Unfortunately, when this measure is not accompanied by thorough interviewing of the study participants, it fails to provide a broad view of the effect of a treatment. We speculate that if disulfiram primarily induces an increase in the aversive effects of cocaine, measures of frequency and amount of cocaine used would gradually decrease, as drug addicts would titrate drug intake to achieve the desired subjective effects. Conversely, if disulfiram primarily prevents relapse by blunting the drive to take drug following environmental stressors or cues, one might expect to observe a decrease in latency to abstinence and/or extended periods of abstinence. Carroll and colleagues reported that more study participants assigned to the disulfiram/CBT treatment group achieved complete abstinence from cocaine during the following year than those assigned to the placebo/CBT group. We urge that future clinical studies include interviews with participants and measures that can distinguish between abstinence due to altered subjective drug effects vs healthier responses to environmental triggers.
Conclusion
Disulfiram has been used as an alcohol deterrent for decades, and recent studies indicate that it is also an effective pharmacotherapy for the treatment of cocaine dependence; however, the mechanisms behind its efficacy for alcohol and for cocaine addiction are distinct. Whereas aldehyde dehydrogenase is the primary target in treating alcoholism, human laboratory, genetic, and preclinical animal studies indicate that its beneficial effects on cocaine use result from the inhibition of DBH. Despite the potential of DBH and its inhibition to modulate cocaine reward and aversion, we argue that the most important clinical effect of disulfiram-mediated DBH inhibition arises from the drug’s ability to reduce relapse, particularly, relapse precipitated by stress.
This hypothesis will require further studies in preclinical models of addiction. First, we must explore the effect of acute and chronic disulfiram treatment on several aspects of dopamine transmission, such as neurochemical and behavioral responses to psychostimulants. Second, we need to know the effects of disulfiram on stress-induced, cue-induced, and drug-primed reinstatement of cocaine seeking, as well as the brain region(s) critical for these effects. Third, disulfiram as a treatment for dependence on psychostimulants other than cocaine should also be investigated, as has been suggested by human laboratory studies of amphetamine-like drugs. Pharmacogenetic interactions between disulfiram and DBH genotype may also yield valuable information. Finally, the knowledge acquired by studying disulfiram could be translated into safer and more effective pharmacotherapies for the treatment of cocaine dependence. Because disulfiram use is limited by its non-specificity, side effects, and toxicity, the development and testing of selective DBH inhibitors will be essential to mechanistic studies as well as to improved therapeutics.

Meriem Gaval-Cruz, BS, is a graduate student in the Division of Biological and Biomedical Sciences at Emory University. Her research interests focus on the neurobiology of drug addiction, with an emphasis on cocaine pharmacotherapies. Her activities include neuroscience outreach to K-12 students and the laboratory mentoring of undergraduates.
David Weinshenker, PhD, is Associate Professor in the Department of Human Genetics at Emory University. He has pursued model systems to better understand genes involved in human disease. He applies genetic models combined with pharmacological tools to investigate various aspects of neurobiology, with a particular focus on the role of norepinephrine in brain function and disease. Send correspondence to DW. E-mail dweinshenker@genetics.emory.edu; fax 404-727-3949.
Acknowledgments
We thank Cheryl Strauss for assistance with text editing, Robert Malison for sharing unpublished results, and the National Institutes of Health and the National Institute of Drug Abuse for financial support. This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grants DA017963, DA25040].
