STRUCTURED ABSTRACT
Objectives
We evaluated whether carotid intima-media thickness (C-IMT) and the presence or absence of plaque improved coronary heart disease (CHD) risk prediction when added to traditional risk factors (TRF).
Background
Traditional CHD risk prediction schemes need further improvement as the majority of the CHD events occur in the “low” and “intermediate” risk groups. C-IMT and presence of plaque on an ultrasound are associated with CHD and therefore could potentially help improve CHD risk prediction.
Methods
Risk prediction models (overall, in men and women) considered included TRF-only, TRF+C-IMT, TRF+plaque, and TRF+C-IMT+ plaque. Model predictivity was determined by calculating the area under the receiver operating characteristic curve (AUC) adjusted for optimism. Cox-proportional hazards models were used to estimate 10-year CHD risk for each model, and the number of individuals reclassified determined. Observed events were compared with expected events; and, the net reclassification index (NRI) was calculated.
Results
Of 13,145 eligible individuals (5,682 men; 7,463 women), ~23% were reclassified by adding C-IMT+plaque information. Overall, the addition of C-IMT and plaque separately or together to the TRF model improved the AUC which increased from 0.742 to 0.750, 0.751 and 0.755 for the TRF-only, TRF+C-IMT, TRF+plaque and TRF+C-IMT+plaque model respectively. The C-IMT+TRF+plaque model had a NRI of 9.9% when compared to TRF-only in the overall population. However, comparison of TRF+C-IMT+plaque with TRF+C-IMT or TRF+plaque only resulted in non-significant or modestly significant changes of the various statistical tests. Sex-specific analyses are presented in the manuscript.
Conclusion
Adding plaque and C-IMT to TRF improves CHD risk prediction in the ARIC study.
Keywords: C-IMT, plaque, risk prediction
INTRODUCTION
Traditional risk prediction scores such as the Framingham risk score have proven very useful in identifying individuals at risk for coronary heart disease (CHD), but such risk scores have limitations. Biomarkers, imaging and genotypes are being examined to try to improve CHD risk prediction (1–6).
Carotid intima-media thickness (C-IMT) is a well-described surrogate marker for cardiovascular disease, and increased C-IMT has been associated with prevalent and incident CHD and stroke (7,8). Further, statins, which reduce major adverse cardiovascular events (9) have been shown to stabilize and regress C-IMT. Although reports (3,4) have suggested that adding C-IMT, by improving the area under the receiver operating characteristics curve, can improve risk prediction from a clinical decision-making standpoint, the ability of a marker to reclassify an individual’s risk group is critical (10).
Furthermore, plaque presence, which has been shown to be associated with CHD independent of C-IMT measurements in several studies, (11) has not been adequately evaluated in risk classification especially using contemporary criteria for evaluating novel cardiovascular risk markers (12). We investigated whether C-IMT and information about the presence or absence of plaque improves CHD risk prediction in the ARIC study.
METHODS
Subjects
The ARIC study is an epidemiologic study of cardiovascular disease incidence which recruited a population-based cohort of 15,792 individuals aged between 45–64 years from 4 U.S. communities between 1987 and 1989. A complete description of the study design, objectives and sampling strategy have been previously described (13). For this analysis, we excluded individuals with prevalent CHD or prevalent stroke (n= 763), missing prevalent CHD data (n= 339), missing C-IMT or plaque data (n= 909), missing information on traditional CHD risk factors (TRF) (n = 533), races other than “black” or “white” (n=48) and black participants from the Minnesota or Washington field center (n=55) which provided us with a sample of 13,145 individuals for the analysis.
