Abstract
Accelerator mass spectrometry is a detection platform with exceptional sensitivity compared with other bioanalytical platforms. Accelerator mass spectrometry (AMS) is widely used in archeology for radiocarbon dating applications. Early exploration of the biological and pharmaceutical applications of AMS began in the early 1990s. AMS has since demonstrated unique problem-solving ability in nutrition science, toxicology and pharmacology. AMS has also enabled the development of new applications, such as Phase 0 microdosing. Recent development of AMS-enabled applications has transformed this novelty research instrument to a valuable tool within the pharmaceutical industry. Although there is now greater awareness of AMS technology, recognition and appreciation of the range of AMS-enabled applications is still lacking, including study-design strategies. This review aims to provide further insight into the wide range of AMS-enabled applications. Examples of studies conducted over the past two decades will be presented, as well as prospects for the future of AMS.
The Ebers papyrus, written in Egypt in the 16th Century BC, lists the extensive pharmacopeia of that civilization. Included in the writings are beer, turpentine, myrrh, juniper berries, poppy, lead, salt and crushed precious stones. Also included were products derived from animals, such as lizard’s blood, swine teeth and goose grease. From ancient China comes evidence of that culture’s extensive efforts to heal through the use of natural products. The Pen Tsao, or Great Herbal, comprised 40 volumes describing thousands of prescriptions [1].
It is curious to note that the same techniques that established the age of these written artifacts are now being used to quantify the kinetics and distribution of the pharmacopeia of this civilization. Accelerator mass spectrometry (AMS) established itself as an indispensable tool in archeology and radiocarbon dating in the 1980s. This article summarizes two decades of AMS research applied to the biomedical and pharmaceutical fields, with special focus on radiocarbon-based AMS applications.
Review of published work
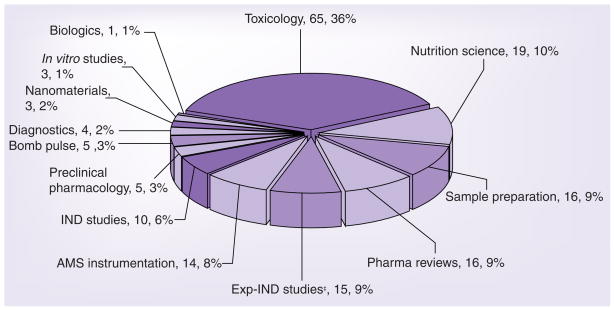
A Medline (US National Library of Medicine) search was performed in September 2009 using the search phrase ‘accelerator mass spectrometry’. References to nonbiological applications were excluded from the search results. Biological studies utilizing isotopes other than 14C, such as 26Al, 36Cl and 41Ca, were also excluded from the search results and are not part of this review. The remaining references were categorized into various applications and are summarized in Table 1 and shown in Figure 1.
Table 1.
Summary of accelerator mass spectrometry-enabled publications.
| Publication focus | Number of publications | First appearance | Ref. |
|---|---|---|---|
| AMS instrumentation | 14 | 1987 | [2,4–13,15–17] |
| Sample preparation | 16 | 1990 | [14,18–26,109–115] |
| in vitro studies | 3 | 1998 | [28,29,116] |
| Nutrition science | 19 | 1996 | [31–42] |
| Toxicology | 65 | 1990 | [43–50,52,53,91,95,117–149] |
| Preclinical pharmacology | 5 | 1998 | [51,54,55,69,76] |
| Exp-IND‡ (Phase 0 or microdosing) | 15 | 2003 | [58–68,70,71,73,150] |
| IND (Phase 1) | 10 | 2001 | [74–78,80–82,151,152] |
| Biologics | 1 | 2006 | [83] |
| Pharmaceutical reviews | 16 | 1994 | [27,84–98] |
| Diagnostics | 4 | 1999 | [56,57,99,100] |
| Bomb pulse | 5 | 1991 | [101–105] |
| Nanomaterials | 3 | 2001 | [106–108] |
Included in Exp-IND studies are five non-AMS publications and seven strategy/review publications.
Exp-IND: Exploratory investigational new drug.
Figure 1. Categories of accelerator mass spectrometry-enabled applications and number of publications.
‡Included in Exp-IND studies are five non-AMS publications and seven strategy/review publications. AMS: Accelerator mass spectrometry.
Accelerator mass spectrometry-enabled studies are described in this review in an effort to convey the wide range of applications and challenges that have been overcome by this approach. The common denominator in these applications is employing this ultra-sensitive platform to address analytical, safety and strategic challenges. Some of the reviewed publications are proof-of-concept studies, while others are research papers reporting solutions to actual problems. Details of selected references are provided in Table 2, which lists publications in chronological order, along with the intended purpose, the model organism, the number of animals or human subjects, the chemical agent, the chemical dose size, the route of administration and the radiochemical dose size.
Table 2.
Design considerations of selected AMS publications‡.
| Lead (year) | Platform | Purpose | Organism | Number of subjects | Compound | Route | Chemical dose | Radiochemical dose | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| kBq | nCi | |||||||||
| Lund University (1996) | AMS | Long-term nutrient metabolism | Human | 3 male | Triolein | Oral | Meal containing 20 g fat | 74 | 2000 | [31] |
| UCSF, LLNL (1997) | AMS | Tissue distribution and macromolecular binding | Mice | Multiple groups | Benzene | i.p. | 500 mg/kg; 15.78 mg/kg | 0.00029–0.37 | 0.008–10 | [120] |
| Pfizer, York, LLNL (1997) | AMS; LSC | Preliminary evaluation of AMS using archived samples from traditional radiocarbon study | Human; rat | NA | Candoxatril; dofetilide | Oral | 200 mg; 5 mg/kg | 1851; 296; 0.00096 | 50,000; 8000; 0.026 | [85] |
| UCD (1998) | AMS | Nutrient kinetics, compartmental modeling | Human | 1 | Folic acid | Oral | 35 μg | 3.7 | 100 | [32] |
| LLNL (1998) | AMS | Evaluate exposure assays proposed by US EPA | In vitro | Multiple groups | Trichloroethylene | Dermal model | ~5 ppb | [116] | ||
| LLNL (1998) | AMS; LSC | Evaluate analytical performance of AMS against LSC | Human | 10 | Atrazine | Dermal | [4] | |||
| National Cancer Institute (US), LLNL (1998) | AMS | Distribution and metabolism | Rat | 3 in each group | 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) | Oral | 50 ng/kg; 500 ng/kg; 1000 ng/kg | 0.814 | 22 | [51] |
| UCD (1999) | AMS | Nutrient kinetics, compartmental modeling | Human | 1 | Folic acid | oral | 35 μg | 3.7 | 100 | [33] |
| LLNL (1999) | AMS | Monitor exposure | Human | 1 | Atrazine | dermal | 0.167 mg; 1.98 mg | 238; 915 | 6450; 24,700 | [124] |
| Veterans Administration, LLNL (1999) | AMS; LSC | Metabolism in plasma and urine | Human | 5 male patients scheduled for colon resection | 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) | Oral | 70–84 μg | 648 | 17,500 | [126] |
| Lund University (1999) | AMS; LSC | Diagnostic test for Helicobacter pylori infection | Human | 9 adults and 8 children | Urea | Oral | 110 (adults); 55 (children) | 2970; 1480 | [56] | |
| UCD (2000) | AMS | Nutrient kinetics, metabolism, compartmental modeling | Human | 1 male | β-carotene | Oral | 306 μg | 7.4 | 200 | [35] |
| CBAMS, University of York, Pfizer, Glaxo Wellcome (2000) | AMS | Validation of AMS | Rat | 15 in each treatment group | Fluticasone propionate | i.v. | 83.6 μg/kg 134 μg/kg, |
426 kBq/kg; 0.69 kBq/kg | 11,500 nCi/kg; 18.1 nCi/kg | [5] |
| Glaxo Welcome (2001) | AMS | Pharamacokinetics and mass balance | Human | 6 male | GI1817771 | Oral | 2.7 mg | 0.122 | 3.3 | [74] |
| CBAMS (2002) | AMS; LSC | Absorption, formulation | Human | 12 male | Diosmin | Oral | 500 mg | 0.925 | 25 | [75] |
| CBAMS, Janssen Pharma. (2002) | AMS | Evaluation of AMS for human mass balance studies | Human | 4 male | R115777 | Oral | 50 mg | 1.25 | 34 | [77] |
| UCD (2002) | AMS; GC–MS | Nutrient metabolism | Human | 1 male | β-carotene | Oral | 0.27 μg | 1.85 | 50 | [36] |
| LLNL (2002) | AMS | Toxicology and exposure assessment | Mice | Multiple groups | Diisopropyl-fluorophosphate; parathion; permethrin | i.p. | 100 ng/kg to 500 μg/kg | 0.081 | 2.2 | [130] |
| UCSF (2002) | AMS; LC–MS/MS; MALDI-TOF-MS; LC–ESI-MS | Toxicology and metabolism | Mice | Multiple groups | Benzene | i.p. | 155 μg/kg; 800 μg/kg | Not reported | Not reported | [131] |
| UCD (2003) | AMS | Nutrient kinetics, metabolism, compartmental modeling | Human | 2 female | β-carotene | Oral | 306 μg | 3.7 (each phase) | 100 (each phase) | [37] |
| Peking University (2003) | AMS | Assessment of nutrient protection against a toxin | Mice | 6 mice/dose or 2 mice/sample | Nicotine | i.p. | 18.2 μg/kg | Not reported | Not reported | [52] |
| Lund University (2003) | AMS | Assessment of fat malabsorption | Human | 2 male | Triolein | Oral | [99] | |||
| UCD (2004) | AMS | Nutrient kinetics, compartmental modeling | Human | 7 male and 6 female | Folic acid | Oral | 35 μg | 3.7 | 100 | [34] |
| University Hospital of Lausanne, Switzerland (2004) | GC–/MS | Probe CYP3A activity | Human | 13 | Midazolam | Oral | 75 μg | NA | NA | [150] |
| Central Science Laboratory, UK (2004) | AMS and GC-MS | Assessment of exposure to pesticides | None | NA | Hexaconazole | Oral | 0–1500 ng/ml | 0–3.353 mBq/ml | 0–124 μCi/ml | [6] |
| University of York and others, LLNL (2004) | AMS | Exposure and formation of macromolecular adducts | Rat and Human | Multiple rat groups, 7 humans | Aflatoxin B1 | Oral | 0.16 ng/kg to 12.3 mg/kg (rats); 1 μg (humans) | 116.9; 9.84 | 3156; 265 | [47] |
| Merck, LLNL (2004) | AMS; LC–MS/MS | Evaluate linearity between microdose and pharmacologic dose | Dog | 2 in each group | 7-deaza-2′-C-methyl-adenosine | Oral; i.v. | 1 mg/kg; 0.02 mg/kg; 0.02 mg/kg | 6.29; 4; 3.14 | 170; 109; 85 | [69] |
| UCD (2005) | AMS | Nutrient kinetics | Human | 1 female | Lutein | Oral | 71 μg | 1.33 | 36 | [40] |
| LLNL (2005) | AMS | Tissue distribution and excretion | Dog | Multiple groups | Moli1901 | Intratracheal instillation | ~100 μg | 1.66 | 45 | [54] |
| Pfizer, Xceleron (2005) | AMS, LC–MS/MS | Absolute bioavailability | Human | 7 male | Nelfinavir | Oral and i.v. (concurrently) | 250 mg oral; 1 mg i.v. | 1.85 | 50 (i.v. only) | [78] |
| UCD (2006) | AMS | Tissue distribution | Mice | Multiple groups | Doxorubicin | i.v. | 0.1 mg/kg; 1 mg/kg | 0.7 kBq/kg | 19 nCi/kg | [55] |
| Medical University of Vienna, Austria; Research Centers GmbH (2006) | PET | Microdosing | Human | 3 healthy; 6 Alzheimer’s patients | ST1859 | i.v. | <12 μg | 400,000 | 10,810,800 | [67] |
| UCD (2006) | AMS | Nutrient kinetics | Human | 1 male | Vitamin B12 | Oral | 1.5 μg | 2.2 | 59 | [41] |
| Millennium Pharma (2006) | LC–MS/MS | Establish linearity between microdose and pharmacologic dose in animal model | Rat | Multiple groups | Fluconazole; tolbutamide; MLNX | Oral | 0.001 mg/kg 0.001 mg/kg 0.01 mg/kg |
NA | NA | [66] |
| PhotoCure (2006) | AMS | Bioavailability | Human | 8 male | Hexaminolevulinate | i.v. and intravesical | 0.15 mg/kg; 100 mg | 3.7; 11.1 | 100; 300 | [80] |
| Cambridge Antibody Technology, Xceleron (2006) | AMS; ELISA | Quantitation of low levels of systemic therapeutic recombinant protein | Rats | 12 | CAT-192 (metelimumab) | i.v. | 1 mg/kg | 3.7, 0.37 and 0.037 Bq/kg | 0.1 nCi/kg; 0.01 nCi/kg; 0.001 nCi/kg | [83] |
| Xceleron, Eli Lilly, Schering, Servier, Hoffmann-La Roche, Pharma Bio-Research Group (2006) | AMS; LC–MS/MS | Compare microdose and pharmacologic dose bioavailability and PK | Human | 6 subjects per compound | Warfarin; ZK253; diazepam; midazolam; erythromycin | Oral and i.v. | 97.4 μg and 5.3 mg; 102.1 μg and 50 mg; 96.0 μg and 10 mg; 95.4 μg and 7.5 mg; 96.1 μg and 250 mg | 7.4 each | 200 each | [70] |
| UCD (2007) | AMS | Nutrient kinetics, metabolism, compartmental modeling | Human | 1 male | β-carotene | Oral | 40 μg | 3.7 | 100 | [38] |
| Bristol-Myers Squibb (2007) | AMS | Phase I mass balance | Human (cancer patients) | 3 male, 5 female | Ixabepilone | i.v. | 70 mg | 2.96 | 80 | [81] |
| Novartis (2007) | AMS; LC–MS | Investigate pharmacologic variation in resistance to therapy | Human (chronic myeloid leukemia patients) | 3 male, 3 female | Imatinib (Gleevec®) | Oral | 100 mg | 13.6 | 368 | [76] |
| Lund University (2007) | AMS | Long-term kinetics | Human | 9 for each compound | Glycocholic acid, xylose | Oral | 1 g | 200; 74 | 5400; 2000 | [100] |
| Accium BioSciences, Ceptyr (2007) | AMS | PK/ADME | Mice | 36 mice | CPT 377 | Oral and i.v. | 2 mg/kg | 0.43 | 11.6 | [10] |
| La Trobe University (2008) | AMS | Drug–DNA interaction and mechanism of action | In vitro | NA | Adriamycin | Cell culture | 25–500 nM | Not reported | Not reported | [146] |
| Vitalea Science (2008) | AMS | Plasma PK and mononuclear cell uptake | Human | 1 male | 3′-azido-3′-deoxythymidine | Oral | 0.520 μg | 3.77 | 102 | [71] |
| Peking University (2008) | AMS | Toxicology | Mice | 20 per group | Acrylamide | i.v. | 10 μg/kg | 0.651 | 17.6 | [148] |
| UCD (2009) | AMS | Nutrient metabolism | Human | 8 males | β-carotene | Oral | 0.536 μg | 3.7 | 100 | [39] |
| Daiichi-Sankyo, IAA; Sekisui Medical, Yokohama College of Pharmacy (2009) | AMS; LC–MS/MS | Absolute bioavailability | Dog | 3 in each group | R-142086 | Oral and i.v. | 1.5 μg/kg (14C i.v.); 0.3 mg/kg (14C i.v.); 1 mg/kg (unlabeled, oral); | 2.61 kBq/kg | 71.25 nCi/kg | [79] |
| Idenix Pharma. (2009) | AMS | Microdosing | Human | 4 males per compound | IDX899 and IDX989 | Oral | 100 μg | 3.7 | 100 | [73] |
For comparison, several references are provided to other detection platforms, such as PET and LC–MS/MS.