References
- 1.Kitson TM. The disulfiram–ethanol reaction. J Stud Alcohol 38 96–113 (1977). [DOI] [PubMed] [Google Scholar]
- 2.George TP, Chawarski, MC, Pakes, J, Carroll, KM, Kosten, TR and Schottenfeld, RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: A preliminary trial. Biol Psychiatry 47 1080–1086 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Petrakis IL, Carroll, KM, Nich, C, Gordon, LT, McCance-Katz, EF, Frankforter, T and Rounsaville, BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction 95 219–228 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Carroll KM, Fenton, LR, Ball, SA, Nich, C, Frankforter, TL, Shi, J and Rounsaville, BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: A randomized placebo-controlled trial. Arch Gen Psychiatry 61 264–272 (2004). This paper reports the first systematic study of the effect of disulfiram on cocaine use in a general population of cocaine users, regardless of concurrent ethanol intake. It put forth the notion that disulfiram’s mechanism of action in curbing cocaine intake must be independent of its effects on alcohol metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin EY, Levenberg, B and Kaufman, S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem 235 2080–2086 (1960). [PubMed] [Google Scholar]
- 6.Deitrich RA and Erwin, VG. Mechanism of the inhibition of aldehyde dehydrogenase in vivo by disulfiram and diethyldithiocarbamate. Mol Pharmacol 7 301–307 (1971). [PubMed] [Google Scholar]
- 7.Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl 369 15–26 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Hald J and Jacobsen, E. A drug sensitizing the organism to ethyl alcohol. Lancet 2 1001–1004 (1948). [DOI] [PubMed] [Google Scholar]
- 9.Johnston CD. The in vitro reaction between tetraethylthiuram disulfide (antabuse) and glutathione. Arch Biochem Biophys 44 249–251 (1953). [DOI] [PubMed] [Google Scholar]
- 10.Torronen R and Marselos, M. Changes in the hepatic copper conent after treatment with foreign compounds. Arch Toxicol Suppl 247–249 (1978). [DOI] [PubMed]
- 11.Suh JJ, Pettinati, HM, Kampman, KM and O’Brien, CP. The status of disulfiram: A half of a century later. J Clin Psychopharmacol 26 290–302 (2006). This paper provides a thorough summary of recent supervised clinical trials testing disulfiram in the treatment of alcoholics. [DOI] [PubMed] [Google Scholar]
- 12.Chick J, Gough, K, Falkowski, W, Kershaw, P, Hore, B, Mehta, B, Ritson, B, Ropner, R and Torley, D. Disulfiram treatment of alcoholism. Br J Psychiatry 161 84–89 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Gerrein JR, Rosenberg, CM and Manohar, V. Disulfiram maintenance in outpatient treatment of alcoholism. Arch Gen Psychiatry 28 798–802 (1973). [DOI] [PubMed] [Google Scholar]
- 14.Azrin NH. Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther 14 339–348 (1976). [DOI] [PubMed] [Google Scholar]
- 15.Keane TM, Foy, DW, Nunn, B and Rychtarik, RG. Spouse contracting to increase antabuse compliance in alcoholic veterans. J Clin Psychol 40 340–344 (1984). [DOI] [PubMed] [Google Scholar]
- 16.Robichaud C, Strickler, D, Bigelow, G and Liebson, I. Disulfiram maintenance employee alcoholism treatment: A three-phase evaluation. Behav Res Ther 17 618–621 (1979). [DOI] [PubMed] [Google Scholar]
- 17.Sereny G, Sharma, V, Holt, J and Gordis, E. Mandatory supervised antabuse therapy in an outpatient alcoholism program: A pilot study. Alcohol Clin Exp Res 10 290–292 (1986). [DOI] [PubMed] [Google Scholar]
- 18.Brewer C. Patterns of compliance and evasion in treatment programmes which include supervised disulfiram. Alcohol Alcohol 21 385–388 (1986). [PubMed] [Google Scholar]
- 19.Besson J, Aeby, F, Kasas, A, Lehert, P and Potgieter, A. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: A controlled study. Alcohol Clin Exp Res 22 573–579 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Frisoni GB and Di Monda, V. Disulfiram neuropathy. (1971–1988) and report of a case. Alcohol Alcohol 24 429–437 (1989). [PubMed] [Google Scholar]
- 21.Kristenson H. How to get the best out of antabuse. Alcohol Alcohol 30 775–783 (1995). [PubMed] [Google Scholar]
- 22.Wilson H. Side effects of disulfiram. Br Med J 2 1610–1611 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupuy O, Flocard, F, Vial, C, Rode, G, Charles, N, Boisson, D and Flechaire, A. Disulfiram (Esperal) toxicity. Apropos of 3 original cases. Rev Med Interne 16 67–72 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Closser MH and Kosten, TR. Alcohol and cocaine abuse. A comparison of epidemiology and clinical characteristics. Recent Dev Alcohol 10 115–128 (1992). [PubMed] [Google Scholar]