Ultrasound measurement
The ultrasound procedure in the ARIC study has been previously described (14–17). Briefly, a Biosound 2000IISA system was used and images recorded on a VHS tape. C-IMT was measured centrally by trained readers at the ARIC Ultrasound Reading Center. The C-IMT was assessed in 3 segments: the distal common carotid (1 cm proximal to dilation of the carotid bulb), the carotid artery bifurcation (1 cm proximal to the flow divider) and the proximal internal carotid arteries (1 cm section of the internal carotid artery immediately distal to the flow divider). At each of these segments, 11 measurements of the far wall (in 1 mm increments) were attempted. The mean of the mean measurements across these segments of both right and the left sides were estimated. Trained readers adjudicated plaque presence or absence if 2 of the following 3 criteria were met: abnormal wall thickness (defined as C-IMT >1.5mm), abnormal shape (protrusion into the lumen, loss of alignment with adjacent arterial wall boundary), and abnormal wall texture (brighter echoes than adjacent boundaries) (11,15). The reproducibility and variation of C-IMT and plaque measurements in the ARIC study have been previously published (15,18). The site-specific reliability coefficients was estimated as 0.77, 0.73 and 0.70 for the mean carotid far wall IMT at the carotid bifurcation, internal carotid arteries, and common carotid arteries, respectively. For the presence or absence of plaque, the intra-reader agreement was associated with a κ statistic of 0.76, while the inter-reader agreement was 0.56, which suggests good agreement beyond chance.
Ascertainment of incident CHD events
Incident CHD events included definite or probable myocardial infarction (MI), silent MI between examinations indicated by electrocardiograms, definite CHD death, or coronary revascularization. The methods by which the incident CHD events were ascertained and classified and the details of quality assurance have been previously published (19). Briefly, participants were contacted annually, and discharge lists from local hospitals and death certificates were surveyed to look for incident CHD events. Follow-up for this analysis was until December 31st 2005.
Statistical analysis
The analyses were performed in the entire study sample and then by gender. The ARIC coronary risk score (ACRS) developed by Chambless et al (4) in the ARIC cohort, is similar to the Framingham risk score and includes age, age2, sex, systolic blood pressure, antihypertensive medication use, total cholesterol, high-density lipoprotein cholesterol (HDL-C), gender, diabetes, and smoking status. The ACRS variables were used in the “TRF-only” risk prediction model in our analysis as it would represent the best TRF-based model in the ARIC study for CHD prediction. However, we also evaluated adding C-IMT and plaque to a Framingham risk score (FRS)-based TRF model since the FRS is traditionally used by most clinicians.
Several models were considered: 1) TRF+(sex-specific) C-IMT, categorized as <25th percentile, 25–75th percentile, >75th percentile; 2) TRF+plaque; and, 3) TRF+C-IMT (sex-specific and categorized as previously stated)+plaque. We described the area under the receiver operator characteristic curve (AUC) for 10-year risk using methods which accounted for censoring (20) for each of the models to describe the model predictivity. Bootstrapping was performed to obtain confidence intervals for the differences in adjusted AUC between the models and to adjust for the over-optimism that can occur when the fit of the model is tested using the same data in which it was described (21–23).
Using Cox-proportional hazards, the 10-year CHD risk for each of the models was calculated, and individuals classified into 0–5% (“low” risk), 5–10% (“low-intermediate” risk), 10–20% (“intermediate-high” risk) and >20% (“high”) risk. The number of individuals who changed risk groups (i.e., reclassified after adding C-IMT and plaque data) was then described. To test the model calibration, we compared the “goodness-of-fit” of the observed and expected number of events within estimated risk decile groups using the Grønnesby-Borgan statistic (24). Large values of the test statistic (i.e., significant ‘p’ values) suggest poor model fit. We then calculated the net reclassification index (NRI) which examines the net effect of adding a marker to the risk prediction scheme using a statistic described by Pencina et al (25) except estimated by a method accounting for censoring (Personal Communication). We also described the “clinical NRI” or the NRI in the groups defined as intermediate (5–10% and 10–20% estimated CHD risk based on the model prior to reclassification) in risk (i.e., the groups in which the addition of a marker may be of most use). Finally, we also estimated the “integrated discrimination improvement” (IDI) (25) (again accounting for censoring) which is the difference in an R2-like statistic between the traditional and expanded models. AUC, NRI, and IDI were calculated for 10-year follow-up and confidence intervals were furnished by bootstraping.
RESULTS
The study sample’s baseline characteristics are listed in Table 1. The 25th and 75th percentile C-IMT of the 5,682 men and 7,463 women (13,145 total individuals) were 0.65 mm and 0.84 mm for men and 0.58 mm and 0.74 mm for women, respectively. Atherosclerotic plaque presence increased from 13.6% in the overall population with a C-IMT<25th percentile (17.4% in men, 10.7% in women), to 26.2% in those with a C-IMT between 25–75th percentile (33.5% for men and 20.7% for women), and to 65.3% in those with a C-IMT > 75th percentile (73.1% in men and 59.5% in women) respectively. When evaluated by risk groups, plaque prevalence increased from 24% in the 0–5% risk group to 34.3% in the 5–10%, 46.5% in the 10–20% and 54.6% in the >20%, 10-year CHD (high) risk groups, respectively.
