Abstract
An efficient total synthesis of notoamide J, a new prenylated indole alkaloid and potential biosynthetic precursor, is described herein. Starting from L-proline and a substituted tryptophan derivative, this synthesis also employs an oxidation and pinacol rearrangment for the formation of the oxindole in the final step.
Introduction
Over the past decade our laboratory has extensively studied the biosynthesis of naturally occurring prenylated indole alkaloids,1 specifically the paraherquamides,2 brevianamides,3 notoamides4 and stephacidins,5 amongst others. These families of secondary metabolites have been isolated from various fungi of the genera Aspergillus and Penicillium and have exhibited a wide range of biological activity, including insecticidal, antitumor, anthelmintic and antibacterial activity.6 Their desirable biological activity, as well as the complex amino acid skeleton7 and bicyclo[2.2.2]diazaoctane ring, make these prenylated indole alkaloids attractive targets for total synthesis.8
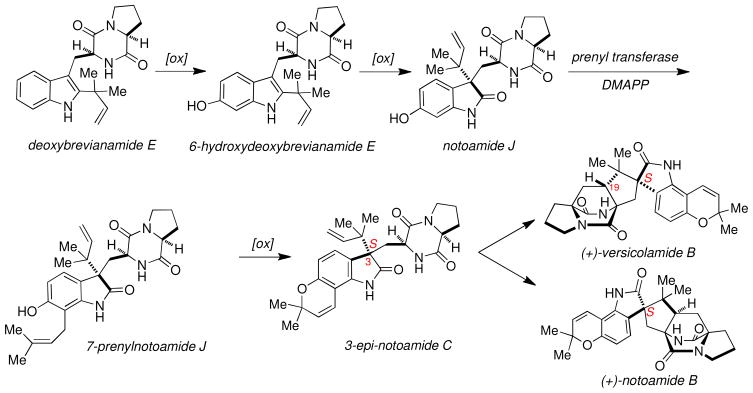
Over the past several years the number of new prenylated indole alkaloids isolated from fungi of both marine and terrestrial origin has greatly increased. Recently, Tsukamoto and co-workers isolated notoamide J (1),4b one of six novel secondary metabolites, from a marine-derived Aspergillus sp. that was collected in the Sea of Japan off the Noto Peninsula. Like the rest of the notoamides, 1 contains both tryptophan and proline, as well as an isoprene unit; however, this natural product lacks the bridged bicyclo[2.2.2]diazaoctane ring system, a distinguishing characteristic observed in many of the prenylated indole alkaloids such as the paraherquamides and brevianamides. Notoamide J also lacks the pyranoindole ring system often found in the notoamides, norgemides9 and stephacidins. However, notoamide J does contain two notable structural features, both of which may allow 1 to serve as an advanced intermediate along the biosynthetic pathway to other notoamides. First, notoamide J is only one of a handful of metabolites to contain an oxindole ring system, in which one could envision the oxindole moiety to serve as a biosynthetic precursor to the numerous natural products containing the spirooxindole ring system. Notoamide J is unique among the alkaloids in these families of natural products as it displays substitution only at the C-6 position of the indole. This feature allows one to envisage that the unsubstitued hydroxy group at this position could lead to the biosynthetic formation of the pyranoindole ring system. These two structural characteristics provide provocative hints that notoamide J could be a biosynthetic precursor to notoamide B and/or versicolamide B. A possible series of biogenetic relationships between notoamide J and the more advanced metabolites (+)-versicolamide B and (+)-notoamide B are illustrated in Scheme 1. It is reasonably assumed that notoamide J is fashioned from the common biosynthetic precursor, deoxybrevianamide E by aromatic hydroxylation at the 6-position of the indole ring. A subsequent oxidation and pinacol-type rearrangement, furnishes the oxindole notoamide J. Subsequent prenylation of notoamide J at the C-7 position yields 7-prenylnotoamide J that would serve as the key precursor for biosynthetic construction of the pyran ring system yielding 3-epi-notoamide C; a natural metabolite identified by Tsukamoto and co-workers. Final oxidation of 3-epi-notoamide C to an azadiene and intramolecular Diels- Alder cycloaddition could in principle, lead to (+)-versicolamide B and/or (+)-notoamide B. The former conversion has recently been experimentally verified by this laboratory.10
Scheme 1.