- 25.Grant BF and Harford, TC. Concurrent and simultaneous use of alcohol with cocaine: Results of national survey. Drug Alcohol Depend 25 97–104 (1990). [DOI] [PubMed] [Google Scholar]
- 26.Weiss RD, Mirin, SM, Griffin, ML and Michael, JL. Psychopathology in cocaine abusers. Changing trends. J Nerv Ment Dis 176 719–725 (1988). [DOI] [PubMed] [Google Scholar]
- 27.Khalsa H, Paredes, A and Anglin, MD. The role of alcohol in cocaine dependence. Recent Dev Alcohol 10 7–35 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Dean RA, Christian, CD, Sample, RH and Bosron, WF. Human liver cocaine esterases: Ethanol-mediated formation of ethylcocaine. FASEB J 5 2735–2739 (1991). [DOI] [PubMed] [Google Scholar]
- 29.Perez-Reyes M and Jeffcoat, AR. Ethanol/cocaine interaction: Cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sci 51 553–563 (1992). [DOI] [PubMed] [Google Scholar]
- 30.McCance-Katz EF, Price, LH, McDougle, CJ, Kosten, TR, Black, JE and Jatlow, PI. Concurrent cocaine-ethanol ingestion in humans: Pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology (Berl) 111 39–46 (1993). [DOI] [PubMed] [Google Scholar]
- 31.Rose JS. Cocaethylene: A current understanding of the active metabolite of cocaine and ethanol. Am J Emerg Med 12 489–490 (1994). [DOI] [PubMed] [Google Scholar]
- 32.Farre M, de la Torre, R, Gonzalez, ML, Teran, MT, Roset, PN, Menoyo, E and Cami, J. Cocaine and alcohol interactions in humans: Neuroendocrine effects and cocaethylene metabolism. J Pharmacol Exp Ther 283 164–176 (1997). [PubMed] [Google Scholar]
- 33.Jatlow P, Hearn, WL, Elsworth, JD, Roth, RH, Bradberry, CW and Taylor, JR. Cocaethylene inhibits uptake of dopamine and can reach high plasma concentrations following combined cocaine and ethanol use. NIDA Res Monogr 105 572–573 (1990). [PubMed] [Google Scholar]
- 34.Hearn WL, Flynn, DD, Hime, GW, Rose, S, Cofino, JC, Mantero-Atienza, E, Wetli, CV and Mash, DC. Cocaethylene: A unique cocaine metabolite displays high affinity for the dopamine transporter. J Neurochem 56 698–701 (1991). [DOI] [PubMed] [Google Scholar]
- 35.Jatlow P, Elsworth, JD, Bradberry, CW, Winger, G, Taylor, JR, Russell, R and Roth, RH. Cocaethylene: A neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci 48 1787–1794 (1991). [DOI] [PubMed] [Google Scholar]
- 36.Bunney EB, Appel, SB and Brodie, MS. Electrophysiological effects of cocaethylene, cocaine, and ethanol on dopaminergic neurons of the ventral tegmental area. J Pharmacol Exp Ther 297 696–703 (2001). [PubMed] [Google Scholar]
- 37.Hart CL, Jatlow, P, Sevarino, KA and McCance-Katz, EF. Comparison of intravenous cocaethylene and cocaine in humans. Psychopharmacology (Berl) 149 153–162 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Raven MA, Necessary, BD, Danluck, DA and Ettenberg, A. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol 8 117–124 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Schechter MD and Meehan, SM. The lethal effects of ethanol and cocaine and their combination in mice: implications for cocaethylene formation. Pharmacol Biochem Behav 52 245–248 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Higgins ST, Budney, AJ, Bickel, WK, Hughes, JR and Foerg, F. Disulfiram therapy in patients abusing cocaine and alcohol. Am J Psychiatry 150 675–676 (1993). This was the first published study to show disulfiram therapy significantly decreased both cocaine and alcohol use in comorbid abusers. It laid the foundation for future studies to examine the direct contribution of disulfiram to each of these outcomes. [DOI] [PubMed] [Google Scholar]
- 41.Carroll K, Ziedonis, D, O’Malley, S, McCance-Katez, E, Gordon, L and Rounsaville, B. Pharmacologic interventions for alcohol-and-cocaine-abusing individuals. Am J Addictions 2 77–79 (1993). [Google Scholar]
- 42.Carroll KM, Nich, C, Ball, SA, McCance, E and Rounsavile, BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 93 713–727 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Carroll KM, Nich, C, Ball, SA, McCance, E, Frankforter, TL and Rounsaville, BJ. One-year follow-up of disulfiram and psychotherapy for cocaine-alcohol users: Sustained effects of treatment. Addiction 95 1335–1349 (2000). This paper is the follow-up report to a clinical trial testing disulfiram on cocaine and alcohol users. It was the first to show that disulfiram treatment promotes cocaine abstinence and the effect is sustained for at least one year. [DOI] [PubMed] [Google Scholar]