ADME: Absorption, distribution, metabilism and excretion; AMS: Accelerator mass spectrometry; CBAMS: The Centre for Biomedical Accelerator Mass Spectrometry; EPA: Environmental Protection Agency; i.p.: Intraperitoneal; i.v.: Intravenous; LLNL: Lawrence Livermore National Laboratory; LSC: Liquid scintillation counter; NA: Not applicable; PK: Pharmacokinetics; UCD: University of California, Davis, USA; UCSF: University of California, CA, USA.
AMS instrumentation
Accelerator mass spectrometry quantifies long-lived isotopes present at very low abundance ratios [2,3]. 10Be, 14C, 26Al, 36Cl, 41Ca and 129I can be measured in small natural samples, requiring as few as 105 atoms of the rare isotope. The 1980s were marked by the application of AMS to the earth sciences, archeology (radiocarbon dating) and physics (searches for exotic particles). As AMS utility was further explored in the 1990s, several teams sought to evaluate its validity against platforms more common to bioanalytical scientists. AMS was successfully evaluated against liquid scintillation counting using 14C-atrazine [4] and 14C-fluconazole [5] for biological applications. AMS was also compared with GC using 14C-hexaconazole [6].
Separation techniques, such as LC, allow association of the measured 14C with various known and unknown analytes. In contrast to LC–MS/MS systems, no LC system has been directly and efficiently coupled to an AMS instrument. Samples are fractionated offline and one or more fractions are processed and measured by AMS.
Several approaches have been employed to circumvent this limitation. These include an interface for direct analysis of nonvolatile constituents [7], development of a hybrid GC–AMS system [8], and a chromatography–combustion system that introduces CO2 into a specialized AMS instrument [9].
Several 14C AMS instrument designs are now available and all have moved away from the traditionally large million-plus volt instruments. Installation and validation of these instruments has been reported by a commercial service provider, a pharmaceutical company and an academic institution. Accium BioSciences demonstrated the performance capabilities of the National Electrostatics Corporation (NEC) 500-kV tandem AMS instrument under a GLP environment [10]. GlaxoSmithKline demonstrated that the NEC 250-kV single-stage instrument has comparable performance to the much larger 5-MV instrument housed at Xceleron in the UK [11]. A similar 5-MV instrument is also available at Uppsala University, where performance capabilities were recently reported [12–14]. Several reviews of AMS instrumentation and the underlying physics behind the technique have been published [15–17].
Sample preparation
The most common approach for biological sample preparation is the conversion of organic samples to a thermally and electrically conductive solid known as graphite. Graphite generates an intense ion beam when exposed to a cesium sputter ion source. The 12C, 13C and 14C beams are then separated by the instrument to generate the 14C/12C ratios. Two common methods are available to produce graphite. An older method, developed originally for archeological applications, was applied to graphite production of biomedical samples [18]. An alternative method was developed specifically for biological samples and uses septa-sealed vials for faster graphite production, while using less disposable materials [19]. Both methods are time consuming and require an overnight combustion step to generate CO2 from the dried sample. Kim et al. investigated the graphitization process and the physical properties of the graphite [20–22].
Extremely small amounts of sample can be processed for AMS measurement. For example, less than 1 μl of plasma or a single cell may be processed as long as there is approximately 1-mg total carbon during the graphitization procedure. External carbon may be added to samples with insufficient carbon to facilitate graphitization. Recent sample-preparation methods have reduced the required amount of carbon to the low microgram range [23].
Direct graphitization does not provide structure-related 14C information but simply the total amount of 14C in the sample. All structural information is lost during sample combustion. HPLC is often used to associate AMS measurements with specific analytes in a sample, such as parent drug or metabolites. Individual fractions may be collected around the desired peaks or across the entire chromatogram (metabolite profiling). Two publications provide further details of HPLC fractionation prior to AMS measurement [24,25].
Tissue samples collected from animals or biopsies obtained from humans are generally homogenized in water or a suitable buffer to form a near homogenous product. The homogenized sample is simply aliquotted, dried and graphitized. Homogenates may also undergo extraction and HPLC fractionation to provide metabolite information. Investigating the interaction of the 14C-labeled dose with protein or DNA requires further purification of protein or DNA in the homogenate [26].
Finally, it is important to know the percentage of carbon in a sample so the concentration of 14C/ml sample, expressed as disintegrations per min (DPM)/ml or nCi/ml, can be calculated from the 14C:C ratio determined by AMS. Plasma and whole blood have consistent carbon concentrations over time and across subjects, so it is possible to use a reference value for the percent of carbon in these samples. Urine, fecal blend and tissue homogenates have highly variable carbon content and should be quantified analytically by a carbon analyzer such as the Shimadzu TOC-V Series Total Organic Carbon Analyzer. HPLC fractions contain essentially no carbon once they are dried. A known amount of carbon carrier is added to these samples to facilitate graphitization and calculation of 14C concentration in the fraction [27].
AMS in vitro studies
The application of AMS to in vitro studies is limited, as there is little constraint on the amount of isotope that can be employed in an in vitro assay. Competing techniques provide sufficient sensitivity, are high-throughput and can be performed at lower cost. That said, an assay system was developed for quantitation of 14C-labeled antibodies, similar to ELISA, with the exception that bound 14C-antibodies were quantified by AMS [28]. The true sensitivity of this immunoassay, however, was limited by the Kd of the antibody and not by the detection system. Detection limits for atrazine and 2,3,7,8-tetrachlorodibenzo-p-dioxin were 2.0 × 10−10 M and 2.0 × 10−11 M, respectively, an order of magnitude improvement compared with the standard ELISA.
Accelerator mass spectrometry sensitivity has been used to extend the range of possible studies in cell-culture systems. Investigation of 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-oxodG) metabolism and repair is hampered by poor analytical sensitivity. MCF-7 human breast cancer cells were exposed to 14C-8-oxodG at concentrations up to 2 pmol/ml [29]. The radiotracer was taken up by the cells, phosphorylated and incorporated into DNA. Oxidative stress induced by 17β-estradiol exposure reduced 14C-8-oxodG DNA incorporation. AMS permitted quantitation of 14C-8-oxodGTP in the nucleotide pool and provided evidence that DNA incorporation utilizes a mechanism that can lead to mutations in the presence of this oxidized nucleoside.
Nutrition science
Nutrition studies have a particularly unique challenge. First, the dose is typically administered in the microgram range to mimic typical daily consumption of the nutrient. Second, in contrast to pharmaceutical applications, quantitation of the administered nutrient must distinguish between the administered dose and the nutrient already in the body. As such, isotope labeling is often used to uniquely distinguish between the two. However, stable isotopes such as 13C and 2H, do not provide sufficient enrichment of the sample above background when administered at dietary-relevant doses. At best, an approximately estimation of nutrient kinetics can be derived using stable-isotope labeled nutrients [30]. AMS-based methods overcome these limitations, allowing quantitation of dietary-relevant doses (or less) of a nutrient for prolonged periods. Examples of AMS-based clinical studies of triolein [31], folic acid [32–34], β-carotene [35–39], lutein [40], cobalamin (vitamin B12) [41] and α-tocopherol (vitamin E) [42] are provided below.
A long-term study was performed to demonstrate the degree of fat malabsorption in three healthy male volunteers [31]. Each subject received 14C-triolein orally (2000 nCi) in a meal containing 20 g of fat. AMS was used to follow the long-term elimination of 14 C-CO2 in expired air. Approximately 30% of the administered dose was catabolized rapidly with the remaining 70% turning over very slowly. The study differed from most current AMS-based study designs in that the radiocarbon dose was approximately tenfold higher and no plasma, urine or feces samples were collected for quantitation of systemic or excretory components of the dose.
The first comprehensive AMS-enabled clinical study investigated folic acid absorption and kinetics. Folic acid is an essential nutrient with numerous functions that include nucleotide biosynthesis and the production and maintenance of new cells. A healthy male subject received 14C-folic acid (35 μg; 100 nCi), equivalent to a sixth of the recommended daily intake of folic acid [32]. Plasma and red blood cells were collected over a 6-month period and separately measured by AMS. All urine and feces samples were collected for 42 days postdose and measured by AMS. Elimination in feces accounted for 9% of the dose in the first 24 h, with a steady loss of approximately 0.1% over the following 41 days. Elimination in urine accounted for another 10% of the administered dose over the 42-day collection period. Plasma kinetics showed rapid oral bioavailability and half-life. Interestingly, little or no 14C appeared in red blood cells up to 4 days postdose. This was followed by rapid appearance of 14C in red blood cells that remained unchanged for the following 100 days, after which the 14C signal quickly dissipated [33]. This observation demonstrated the transfer of a small portion of the 14C-folic acid dose to marrow, where it was incorporated into newly formed red blood cells and released into circulation. Folic acid polyglutamation prevented 14C cell efflux while in circulation. Red blood cell survival time was demonstrated to be approximately 100 days. A compartmental model was developed describing for the first time the uptake, distribution and elimination of folic acid administered at a dietary-relevant dose.
A follow-up study was conducted in 13 women with the primary purpose of revealing any association between folic acid kinetic parameters and genetic polymorphisms in folic acid-metabolizing pathways [34].
Plant carotenoids are the primary dietary source of provitamin A worldwide, with β-carotene the most well-known provitamin A. A dietary-relevant amount of β-carotene (306 μg; 200 nCi) was administered orally to a healthy male subject [35]. Extraction and chromatographic separation by HPLC permitted AMS quantitation of 14C-β-carotene, 14C-retinyl esters, 14C-retinol and several 14C-retinoids in plasma, collected up to 96 h postdose. The results demonstrated a 5.5-h lag between dosing and the appearance of 14C in plasma, after which 14C-β-carotene and 14C-retinyl esters displayed several maxima with virtually identical kinetic profiles up to 25 h postdose. 14C-retinol concentration rose linearly from 5 to 28 h postdose before declining up to 96 h postdose. Cumulative losses in feces were 57.4% of the dose at 48 h postdose. Area under the curve analysis of plasma suggested that 53% of the absorbed β-carotene was cleaved to vitamin A. It was proposed that retinyl esters derived from β-carotene undergo hepatic resecretion with VLDL similar to β-carotene.
Lutein is a dietary constituent with anti-oxidant properties. A healthy female subject was orally administered 14C-lutein (71 μg; 36 nCi) dissolved in olive oil [40]. 14C first appeared in plasma 1 h after dosing and reached a maximum at 14 h postdose with a concentration equivalent to 2.08% of the dose/l plasma. Lutein elimination half-life was approximately 10 days. In total, 45% of the administered 14C dose was eliminated in feces and 10% in urine within 48 h postdose. Plasma 14C kinetics did not follow the chylomicron/VLDL pattern previously observed for 14C-β-carotene [35], suggesting a different mechanism for lutein distribution.
Vitamin B12 is a water-soluble vitamin with a key role in the normal functioning of the brain and nervous systems and the formation of blood cells. AMS methodology was employed to help identify poor vitamin B12 absorbers, so dietary or pharmacologic intervention could be provided [41]. 14 C-labeled vitamin B12 was biosynthesized with the aid of Salmonella enterica. The organism was cultured aerobically in the presence of two vitamin B12 precursors, cobinamide and 14C-dimethylbenzimidazole. Purified 14C-labeled vitamin B12 was administered to a healthy male subject at dietary-relevant dose (1.5 μg; 59 nCi). Plasma 14C concentration demonstrated a maximum at 7 h postdose. Cumulative losses of 14C in urine and feces totaled 15.9% of the administered dose at 7 days postdose. Total 14C kinetics in plasma were consistent with the predicted behavior of the pure vitamin.
