Abstract
In vivo studies suggest that replication forks are arrested due to encounters with head-on transcription complexes. Yet, the fate of the replisome and RNA polymerase (RNAP) following a head-on collision is unknown. Here, we find that the E. coli replisome stalls upon collision with a head-on transcription complex, but instead of collapsing, the replication fork remains highly stable and eventually resumes elongation after displacing the RNAP from DNA. We also find that the transcription-repair coupling factor, Mfd, promotes direct restart of the fork following the collision by facilitating displacement of the RNAP. These findings demonstrate the intrinsic stability of the replication apparatus and a novel role for the transcription-coupled repair pathway in promoting replication past a RNAP block.
In vivo studies suggest that replication forks are arrested by head-on transcription complexes, but are unaffected by co-directional transcription complexes (1)[supporting online material (SOM) text S1]. Mechanisms that resolve head-on collisions in favor of the replisome are therefore necessary for chromosome duplication and may preserve genomic integrity by preventing fork collapse. In vivo data indicate that head-on replisome-RNA polymerase (RNAP) collisions cause chromosomal deletions which suggests dissociation of the replisome (2). Genetic studies implicate recombinational repair in resolving conflicts between replication and transcription which also suggests the possibility of fork collapse (3, 4). Similarly, in vitro data imply that the replisome dissociates after encountering a lac repressor which arrests the fork (5). In contrast, several in vivo studies indicate that although replication forks stall at protein barriers, the replisome remains stable and resumes elongation following removal of the block (6). Thus, replisome stalling may not necessitate fork collapse (7). We investigate the stability of the E. coli replisome after it encounters a head-on RNAP in vitro.
The E. coli replisome is a multi-protein complex that copies DNA with high speed (~630 nt s−1) and processivity (~50 kb)(8). Fig. 1A illustrates a solid-phase assay used to study a replisome-RNAP head-on collision. A RNAP halted elongation complex was assembled on linear DNA, immobilized to streptavidin beads, then washed with high salt to remove unstable RNAP-DNA complexes (fig. S1)(9). Next, the replisome was assembled in two steps: first, the replicative DnaB helicase was added which encircles the lagging strand; second, DNA polymerase III (Pol III), the clamp-loader, and the β-clamp were added along with ATP, 32P-α-dCTP, and 32P-α-dGTP. Fork movement was initiated by adding 32P-α-dATP and 32P-α-dTTP with single-strand DNA binding protein (SSB). We observe a 2.5 kb product equal to the distance from the fork to the promoter indicating that the replisome is impeded by the RNAP (Fig. 1B, lane 2). Full-length DNA (3.6 kb) is also produced suggesting incomplete promoter occupancy by RNAP or replisome read-through of the transcription complex (lane 2). Omitting the promoter specificity factor, σ70, results in only full-length DNA (lane 1). An average of 50% (n=8) of the replisomes produce full-length DNA in the presence of a head-on RNAP (right) which exceeds the number of templates that lack RNAP (24%; fig. S2). This suggests that ~26% of the replisomes pass a head-on RNAP during the 10 min timecourse. In Fig. 1c we determined whether the replisome reads-through RNAP directly by performing a pulse-chase experiment in which cold dNTPs were added after 5 min, and extension of the 2.5 kb product was monitored. A steady increase in the ratio of full-length to intermediate-length product is observed indicating that the replisome passes the RNAP, albeit after pausing for a considerable duration (left, right plot). The relatively large amount of full-length product (39%) observed after 5 min suggests that some replisomes might pass the RNAP with high efficiency. Replisome read-through of RNAP on a different template is demonstrated to rule out any sequence specific effects (fig. S3). Fig. 1C demonstrates that the stalled replisome remains active for 60 min following the collision without need for primosomal proteins, such as PriA/C, that are necessary to re-load DnaB onto SSB coated single-strand DNA (ssDNA)(10, 11). DnaB therefore stays bound to the stalled fork. Consistent with previous studies, we demonstrate that DnaB is required for replication and that SSB prevents DnaB loading in the absence of primosomal proteins (fig. S4). Thus, although the replication fork stalls upon encountering a head-on RNAP, the replisome remains intact and resumes elongation, presumably after displacing the transcription complex.
Fig. 1. The replisome slowly passes a head-on RNAP.
(A) A head-on RNAP was assembled on immobilized DNA. DnaB was added, then the replisome was assembled and replication initiated. Replisome component functions (8): Pol III core (orange), synthesizes DNA; β-clamp (dark blue), confers processivity to Pol III; clamp-loader (light blue), assembles β-clamps onto primed sites; DnaB (yellow), unwinds DNA. (B) Leading strand synthesis was performed in the presence (lane 2) or absence (lane 1) of a head-on RNAP. The mean fraction of full-length DNA (0.5, n=8) produced in the presence of a head-on RNAP is plotted with standard error (right). (C) Time course of leading strand synthesis on DNA containing a head-on RNAP. The ratio of full-length to intermediate-length DNA (IFL/II) is shown in the right plot (IFL = intensity of full-length DNA, II = intensity of intermediate-length DNA).