- 44.Grassi MC, Cioce, AM, Giudici, FD, Antonilli, L and Nencini, P. Short-term efficacy of disulfiram or naltrexone in reducing positive urinalysis for both cocaine and cocaethylene in cocaine abusers: A pilot study. Pharmacol Res 55 117–121 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Pettinati HM, Kampman, KM, Lynch, KG, Xie, H, Dackis, C, Rabinowitz, AR and O’Brien, CP. A double blind, placebo-controlled trial that combines disulfiram and naltrexone for treating co-occurring cocaine and alcohol dependence. Addict Behav 33 651–667 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nich C, McCance-Katz, EF, Petrakis, IL, Cubells, JF, Rounsaville, BJ and Carroll, KM. Sex differences in cocaine-dependent individuals’ response to disulfiram treatment. Addict Behav 29 1123–1128 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCance-Katz EF, Kosten, TR and Jatlow, P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend 52 27–39 (1998). [DOI] [PubMed] [Google Scholar]
- 48.McCance-Katz EF, Kosten, TR and Jatlow, P. Chronic disulfiram treatment effects on intranasal cocaine administration: Initial results. Biol Psychiatry 43 540–543 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Hameedi FA, Rosen, MI, McCance-Katz, EF, McMahon, TJ, Price, LH, Jatlow, PI, Woods, SW and Kosten, TR. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol Psychiatry 37 560–563 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Mutschler J, Diehl, A and Kiefer, F. Pronounced paranoia as a result of cocaine-disulfiram interaction: Case report and mode of action. J Clin Psychopharmacol 29 99–101 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Murthy KK. Psychosis during disulfiram therapy for alcoholism. J Indian Med Assoc 95 80–81 (1997). [PubMed] [Google Scholar]
- 52.Ceylan ME, Turkcan, A, Mutlu, E and Onal, O. Manic episode with psychotic symptoms associated with high dose of disulfiram: A case report. J Clin Psychopharmacol 27 224–225 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Baker JR, Jatlow, P and McCance-Katz, EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend 87 202–209 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sofuoglu M, Poling, J, Waters, A, Sewell, A, Hill, K and Kosten, T. Disulfiram enhances subjective effects of dextroamphetamine in humans. Pharmacol Biochem Behav 90 394–398 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malcolm R, Olive, MF and Lechner, W. The safety of disulfiram for the treatment of alcohol and cocaine dependence in randomized clinical trials: Guidance for clinical practice. Expert Opin Drug Saf 7 459–72 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Maj J and Przegalinski, E. Disulfiram and some effects of amphetamine in mice and rats. J Pharm Pharmacol 19 341–342 (1967). [DOI] [PubMed] [Google Scholar]
- 57.Maj J, Przegalinski, E and Wielosz, M. Disulfiram and the drug-induced effects on motility. J Pharm Pharmacol 20 247–248 (1968). [DOI] [PubMed] [Google Scholar]
- 58.Haile CN, During, MJ, Jatlow, PI, Kosten, TR and Kosten, TA. Disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats. Biol Psychiatry 54 915–921 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Gaval-Cruz M, Schroeder, JP, Liles, LC, Javors, MA and Weinshenker, D. Effects of disulfiram and dopamine beta-hydroxylase knockout on cocaine-induced seizures. Pharmacol Biochem Behav 89 556–562 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood PL and Altar, CA. Dopamine release in vivo from nigrostriatal, mesolimbic, and mesocortical neurons: Utility of 3-methoxytyramine measurements. Pharmacol Rev 40 163–187 (1988). [PubMed] [Google Scholar]
- 61.Burke WJ, Li, SW, Williams, EA, Nonneman, R and Zahm, DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: Implications for Parkinson’s disease pathogenesis. Brain Res 989 205–213 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Vaccari A, Saba, PL, Ruiu, S, Collu, M and Devoto, P. Disulfiram and diethyldithiocarbamate intoxication affects the storage and release of striatal dopamine. Toxicol Appl Pharmacol 139 102–108 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Musacchio J, Kopin, IJ and Snyder, S. Effects of disulfiram on tissue norepinephrine content and subcellular distribution of dopamine, tyramine and their beta-hydroxylated metabolites. Life Sci 3 769–775 (1964). [DOI] [PubMed] [Google Scholar]
- 64.Musacchio JM, Goldstein, M, Anagnoste, B, Poch, G and Kopin, IJ. Inhibition of dopamine-beta-hydroxylase by disulfiram in vivo. J Pharmacol Exp Ther 152 56–61 (1966). This was the first demonstration that disulfiram can inhibit dopamine beta-hydroxylase in vivo. [PubMed] [Google Scholar]
- 65.Goldstein M. Inhibition of norepinephrine biosynthesis at the dopamine-beta-hydroxylation stage. Pharmacol Rev 18 77–82 (1966). [PubMed] [Google Scholar]