A final example of an AMS-enabled nutrition study is the quantitation of human vitamin E (α-tocopherol) metabolism [42]. Naturally occurring α-tocopherol is a single stereoisomer known as RRR-α-tocopherol. Chemically synthesized α-tocopherol is an equal mix of eight stereoisomers known as all-rac-α-tocopherol. It is important to determine whether synthetic α-tocopherol (vitamin supplements) has the same kinetic properties as the naturally occurring form of the vitamin. The two forms were administered orally in a crossover design consisting of [5-14CH3]-RRR-α-tocopheryl acetate (0.78 μg; 101.5 nCi) followed by a wash-out period and subsequent administration of [5-14CH3]-all-rac-α-tocopheryl acetate (0.72 μg; 99.98 nCi). Both forms of the vitamin were absorbed equally well with a fractional absorption of approximately 0.775. Urine was the main route of elimination, with approximately 90% of the absorbed dose lost as α-2(2′-carboxyethyl)-6-hydroxychroman. Liver had two kinetically distinct α-tocopherol pools. AMS methodology helped to determine that both isomers were well absorbed. Synthetic all-rac-α-tocopherol was preferentially degraded and eliminated in urine. Naturally occurring RRR-α-tocopherol had a longer residence time and larger distribution than the synthetic form.
Toxicology
It may be surprising to observe that the largest single category of AMS-enabled publications is in the area of toxicology (Figure 1). The basic design of these mostly animal studies is the administration of a 14C-labeled agent known to have toxic or carcinogenic properties. The dose size is decreased to mirror actual environmental or dietary exposure levels, thus the need for AMS. Distribution of the 14C-labeled agent is followed in plasma and urine and, more importantly, in target tissue. Especially revealing is the quantitation of covalent adducts formed between the 14C-labeled agent and host macromolecules, such as proteins or DNA.
A detailed review of AMS-enabled toxicology studies is beyond the scope of this review. A summary of these toxicology studies is presented in Table 3. Importantly, studies have been conducted in humans using 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) [43–46], aflatoxin B(1) [47] and MeIQx [43,45,48–50]. Very few alternative approaches are available to investigate these known carcinogens and mutagens in humans.
Table 3.
Accelerator mass spectrometry-enabled studies of environmental and dietary toxicants.
| Agent | Organism | Ref. |
|---|---|---|
| 1,2-dibromoethane | Mice, rats | [144] |
| 1,2-dichloroethane | Mice, rats | [144] |
| 11β-dichloro | Mice | [139] |
| 2,6-dimethyl-ethylaniline | Mice | [140] |
| 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) | Mice, rats, humans | [44–46,51,117,126,137] |
| 3-(2-deoxy-β-D-erythro-pentofuranosyl) pyrimido[1,2-α]purin-10(3H)-one (M1dG) | Rats | [147] |
| 3,5-dimethyl-ethylaniline | Mice | [140] |
| 3-ethylaniline | Mice | [140] |
| Acrylamide | Mice | [141,148] |
| Aflatoxin B(1) | Rats, humans | [47,123] |
| Atrazine | Humans | [4,124] |
| Benzene | Mice | [120,127,131,134] |
| Benzopyrene | Not available | [128] |
| Dibromomethane | Mice, rats | [144] |
| Dichloromethane | Mice, rats | [144] |
| Ethylene oxide | Rats | [149] |
| Isofluorophate | Mice | [130] |
| MeIQx | Rats, humans | [43,45,48–50,118,119] |
| MTBE | Mice | [136,145] |
| Nicotine | Mice | [52] |
| Nitrobenzene | Mice | [53,132] |
| Ochratoxin A | Rats | [135,138] |
| Ortho-phenylphenol | Rats | [125] |
| Oxaliplatin | Human breast and bladder cancer cells | [143] |
| Tamoxifen | Mice, rats, humans | [121,122,129,133,142] |
| Tert-butyl alcohol | Mice | [145] |
| Toremifene | Rats | [122] |
2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) is a known mammary carcinogen in female rats. It is present in a variety of cooked meats at doses too low for in vivo quantitation by traditional analytical methods. AMS was used to quantify the excretion of PhIP and its metabolites in breast milk of lactating rats [51]. Lactating female F344 rats with suckling pups were orally administered 50, 500 and 1000 ng 14C-PhIP/kg body weight. Distribution into mammary tissue and liver, the blood of the dam, stomach contents and liver of their suckling pups, as well as excretion into milk was determined by AMS. PhIP and several known metabolites were found in the milk at all doses. Another group of rats were co-administered chlorophyllin, a food derivative with chemopreventive properties, to investigate modulation of PhIP concentration in tissue samples. Chlorophyllin (500 μg/kg) co-administered with PhIP (500 ng/kg) resulted in a 32% increase in 14C-PhIP in the milk and a 35% increase in stomach contents. Blood and mammary tissue 14C-PhIP concentration were 47 and 68% lower, respectively, in chlorophyllin-treated animals. The results demonstrated that PhIP and its metabolites are present in the breast milk of lactating rats at exposure levels that are relevant to human dietary intake. Furthermore, other dietary components affected PhIP distribution and excretion in breast-feeding offspring.
An interesting variation to these studies is the modulation of host–toxin interactions with exposure to potentially protective agents. For example, mice were first exposed to vitamin C, vitamin E, tea polyphenols, garlic squeeze, curcumin or grapestone extract. The animals were then exposed to 14C-nicotine or 14C-nitrobenzene to examine the protective effects of the dietary constituents against the formation of 14C-labeled adducts with hemoglobin or DNA [52,53]. All dietary constituents induced a marked dose-dependent decrease in 14C-nicotine or 14C-nitrobenzene adduct formation, as compared with controls.
Preclinical pharmacology
Moli1901 is a 19-residue polycyclic peptide antibiotic and may be useful for treatment of cystic fibrosis. 14C-Moli1901 (~100 μg; 45 nCi) was administered to beagle dogs after intra-tracheal instillation [54]. There was 64% retention of the dose in the left cranial lobe of the lung 28 days after administration. Whole blood and plasma concentrations of 14C remained below 5 ng/ml at all times. Approximately 13% of the dose was eliminated in urine and feces in 28 days, with fecal elimination accounting for approximately 10% of the dose. A Phase II randomized, double-blind, placebo-controlled study was successfully completed in 2007 using aerosolized Moli1901 in adolescents and adults with cystic fibrosis.
A significant advantage of performing studies in animals is the opportunity to investigate drug distribution to target tissues and to report tumor drug uptake, for example. Researchers used AMS to quantify total cellular 14C-anthracycline concentrations, following administration of doxorubicin, an anthracycline chemotherapeutic agent [55]. Two different mouse models were used:
A nude mouse xenograft model bearing subcutaneously-implanted breast cancer cells;
A nude mouse xenograft model bearing wild type and MDR+ cells on opposite flanks (a model for multidrug resistance).
14C-anthracycline concentrations were significantly higher in the wild-type tumors compared with the MDR+ tumors, consistent with the MDR model. AMS was over five orders of magnitude more sensitive than the standard fluorescence-based HPLC method.
In summary, the primary advantage of AMS-based animal studies is the ability to quantify extremely low concentrations of 14C-labeled molecules in small samples, especially target tissue such as tumor xenografts. Furthermore, AMS eliminates tissue matrix effects that hinder bioanalysis based on other approaches, such as ELISA.
AMS-enabled drug development
Early clinical development is mainly focused on addressing safety, tolerability and pharmacology of a new chemical entity. Clinical studies that employ radiolabeled strategies can be divided into two categories, those that administer a traditional amount of radioisotope (typically >20 μCi) and those that administer a lightly labeled dose (typically <250 nCi).
In the first case noted above, AMS is used to quantify samples that have insufficient 14C for reliable detection by liquid scintillation counters (LSC). These samples typically possess less than 70–200 DPM/ml, based on various definitions of background and limit of detection. Whole blood and plasma obtained from later timepoints fall into this category. AMS easily quantifies 14C concentration in these samples and often requires a 100- to 1000-fold dilution of the sample to bring the signal down to the AMS detection range.
In the second case noted above, the amount of radiolabel administered to each subject is limited to approximately 250 nCi or less. Again, AMS easily quantifies 14C concentration in these samples and may require a ten- to 100-fold dilution of certain samples. Samples from later timepoints may eventually reach baseline values as determined by the limit of quantitation (LOQ) for that sample type. Lightly labeled studies provide practical, ethical and design advantages over traditional radiolabeled studies.
From a practical point of view, lightly labeled studies reduce the need to synthesize radiolabeled compounds with highly specific activities. Often, an initial synthesis batch with minimal 14C incorporation is more than sufficient to conduct a lightly labeled study. This reduces the overall cost and time required to produce a batch of material. In other cases, certain chemical structures may undergo radiolysis and degradation, due to the high specific activity of the molecule. This problem is alleviated entirely with lightly labeled strategies. Finally, sample handling, shipment to various laboratories and final disposition are greatly simplified when dosing lightly labeled compounds. It is rare to generate a sample that is categorized as radioactive material when less than approximately 250 nCi is administered to human subjects. Lightly labeled studies have other practical advantage when it comes to sample preparation and processing. Many of the laboratory steps, such as extraction and HPLC fractionation, can be performed in a laboratory that is not licensed to handle radioactive material. In fact, it is advantageous to perform this work in such a laboratory to reduce the likelihood of laboratory-to-sample cross-contamination.
Dosing radiolabeled compounds to human subjects requires ethical considerations above and beyond standard safety concerns. Traditional methods that administer microCurie doses require dosimetry assessment in animals to ensure the radioisotope is eliminated from the subject and does not accumulate in sensitive organs. Administering nanoCurie doses eliminates dosimetry assessment in animals in most cases [56,57].
The second component of designing and conducting AMS-enabled clinical studies is the size of the administered chemical dose, which is primarily driven by the development stage for the drug candidate. The key factor to consider is whether an investigational new drug (IND) has been obtained. If so, the drug candidate may be administered at or near to the expected pharmacologic dose. The size of the chemical dose is then highly dependent on the particular compound, based on preclinical data and other supporting evidence. If an IND has not been obtained, the drug candidate may be administered to human subjects using an exploratory IND (Exp-IND). The chemical dose is limited to 100 μg or 1% of the expected pharmacologic dose. The advantages and disadvantages of Exp-IND studies, also known as microdosing or Phase 0 studies, are reviewed elsewhere [58–65].
It is important to emphasize the distinction between chemical and radiochemical doses in AMS-enabled studies. An often-made misunderstanding is to refer to all AMS-enabled studies, those that administer <250 nCi radiochemical, as microdosing studies. A more accurate and less misleading terminology is to refer to these as lightly labeled studies. By inference, these studies require AMS as the detection platform. Importantly, AMS-enabled studies do not restrict the size of the chemical dose, which depends on the candidate’s developmental stage and the strategy chosen to enter clinical evaluation.
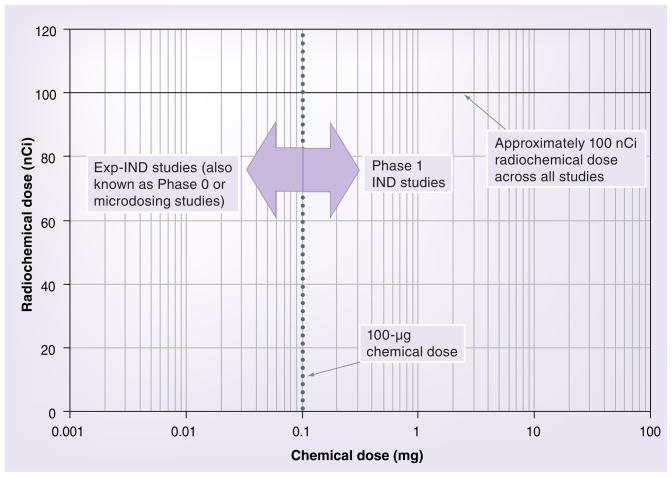
Figure 2 depicts the chemical dose size on the x-axis and the radiochemical dose size on the y-axis. As stated above, clinical studies may be performed with an Exp-IND if the chemical dose is limited to 100 μg or 1% the size of the expected pharmacologic dose. These studies are shown in Figure 2 as Exp-IND, Phase 0 or microdosing studies. Studies that expose subjects to greater than 100 μg chemical dose require an IND and are classified as Phase I studies. In all cases (Exp-IND and IND), AMS-enabled studies typically provide a radiochemical dose of <250 nCi (100 nCi is shown in Figure 2). All the studies in Figure 2 should, therefore, be referred to as lightly labeled studies, whereas only Exp-IND studies should be referred to as microdosing studies.
Figure 2. Chemical and radiochemical doses used in AMS-based exploratory-IND and IND studies.
The radiochemical component typically ranges from 50 to 250 nCi (100 nCi is shown below). Note that all the studies should be referred to as lightly labeled studies, whereas only exploratory-IND studies should be referred to as microdosing studies.
AMS: Accelerator mass spectrometry; IND: Investigational new drug.
An important note that should be made is that all lightly labeled studies require AMS but not all microdosing studies do. In fact, microdose studies have been performed using LC–MS/MS [66] and PET [62,67,68]. Further insight into the design components of AMS-enabled studies as well as their intended purpose is provided in Table 2. For comparison, several examples listed in the table employed detection platforms other than AMS [66–68].