Possible Biogenetic Relationships via Notoamide J
As part of our program aimed at the elucidation of the entire notoamide/stephacidin biosynthetic pathway, we desired a synthesis of notoamide J that was readily amenable to the incorporation of stable isotopes from which probe substrates could be interrogated. We report here, the first synthesis of notoamide J which also serves to corroborate the structural assignment recently published by Tsukamoto and co-workers.
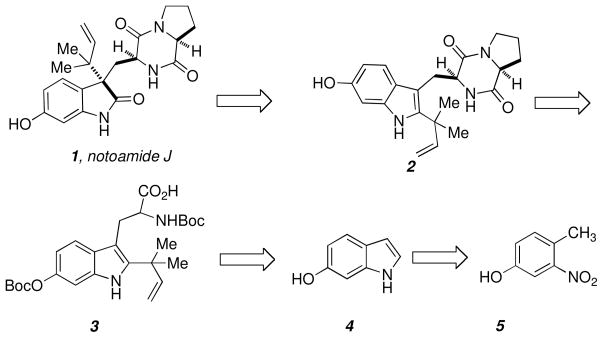
Following established chemistry deployed in our laboratory,8 we planned notoamide J arising from an oxidation and pinacol rearrangement of 2 (Scheme 2). The dioxopiperazine 2 could be obtained by coupling and cyclizing L-proline ethyl ester with the reverse prenylated tryptophan derivative 3, which we could easily obtain from 6-hydroxyindole 4. Formation of 4 could be achieved from 5 following the Leimgruber-Batcho protocol.11
Scheme 2.
Retrosynthetic plan
Results and Discussion
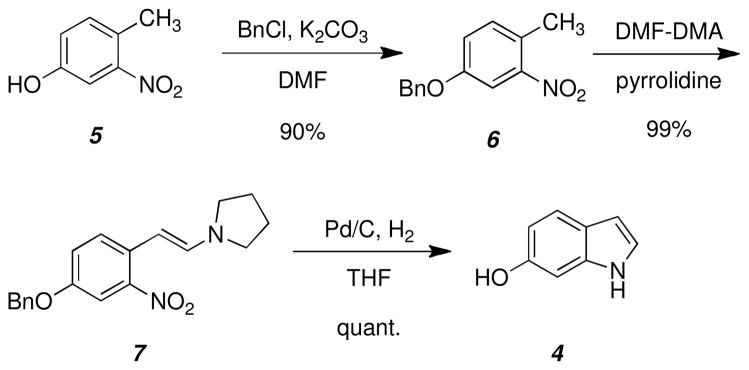
Following a modified Leimgruber-Batcho indole synthesis (Scheme 3), commercially available nitrophenol was readily converted to the desired 6-hydroxyindole. 4-Methyl-3-nitrophenol (5) was first protected as benzyl ether 6, followed by condensation of the nitrotoluene with N,N-dimethylformamide dimethyl acetal and pyrrolidine to afford 7. Reductive cyclization and benzyl deprotection using hydrogenation was achieved in one step to yield the desired 6-hydroxyindole (4).
Scheme 3.