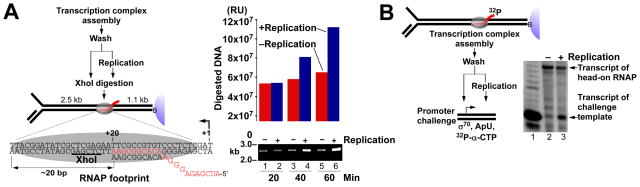
We determined whether the replisome displaces the RNAP from DNA by XhoI digestion of the promoter-proximal sequence, which is protected by RNAP (Fig. 2A). RNAP occupancy of the promoter blocks digestion, whereas RNAP displacement allows digestion. Removing ribonucleotides (NTPs) by washing prevents re-assembly of the halted RNAP. A significant increase in digestion is observed only when replication is performed (compare blue and red bars). Little RNAP displacement is detected at 20 min which is likely due to replication of only 8.5% of the DNA, probably as a result of RNAP binding to the primer-template (12) (fig. S5). Nevertheless, the large increase in digestion due to replication indicates that the replisome displaces the RNAP. Similar results are observed in the presence of GreB and limiting NTPs which inhibit RNAP backtracking (fig. S6). RNAP displacement was further investigated by monitoring transcription of a challenge template in the presence of σ70 and limiting NTPs (Fig. 2B). Transcription of the challenge template is only observed following replication which indicates that the replisome displaces the RNAP (compare lanes 2 and 3). These results are consistent with the ability of DnaB to displace a protein block during DNA unwinding (13).
Fig. 2. The replisome displaces a head-on RNAP from DNA.
(A) Replisome displacement of RNAP was probed by XhoI digestion of the promoter-proximal sequence either in the presence or absence of replication. A halted RNAP was assembled on DNA and the reaction was divided. Replication was (lanes 2, 4, 6, blue bars) or was not (lanes 1, 3, 5, red bars) initiated and aliquots were treated with XhoI at the indicated times. RU = relative units. (B) RNAP displacement in the presence (lane 3) or absence (lane 2) of replication was determined by monitoring transcription of a challenge template. Transcription of challenge template control (lane 1).
Genetic data implicate RNAP modulators such as Mfd in resolving conflicts between replication and transcription (3). Mfd displaces a halted RNAP from DNA and recruits the nucleotide excision repair machinery to the site which results in preferential repair of the transcribed strand when RNAP stalls at lesion—referred to as transcription-coupled repair (TCR)(14–18). TCR has been postulated to promote fork progression by displacing RNAP blocks from DNA (16). In Fig. 3A we examined whether Mfd facilitates restart of the fork following a head-on collision. After providing 5 min to allow the replisome to collide with RNAP, the reaction was divided and treated with either Mfd or buffer for a further 5 min. Nearly full extension of the 2.5 kb product is observed upon addition of Mfd (compare lanes 1 and 2) which removes the RNAP block (fig. S7). We determined whether Mfd mediated replication restart requires a supply of DnaB in solution (Fig. 3B). Here, the beads were washed after the collision then resuspended in buffer containing dNTPs and all the replication proteins except DnaB either in the presence or absence of Mfd. The addition of Mfd again results in extension of the 2.5 kb product, providing yet further evidence that DnaB stays bound to the stalled fork. Next, the experiment of Fig. 3A was repeated using Mfd K634N which is defective in ATP hydrolysis and can no longer dislodge RNAP (17) (Fig. 3C). Mfd K634N fails to promote extension of the 2.5 kb product (compare lanes 1 and 2). These results indicate that Mfd promotes fork progression following the collision by utilizing the energy of ATP, and hence translocase activity, to dissociate the transcription complex ahead of the stalled fork. Lastly, we demonstrate that the ability of Mfd to promote replication past a head-on RNAP is unaffected by the addition of all four NTPs and GreB which promote transcription elongation (fig. S8).
Fig. 3. Mfd promotes fork progression following a head-on collision.
Leading strand synthesis was performed on DNA containing a head-on RNAP. Reactions were divided prior to (B) or after (A,C) replication was performed for 5 min. (B) DnaB was removed from solution by washing after the collision was formed. Buffer (lanes 1) or either wild-type (A,B, lane 2) or K634N Mfd (C, lane 2) was then added for a further 5 min.