Exploratory IND studies (Phase 0 or microdosing)
A 2001 workshop, organized by Volunteers in Research and Testing in the UK, addressed the possibility of conducting early human studies using microdosing techniques [58]. AMS, NMR spectroscopy and PET were presented as potentially useful spectrometric and imaging methods that enable microdosing studies. The regulatory authorities in Europe, the USA and Japan have since introduced new guidelines describing the steps necessary to conduct human microdosing studies with limited safety studies in animals (Japanese Ministry of Health and Welfare) [201,202]. These efforts are currently being standardized through the International Conference on Harmonisation.
Microdosing efforts by pharmaceutical companies are divided into two categories:
Those that aim to answer linearity questions between a subpharmacologic and pharmacologic dose;
Those that report microdosing results for a new chemical entity.
Research teams at Merck used AMS to examine whether a preclinical drug candidate, 7-deaza-2′-C-methyl-adenosine (compound A), displayed linear kinetics between the sub-pharmacologic (0.02 mg/kg) and pharmacological (1 mg/kg) doses after oral and intravenous administration [69]. The pharmacokinetics and disposition of Compound A were evaluated in dogs to help decide whether a micro-dosing study in humans should be initiated. At the time, no other published comparisons were available investigating the kinetics of a pharmaceutical compound at both subpharmacological and pharmacological doses. Compound A displayed multiphasic kinetics and exhibited low plasma clearance (5.8 ml/min/kg), a long terminal elimination half-life (17.5 h) and high oral bioavailability (103%). The pharmacokinetic properties of Compound A were similar at both doses in dogs, demonstrating linearity across a 50-fold dose range. AMS sensitivity generated the required pharmacokinetics properties at the microdose level, revealing aspects of drug disposition that were unattainable by conventional analytical approaches.
In another study, human pharmacokinetic properties of five drugs were compared at sub-pharmacologic and pharmacologic doses [70]. Warfarin, ZK253 (Schering Plough), diazepam, midazolam, and erythromycin were selected due to challenges predicting human pharmacokinetics based on animal or in vitro studies. Each subject received a microdose and a pharmacologic dose of one of the five compounds in a crossover design. On a separate occasion, subjects were simultaneously administered 14C-labeled ZK253, midazolam or erythromycin intravenously, with the counterpart nonlabeled compound administered orally at therapeutic doses. Diazepam, midazolam and ZK253 showed good pharmacokinetic concordance between the microdose and pharmacologic dose. Warfarin clearance was reasonably well predicted with the discrepancy observed in distribution likely to be a result of high-affinity, low-capacity tissue binding. The erythromycin oral microdose failed to provide detectable plasma levels, perhaps due to stomach acid lability. Absolute bio-availability examined for three compounds was concordant with the existing literature or with data generated in house. The authors concluded that overall, microdosing offers the potential to aid in early drug candidate selection when used appropriately.
As mentioned earlier, not all microdosing studies require AMS for quantitation of drug concentration in biological samples. Researchers at Millennium Pharmaceuticals described a feasibility study using conventional LC–MS/MS technology to quantify the concentration of an oral microdose in rats [66]. Nonlabeled fluconazole and tolbutamide were selected due to their similar pharmacokinetics between rats and humans. An investigational compound, MLNX, was also selected for its demonstrated nonlinearity. LC–MS/MS successfully characterized the pharmacokinetics of the compounds dosed at 1 μg/kg. Fluconazole and tolbutamide demonstrated linearity in exposure across a 1000-fold dose range. MLNX exhibited nonlinear kinetics, suggesting that human MLNX microdosing might not reflect linear kinetics either. The authors concluded that LC–MS/MS should be adequate for microdosing studies in humans.
The pharmacokinetics and mass balance of a model agent were investigated in another study performed at subpharmacologic doses. Zidovudine was approved by the FDA in 1987 for the treatment of HIV. A subpharmacologic dose of 14C-zidovudine (0.52 μg; 102 nCi) was administered orally to a healthy male subject [71]. AMS was used to determine the concentration of total 14C in plasma, urine, feces, saliva, and in peripheral blood mononuclear cells, which the drug targets. Recovery of 14C in urine and feces totaled 94% of the dose 96 h postdose. In most cases, zidovudine microdose pharmacokinetic parameters were within the published values for therapeutic doses at 95% confidence intervals or standard deviations.
The EU Microdosing AMS Partnership Program (EUMAPP) is a €2 million program funded by the EU to evaluate microdosing for drug development and to arrive at recommendations about how and when it could and should be applied [72]. Seven compounds were selected for specific pharmacologic challenges. These were paracetamol and sumatriptan (metabolism predominantly not mediated by CYP enzymes), fexofenadine (transporter-dependent pharmacokinetics), phenobarbital (very low metabolic clearance), clarithromycin (transporter and metabolism are coupled), propafenone (dose-dependent pharmacokinetics) and S-19812 (highly metabolized). Publication of the results is expected once analysis and interpretation is completed.
A sufficiently large body of data now exists to support linearity for most chemical entities, helping microdosing studies transition from a curiosity to a decision-making tool within drug development. Although the tool has been available for more than 5 years, precious few studies have been published to describe its actual use within a pharmaceutical setting. Much of the work performed to date remains confidential, due to the proprietary nature of drug development.
An exception to this is a recent publication by Zhou et al. from Idenix Pharmaceuticals [73]. Two new inhibitors were identified that exhibited potent inhibition of HIV-1 replication. IDX899 and IDX989 were each administered to four healthy male subjects as a single oral and intravenous dose (100 μg; 100 nCi) in a randomized crossover design. Total 14C measurements in plasma demonstrated near complete oral absorption for both compounds. Mean absolute bio-availability for the parent compound was 61% for IDX899 and 65% for IDX989. Both compounds underwent extensive metabolism, especially when administered orally. The compounds exhibited comparable terminal-phase half-lives ranging from 4 to 10 h. The study provided the first human-based evidence that IDX899 and IDX989 have favorable pharmacokinetic properties. Based on its initial pharmacokinetic assessments and other criteria, IDX899 was selected for further clinical development.
Some of the limiting factors that have slowed the adoption of Exp-IND studies are organizational rather than technical. Preclinical teams within pharmaceutical companies are tasked with candidate selection. Meanwhile, clinical teams are tasked with the design and conduct of clinical studies. The challenge is that Exp-IND studies are a candidate selection process; however, they are conducted in human subjects. Most preclinical teams are not experienced in conducting clinical studies and most clinical teams are not experienced in candidate selection. As such, a new organizational paradigm may be necessary, such as a multidisciplinary translational team, to permit smooth planning and execution of Exp-IND studies when and where it is warranted.
IND studies (Phase I)
In 2001, researchers at Glaxo Wellcome published an early assessment of AMS methodology as applied to Phase I drug development. Six healthy male volunteers were administered 14C-GI1817771, a new chemical entity (2.7 mg; 3.3 nCi) [74]. A mean of 92% of the dose was recovered in the feces after 5 days. All urine measurements fell below the limit of quantitation for these samples. Animal studies also showed low recovery of 14C-GI1817771 in urine. All serum samples were below the calculated limit of quantitation (161 ng/ml). Serum proteins were removed by precipitation which improved the limit of quantitation to approximately 2.0 ng/ml. One fourth of the serum samples were now reportable. HPLC fractionation revealed that the majority of the signal was associated with the parent drug and a minor signal with a glucuronide metabolite. GlaxoSmithKline subsequently invested in two AMS facilities for internal-development efforts.
Phase I studies may also use AMS to compare the bioavailability of two different formulations. Daflon is used clinically to treat chronic venous insufficiency and hemorrhoidal disease. A double-blinded crossover study was conducted to investigate the influence of particle size on oral absorption of micronized (1.79 μm particles) and nonmicronized (36.5 μm particles) [75]. 14C-diosmin (500-mg tablets; 25 nCi) was administered orally to 12 healthy male volunteers. Absorption, determined by measuring urinary excretion of total 14C, was significantly improved with the micronized (57.9 ± 20.2%) versus nonmicronized formulation (32.7 ± 18.8%).
In another recent example of an AMS-enabled study, six patients received 14C-imatinib (100 mg; 368 nCi), also known as Gleevec® [76]. The patients were receiving 400 mg/day imatinib as part of their ongoing treatment for chronic myeloid leukemia. AMS results demonstrated rapid absorption of imatinib, which remained detectable in plasma 72 h postdose. Imatinib was also detected in peripheral blood lymphocytes 24 h postdose. Plasma concentration area under the curve, as determined by AMS and LC–MS, were comparable, 26 ± 3 versus 27 ± 11 μg/ml•h, respectively. The study confirmed that AMS could generate single-dose imatinib pharmacokinetics in patients undergoing chronic dosing. Importantly, this study enabled the pharmacologic assessment of a cancer drug in patients. The authors concluded that this approach could reveal the possible pharmacologic basis for resistance to imatinib in these patients.
Another example of a Phase I study is the investigation of the mass balance and metabolism of a farnesyl transferase inhibitor. 14C-R115777 (50 mg; 34 nCi) was administered to four healthy volunteers [77]. Drug-related 14C had a Cmax ranging from 1.6055 to 2.9074 DPM/ml at tmax of 2–3 h. Drug-related 14C was eliminated from the body with a mean total recovery of 79.8 ± 12.9% in the feces and 13.7 ± 6.2% in the urine. HPLC was performed using no more than five DPM per column injection. The metabolite profiles obtained by AMS compared well with those obtained by LC–MS/MS. The study demonstrated AMS utility in Phase I studies with a 1000-fold reduction in radioisotope exposure compared with conventional radioactive studies.
Traditional absolute bioavailability studies are performed in a crossover design to compare plasma area under the curve of the intravenous route against the intended route of administration, such as oral, dermal or pulmonary. This requires formulation of an intravenous dose, even though the formulation will not have any commercial value. Furthermore, formulating an intravenous dose can be challenging for some chemical entities, due to the physical property and solubility of the agent. A novel approach to eliminate these developmental challenges is the application of AMS to absolute bioavailability studies.
Accelerator mass spectrometry-based absolute bioavailability studies allow concomitant administration of the intravenous and oral dose (or other routes). To differentiate between each route of administration, the intravenous dose is lightly labeled with 14C. To minimize or eliminate kinetic interference between the two routes, the size of the intravenous dose is lowered to subpharmacologic levels. This approach was successfully demonstrated by Sarapa et al., who concomitantly administered 1250 mg oral nelfinavir (Viracept, Pfizer) with 14C-nelfinavir mesylate (1 mg; 50 nCi) by intravenous infusion in six healthy volunteers [78]. Steady state was maintained from day 2 to 10 by twice-daily oral intake of 1250 mg nelfinavir. Plasma concentration of orally derived nelfinavir was determined by HPLC–UV. The plasma concentration of intravenously-derived 14C-nelfinavir was determined by AMS after HPLC fractionation. Oral bioavailability decreased from 0.88 to 0.47 over the 11-day study period. The moderate bioavailability of nelfinavir was determined to be due to significant first-pass metabolism and not due to poor absorption. Efforts to increase bioavailability by using different formulations would therefore have limited impact on lowering dose size. AMS enabled a several 1000-fold reduction in 14C exposure, relative to that required for liquid scintillation counting and eliminated the need to formulate concentrated doses for intravenous administration.
This technique was recently evaluated with R-142086, a highly hydrophilic and poorly absorbed agent. Three male dogs were administered 14C-R-142086 (1.5 μg/kg; 71.25 nCi/kg) intravenously over a period of 1 h as well as nonlabeled R-142086 (1 mg/kg) orally at the start of the intravenous infusion [79]. Plasma concentrations of orally administered R-142086 were measured by LC–MS/MS and intravenously administered 14C-R-142086 by AMS. The absence of metabolites was confirmed by observation of 14C associated with only a single HPLC fraction. R-142086 oral bioavailability was determined to be 16.1%, slightly higher than the observed 12.5% obtained by separate dosing of each route. The authors concluded that bioavailability in humans may be approximated at an earlier stage and a lower cost.
As with new therapeutic entities, the clinical pharmacology of imaging agents must also be characterized in clinical studies. For example, hexaminolevulinate (HAL) is a diagnostic agent that provides visualization of tumor tissue in the bladder by fluorescence cystoscopy. As HAL is applied locally, demonstrating minimum systemic distribution would streamline further clinical development. 14C-HAL was administered to eight human volunteers by intravesical (100 mg; 300 nCi) and intravenous (0.15 mg/kg; 100 nCi) routes [80]. The mean bioavailability of 14C-HAL was determined to be 7% based on total radioactivity. Systemic absorption of 14C-HAL after intravesical administration was low and supported previous clinical experience showing HAL has no systemic side effects.
Ixabepilone (BMS-247550) is a semi-synthetic, microtubule-stabilizing epothilone B analogue with greater potency than taxanes. Excretory pathways and the degree to which elimination contributed to metabolism were unknown. A mass-balance study was initiated in cancer patients. However, ixabepilone was very unstable when radiolabeled using conventional specific activities (100 μCi/70 mg) due to autoradiolysis. Lightly labeled 14C-ixabepilone (100 nCi/70 mg) was stable at the desired dose range. 14C-ixabepilone (70 mg; 80 nCi) was administered intravenously to eight patients with advanced cancer over a 3-h period [81]. ixabepilone-derived recovery was 52.2% in the feces and 25.1% in the urine. Unchanged ixabepilone, determined by AMS, represented only a minor component of total 14C in plasma and urine, indicating metabolism was a major mechanism for drug elimination.
Another significant application of AMS to Phase I studies is in the area of metabolite profiling. This has received recent attention by regulatory authorities [203]. Prakash et al. simulated an AMS-based metabolite profiling study using urine and plasma samples obtained from a conventional radiolabeled human ADME study [82]. Urine and plasma samples were diluted 100- and 1000-fold, respectively, to bring the 14C signal into a suitable range for AMS. LC-fractionated samples were measured by AMS without conversion to graphite [7]. The authors concluded that a simulated clinical study performed with a 100 nCi dose would provide sufficient signal for 14C metabolite profiling in plasma and urine. This approach may be used to generate high resolution metabolite profiles of complex mixtures at potentially lower cost when compared with methods that convert fractions to graphite [18,19].