Table 1.
Baseline characteristics [means (SD) or prevalence %] after exclusions: ARIC study, 1987–89
| Variable | Men (n= 5,682) |
Women (n= 7,463) |
Entire Sample (n=13,145) |
|---|---|---|---|
| Age (years) | 54.42 (5.8) | 53.75 (5.7) | 54.0 (5.8) |
| Body mass index (kg/m2) | 27.23 (4.0) | 27.46 (5.8) | 27.36 (5.1) |
| Systolic blood pressure (mmHg) |
122.1 (17.7) | 119.7 (19.1) | 120.72 (18.6) |
| Diastolic blood pressure (mmHg) |
75.5 (11.2) | 71.9 (10.9) | 73.46 (11.2) |
| Total cholesterol (mg/dl) | 210.2 (39.4) | 217.0 (42.1) | 214.0 (41.1) |
| Triglycerides (mg/dl) | 130.4 (67.0) | 117.1 (60.5) | 122.9 (63.7) |
| High density lipoprotein cholesterol (mg/dl) |
45.3 (13.9) | 58.2 (17.2) | 52.6 (17.1) |
| Low density lipoprotein cholesterol (mg/dl) |
138.8 (37.2) | 135.4 (40.2) | 136.8 (39.0) |
| C-IMT 25th percentile (unadjusted) (mm) |
0.65 | 0.58 | 0.61 |
| C-IMT 75th percentile (unadjusted) (mm) |
0.84 | 0.74 | 0.78 |
| Fasting Glucose (md/dl) | 106.3 (28.0) | 104.1 (32.6) | 105.0 (30.7) |
| Whites (%) | 77.7% | 72.6% | 74.8% |
| Diabetes (%) | 10.3% | 10.0% | 10.1% |
| Current tobacco use (%) | 27.6% | 25.0% | 26.1% |
| Former tobacco use (%) | 43.2% | 22.48% | 31.5% |
| Cholesterol Lowering Medication Use (%) | 2.3% | 2.6% | 2.4% |
| Aspirin Use (%) | 41.1% | 49.4% | 45.8% |
| Statin Use (%) | 0.3% | 0.6% | 0.5% |
Over a mean follow-up period of 15.1 years (men=14.4 years, women=15.7 years), there were 1,812 incident CHD events (867 definite or probable MI’s, 159 CHD deaths, 688 coronary revascularizations, and 98 silent [ECG-confirmed] MI’s).
When examining the AUC, adding C-IMT and/or plaque information (individually and together) to TRF improved the AUC significantly (even after adjustment for optimism) in both men and women, except that adding C-IMT alone in women was not significant (Table 2). Adding plaque to TRF had a more pronounced effect than adding C-IMT to TRF on the AUC in women. In women, the AUC increased from 0.759 (TRF alone) to 0.762 (95% confidence interval [CI] for the difference in adjusted AUC, −0.002, 0.006) when C-IMT was added to TRF while the AUC increased to 0.770 (95% CI for the difference in adjusted AUC, 0.005, 0.016) for plaque alone + TRF. The TRF + C-IMT + plaque model was associated with a similar AUC of 0.770 (0.005, 0.017). On the other hand, adding C-IMT had a more pronounced effect than adding plaque to TRF on the AUC in men. In men, the AUC increased from 0.674 (TRF alone) to 0.690 (95% CI for the difference in adjusted AUC 0.009, 0.022) when C-IMT was added to TRF while the AUC increased to 0.686 (95% CI 0.005, 0.017) for plaque alone+TRF. The TRF+C-IMT+plaque model was associated with the most increase in AUC which increased to 0.694 (95% C.I. 0.011, 0.027). When we considered the addition of plaque to a model that included TRF+C-IMT, it significantly improved the AUC in women by 0.009 (95% CI 0.003, 0.012), while in men, the increase in AUC by 0.004 (95% CI −0.001, 0.006) was non-significant. On the other hand, when we considered the addition of C-IMT to a model that included TRF+plaque, it improved the AUC in men by 0.008 (95% CI 0.002, 0.011), while in women, the increase in AUC by 0.000 (95% CI −0.002, 0.002) was non-significant.