Leimgruber-Batcho synthesis of indole 4
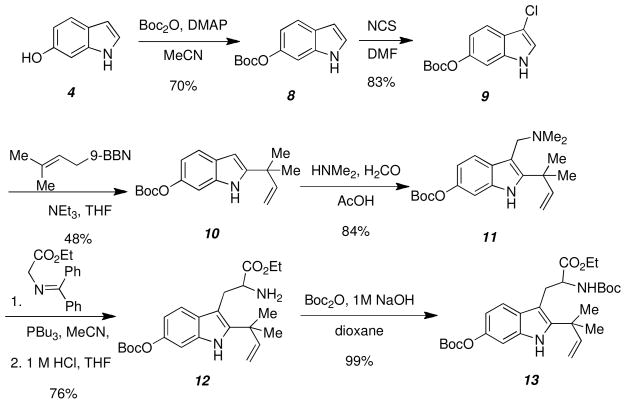
Following Boc protection of the 6-hydroxyindole (Scheme 4), formation of the C-2 reverse prenylated indole was carried out following the excellent procedure established by Danishefsky and co-workers.12 The C-3 indole position of 8 was chlorinated using NCS in DMF to afford 9, which was treated with prenyl-9-BBN in the presence of NEt3 to yield the reverse prenylated indole 10. Pure 10 was obtained in 48% yield due to the complication of removing excess 9-BBN from the reaction mixture. The gramine 11 was formed by treatment of 10 with dimethylamine and formaldehyde, which was coupled with the benzophenone imine of glycine by a modified Somei-Kametani coupling protocol.13 Imine hydrolysis afforded the tryptophan derivative 12, which was readily converted to the corresponding N-Boc carbamate 13.
Scheme 4.
Synthesis of the reverse prenylated tryptophan derivative 13
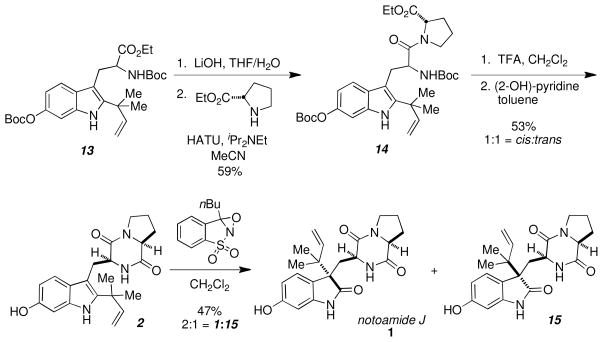
Following standard saponification conditions of 13, the N-Boc acid was achieved in good yield, which was subsequently coupled with L-proline ethyl ester in the presence of HATU and DIPEA to provide the amide 14 (Scheme 5). Treatment of 14 with TFA removed both Boc protecting groups to afford the free amine, which was subsequently cyclized with 2-hydroxypyridine to provide both the cis and trans dioxopiperazines 2 as a 1:1 ratio, which were readily separated by column chromatography.
Scheme 5.
Assembly of notoamide J
Completion of the synthesis required the treatment of the cis dioxopiperazine 2 with excess Davis oxaziridine14 to afford a 2:1 ratio of notoamide J and 3-epi-notoamide J, which were readily separable by chromatography. Synthetic 1 was identical to the natural product in all respects (1H, 13C, HRMS, CD). 4b
In summary, the first total synthesis of notoamide J was completed in fifteen steps and 0.78% overall yield. Research is currently underway to establish both the biosynthesis of notoamide J, and its role in the biosynthetic pathway of notoamide B and versicolamide B. The synthesis recorded here allows for easy access to isotopomers of notoamide J, which allow us to interrogate the potential role of this species as a potential biosynthetic precursor to more complex natural congeners. Work along these lines is in progress and will be reported on in due course.
Experimental Section
1H and 13C spectra were obtained using 300 MHz or 400 MHz spectrometers. The chemical shifts are given in parts per million (ppm) relative to TMS at δ 0.00 ppm or to residual CDCl3 δ 7.26 ppm for proton spectra and relative to CDCl3 at δ 77.23 ppm for carbon spectra. IR spectra were recorded on an FT-IR spectrometer as thin films. Mass spectra were obtained using a high/low resolution magnetic sector mass spectrometer. Flash column chromatography was performed with silica gel grade 60 (230–400 mesh). Unless otherwise noted materials were obtained from commercially available sources and used without further purification. Dichloromethane (CH2Cl2), tetrahydrofuran (THF), toluene (PhMe), N, N-dimethylformamide (DMF), acetonitrile (CH3CN), triethylamine (Et3N), and methanol (MeOH) were all degassed with argon and passed through a solvent purification system containing alumina or molecular sieves.