Biologics
Although 14C-labeling of small molecules is routinely employed in clinical studies, there are limited publications describing this approach for therapeutic proteins. One suggested challenge is the difficulty of producing radiolabeled proteins with sufficiently high specific activity. The sensitivity of AMS significantly lowers the specific activity required for these studies.
CAT-192 (metelimumab), a human anti-TGFb1 monoclonal antibody, was manufactured in the presence of 14C-precursors resulting in a low specific activity product [83]. 14C-CAT-192 was administered to rats at a dose of 1 mg/kg containing 2.2, 22 or 222 DPM/kg. Serum samples from the 22 and 222 DPM/kg groups had sufficient signal for AMS measurement. The half-life was approximately 140 h. Clearance was estimated to be 7.3 × 10−4 ml/h/g (determined by ELISA) and 4.6 × 10−4 ml/h/g (determined by AMS). The estimated limit of quantification for AMS was approximately 1 ng/ml, approximately 15-times lower than the ELISA LOQ of 15.6 ng/ml. The LOQ may be lowered further for AMS by increasing the degree of 14C incorporation in the molecule.
Pharmaceutical reviews
A detailed review of publications with a pharmaceutical industry perspective is beyond the scope of this article. A number of reviews are available describing the technical and strategic advantages of AMS as applied to pharmaceutical development [27,84–98].
Diagnostics
Leide-Svegborn et al. published an early paper describing the administration of a 14C-labeled dose with detection of 14C in expired air and urine by AMS and liquid scintillation counting [56]. Nine adults and eight children were administered 14C-urea (2970 nCi for adults; 1480 nCi for children) orally to determine the presence of gut Helicobacter pylori (HP) infection. Samples of exhaled air were taken up to 180 days after administration and samples of urine were collected for up to 40 days. A total of 16 of the subjects were found to be HP-negative. These subjects eliminated 91.1 ± 3.9% of the administered 14C in expired air and urine over the collection period with the majority excreted via the urine within the first 72 h. The authors concluded that, from a radiation protection point of view, there is no reason to restrict 14C dose administration in adults and children, even on repeated investigations. This corresponds to approximately 1 day of exposure to natural radiation from the environment, excluding exposure to radon progeny. A similar study was later conducted in seven children, aged 3–6 years [57].
Another diagnostic application of AMS was the investigation of fat malabsorption. Glycerol tri[1–14C]olein was administered to two male volunteers and expired air, urine and feces collected along with biopsy samples of abdominal fat [99]. Expired air accounted for 73 and 55% of the administered dose for the two subjects. This was described by three components with half-times of 1 h, 2 days and 150 days. Almost all the recovery in urine occurred in the first 24 h after administration, accounting for 24% of the dose. Approximately 2% was excreted in the feces within 48 h. Half-time retention of 14C in fat ranged from 137 to 620 days. Again, it was concluded that no safety restrictions are needed to protect against the radiation in the triolein breath test.
The 14C-glycocholic acid and 14C-xylose breath tests are clinically useful for diagnosing intestinal diseases such as bacterial overgrowth. A total of 18 subjects were administered one of the test agents. Exhaled air, urine and feces were analyzed by liquid scintillation counting and AMS [100]. One year after 14C-glycocholic acid administration, 67% of the dose was recovered in exhaled air, 2.4% in urine and 7.6% in feces. In the xylose study, 66% of the dose was recovered in urine and 28% in expired air.
Bomb pulse
Accelerator mass spectrometry can estimate the turnover rate of carbon in biomolecules or cells by quantifying loss of 14C enrichment that originated from 1950s-era nuclear weapons testing and atmospheric 14C enrichment (bomb pulse). Although the amount of exposure is not controlled as it would be in a traditional clinical trial, a good estimate of turnover rates can be achieved.
Turnover of elastin in the lung was determined by measuring the amount of bomb-pulse 14C remaining in the protein. The amount of 14C in D-aspartate isolated from elastin increased linearly with age, indicating that the age of lung parenchymal elastin corresponded with the age of the subject [101]. The calculated mean carbon residence time in elastin was 74 years. The study was the first tissue-specific demonstration of extracellular matrix turnover in humans and underscored the importance of elastin for maintenance of normal lung structure.
More recent publications determined the turnover of human cardiomyocytes [102], neurons [103], fat cells [104] and teeth [105].
Nanomaterials
Nanomaterial applications of AMS to date fall into two categories: quantifying the degradation and elimination of biomaterial implants and determining drug release by microelectromechanical systems devices.
The rate of in vivo degradation of a naturally occurring 14C-labeled biomaterial was determined after implantation in dogs [106]. The bioscaffold provided a temporary structure for tissue remodeling with rapid degradation and elimination of the structure through urine. Using the same approach, the degradation rate of a 14C-labeled naturally occurring extracellular matrix was quantified by AMS in a swine model. Degradation products were detected in the heart, liver, trachea, pancreas, small intestine and urinary bladder tissue [107].
A drug-delivery device was designed to release multiple substances in order to maximize the effectiveness of therapeutic agents. The device contained microresevoirs etched into a silicon substrate that contain individual doses of a drug. Subcutaneous release was demonstrated in rats using fluorescein dye, detected by fluorimetry, 14C-mannitol, detected by liquid scintillation counting, and 14C-carmustine, detected by AMS [108].
Future perspective
Accelerator mass spectrometry is well positioned for broader and deeper adoption by biomedical researchers, especially for clinical-stage drug development. Given the competitive nature of the pharmaceutical industry, there has been limited public dissemination of AMS productivity with the pharmaceutical industry and guarded discussion of its utility. AMS-enabled Phase I studies have demonstrated their value for assessment of absolute bioavailability and metabolite profiling. To date, these two applications have been applied to hand-picked programs that faced specific challenges. It is expected that a wider range of programs will be served by AMS methodology and, possibly, a majority of programs will undergo metabolite profiling by AMS. Mass-balance studies for certain programs will advance with the aid of AMS, although only in cases faced with specific challenges that cannot be resolved by conventional approaches.
The current body of knowledge suggests that microdosing studies may predict the pharmacokinetic properties of pre-IND drug candidates when applied appropriately. Publication of results from additional comprehensive microdosing studies are expected to further reduce concerns around linearity and predictability of microdosing studies and better define which compounds may benefit from this approach. Early-stage companies, as well as some large pharmaceutical companies, may adopt microdosing strategies as confidence increases in this approach. Wider adoption of microdosing strategies may ultimately hinge on licensing and financing deals supported primarily by microdosing data.
Potential opportunities exist for the development of AMS-based applications that assess the pharmacologic properties of drugs in patients rather than healthy volunteers. AMS may be used to segregate patients based on a desired metabolic profile or target-tissue uptake, as determined by a small biopsy. Such evaluation may serve as a screening tool to identify patients most likely to respond positively in a clinical trial. The same approach may be applied to marketed drugs where AMS-based evaluation may help to stratify the patient population based on individual pharmacologic response. Personalized treatment strategies can be developed based on the predicted response in each patient. This evaluation may be performed using subpharmacologic doses that further enhance patient safety. As such, the dose may be considered a probe and the evaluation may be part of an AMS-based diagnostic test.
Ultimately, widespread adoption of AMS-based approaches will be driven by the development of compelling applications that address unresolved challenges in medicine and health. Once these applications advance beyond the proof-of-principle stage, significant commercialization opportunities will drive the evolution of industrial-scale hardware, instruments and sample preparation procedures. The past two decades have laid the foundation for such transformational advancements in the forthcoming decade.
Executive summary
Accelerator mass spectrometry
Radioisotope-based method for quantifying isotope enrichment.
Most common biological application is to quantify 14C-labeled molecules administered to animals and humans.
-
Advantages
▪ Ultrasensitive method with attomole (10−18) sensitivity in a clinical setting.
▪ Requires very little sample for quantitation.
▪ Sample preparation requires little or no method development and measurement is free of ion suppression or matrix effects.
▪ Reduces radioisotope exposure to study subjects and often eliminates the need to handle samples and waste as radioactive material.
-
Disadvantages
▪ Requires 14C-labeled molecules.
▪ Accelerator mass spectrometry (AMS) facilities are expensive to own and operate.
▪ HPLC fractionation is necessary if quantitation of a specific 14C chemical structure is required.
▪ Sample preparation is not amenable to automation and suffers from long turnaround compared with other analytical platforms.
▪ Industry scientists have modest applied experience with this innovative technology.
Nutrition & toxicology studies
These have been basic research studies historically conducted at academic institutions.
Fundamental nutrient bioavailability and metabolism questions have been answered for the first time.
AMS has enabled exposure quantitation and identification of targets for a wide range of toxins in animals and humans.
Microdosing studies
Clinical evaluation of pharmacokinetic properties can be conducted with reduced safety and toxicology studies.
Permits candidate selection based on in vitro, preclinical and early clinical data.
May help reduce Phase I failures resulting from unexpected human bioavailability or pharmacokinetics.
Phase I studies
Significantly lowers radiochemical exposure to healthy volunteers as compared with traditional radioactive studies.
Absolute bioavailability studies are now performed by concomitant administration of nonlabeled oral dose and 14C-labeled intravenous dose at a subpharmacologic dose.
AMS-based metabolite profiling studies are now used to unequivocally demonstrate the presence or absence of unique human metabolites.
Future prospects
Increased awareness and appreciation for AMS-enabled studies.
Investigate metabolism and target tissue uptake in patients.
Stratify patient populations for personalized treatment and medicine.