- 66.Goldstein M and Nakajima, K. The effects of disulfiram on the repletion of brain catecholamine stores. Life Sci 5 1133–1138 (1966). [DOI] [PubMed] [Google Scholar]
- 67.Sesack SR, Carr, DB, Omelchenko, N and Pinto, A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci 1003 36–52 (2003). This is a comprehensive review of dopamine and glutamate interactions in the cortex, ventral tegmental area, basal ganglia and amygdala. It focuses on tract-tracing and immunocytochemical evidence of reciprocal anatomical connections and common target regions. [DOI] [PubMed] [Google Scholar]
- 68.Gass JT and Olive, MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75 218–265 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pulvirenti L and Diana, M. Drug dependence as a disorder of neural plasticity: Focus on dopamine and glutamate. Rev Neurosci 12 141–158 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Tzschentke TM and Schmidt, WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry 8 373–382 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Lapish CC, Seamans, JK and Chandler, LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res 30 1451–1465 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Kotlinska J and Biala, G. Memantine and ACPC affect conditioned place preference induced by cocaine in rats. Pol J Pharmacol 52 179–185 (2000). [PubMed] [Google Scholar]
- 73.Maldonado C, Rodriguez-Arias, M, Castillo, A, Aguilar, MA and Minarro, J. Effect of memantine and CNQX in the acquisition, expression and reinstatement of cocaine-induced conditioned place preference. Prog Neuropsychopharmacol Biol Psychiatry 31 932–939 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Pulvirenti L, Balducci, C and Koob, GF. Dextromethorphan reduces intravenous cocaine self-administration in the rat. Eur J Pharmacol 321 279–283 (1997). [DOI] [PubMed] [Google Scholar]
- 75.Hyytia P, Backstrom, P and Liljequist, S. Site-specific NMDA receptor antagonists produce differential effects on cocaine self-administration in rats. Eur J Pharmacol 378 9–16 (1999). [DOI] [PubMed] [Google Scholar]
- 76.Papp M, Gruca, P and Willner, P. Selective blockade of drug-induced place preference conditioning by ACPC, a functional NDMA-receptor antagonist. Neuropsychopharmacology 27 727–743 (2002). [DOI] [PubMed] [Google Scholar]
- 77.Blokhina EA, Kashkin, VA, Zvartau, EE, Danysz, W and Bespalov, AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol 15 219–225 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Backstrom P and Hyytia, P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology 31 778–786 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Backstrom P and Hyytia, P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 192 571–580 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Nagendra SN, Faiman, MD, Davis, K, Wu, JY, Newby, X and Schloss, JV. Carbamoylation of brain glutamate receptors by a disulfiram metabolite. J Biol Chem 272 24247–24251 (1997). [DOI] [PubMed] [Google Scholar]
- 81.Mamatha RK and Nagendra, SN. Effect of disulfiram administration on glutamate uptake by synaptosomes in the rat brain. Eur J Pharmacol 292 89–94 (1994). [DOI] [PubMed] [Google Scholar]
- 82.Vaccari A, Ferraro, L, Saba, P, Ruiu, S, Mocci, I, Antonelli, T and Tanganelli, S. Differential mechanisms in the effects of disulfiram and diethyldithiocarbamate intoxication on striatal release and vesicular transport of glutamate. J Pharmacol Exp Ther 285 961–967 (1998). [PubMed] [Google Scholar]
- 83.Smith JA, Mo, Q, Guo, H, Kunko, PM and Robinson, SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res 683 264–269 (1995). [DOI] [PubMed] [Google Scholar]
- 84.Meshul CK, Noguchi, K, Emre, N and Ellison, G. Cocaine-induced changes in glutamate and GABA immunolabeling within rat habenula and nucleus accumbens. Synapse 30 211–220 (1998). [DOI] [PubMed] [Google Scholar]
- 85.Keys AS, Mark, GP, Emre, N and Meshul, CK. Reduced glutamate immunolabeling in the nucleus accumbens following extended withdrawal from self-administered cocaine. Synapse 30 393–401 (1998). [DOI] [PubMed] [Google Scholar]
- 86.Williams JM and Steketee, JD. Cocaine increases medial prefrontal cortical glutamate overflow in cocaine-sensitized rats: A time course study. Eur J Neurosci 20 1639–1646 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Kalivas PW. Neurobiology of cocaine addiction: Implications for new pharmacotherapy. Am J Addict 16 71–78 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Faiman MD. 1979. Biochemical pharmacology of disulfiram. In: E Majchowicz and EP Novel (Eds.), Biochemistry and Pharmacology of Ethanol. Plenum, New York, pp. 325–348.