In conclusion, we find that although the replication fork stalls upon collision with a head-on transcription complex, the replisome remains stable and resumes elongation after displacing the RNAP (fig. S9, left). It is conceivable that the collision may induce RNAP backtracking. However, the lack of stimulation of RNAP endonuclease activity following the collision suggests that this is not the case (fig. S10). Moreover, the addition of GreB and NTPs, which inhibit backtracking, have no effect on our assays (fig. S6, S8, S11). A previous study of the T4 replisome reported that a head-on RNAP remains bound to the DNA, but the RNAP and transcript switch strands as the replication fork passes (19). This result is difficult to reconcile with the current view of transcription. We find that Mfd promotes direct restart of the fork following the collision by facilitating displacement of the RNAP (fig. S9, right). Co-directional collisions are resolved without auxiliary factors; the replisome uses mRNA as a primer to reinitiate leading strand synthesis after displacing a co-directional RNAP from DNA (9). Pol III extension of the RNA is not observed herein, probably due to displacement of the transcript. Genetic data suggest that recombinational repair and other RNAP modulators help resolve replisome-RNAP conflicts, thus explaining the normal growth rate of cells lacking Mfd (3). mfd cells, however, demonstrate a greater lapse in replication and cell growth following UV irradiation which supports a role for Mfd in facilitating replication through transcription complexes arrested by lesions in vivo (20). Our data demonstrate a new role for TCR in promoting replication past a RNAP block and may have implications for human disorders that result from deficient TCR, such as Cockayne Syndrome (15, 16).
Footnotes
Supporting Online Material
References
- 1.Rudolph CJ, Dhillon P, Moore T, Lloyd RG. DNA Repair (Amst) 2007 Jul 1;6:981. doi: 10.1016/j.dnarep.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Vilette D, Ehrlich SD, Michel B. Mol Gen Genet. 1996 Sep 25;252:398. doi: 10.1007/BF02173004. [DOI] [PubMed] [Google Scholar]
- 3.Trautinger BW, Jaktaji RP, Rusakova E, Lloyd RG. Mol Cell. 2005 Jul 22;19:247. doi: 10.1016/j.molcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn P, Lloyd RG. Cell. 2000 Mar 31;101:35. doi: 10.1016/S0092-8674(00)80621-2. [DOI] [PubMed] [Google Scholar]
- 5.McGlynn P, Guy CP. J Mol Biol. 2008 Aug 29;381:249. doi: 10.1016/j.jmb.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 6.Labib K, Hodgson B. EMBO Rep. 2007 Apr;8:346. doi: 10.1038/sj.embor.7400940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bidnenko V, Ehrlich SD, Michel B. Embo J. 2002 Jul 15;21:3898. doi: 10.1093/emboj/cdf369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomerantz RT, O’Donnell M. Trends Microbiol. 2007 Apr;15:156. doi: 10.1016/j.tim.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Pomerantz RT, O’Donnell M. Nature. 2008 Dec 11;456:762. doi: 10.1038/nature07527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller RC, Marians KJ. Nature. 2006 Feb 2;439:557. doi: 10.1038/nature04329. [DOI] [PubMed] [Google Scholar]
- 11.Yuzhakov A, Turner J, O’Donnell M. Cell. 1996 Sep 20;86:877. doi: 10.1016/s0092-8674(00)80163-4. [DOI] [PubMed] [Google Scholar]
- 12.Hinkle DC, Ring J, Chamberlin MJ. J Mol Biol. 1972 Sep 28;70:197. doi: 10.1016/0022-2836(72)90533-5. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan DL, O’Donnell M. Mol Cell. 2004 Aug 13;15:453. doi: 10.1016/j.molcel.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 14.Park JS, Marr MT, Roberts JW. Cell. 2002 Jun 14;109:757. doi: 10.1016/s0092-8674(02)00769-9. [DOI] [PubMed] [Google Scholar]
- 15.Hanawalt PC, Spivak G. Nat Rev Mol Cell Biol. 2008 Dec;9:958. doi: 10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- 16.Hanawalt PC. Science. 1994 Dec 23;266:1957. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- 17.Selby CP, Sancar A. J Biol Chem. 1995 Mar 3;270:4882. doi: 10.1074/jbc.270.9.4882. [DOI] [PubMed] [Google Scholar]
- 18.Deaconescu AM, et al. Cell. 2006 Feb 10;124:507. doi: 10.1016/j.cell.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Alberts BM. Science. 1995 Feb 24;267:1131. doi: 10.1126/science.7855590. [DOI] [PubMed] [Google Scholar]
- 20.George DL, Witkin EM. Mol Gen Genet. 1974;133:283. doi: 10.1007/BF00332704. [DOI] [PubMed] [Google Scholar]
- 21.We are grateful to Seth Darst for providing RNAP and Mfd proteins and expression vectors. We also thank Sergei Borukhov for providing GreB and Dan Zhang for technical support. This work was supported by a grant from the National Institutes of Health (M.O.D.) and by a Marie-Josee and Henry Kravis Fellowship at the Rockefeller University (R.T.P.).