- Accelerator Mass Spectrometry
Isotope ratio technique for quantifying rare, long-lived isotopes with the measurement of 14C as its primary biological application
- Metabolite Profiling
Studies that demonstrate the presence or absence of unique human metabolites
- Microdose
1/100th of the dose calculated to yield a pharmacologic effect, or 100 μg, whichever is the lower
Footnotes
Financial & competing interests disclosure
The author is employed by Accium BioSciences, a provider of accelerator mass spectrometry services to the pharmaceutical industry, where he has stock ownership, stock options and patents pending. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Frey EF. The earliest medical texts. Clio Med. 1985;20(1–4):79–90. [PubMed] [Google Scholar]
- 2.Elmore D, Phillips FM. Accelerator mass spectrometry for measurement of long-lived radioisotopes. Science. 1987;236(4801):543–550. doi: 10.1126/science.236.4801.543. [DOI] [PubMed] [Google Scholar]
- 3.Turteltaub KW, Felton JS, Gledhill BL, et al. Accelerator mass spectrometry in biomedical dosimetry. relationship between low-level exposure and covalent binding of heterocyclic amine carcinogens to DNA. Proc Natl Acad Sci USA. 1990;87(14):5288–5292. doi: 10.1073/pnas.87.14.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilman SD, Gee SJ, Hammock BD, et al. Analytical performance of accelerator mass spectrometry and liquid scintillation counting for detection of 14C-labeled atrazine metabolites in human urine. Anal Chem. 1998;70(16):3463–3469. doi: 10.1021/ac971383v. [DOI] [PubMed] [Google Scholar]
- 5.Garner RC, Barker J, Flavell C, et al. A validation study comparing accelerator MS and liquid scintillation counting for analysis of 14C-labelled drugs in plasma, urine and faecal extracts. J Pharm Biomed Anal. 2000;24(2):197–209. doi: 10.1016/s0731-7085(00)00397-6. [DOI] [PubMed] [Google Scholar]
- 6.Brown P, Garner C, Glass R, Ridgway C, Hart A. Comparison of accelerator mass spectrometry with gas chromatography for the determination of pesticide residues in individual items in the diets of wild birds and mammals. J Agric Food Chem. 2004;52(12):3693–3701. doi: 10.1021/jf040012u. [DOI] [PubMed] [Google Scholar]
- 7▪.Liberman RG, Tannenbaum SR, Hughey BJ, et al. An interface for direct analysis of 14C in nonvolatile samples by accelerator mass spectrometry. Anal Chem. 2004;76(2):328–334. doi: 10.1021/ac030181y. Demonstrates the potential feasibility of integrating LC with accelerator mass spectrometry (AMS) to allow direct and near real-time anlaysis of chromatographically separated molecules by AMS. [DOI] [PubMed] [Google Scholar]
- 8.Flarakos J, Liberman RG, Tannenbaum SR, Skipper PL. Integration of continuous-flow accelerator mass spectrometry with chromatography and mass-selective detection. Anal Chem. 2008;80(13):5079–5085. doi: 10.1021/ac800286g. [DOI] [PubMed] [Google Scholar]
- 9.Mcintyre CP, Sylva SP, Roberts ML. Gas chromatograph–combustion system for (14) C-accelerator mass spectrometry. Anal Chem. 2009;81(15):6. doi: 10.1021/ac900958m. [DOI] [PubMed] [Google Scholar]
- 10.Zoppi U, Crye J, Song Q, Arjomand A. Performance evaluation of the new AMS system at Accium BioSciences. Radiocarbon. 2007;49(1):10. [Google Scholar]
- 11.Young GC, Corless S, Felgate CC, Colthup PV. Comparison of a 250 KV single-stage accelerator mass spectrometer with a 5 MV tandem accelerator mass spectrometer – fitness for purpose in bioanalysis. Rapid Commun Mass Spectrom. 2008;22(24):4035–4042. doi: 10.1002/rcm.3829. [DOI] [PubMed] [Google Scholar]
- 12.Salehpour M, Possnert G, Bryhni H. Subattomole sensitivity in biological accelerator mass spectrometry. Anal Chem. 2008;80(10):3515–3521. doi: 10.1021/ac800174j. [DOI] [PubMed] [Google Scholar]
- 13.Salehpour M, Possnert G, Bryhni H, Palminger-Hallen I, Stahle L. Biological accelerator mass spectrometry at Uppsala University. Appl Radiat Isot. 2009;67(3):495–499. doi: 10.1016/j.apradiso.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Salehpour M, Forsgard N, Possnert G. Femtomolar measurements using accelerator mass spectrometry. Rapid Commun Mass Spectrom. 2009;23(5):557–563. doi: 10.1002/rcm.3903. [DOI] [PubMed] [Google Scholar]
- 15.Vogel JS, Turteltaub KW, Finkel R, Nelson DE. Accelerator mass spectrometry. Anal Chem. 1995;67(11):353A–359A. doi: 10.1021/ac00107a001. [DOI] [PubMed] [Google Scholar]
- 16.Brown K, Dingley KH, Turteltaub KW. Accelerator mass spectrometry for biomedical research. Methods Enzymol. 2005;402:423–443. doi: 10.1016/S0076-6879(05)02014-8. [DOI] [PubMed] [Google Scholar]
- 17.Hellborg R, Skog G. Accelerator mass spectrometry. Mass Spectrom Rev. 2008;27(5):398–427. doi: 10.1002/mas.20172. [DOI] [PubMed] [Google Scholar]
- 18.Vogel JS. Rapid production of graphite without contamination for biomedical AMS. Radiocarbon. 1992;34:344–350. [Google Scholar]
- 19.Ognibene TJ, Bench G, Vogel JS, Peaslee GF, Murov S. A high-throughput method for the obtained from biochemical conversion of CO2 samples to graphite in septa-sealed vials for quantification of 14C via accelerator mass spectrometry. Anal Chem. 2003;75(9):2192–2196. doi: 10.1021/ac026334j. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Kelly PB, Clifford AJ. Biological/biomedical accelerator mass spectrometry targets. 2 Physical, morphological, and structural characteristics. Anal Chem. 2008;80(20):7661–7669. doi: 10.1021/ac801228t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Kelly PB, Clifford AJ. Biological/biomedical accelerator mass spectrometry reduction step targets. 1 Optimizing the CO2 using zinc dust. Anal Chem. 2008;80(20):7651–7660. doi: 10.1021/ac801226g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Kelly PB, Clifford AJ. Accelerator mass spectrometry targets of submilligram carbonaceous samples using the high-throughput Zn reduction method. Anal Chem. 2009;81(14):5949–5954. doi: 10.1021/ac900406r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehpour M, Forsgard N, Possnert G. Accelerator mass spectrometry of small biological samples. Rapid Commun Mass Spectrom. 2008;22(23):3928–3934. doi: 10.1002/rcm.3808. [DOI] [PubMed] [Google Scholar]
- 24.Buchholz BA, Dueker SR, Lin Y, Clifford AJ, Vogel J. Methods and applications of HPLC-AMS. Nucl Instrum Methods Phys Res B. 2000;(192):5. [Google Scholar]
- 25.Lappin G, Simpson M, Shishikura Y, Garner C. High-performance liquid chromatography accelerator mass spectrometry. Correcting for losses during analysis by internal standardization. Anal Biochem. 2008;378(1):93–95. doi: 10.1016/j.ab.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Dingley KH, Ubick EA, Vogel JS, Haack KW. DNA isolation and sample preparation for quantification of adduct levels by accelerator mass spectrometry. Methods Mol Biol. 2005;291:21–27. doi: 10.1385/1-59259-840-4:021. [DOI] [PubMed] [Google Scholar]
- 27.Vogel JS, Love AH. Quantitating isotopic molecular labels with accelerator mass spectrometry. Methods Enzymol. 2005;402:402–422. doi: 10.1016/S0076-6879(05)02013-6. [DOI] [PubMed] [Google Scholar]
- 28.Shan G, Huang W, Gee SJ, Buchholz BA, Vogel JS, Hammock BD. Isotope-labeled immunoassays without radiation waste. Proc Natl Acad Sci USA. 2000;97(6):2445–2449. doi: 10.1073/pnas.040575997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hah SS, Mundt JM, Kim HM, Sumbad RA, Turteltaub KW, Henderson PT. Measurement of 7,8-dihydro-8-oxo-2′-deoxyguanosine metabolism in MCF-7 cells at low concentrations using accelerator mass spectrometry. Proc Natl Acad Sci USA. 2007;104(27):11203–11208. doi: 10.1073/pnas.0701733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stites TE, Bailey LB, Scott KC, Toth JP, Fisher WP, Gregory JF., 3rd Kinetic modeling of folate metabolism through use of chronic administration of deuterium-labeled folic acid in men. Am J Clin Nutr. 1997;65(1):53–60. doi: 10.1093/ajcn/65.1.53. [DOI] [PubMed] [Google Scholar]
- 31.Stenstrom K, Leide-Svegborn S, Erlandsson B, et al. Application of accelerator mass spectrometry (AMS) for high-sensitivity measurements of 14 in long-term studies CO2 of fat metabolism. Appl Radiat Isot. 1996;47(4):417–422. doi: 10.1016/0969-8043(96)82298-6. [DOI] [PubMed] [Google Scholar]
- 32▪.Clifford AJ, Arjomand A, Dueker SR, Schneider PD, Buchholz BA, Vogel JS. The dynamics of folic acid metabolism in an adult given a small tracer dose of 14C-folic acid. Adv Exp Med Biol. 1998;445:239–251. doi: 10.1007/978-1-4899-1959-5_15. Describes the first clinical study that employed AMS to study the metabolism, plasma kinetics and excretion of a lightly labeled agent. [DOI] [PubMed] [Google Scholar]
- 33.Buchholz BA, Arjomand A, Dueker SR, Schneider PD, Clifford AJ, Vogel JS. Intrinsic erythrocyte labeling and attomole pharmacokinetic tracing of 14C-labeled folic acid with accelerator mass spectrometry. Anal Biochem. 1999;269(2):348–352. doi: 10.1006/abio.1999.4041. [DOI] [PubMed] [Google Scholar]
- 34.Lin Y, Dueker SR, Follett JR, et al. Quantitation of in vivo human folate metabolism. Am J Clin Nutr. 2004;80(3):680–691. doi: 10.1093/ajcn/80.3.680. [DOI] [PubMed] [Google Scholar]
- 35.Dueker SR, Lin Y, Buchholz BA, et al. Long-term kinetic study of β-carotene, using accelerator mass spectrometry in an adult volunteer. J Lipid Res. 2000;41(11):1790–1800. [PubMed] [Google Scholar]
- 36.Hickenbottom SJ, Lemke SL, Dueker SR, et al. Dual isotope test for assessing β-carotene cleavage to vitamin A in humans. Eur J Nutr. 2002;41(4):141–147. doi: 10.1007/s00394-002-0368-0. [DOI] [PubMed] [Google Scholar]
- 37.Lemke SL, Dueker SR, Follett JR, et al. Absorption and retinol equivalence of β-carotene in humans is influenced by dietary vitamin A intake. J Lipid Res. 2003;44(8):1591–1600. doi: 10.1194/jlr.M300116-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Ho CC, DE Moura FF, Kim SH, Clifford AJ. Excentral cleavage of β-carotene in vivo in a healthy man. Am J Clin Nutr. 2007;85(3):770–777. doi: 10.1093/ajcn/85.3.770. [DOI] [PubMed] [Google Scholar]
- 39.Ho CC, De Moura FF, Kim SH, Burri BJ, Clifford AJ. A minute dose of 14C–β-carotene is absorbed and converted to retinoids in humans. J Nutr. 2009;139(8):1480–1486. doi: 10.3945/jn.109.105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Moura FF, Ho CC, Getachew G, Hickenbottom S, Clifford AJ. Kinetics of 14C distribution after tracer dose of 14C-lutein in an adult woman. Lipids. 2005;40(10):1069–1073. doi: 10.1007/s11745-005-1471-4. [DOI] [PubMed] [Google Scholar]
- 41.Carkeet C, Dueker SR, Lango J, et al. Human vitamin B12 absorption measurement by accelerator mass spectrometry using specifically labeled (14)C-cobalamin. Proc Natl Acad Sci USA. 2006;103(15):5694–5699. doi: 10.1073/pnas.0601251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clifford AJ, De Moura FF, Ho CC, et al. A feasibility study quantifying in vivo human α-tocopherol metabolism. Am J Clin Nutr. 2006;84(6):1430–1441. doi: 10.1093/ajcn/84.6.1430. [DOI] [PubMed] [Google Scholar]
- 43.Garner RC, Lightfoot TJ, Cupid BC, et al. Comparative biotransformation studies of MeIQx and PhIP in animal models and humans. Cancer Lett. 1999;143(2):161–165. doi: 10.1016/s0304-3835(99)00118-4. [DOI] [PubMed] [Google Scholar]
- 44.Dingley KH, Curtis KD, Nowell S, Felton JS, Lang NP, Turteltaub KW. DNA and protein adduct formation in the colon and blood of humans after exposure to a dietary-relevant dose of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Epidemiol Biomarkers Prev. 1999;8(6):507–512. [PubMed] [Google Scholar]
- 45.Turteltaub KW, Dingley KH, Curtis KD, et al. Macromolecular adduct formation and metabolism of heterocyclic amines in humans and rodents at low doses. Cancer Lett. 1999;143(2):149–155. doi: 10.1016/s0304-3835(99)00116-0. [DOI] [PubMed] [Google Scholar]
- 46▪.Malfatti MA, Dingley KH, Nowell-Kadlubar S, et al. The urinary metabolite profile of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine is predictive of colon DNA adducts after a low-dose exposure in humans. Cancer Res. 2006;66(21):10541–10547. doi: 10.1158/0008-5472.CAN-06-1573. Study that employed a 14C-labeled probe and urinary biomarkers to predict colon DNA-adduct formation with possible applications to risk assessment and diagnostics. [DOI] [PubMed] [Google Scholar]
- 47.Cupid BC, Lightfoot TJ, Russell D, et al. The formation of AFB(1)-macromolecular adducts in rats and humans at dietary levels of exposure. Food Chem Toxicol. 2004;42(4):559–569. doi: 10.1016/j.fct.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Mauthe RJ, Dingley KH, Leveson SH, et al. Comparison of DNA-adduct and tissue-available dose levels of MeIQx in human and rodent colon following administration of a very low dose. Int J Cancer. 1999;80(4):539–545. doi: 10.1002/(sici)1097-0215(19990209)80:4<539::aid-ijc10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 49.Dingley KH, Freeman SP, Nelson DO, Garner RC, Turteltaub KW. Covalent binding of 2-amino-3,8-dimethylimidazo[4,5-f] quinoxaline to albumin and hemoglobin at environmentally relevant doses. Comparison of human subjects and F344 rats. Drug Metab Dispos. 1998;26(8):825–828. [PubMed] [Google Scholar]