(E)-4-Benzyloxy-2-nitro-β-pyrrolidinostyrene 7 was synthesized by a known method established within the literature.11 Compounds 9 and 10 were synthesized via literature methods.8a
tert-Butyl 1H-indol-6-yl carbonate (8)
Enamine 7 (24.3 g, 74.9 mmol) was combined with 10% Pd/C (2.43 g) and dissolved in THF (250 mL). The reaction mixture was stirred under H2 at 40 psi for 2 hours. The reaction mixture was filtered through a pad of silica gel and the catalyst was washed with diethyl ether. The filtrate was concentrated and crude 4 was immediately taken up in acetonitrile (150 mL) and cooled to 0°C. Di-tert- butyl dicarbonate (14.7 g, 67.4 mmol) and a catalytic amount of DMAP were added and the reaction mixture stirred for 2 hours at room temperature. The reaction was concentrated and purified via flash column chromatography in 95:5 hexanes/ethyl acetate. The product was collected as a white solid in 70% yield (12.2 g). White crystalline solid (mp 141–143 OC). 1H-NMR (300 MHz, CDCl3) δ 8.16 (bs, 1H), 7.60 (d, J = 8.4 Hz, 1H), 7.21 (m, 1H), 7.18 (t, J = 5.7 Hz, 1H) 6.93 (dd, J = 8.7, 2.1 1H), 6.52 (m, 1H), 1.58 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 153.0, 147.0, 135.7, 126.0, 125.2, 121.2, 114.2, 104.0, 102.7, 83.5, 28.0; IR (neat) 3413, 2981, 1738, 1457, 1395, 883, 722 cm−1; HRMS (ESI/APCI) calcd for C13H15NO3Na (M+Na) 256.0944, found 256.0944.
tert-Butyl-3-((dimethylamino)methyl)-2-(2-methylbut-3-en-2-yl)-1H-indol-6-yl carbonate (11)
Aqueous formaldehyde (1.26 mL, 15.5 mmol) was diluted with glacial acetic acid (35 mL). Aqueous dimethylamine (6.5 mL, 58.5 mmol) was added, followed by addition of indole 10 (4.15 g, 13.7 mmol) diluted in glacial acetic acid (10 mL). The reaction stirred at room temperature for 14 hours. The reaction mixture was diluted with 1M NaOH to a pH>10. The aqueous layer was extracted with diethyl ether and the combined organic layer was dried over Na2SO4 and concentrated to afford 4.14 g (84%) of an amber oil that was taken on to the next reaction without further purification. 1H-NMR (300 MHz, CDCl3) δ 7.99 (bs, 1H), 7.62 (d, J = 8.7 Hz, 1H), 7.08 (d, J = 2.1 Hz, 1H), 6.87 (dd, J = 8.4, 2.1 Hz, 1H), 6.12 (dd, J = 17.7, 11.1 Hz, 1H), 5.16 (dd, J = 6.0, 0.9 Hz, 1H), 5.12 (d, J = 0.3 Hz, 1H), 3.55 (s, 2H), 2.19 (s, 6H), 1.56 (s, 9H), 1.53 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 152.9, 146.5, 146.3, 142.0, 133.7, 128.6, 119.7, 113.4, 112.32, 109.0, 103.1, 83.3, 54.1, 45.5, 39.6, 28.0, 27.3; IR (neat) 3385, 2933, 1734, 1466, 1142, 1013, 886 cm−1; IR (neat) 3375, 2925, 1756, 1455 cm−1; HRMS (ESI/APCI) calcd for C21H31N2O3 (M+H) 359.2329, found 359.2324.