- 89.Stewart DJ, Inaba, T, Lucassen, M and Kalow, W. Cocaine metabolism: Cocaine and norcocaine hydrolysis by liver and serum esterases. Clin Pharmacol Ther 25 464–468 (1979). [DOI] [PubMed] [Google Scholar]
- 90.Benowitz NL. Clinical pharmacology and toxicology of cocaine. Pharmacol Toxicol 72 3–12 (1993). [DOI] [PubMed] [Google Scholar]
- 91.Heikkila RE, Orlansky, H and Cohen, G. Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol 24 847–852 (1975). [DOI] [PubMed] [Google Scholar]
- 92.Reith ME, Meisler, BE, Sershen, H and Lajtha, A. Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem Pharmacol 35 1123–1129 (1986). [DOI] [PubMed] [Google Scholar]
- 93.Ritz MC, Lamb, RJ, Goldberg, SR and Kuhar, MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237 1219–1223 (1987). [DOI] [PubMed] [Google Scholar]
- 94.Lake CR, Major, LF, Ziegler, MG and Kopin, IJ. Increased sympathetic nervous system activity in alcoholic patients treated with disulfiram. Am J Psychiatry 134 1411–1414 (1977). [DOI] [PubMed] [Google Scholar]
- 95.Frigon RP, Converse, JL and Stone, RA. Plasma dopamine beta-hydroxylase species dependence and in the vitro influence of NEM, copper, and pH. Biochem Med 19 1–15 (1978). [DOI] [PubMed] [Google Scholar]
- 96.Green AL. Inhibition of DBH by chelating agents. Biochim Biophys Acta 81 (1964).
- 97.Bourdelat-Parks BN, Anderson, GM, Donaldson, ZR, Weiss, JM, Bonsall, RW, Emery, MS, Liles, LC and Weinshenker, D. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl) 183 72–80 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Grabowski J, Shearer, J, Merrill, J and Negus, SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29 1439–1464 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Sofuoglu M and Kosten, TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opin Emerg Drugs 11 91–98 (2006). [DOI] [PubMed] [Google Scholar]
- 100.Weinshenker D and Schroeder, JP. There and back again: A tale of nor-epinephrine and drug addiction. Neuropsychopharmacology 32 1433–1451 (2007). This review focuses on the role of norepinephrine in reward, dopamine signaling and drug addiction. It thoroughly summarizes clinical and animal data from an array of behavioral paradigms used to test drug responses. [DOI] [PubMed] [Google Scholar]
- 101.Mejias-Aponte CA, Drouin, C and Aston-Jones, G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: Prominent inputs from medullary homeostatic centers. J Neurosci 29 3613–3626 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grenhoff J, Nisell, M, Ferre, S, Aston-Jones, G and Svensson, TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect 93 11–25 (1993). [DOI] [PubMed] [Google Scholar]
- 103.Grenhoff J and Svensson, TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol 233 79–84 (1993). [DOI] [PubMed] [Google Scholar]
- 104.Lategan AJ, Marien, MR and Colpaert, FC. Effects of locus coeruleus lesions on the release of endogenous dopamine in the rat nucleus accumbens and caudate nucleus as determined by intracerebral microdialysis. Brain Res 523 134–138 (1990). [DOI] [PubMed] [Google Scholar]
- 105.Ventura R, Cabib, S, Alcaro, A, Orsini, C and Puglisi-Allegra, S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci 23 1879–1885 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ventura R, Morrone, C and Puglisi-Allegra, S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci USA 104 5181–5186 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schank JR, Ventura, R, Puglisi-Allegra, S, Alcaro, A, Cole, CD, Liles, LC, Seeman, P and Weinshenker, D. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology 31 2221–2230 (2006). This paper reports neurochemical changes and behavioral responses to cocaine in mice dopamine beta-hydroxylase knockout mice, providing strong evidence for the role of norepinephrine and norepinephrine-dopamine interactions in reward. [DOI] [PubMed] [Google Scholar]
- 108.Weinshenker D, Ferrucci, M, Busceti, CL, et al. Genetic or pharmacological blockade of noradrenaline synthesis enhances the neurochemical, behavioral, and neurotoxic effects of methamphetamine. J Neurochem 105 471–483 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Snoddy AM and Tessel, RE. Prazosin: Effect on psychomotor-stimulant cues and locomotor activity in mice. Eur J Pharmacol 116 221–228 (1985). [DOI] [PubMed] [Google Scholar]