- 50.Turteltaub KW, Mauthe RJ, Dingley KH, et al. MeIQx-DNA adduct formation in rodent and human tissues at low doses. Mutat Res. 1997;376(1–2):243–252. doi: 10.1016/s0027-5107(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 51.Mauthe RJ, Snyderwine EG, Ghoshal A, Freeman SP, Turteltaub KW. Distribution and metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PHiP) in female rats and their pups at dietary doses. Carcinogenesis. 1998;19(5):919–924. doi: 10.1093/carcin/19.5.919. [DOI] [PubMed] [Google Scholar]
- 52.Cheng Y, Li HL, Wang HF, et al. Inhibition of nicotine-DNA adduct formation in mice by six dietary constituents. Food Chem Toxicol. 2003;41(7):1045–1050. doi: 10.1016/s0278-6915(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Cheng Y, Wang H, et al. Inhibition of nitrobenzene-induced DNA and hemoglobin adductions by dietary constituents. Appl Radiat Isot. 2003;58(3):291–298. doi: 10.1016/s0969-8043(02)00315-9. [DOI] [PubMed] [Google Scholar]
- 54.Rickert DE, Dingley K, Ubick E, Dix KJ, Molina L. Determination of the tissue distribution and excretion by accelerator mass spectrometry of the nonadecapeptide 14C-Moli1901 in beagle dogs after intratracheal instillation. Chem Biol Interact. 2005;155(1–2):55–61. doi: 10.1016/j.cbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 55.DeGregorio MW, Dingley KH, Wurz GT, Ubick E, Turteltaub KW. Accelerator mass spectrometry allows for cellular quantification of doxorubicin at femtomolar concentrations. Cancer Chemother Pharmacol. 2006;57(3):335–342. doi: 10.1007/s00280-005-0060-1. [DOI] [PubMed] [Google Scholar]
- 56.Leide-Svegborn S, Stenstrom K, Olofsson M, et al. Biokinetics and radiation doses for carbon-14 urea in adults and children undergoing the Helicobacter pylori breath test. Eur J Nucl Med. 1999;26(6):573–580. doi: 10.1007/s002590050424. [DOI] [PubMed] [Google Scholar]
- 57.Gunnarsson M, Leide-Svegborn S, Stenstrom K, et al. No radiation protection reasons for restrictions on 14C urea breath tests in children. Br J Radiol. 2002;75(900):982–986. doi: 10.1259/bjr.75.900.750982. [DOI] [PubMed] [Google Scholar]
- 58.Combes RD, Berridge T, Connelly J, et al. Early microdose drug studies in human volunteers can minimise animal testing. Proceedings of a workshop organised by volunteers in research and testing. Eur J Pharm Sci. 2003;19(1):1–11. doi: 10.1016/s0928-0987(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 59.Lappin G, Garner RC. Big physics, small doses. The use of AMS and PET in human microdosing of development drugs. Nat Rev Drug Discov. 2003;2(3):233–240. doi: 10.1038/nrd1037. [DOI] [PubMed] [Google Scholar]
- 60.Buchan P. Smarter candidate selection – utilizing microdosing in exploratory clinical studies. Ernst Schering Res Found Workshop. 2007;59:7–27. doi: 10.1007/978-3-540-49529-1_2. [DOI] [PubMed] [Google Scholar]
- 61.Butz RF, Morelli G. Innovative strategies for early clinical R&D. IDrugs. 2008;11(1):36–41. [PubMed] [Google Scholar]
- 62.Carpenter AP, Pontecorvo MJ, Hefti FF, Skovronsky DM. The use of the exploratory IND in the evaluation and development of 18 F-PET radiopharmaceuticals for amyloid imaging in the brain. A review of one company’s experience. Q J Nucl Med Mol Imaging. 2009;53(4):387–393. [PubMed] [Google Scholar]
- 63.Jacobson-Kram D, Mills G. Leveraging exploratory investigational new drug studies to accelerate drug development. Clin Cancer Res. 2008;14(12):3670–3674. doi: 10.1158/1078-0432.CCR-07-4558. [DOI] [PubMed] [Google Scholar]
- 64.Kummar S, Rubinstein L, Kinders R, et al. Phase 0 clinical trials. Conceptions and misconceptions. Cancer J. 2008;14(3):133–137. doi: 10.1097/PPO.0b013e318172d6f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarapa N, Exploratory IND. A new regulatory strategy for early clinical drug development in the United States. Ernst Schering Res Found Workshop. 2007;(59):151–163. doi: 10.1007/978-3-540-49529-1_11. [DOI] [PubMed] [Google Scholar]
- 66.Balani SK, Nagaraja NV, Qian MG, et al. Evaluation of microdosing to assess pharmacokinetic linearity in rats using liquid chromatography–tandem mass spectrometry. Drug Metab Dispos. 2006;34(3):384–388. doi: 10.1124/dmd.105.007195. [DOI] [PubMed] [Google Scholar]
- 67.Bauer M, Langer O, Dal-Bianco P, et al. A positron emission tomography microdosing study with a potential antiamyloid drug in healthy volunteers and patients with Alzheimer’s disease. Clin Pharmacol Ther. 2006;80(3):216–227. doi: 10.1016/j.clpt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Bauer M, Wagner CC, Langer O. Microdosing studies in humans. The role of positron emission tomography. Drugs RD. 2008;9(2):73–81. doi: 10.2165/00126839-200809020-00002. [DOI] [PubMed] [Google Scholar]
- 69.Sandhu P, Vogel JS, Rose MJ, et al. Evaluation of microdosing strategies for studies in preclinical drug development. Demonstration of linear pharmacokinetics in dogs of a nucleoside analog over a 50-fold dose range. Drug Metab Dispos. 2004;32(11):1254–1259. doi: 10.1124/dmd.104.000422. [DOI] [PubMed] [Google Scholar]
- 70▪▪.Lappin G, Kuhnz W, Jochemsen R, et al. Use of microdosing to predict pharmacokinetics at the therapeutic dose. Experience with 5 drugs. Clin Pharmacol Ther. 2006;80(3):203–215. doi: 10.1016/j.clpt.2006.05.008. First comprehensive study to evaluate the value of human microdosing to predict the pharmacology of the corresponding therapeutic dose. [DOI] [PubMed] [Google Scholar]
- 71.Vuong L, Ruckle JL, Blood AB, et al. Use of accelerator mass spectrometry to measure the pharmacokinetics and peripheral blood mononuclear cell concentrations of zidovudine. J Pharm Sci. 2008;97(7):2833–2843. doi: 10.1002/jps.21160. [DOI] [PubMed] [Google Scholar]
- 72.Garner C. New paradigm of early clinical research – EUMAPP. Parliament Magazine. 2007;238:1. [Google Scholar]
- 73.Zhou XJ, Garner RC, Nicholson S, Kissling CJ, Mayers D. Microdose pharmacokinetics of IDX899 and IDX989, candidate HIV-1 non-nucleoside reverse transcriptase inhibitors, following oral and intravenous administration in healthy male subjects. J Clin Pharmacol. 2009 doi: 10.1177/0091270009343698. [DOI] [PubMed] [Google Scholar]
- 74.Young G, Ellis W, Ayrton J, Hussey E, Adamkiewicz B. Accelerator mass spectrometry (AMS). Recent experience of its use in a clinical study and the potential future of the technique. Xenobiotica. 2001;31(8–9):619–632. doi: 10.1080/00498250110052724. [DOI] [PubMed] [Google Scholar]
- 75.Garner RC, Garner JV, Gregory S, Whattam M, Calam A, Leong D. Comparison of the absorption of micronized (Daflon 500 mg) and nonmicronized 14C-diosmin tablets after oral administration to healthy volunteers by accelerator mass spectrometry and liquid scintillation counting. J Pharm Sci. 2002;91(1):32–40. doi: 10.1002/jps.1168. [DOI] [PubMed] [Google Scholar]
- 76.Boddy AV, Sludden J, Griffin MJ, et al. Pharmacokinetic investigation of imatinib using accelerator mass spectrometry in patients with chronic myeloid leukemia. Clin Cancer Res. 2007;13(14):4164–4169. doi: 10.1158/1078-0432.CCR-06-2179. [DOI] [PubMed] [Google Scholar]
- 77.Garner RC, Goris I, Laenen AA, et al. Evaluation of accelerator mass spectrometry in a human mass balance and pharmacokinetic study-experience with 14C-labeled (r)-6-[amino(4-chlorophenyl) (1-methyl-1h-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1h)-quinolinone (r115777), a farnesyl transferase inhibitor. Drug Metab Dispos. 2002;30(7):823–830. doi: 10.1124/dmd.30.7.823. [DOI] [PubMed] [Google Scholar]
- 78▪▪.Sarapa N, Hsyu PH, Lappin G, Garner RC. The application of accelerator mass spectrometry to absolute bioavailability studies in humans. Simultaneous administration of an intravenous microdose of 14C-nelfinavir mesylate solution and oral nelfinavir to healthy volunteers. J Clin Pharmacol. 2005;45(10):1198–1205. doi: 10.1177/0091270005280051. Describes an innovative approach towards conducting absolute bioavailability studies in early clinical development, one that has the potential of reducing the time and cost of conducting such studies. [DOI] [PubMed] [Google Scholar]
- 79.Miyaji Y, Ishizuka T, Kawai K, et al. Use of an intravenous microdose of 14C-labeled drug and accelerator mass spectrometry to measure absolute oral bioavailability in dogs; cross-comparison of assay methods by accelerator mass spectrometry and liquid chromatography-tandem mass spectrometry. Drug Metab Pharmacokinet. 2009;24(2):130–138. doi: 10.2133/dmpk.24.130. [DOI] [PubMed] [Google Scholar]
- 80.Klem B, Lappin G, Nicholson S, et al. Determination of the bioavailability of [14C]-hexaminolevulinate using accelerator mass spectrometry after intravesical administration to human volunteers. J Clin Pharmacol. 2006;46(4):456–460. doi: 10.1177/0091270006286849. [DOI] [PubMed] [Google Scholar]
- 81.Beumer JH, Garner RC, Cohen MB, et al. Human mass balance study of the novel anticancer agent ixabepilone using accelerator mass spectrometry. Invest New Drugs. 2007;25(4):327–334. doi: 10.1007/s10637-007-9041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prakash C, Shaffer CL, Tremaine LM, et al. Application of liquid chromatography–accelerator mass spectrometry (LC–AMS) to evaluate the metabolic profiles of a drug candidate in human urine and plasma. Drug Metab Lett. 2007;1(3):226–231. doi: 10.2174/187231207781369735. [DOI] [PubMed] [Google Scholar]
- 83.Lappin G, Garner RC, Meyers T, Powell J, Varley P. Novel use of accelerator mass spectrometry for the quantification of low levels of systemic therapeutic recombinant protein. J Pharm Biomed Anal. 2006;41(4):1299–1302. doi: 10.1016/j.jpba.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 84.Nakamura T. Applications of low-abundance-radioisotope measurements with accelerator mass spectrometry to the biomedical sciences. Tanpakushitsu Kakusan Koso. 1994;39(11):2011–2020. [PubMed] [Google Scholar]
- 85.Kaye B, Garner RC, Mauthe RJ, Freeman SP, Turteltaub KW. A preliminary evaluation of accelerator mass spectrometry in the biomedical field. J Pharm Biomed Anal. 1997;16(3):541–543. doi: 10.1016/s0731-7085(97)00104-0. [DOI] [PubMed] [Google Scholar]
- 86.Barker J, Garner RC. Biomedical applications of accelerator mass spectrometry-isotope measurements at the level of the atom. Rapid Commun Mass Spectrom. 1999;13(4):285–293. doi: 10.1002/(SICI)1097-0231(19990228)13:4<285::AID-RCM469>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 87.Garner RC. Accelerator mass spectrometry in pharmaceutical research and development – a new ultrasensitive analytical method for isotope measurement. Curr Drug Metab. 2000;1(2):205–213. doi: 10.2174/1389200003339054. [DOI] [PubMed] [Google Scholar]
- 88.Turteltaub KW, Vogel JS. Bioanalytical applications of accelerator mass spectrometry for pharmaceutical research. Curr Pharm Des. 2000;6(10):991–1007. doi: 10.2174/1381612003400047. [DOI] [PubMed] [Google Scholar]
- 89.Papac DI, Shahrokh Z. Mass spectrometry innovations in drug discovery and development. Pharm Res. 2001;18(2):131–145. doi: 10.1023/a:1011049231231. [DOI] [PubMed] [Google Scholar]
- 90.Lappin G, Garner RC. Current perspectives of 14C-isotope measurement in biomedical accelerator mass spectrometry. Anal Bioanal Chem. 2004;378(2):356–364. doi: 10.1007/s00216-003-2348-5. [DOI] [PubMed] [Google Scholar]
- 91.White IN, Brown K. Techniques. The application of accelerator mass spectrometry to pharmacology and toxicology. Trends Pharmacol Sci. 2004;25(8):442–447. doi: 10.1016/j.tips.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Lappin G, Garner RC. The use of accelerator mass spectrometry to obtain early human ADME/PK data. Expert Opin Drug Metab Toxicol. 2005;1(1):23–31. doi: 10.1517/17425255.1.1.23. [DOI] [PubMed] [Google Scholar]
- 93.Palmblad M, Buchholz BA, Hillegonds DJ, Vogel JS. Neuroscience and accelerator mass spectrometry. J Mass Spectrom. 2005;40(2):154–159. doi: 10.1002/jms.734. [DOI] [PubMed] [Google Scholar]
- 94.Vogel JS. Accelerator mass spectrometry for quantitative in vivo tracing. Biotechniques. 2005;(Suppl):25–29. doi: 10.2144/05386su04. [DOI] [PubMed] [Google Scholar]
- 95.Brown K, Tompkins EM, White IN. Applications of accelerator mass spectrometry for pharmacological and toxicological research. Mass Spectrom Rev. 2006;25(1):127–145. doi: 10.1002/mas.20059. [DOI] [PubMed] [Google Scholar]
- 96.Lappin G, Stevens L. Biomedical accelerator mass spectrometry. Recent applications in metabolism and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2008;4(8):1021–1033. doi: 10.1517/17425255.4.8.1021. [DOI] [PubMed] [Google Scholar]
- 97.Larsson M. Editorial. Accelerator mass spectrometry. Mass Spectrom Rev. 2008;27(5):397. doi: 10.1002/mas.20171. [DOI] [PubMed] [Google Scholar]