Ethyl 2-amino-3-(6-((tert-butoxycarbonyl)oxy)-2-(2-methylbut-3-en-2-yl)-1H-indol-3-yl)propanoate (12)
Gramine 11 (4.14 g, 11.5 mmol), N-(Diphenylmethylene)glycine ethyl ester (2.80 g, 10.5 mmol), tributylphosphine ( 850 μL, 4.2 mmol) and acetonitrile (53 mL) were combined and stirred for 20 hours at reflux under Ar. The reaction was concentrated and purified via flash column chromatography in 10% ethyl acetate in hexanes to afford 2.76 g of a yellow amorphous solid, which was dissolved in THF (36 mL). 1M HCl (12.0 mL) was added and the reaction mixture stirred for 30 minutes at room temperature. The solvent was removed under reduced pressure and the residue was rediluted with saturated aqueous NaHCO3 until basic. The mixture was extracted with CH2Cl2 (x2), dried over Na2SO4 and concentrated. The crude residue was purified by flash column chromatography (3:1 hexanes/ethyl acetate; 5:95 MeOH/CH2Cl2) to give 1.51 g (76%) of 12 as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.44 (bs, 1H), 7.43 (d, J = 8.7 Hz, 1H), 7.05 (d, J = 1.8 Hz, 1H), 6.83 (dd, J = 8.7, 2.1 Hz, 1H), 6.00 (dd, J = 17.7, 10.2 Hz, 1H), 5.07–5.01 (m, 2H), 4.12–4.00 (m, 2H), 3.75 (dd, J = 9.6, 5.1 Hz, 1H), 3.22 (dd, J = 14.4, 4.8 Hz, 1H), 2.95 (dd, J = 14.4, 9.6 Hz, 1H), 1.53 (s, 9H), 1.42 (s, 6H), 1.12 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 175.6, 153.1, 146.5, 146.1, 141.6, 134.2, 127.9, 119.0, 113.3, 112.0, 106.8, 103.7, 83.4, 61.0, 56.1, 39.3, 31.3, 27.9, 27.8, 14.4, 14.2; IR (neat) 3389, 2977, 1734, 1465, 1243, 1143, 885 cm−1; HRMS (ESI/APCI) calcd for C23H33N2O5 (M+H) 417.2384, found 417.2388.
ethyl 2-((tert-butoxycarbonyl)amino)-3-(6-((tert-butoxycarbonyl)oxy)-2-(2- methylbut-3-en-2-yl)-1H-indol-3-yl)propanoate (13)
Boc2O (824 mg, 3.78 mmol) and 1M NaOH (3.60 mL, 3.60 mmol) were added to a solution of amine 12 (1.50 g, 3.60 mmol) in dioxane (18 mL). The reaction mixture stirred at room temperature for 1 hour, and then concentrated under reduced pressure to remove the dioxane. The resulting slurry was taken up in H2O, acidified to pH 2 with 1M KHSO4, and extracted with EtOAc (3 x 50 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure to afford 1.85 g of 13 as a yellow amorphous solid that was used without further purification. 1H-NMR (300 MHz, CDCl3) δ 8.11 (bs, 1H), 7.42 (d, J = 8.7 Hz, 1H), 7.06 (s, 1H), 6.86 (d, J = 8.4 Hz, 1H), 6.07 (dd, J = 17.7, 10.5 Hz, 1H), 5.17–5.06 (m, 3H), 4.49 (m, 1H), 4.05–3.90 (m, 2H), 3.28–3.12 (m, 2H), 1.55 (s, 9H), 1.52 (s, 9H), 1.32 (s, 6H), 1.02 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 173.1, 152.8, 146.7, 146.0, 141.4, 134.0, 128.0, 119.0, 113.5, 112.4, 106.1, 103.4, 83.3, 79.8, 61.4, 54.8, 39.4, 28.4, 28.0, 27.8, 27.6, 14.4, 14.0; IR (neat) 3388, 2979, 1755, 1464, 1369, 1143 cm−1; HRMS (ESI/APCI) calcd for C28H40N2O7Na (M+Na) 539.2728, found 539.2727.