- 110.Darracq L, Blanc, G, Glowinski, J and Tassin, JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci 18 2729–2739 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Drouin C, Darracq, L, Trovero, F, Blanc, G, Glowinski, J, Cotecchia, S and Tassin, JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci 22 2873–2884 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Auclair A, Drouin, C, Cotecchia, S, Glowinski, J and Tassin, JP. 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci 20 3073–3084 (2004). [DOI] [PubMed] [Google Scholar]
- 113.Maldonado R, Robledo, P, Chover, AJ, Caine, SB and Koob, GF. D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav 45 239–242 (1993). [DOI] [PubMed] [Google Scholar]
- 114.McGregor A and Roberts, DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res 624 245–252 (1993). [DOI] [PubMed] [Google Scholar]
- 115.Epping-Jordan MP, Markou, A and Koob, GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res 784 105–115 (1998). [DOI] [PubMed] [Google Scholar]
- 116.Ahmed SH and Koob, GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology (Berl) 172 450–454 (2004). [DOI] [PubMed] [Google Scholar]
- 117.Thierry AM, Tassin, JP, Blanc, G and Glowinski, J. Selective activation of mesocortical dopamine system by stress. Nature 263 242–244 (1976). [DOI] [PubMed] [Google Scholar]
- 118.Abercrombie ED, Keefe, KA, DiFrischia, DS and Zigmond, MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52 1655–1658 (1989). [DOI] [PubMed] [Google Scholar]
- 119.Jensen PG, Curtis, PD, Dunn, JA, Austic, RE and Richmond, ME. Field evaluation of capsaicin as a rodent aversion agent for poultry feed. Pest Manag Sci 59 1007–1015 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Thomas SA, Matsumoto, AM and Palmiter, RD. Noradrenaline is essential for mouse fetal development. Nature 374 643–646 (1995). [DOI] [PubMed] [Google Scholar]
- 121.Thomas SA, Marck, BT, Palmiter, RD and Matsumoto, AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem 70 2468–2476 (1998). [DOI] [PubMed] [Google Scholar]
- 122.Weinshenker D, Miller, NS, Blizinsky, K, Laughlin, ML and Palmiter, RD. Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci USA 99 13873–13877 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weinshilboum RM, Schorott, HG, Raymond, FA, Weidman, WH and Elveback, LR. Inheritance of very low serum dopamine-beta-hydroxylase activity. Am J Hum Genet 27 573–585 (1975). [PMC free article] [PubMed] [Google Scholar]
- 124.Zabetian CP, Anderson, GM, Buxbaum, SG, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: Evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet 68 515–522 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cubells JF, Kranzler, HR, McCance-Katz, E, Anderson, GM, Malison, RT, Price, LH and Gelernter, J. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry 5 56–63 (2000). [DOI] [PubMed] [Google Scholar]
- 126.Kalayasiri R, Sughondhabirom, A, Gueorguieva, R, Coric, V, Lynch, WJ, Lappalainen, J, Gelernter, J, Cubells, JF and Malison, RT. Dopamine beta-hydroxylase gene (DbetaH) -1021C-->T influences self-reported paranoia during cocaine self-administration. Biol Psychiatry 61 1310–1313 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Mutschler J, Diehl, A and Kiefer, F. Pronounced paranoia as a result of cocaine-disulfiram interaction: Case report and mode of action. J Clin Psychopharmacol 29 99–101 (2009). [DOI] [PubMed] [Google Scholar]
- 128.Yokel RA and Wise, RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology (Berl) 48 311–318 (1976). [DOI] [PubMed] [Google Scholar]
- 129.Risner M and Jones, BE. Role of noradrenergic and dopaminergic processes in amphetamine self-administration. Pharmacol Biochem Behav 5 477–482 (1976). [DOI] [PubMed] [Google Scholar]
- 130.Roberts DC, Corcoran, ME and Fibiger, HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 6 615–620 (1977). [DOI] [PubMed] [Google Scholar]
- 131.Guindalini C, Laranjeira, R, Collier, D, Messas, G, Vallada, H and Breen, G. Dopamine-beta hydroxylase polymorphism and cocaine addiction. Behav Brain Funct 4 1 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schottenfeld RS, Chawarski, MC, George, TPA and Cubells, JF, 2004. Pharmacogenetics of disulfiram for cocaine treatment: Role of DBH genotype. The 66th Annual CPDD Meeting, San Juan, Puerto Rico.
- 133.Oliveto O, Poling, J and Pruzinsky, R, 2004. Efficacy of disulfiram for cocaine abuse in methadone patients: relevance of dopamine beta-hydroxylase. The 43rd American College of Neuropsychopharmacology (ACNP) meeting, San Juan, Puerto Rico.