- 98.Hah SS. Recent advances in biomedical applications of accelerator mass spectrometry. J Biomed Sci. 2009;16:54. doi: 10.1186/1423-0127-16-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gunnarsson M, Stenstrom K, Leide-Svegborn S, et al. Biokinetics and radiation dosimetry for patients undergoing a glycerol tri[1–14C] oleate fat malabsorption breath test. Appl Radiat Isot. 2003;58(4):517–526. doi: 10.1016/s0969-8043(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 100.Gunnarsson M, Leide-Svegborn S, Stenstrom K, et al. Long-term biokinetics and radiation exposure of patients undergoing 14C-glycocholic acid and 14C-xylose breath tests. Cancer Biother Radiopharm. 2007;22(6):762–771. doi: 10.1089/cbr.2007.0350. [DOI] [PubMed] [Google Scholar]
- 101.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87(5):1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhardwaj RD, Curtis MA, Spalding KL, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103(33):12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 105.Spalding KL, Buchholz BA, Bergman LE, Druid H, Frisen J. Forensics. Age written in teeth by nuclear tests. Nature. 2005;437(7057):333–334. doi: 10.1038/437333a. [DOI] [PubMed] [Google Scholar]
- 106.Record RD, Hillegonds D, Simmons C, et al. In vivo degradation of 14C-labeled small intestinal submucosa (sis) when used for urinary bladder repair. Biomaterials. 2001;22(19):2653–2659. doi: 10.1016/s0142-9612(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 107.Gilbert TW, Stewart-Akers AM, Badylak SF. A quantitative method for evaluating the degradation of biologic scaffold materials. Biomaterials. 2007;28(2):147–150. doi: 10.1016/j.biomaterials.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Shawgo RS, Tyler B, et al. In vivo release from a drug delivery MEMS device. J Control Release. 2004;100(2):211–219. doi: 10.1016/j.jconrel.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 109.Buchholz BA, Freeman S, Haack K, Vogel J. Tips and traps in the 14C Bio-AMS preparation laboratory. Nucl Instrum Methods Phys Res B. 2000;172:5. [Google Scholar]
- 110.Hah SS. Determination of protein-ligand interactions using accelerator mass spectrometry. Modified crosslinking assay. Anal Sci. 2009;25(5):731–733. doi: 10.2116/analsci.25.731. [DOI] [PubMed] [Google Scholar]
- 111.Minamimoto R, Hamabe Y, Miyaoka T, et al. Accelerator mass spectrometry analysis of background 14C-concentrations in human blood. Aiming at reference data for further microdosing studies. Ann Nucl Med. 2008;22(10):883–889. doi: 10.1007/s12149-008-0200-x. [DOI] [PubMed] [Google Scholar]
- 112.Nelson DE. A new method for carbon isotopic analysis of protein. Science. 1991;251(4993):552–554. doi: 10.1126/science.1990430. [DOI] [PubMed] [Google Scholar]
- 113.Palmblad M, Bench G, Vogel JS. Mass by energy loss quantitation as a practical submicrogram balance. Anal Chem. 2005;77(3):952–953. doi: 10.1021/ac048560u. [DOI] [PubMed] [Google Scholar]
- 114.Palmblad M, Vogel JS. Quantitation of binding, recovery and desalting efficiency of peptides and proteins in solid phase extraction micropipette tips. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;814(2):309–313. doi: 10.1016/j.jchromb.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 115.Veltkamp AC. Radiochromatography in pharmaceutical and biomedical analysis. J Chromatogr. 1990;531:101–129. doi: 10.1016/s0378-4347(00)82282-3. [DOI] [PubMed] [Google Scholar]
- 116.Bogen KT, Keating GA, Meissner S, Vogel JS. Initial uptake kinetics in human skin exposed to dilute aqueous trichloroethylene in vitro. J Expo Anal Environ Epidemiol. 1998;8(2):253–271. [PubMed] [Google Scholar]
- 117.Turteltaub KW, Vogel JS, Frantz CE, Shen N. Fate and distribution of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in mice at a human dietary equivalent dose. Cancer Res. 1992;52(17):4682–4687. [PubMed] [Google Scholar]
- 118.Frantz CE, Bangerter C, Fultz E, Mayer KM, Vogel JS, Turteltaub KW. Dose-response studies of MeIQx in rat liver and liver DNA at low doses. Carcinogenesis. 1995;16(2):367–373. doi: 10.1093/carcin/16.2.367. [DOI] [PubMed] [Google Scholar]
- 119.Turteltaub KW, Vogel JS, Frantz C, Felton JS, Mcmanus M. Assessment of the DNA adduction and pharmacokinetics of PHiP and MeIQx in rodents at doses approximating human exposure using the technique of accelerator mass spectrometry (AMS) and 32P-postlabeling. Princess Takamatsu Symp. 1995;23:93–102. [PubMed] [Google Scholar]
- 120.Creek MR, Mani C, Vogel JS, Turteltaub KW. Tissue distribution and macromolecular binding of extremely low doses of [14C]-benzene in B6C3F1 mice. Carcinogenesis. 1997;18(12):2421–2427. doi: 10.1093/carcin/18.12.2421. [DOI] [PubMed] [Google Scholar]
- 121.Martin EA, Carthew P, White IN, et al. Investigation of the formation and accumulation of liver DNA adducts in mice chronically exposed to tamoxifen. Carcinogenesis. 1997;18(11):2209–2215. doi: 10.1093/carcin/18.11.2209. [DOI] [PubMed] [Google Scholar]
- 122.White IN, Martin EA, Mauthe RJ, Vogel JS, Turteltaub KW, Smith LI. Comparisons of the binding of [14C]radiolabelled tamoxifen or toremifene to rat DNA using accelerator mass spectrometry. Chem Biol Interact. 1997;106(2):149–160. doi: 10.1016/s0009-2797(97)00063-x. [DOI] [PubMed] [Google Scholar]
- 123.Garner RC. The role of DNA adducts in chemical carcinogenesis. Mutat Res. 1998;402(1–2):67–75. doi: 10.1016/s0027-5107(97)00283-2. [DOI] [PubMed] [Google Scholar]
- 124.Buchholz BA, Fultz E, Haack KW, et al. HPLC-accelerator MS measurement of atrazine metabolites in human urine after dermal exposure. Anal Chem. 1999;71(16):3519–3525. doi: 10.1021/ac990152g. [DOI] [PubMed] [Google Scholar]
- 125.Kwok ES, Buchholz BA, Vogel JS, Turteltaub KW, Eastmond DA. Dose-dependent binding of ortho-phenylphenol to protein but not DNA in the urinary bladder of male F344 rats. Toxicol Appl Pharmacol. 1999;159(1):18–24. doi: 10.1006/taap.1999.8722. [DOI] [PubMed] [Google Scholar]
- 126.Lang NP, Nowell S, Malfatti MA, et al. In vivo human metabolism of [2–14C]2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PHiP) Cancer Lett. 1999;143(2):135–138. doi: 10.1016/s0304-3835(99)00142-1. [DOI] [PubMed] [Google Scholar]
- 127.Mani C, Freeman S, Nelson DO, Vogel JS, Turteltaub KW. Species and strain comparisons in the macromolecular binding of extremely low doses of [14C]benzene in rodents, using accelerator mass spectrometry. Toxicol Appl Pharmacol. 1999;159(2):83–90. doi: 10.1006/taap.1999.8707. [DOI] [PubMed] [Google Scholar]
- 128.Goldman R, Day BW, Carver TA, Mauthe RJ, Turteltaub KW, Shields PG. Quantitation of benzo[a]pyrene-DNA adducts by postlabeling with 14C-acetic anhydride and accelerator mass spectrometry. Chem Biol Interact. 2000;126(3):171–183. doi: 10.1016/s0009-2797(00)00160-5. [DOI] [PubMed] [Google Scholar]
- 129.Boocock DJ, Brown K, Gibbs AH, Sanchez E, Turteltaub KW, White IN. Identification of human CYP forms involved in the activation of tamoxifen and irreversible binding to DNA. Carcinogenesis. 2002;23(11):1897–1901. doi: 10.1093/carcin/23.11.1897. [DOI] [PubMed] [Google Scholar]
- 130.Vogel JS, Keating GA, 2nd, Buchholz BA. Protein binding of isofluorophate in vivo after coexposure to multiple chemicals. Environ Health Perspect. 2002;110(Suppl 6):1031–1036. doi: 10.1289/ehp.02110s61031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williams KE, Carver TA, Miranda JJ, et al. Attomole detection of in vivo protein targets of benzene in mice. Evidence for a highly reactive metabolite. Mol Cell Proteomics. 2002;1(11):885–895. doi: 10.1074/mcp.m200067-mcp200. [DOI] [PubMed] [Google Scholar]
- 132.Li H, Wang H, Sun H, Liu Y, Liu K, Peng S. Binding of nitrobenzene to hepatic DNA and hemoglobin at low doses in mice. Toxicol Lett. 2003;139(1):25–32. doi: 10.1016/s0378-4274(02)00438-1. [DOI] [PubMed] [Google Scholar]
- 133.Martin EA, Brown K, Gaskell M, et al. Tamoxifen DNA damage detected in human endometrium using accelerator mass spectrometry. Cancer Res. 2003;63(23):8461–8465. [PubMed] [Google Scholar]
- 134.Turteltaub KW, Mani C. Benzene metabolism in rodents at doses relevant to human exposure from urban air. Res Rep Health Eff Inst. 2003;(113):1–26. discussion 27–35. [PubMed] [Google Scholar]
- 135.Mally A, Zepnik H, Wanek P, et al. Ochratoxin A. Lack of formation of covalent DNA adducts. Chem Res Toxicol. 2004;17(2):234–242. doi: 10.1021/tx034188m. [DOI] [PubMed] [Google Scholar]
- 136.Du HF, Xu LH, Wang HF, et al. Formation of MTBE-DNA adducts in mice measured with accelerator mass spectrometry. Environ Toxicol. 2005;20(4):397–401. doi: 10.1002/tox.20124. [DOI] [PubMed] [Google Scholar]
- 137.Malfatti MA, Ubick EA, Felton JS. The impact of glucuronidation on the bioactivation and DNA adduction of the cooked-food carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in vivo. Carcinogenesis. 2005;26(11):2019–2028. doi: 10.1093/carcin/bgi151. [DOI] [PubMed] [Google Scholar]
- 138.Mally A, Dekant W. DNA adduct formation by ochratoxin a. Review of the available evidence. Food Addit Contam. 2005;22(Suppl 1):65–74. doi: 10.1080/02652030500317544. [DOI] [PubMed] [Google Scholar]
- 139.Hillier SM, Marquis JC, Zayas B, et al. DNA adducts formed by a novel antitumor agent 11β-dichloro in vitro and in vivo. Mol Cancer Ther. 2006;5(4):977–984. doi: 10.1158/1535-7163.MCT-05-0464. [DOI] [PubMed] [Google Scholar]
- 140.Skipper PL, Trudel LJ, Kensler TW, et al. DNA adduct formation by 2,6-dimethyl-, 3,5-dimethyl-, and 3-ethylaniline in vivo in mice. Chem Res Toxicol. 2006;19(8):1086–1090. doi: 10.1021/tx060082q. [DOI] [PubMed] [Google Scholar]
- 141.Xie Q, Sun H, Liu Y, Ding X, Fu D, Liu K. Adduction of biomacromolecules with acrylamide (AA) in mice at environmental dose levels studied by accelerator mass spectrometry. Toxicol Lett. 2006;163(2):101–108. doi: 10.1016/j.toxlet.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 142.Brown K, Tompkins EM, Boocock DJ, et al. Tamoxifen forms DNA adducts in human colon after administration of a single [14C]-labeled therapeutic dose. Cancer Res. 2007;67(14):6995–7002. doi: 10.1158/0008-5472.CAN-07-0913. [DOI] [PubMed] [Google Scholar]
- 143.Hah SS, Sumbad RA, De Vere White RW, Turteltaub KW, Henderson PT. Characterization of oxaliplatin–DNA adduct formation in DNA and differentiation of cancer cell drug sensitivity at microdose concentrations. Chem Res Toxicol. 2007;20(12):1745–1751. doi: 10.1021/tx700376a. [DOI] [PubMed] [Google Scholar]
- 144.Watanabe K, Liberman RG, Skipper PL, Tannenbaum SR, Guengerich FP. Analysis of DNA adducts formed in vivo in rats and mice from 1,2-dibromoethane, 1,2-dichloroethane, dibromomethane, and dichloromethane using HPLC/accelerator mass spectrometry and relevance to risk estimates. Chem Res Toxicol. 2007;20(11):1594–1600. doi: 10.1021/tx700125p. [DOI] [PubMed] [Google Scholar]
- 145.Yuan Y, Wang HF, Sun HF, et al. Adduction of DNA with MTBE and TBA in mice studied by accelerator mass spectrometry. Environ Toxicol. 2007;22(6):630–635. doi: 10.1002/tox.20295. [DOI] [PubMed] [Google Scholar]
- 146.Coldwell KE, Cutts SM, Ognibene TJ, Henderson PT, Phillips DR. Detection of adriamycin-DNA adducts by accelerator mass spectrometry at clinically relevant adriamycin concentrations. Nucleic Acids Res. 2008;36(16):e100. doi: 10.1093/nar/gkn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Knutson CG, Skipper PL, Liberman RG, Tannenbaum SR, Marnett LJ. Monitoring in vivo metabolism and elimination of the endogenous DNA adduct, M1DG {3-(2-deoxy-β-D-erythro-pentofuranosyl) pyrimido[1,2-α]purin-10(3h)-one}, by accelerator mass spectrometry. Chem Res Toxicol. 2008;21(6):1290–1294. doi: 10.1021/tx800049v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie Q, Liu Y, Sun H, et al. Inhibition of acrylamide toxicity in mice by three dietary constituents. J Agric Food Chem. 2008;56(15):6054–6060. doi: 10.1021/jf0730542. [DOI] [PubMed] [Google Scholar]
- 149.Marsden DA, Jones DJ, Britton RG, et al. Dose-response relationships for n7-(2-hydroxyethyl)guanine induced by low-dose [14C]ethylene oxide. Evidence for a novel mechanism of endogenous adduct formation. Cancer Res. 2009;69(7):3052–3059. doi: 10.1158/0008-5472.CAN-08-4233. [DOI] [PubMed] [Google Scholar]
- 150.Eap CB, Buclin T, Cucchia G, et al. Oral administration of a low dose of midazolam (75 microg) as an in vivo probe for CYP3A activity. Eur J Clin Pharmacol. 2004;60(4):237–246. doi: 10.1007/s00228-004-0762-z. [DOI] [PubMed] [Google Scholar]
- 151.Beumer JH, Beijnen JH, Schellens JH. Mass balance studies, with a focus on anticancer drugs. Clin Pharmacokinet. 2006;45(1):33–58. doi: 10.2165/00003088-200645010-00003. [DOI] [PubMed] [Google Scholar]
- 152.Lappin G, Rowland M, Garner RC. The use of isotopes in the determination of absolute bioavailability of drugs in humans. Expert Opin Drug Metab Toxicol. 2006;2(3):419–427. doi: 10.1517/17425255.2.3.419. [DOI] [PubMed] [Google Scholar]
Websites
- 201.EMEA. Position Paper on Non-Clinical Safety Studies to Support Clinical Trials with a Single Microdose. 2004 June; www.ema.europa.eu/pdfs/human/swp/259902en.pdf.
- 202.US FDA. Guidance for Industry, Investigators, and Reviewers – Exploratory IND Studies. 2006 January; www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM078933.pdf.
- 203.US FDA. Guidance for Industry – Safety Testing of Drug Metabolites. 2008 www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079266.pdf.