(2S)-ethyl-1-(2-((tert-butoxycarbonyl)amino)-3-(6-((tert-butoxycarbonyl)oxy)-2-(2-methylbut-3-en-2-yl)-1H-indol-3-yl)propanoyl)pyrrolidine-2-carboxylate (14)
The ethyl ester tryptophan derivative 13 (1.85 g, 3.60 mmol) was dissolved in 2:1 H2O/THF (36 mL) and LiOH (826 mg, 36.0 mmol) was added. The reaction stirred at room temperature for 20 hours. The solvent was removed under reduced pressure and the resulting slurry was taken up in H2O, acidified to pH 2 with 1M KHSO4, and extracted with CH2Cl2 (2 x 50 mL) and EtOAc (2 x 50 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The resulting crude residue was dissolved in acetonitrile (30 mL) and L-proline ethyl ester (426 mg, 2.98 mmol) was added. HATU (1.70 g, 4.47 mmol) and iPr2NEt (2.0 mL, 11.9 mmol) were added and the reaction stirred for 3 hours at room temperature. The reaction was quenched with 1M HCl (40 mL) and extracted with CH2Cl2 (3 x 75 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The crude residue was purified by flash column chromatography and eluted with 3:1 hexanes/EtOAc to afford 1.08 g (59%) of 14 as a yellow amorphous solid. 1H-NMR (300 MHz, CDCl3) δ 8.35 (m, 1H), 7.48–6.74 (m, 3H), 6.04 (m, 1H), 5.52 (m, 1H), 5.15–5.06 (m, 2H), 4.62–1.98 (m, 9H), 1.51–1.12 (m, 30H); 13C NMR (75 MHz, CDCl3) δ 172.1, 171.3, 154.9, 152.7, 146.6, 145.3, 141.6, 133.6, 127.8, 119.2, 119.1, 113.4, 112.6, 106.0, 103.4, 83.2, 79.4, 61.1, 60.6, 59.3, 53.1, 46.6, 39.2, 28.5, 27.9, 27.4, 14.4, 14.3; IR (neat) 3351, 2979, 1753, 1634, 1497, 1450, 1143 cm−1; HRMS (ESI/APCI) calcd for C33H48N3O8 (M+H) 614.3436, found 614.3434.
(3S,8aS)-3-((6-hydroxy-2-(2-methylbut-3-en-2-yl)-1H-indol-3-yl)methyl)- hexahydropyrrolo[1,2-a]pyrazine-1,4-dione (2)
TFA (0.6 M) was added to a solution of 14 (1.08 g, 1.76 mmol) in CH2Cl2 (0.6 M) at 0°C. The reaction stirred for 3 hours at room temperature. The mixture was quenched with saturated NaHCO3 to a pH 10 and extracted with EtOAc (3 x 20 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The residue was dissolved in toluene (0.2 M) and 2-hydroxypyridine (31 mg, 0.328 mmol) was added. The reaction refluxed for 14 hours under Ar atmosphere, cooled to room temperature, and concentrated under reduced pressure. The residue was rediluted with CH2Cl2 (30 mL) and washed with 1M HCl (30 mL). The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The crude residue was purified via flash column chromatography eluting with 5:95 MeOH/CH2Cl2 to afford 160 mg (27%) of the desired cis diastereomer as a cream foam. The trans diastereomer was isolated in 23% yield (140 mg) as yellow foam. 1H NMR (400 MHz, 20:1 CDCl3/CD3OD) δ 8.15 (m, 1H), 7.17 (d, J = 8.4 Hz, 1H), 6.71 (d, J = 2.4 Hz, 1H), 6.58 (dd, J = 8.4, 2.0 Hz, 1H), 6.04 (dd, J = 17.6, 10.4, 1H), 5.10–5.05 (m, 2H), 4.35 (dd, J = 11.2, 2.4 Hz, 1H), 4.00 (t, J = 7.6 Hz, 1H), 3.86–3.54 (m, 3H), 3.08 (dd, J = 14.8, 11.6 Hz, 1H), 2.30–1.82 (m, 6H), 1.45–1.38 (m, 6H); 13C NMR (75 MHz, 20:1 CDCl3/CD3OD) δ 169.7, 166.3, 153.0, 146.1, 140.3, 135.9, 123.0, 118.3, 112.3, 110.0, 103.9, 96.9, 59.4, 55.1, 45.6, 39.1, 28.5, 28.0, 26.1, 25.9, 22.7; IR (neat) 3353, 2925, 1664, 1457 cm−1; HRMS (ESI/APCI) calcd for C21H26N3O3 (M+H) 368.1969, found 368.1969.