- 134.Leshner AI. Addiction is a brain disease, and it matters. Science 278 45–47 (1997). [DOI] [PubMed] [Google Scholar]
- 135.Hunt WA, Barnett, LW and Branch, LG. Relapse rates in addiction programs. J Clin Psychol 27 455–456 (1971). [DOI] [PubMed] [Google Scholar]
- 136.Kalivas PW and Volkow, ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry 162 1403–1413 (2005). [DOI] [PubMed] [Google Scholar]
- 137.Sinha R and Li, CS. Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug Alcohol Rev 26 25–31 (2007). [DOI] [PubMed] [Google Scholar]
- 138.Drummond DC. What does cue-reactivity have to offer clinical research? Addiction 95 Suppl_2, S129–144 (2000). [DOI] [PubMed] [Google Scholar]
- 139.Sinha R, Garcia, M, Paliwal, P, Kreek, MJ and Rounsaville, BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63 324–331 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Ikemoto S and Wise, RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 47 Suppl 1, 190–201 (2004). [DOI] [PubMed] [Google Scholar]
- 141.Kelley AE, Schiltz, CA and Landry, CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav 86 11–14 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Panlilio LV and Goldberg, SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction 102 1863–1870 (2007). This review summarizes popular drug self-administration paradigms used in animal research, discussing their variations, advantages, limitations and their validity in modeling drug addiction in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Davis WM, Smith, SG and Khalsa, JH. Noradrenergic role in the self-administration of morphine or amphetamine. Pharmacol Biochem Behav 3 477–484 (1975). [DOI] [PubMed] [Google Scholar]
- 144.Leri F, Flores, J, Rodaros, D and Stewart, J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22 5713–5718 (2002). This paper conclusively demonstrated that blockade of norepinephrine transmission in stress-related brain circuits attenuates stress-induced reinstatement of drug seeking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang XY and Kosten, TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry 57 1202–1204 (2005). [DOI] [PubMed] [Google Scholar]
- 146.Lee B, Tiefenbacher, S, Platt, DM and Spealman, RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology 29 686–693 (2004). [DOI] [PubMed] [Google Scholar]
- 147.Platt DM, Rowlett, JK and Spealman, RD. Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther 322 894–902 (2007). [DOI] [PubMed] [Google Scholar]
- 148.Brown ZJ, Tribe, E, D’Souza, NA and Erb, S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 203 121–130 (2009). [DOI] [PubMed] [Google Scholar]
- 149.Sinha R, Catapano, D and O’Malley, S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 142 343–351 (1999). [DOI] [PubMed] [Google Scholar]
- 150.Sinha R, Fuse, T, Aubin, LR and O’Malley, SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 152 140–148 (2000). [DOI] [PubMed] [Google Scholar]
- 151.Brewer DD, Catalano, RF, Haggerty, K, Gainey, RR and Fleming, CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction 93 73–92 (1998). [PubMed] [Google Scholar]
- 152.Kosten TR, Rounsaville, BJ and Kleber, HD. A 2.5-year follow-up of depression, life crises, and treatment effects on abstinence among opioid addicts. Arch Gen Psychiatry 43 733–738 (1986). [DOI] [PubMed] [Google Scholar]
- 153.Piazza PV and Le Moal, ML. Pathophysiological basis of vulnerability to drug abuse: Role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 36 359–378 (1996). [DOI] [PubMed] [Google Scholar]
- 154.Piazza PV and Le Moal, M. The role of stress in drug self-administration. Trends Pharmacol Sci 19 67–74 (1998). [DOI] [PubMed] [Google Scholar]
- 155.Kreek MJ and Koob, GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51 23–47 (1998). [DOI] [PubMed] [Google Scholar]
- 156.Heimer L, Alheid, GF and Zahm, DS, 1993. Basal forebrain organization: An anatomical framework for motor aspects of drive and motivation. In: PW Kalivas and CD Barnes (Eds.), Limbic Motor Circuits and Neuropsychiatry. CRC Press, Boca Raton, pp. 1–43.
- 157.Smith RJ and Aston-Jones, G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct 213 43–61 (2008). This paper gives a detailed analysis of norepinephrine and corticosterone releasing factor interactions in the amygdala and how it mediates behavioral responses to drugs of abuse. It explains how stress response systems affect reward processing during protracted drug abstinence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rudoy CA and Van Bockstaele, EJ. Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Prog Neuropsychopharmacol Biol Psychiatry 31 1119–1129 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schank JR, Liles, LC and Weinshenker, D. Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry 63 1007–1012 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]