Notoamide J (1)
Davis oxaziridine (208 mg, 0.871 mmol) was added to a solution of 2 (160 mg, 0.436 mmol) in CH2Cl2 (9 mL). The reaction mixture was stirred 13 hours at room temperature and concentrated under reduced pressure. The residue was purified via flash column chromatography eluting with MeOH/CH2Cl2 (5:95) to give 52 mg (31%) of 1 as a white amorphous solid and 26 mg (15%) of 15 as a white amorphous solid. 1H-NMR (300 MHz, acetone-d6) δ 9.68 (bs, 1H), 8.59 (bs, 1H), 7.02 (d, J = 8.7 Hz, 1H), 6.49 (dd, J = 8.1, 5.7 Hz, 2H), 6.33 (bs, 1H), 6.12 (dd, J = 17.4, 10.8 Hz, 1H), 5.05 (dd, J = 16.8, 10.8 Hz, 1H), 5.04 (dd, J = 16.8, 10.8 Hz, 1H), 4.00 (t, J = 6.9 Hz, 1H), 3.49–3.34 (m, 2H), 3.28 (d, J = 9.6 Hz, 1H), 3.10 (d, J = 14.7 Hz, 1H), 2.18–2.11 (m, 1H), 2.10 (d, J = 6.6 Hz, 1H), 1.98–1.80 (m, 3H), 1.11 (s, 3H), 1.06 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 183.2, 170.4, 165.7, 157.7, 143.3, 142.9, 126.8, 120.3, 114.5, 109.2, 98.6, 59.0, 57.8, 53.0, 45.9, 42.5, 31.7, 29.9, 28.2, 22.7, 21.8; IR (neat) 3265, 2971, 1670, 1429, 1155 cm−1; HRMS (ESI/APCI) calcd for C21H25N3O4Na (M+Na) 406.1737, found 406.1739.
Supplementary Material
Acknowledgments
This work was financially supported by the National Institutes of Health (CA70375 to RMW). We wish to express our gratitude to Professor Sachiko Tsukamoto of Kumamoto University, for providing comparison spectra of natural notoamide J.
Footnotes
Supporting Information Available: Complete experimental details for the preparation of notoamide J including comparison spectra to natural material. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Williams RM. Chem Pharm Bull. 2002;50:711–740. doi: 10.1248/cpb.50.711. [DOI] [PubMed] [Google Scholar]; (b) Williams RM, Cox RJ. Acc Chem Res. 2003;36:127–139. doi: 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]; (c) Stocking EM, Sanz-Cervera JF, Williams RM. Angew Chem. 2001;113:1336–1338. doi: 10.1002/1521-3773(20010401)40:7<1296::aid-anie1296>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:1296–1298. doi: 10.1002/1521-3773(20010401)40:7<1296::aid-anie1296>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.(a) Yamazaki M, Okuyama E, Kobayashi M, Inoue H. Tetrahedron Lett. 1981;22:135–136. [Google Scholar]; (b) Ondeyka JG, Goegelman RT, Schaeffer JM, Kelemen L, Zitano L. J Antibiot. 1990;43:1375–1379. doi: 10.7164/antibiotics.43.1375. [DOI] [PubMed] [Google Scholar]; (c) Liesch JM, Wichmann CF. J Antibiot. 1990;43:1380–1386. doi: 10.7164/antibiotics.43.1380. [DOI] [PubMed] [Google Scholar]; (d) Banks RM, Blanchflower SE, Everett JR, Manger BR, Reading C. J Antibiot. 1997;50:840–846. doi: 10.7164/antibiotics.50.840. [DOI] [PubMed] [Google Scholar]
- 3.(a) Birch AJ, Wright JJ. Tetrahedron. 1970;26:2329. doi: 10.1016/s0040-4020(01)92812-1. [DOI] [PubMed] [Google Scholar]; (b) Birch AJ, Wright JJS. J Chem Soc, Chem Commun. 1969:644–645. [Google Scholar]; (c) Birch AJ, Russell RA. Tetrahedron. 1972;28:2999. [Google Scholar]
- 4.(a) Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew Chem Int Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]; (b) Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. J Nat Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]; (c) Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew Chem Int Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. J Am Chem Soc. 2009;131:3834–3835. doi: 10.1021/ja810029b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tsukamoto S, Kawabata T, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Org Lett. 2009;11:1297–1300. doi: 10.1021/ol900071c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Qian-Cutrong J, Huang S, Shu YZ, Vyas D, Fairchild C, Menendez A, Krampitz K, Dalterio R, Klohr SE, Gao Q. J Am Chem Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]; (b) Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew Chem Int Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 6.(a) Qian-Cutrong J, Krampitz KD, Shu Y-Z, Chang L-P, Lowe SE. 6,291,461 US Patent. ; (b) Blanchflower SE, Banks RM, Everett JR, Manger BR, Reading C. J Antibiot. 1991;44:492–497. doi: 10.7164/antibiotics.44.492. [DOI] [PubMed] [Google Scholar]; (c) Whyte AC, Gloer JB, Wicklow DT, Dowd PF. J Nat Prod. 1996;59:1093–1095. doi: 10.1021/np960607m. [DOI] [PubMed] [Google Scholar]
- 7.(a) Williams RM, Stocking EM, Sanz-Cervera JF. Top Curr Chem. 2000;209:98–173. [Google Scholar]; (b) Stocking EM, Sanz-Cervera JF, Unkefer CJ, Williams RM. Tetrahedron. 2001;57:5303. [Google Scholar]
- 8.(a) Grubbs AW, Artman GD, III, Williams RM. Tetrahedron Lett. 2005;46:9013–9016. [Google Scholar]; (b) Grubbs AW, Artman GD, III, Tsukamoto S, Williams RM. Angew Chem Int Ed. 2007;46:2257–2261. doi: 10.1002/anie.200604377. [DOI] [PubMed] [Google Scholar]; (c) Miller KA, Welch TR, Greshock TJ, Ding Y, Sherman DH, Williams RM. J Org Chem. 2008;73:3116–3119. doi: 10.1021/jo800116y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The structures of the norgeamides were published via the internet detailing the research performed by the Hans-Knöll Institute. See: http://www.hki-jena.de/index.php
- 10.Miller KA, Tsukamoto S, Williams RM. Nature Chemistry. 2009;1:63–68. doi: 10.1038/nchem.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batcho AD, Leimgruber W. Org Synth. 1985;63:214. [Google Scholar]
- 12.Schkeryantz JM, Woo JCG, Siliphaivanh P, Depew KM, Danishefsky SJ. J Am Chem Soc. 1999;121:11964. [Google Scholar]
- 13.(a) Somei M, Karasawa Y, Kaneko C. Heterocycles. 1981;16:941. [Google Scholar]; (b) Kametani T, Kanaya N, Ihara M. J Chem Soc, Perkin Trans 1. 1981:959. [Google Scholar]
- 14.(a) Davis FA, Townson JC, Vashi DB, Thimma Reddy R, McCauley JP, Harakal ME, Gosciniak D. J Org Chem. 1990;55:1254–1261. [Google Scholar]; (b) Snider BB, Zeng H. J Org Chem. 2003;68:545–563. doi: 10.1021/jo0264980. [DOI] [PubMed] [Google Scholar]
- 15.Kramer GW, Brown HC. J Organomet Chem. 1977;132:9–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.