Table 2.
Adjusted area under the curve (AUC) for different models with confidence intervals for the difference in adjusted AUC
| Model | Overall | Men | Women |
|---|---|---|---|
| AUC for the various models and 95% CI for difference in adjusted AUC comparing the various models with TRF only model | |||
| TRF only | 0.742 | 0.674 | 0.759 |
| TRF+C-IMT | 0.750 (0.005, 0.012) | 0.690 (0.009, 0.022) | 0.762 (−0.002, 0.006) |
| TRF+Plaque | 0.751 (0.006, 0.013) | 0.686 (0.005, 0.017) | 0.770 (0.005, 0.016) |
| TRF+C-IMT+ Plaque |
0.755 (0.008, 0.017) | 0.694 (0.011, 0.027) | 0.770 (0.005, 0.017) |
| TRF+IMT+Plaque vs. TRF+IMT | |||
| (0.001, 0.006) | (−0.001, 0.006) | (0.003, 0.012) | |
| TRF+IMT+Plaque vs. TRF+Plaque | |||
| (0.001, 0.005) | (0.002, 0.011) | (−0.002, 0.002) | |
TRF= traditional risk factors
C-IMT= carotid intima media thickness
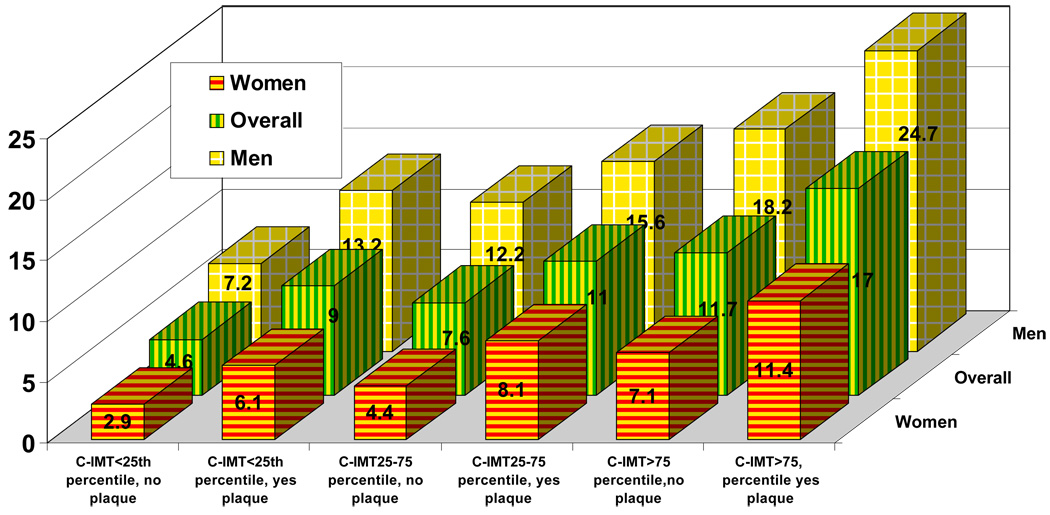
The CHD incidence rate per 1,000 person years in the various C-IMT categories taking into account the presence or absence of plaque is described in Figure 1. In all C-IMT categories, the presence of plaque was associated with a higher incidence of CHD events.
Figure 1.
Adjusted coronary heart disease incidence rate per 1,000 person year adjusted by C-IMT categories (<25th percentile, 25–75th percentile and >75th percentile) with and without plaque
- The figure shows that, at every C-IMT category (i.e., <25th percentile, 25–75th percentile and >75th percentile), for the overall group, men or women, having carotid artery plaque is associated with higher incidence of coronary heart disease.
Adding plaque information along with C-IMT to TRF resulted in the reclassification of 8.6%, 37.5%, 38.3% and 21.5% of the overall sample in the <5%, 5–10%, 10–20% and >20% 10-year estimated risk groups, respectively (Table 3A), while adding plaque and C-IMT reclassified 17.4%, 32.8%, 36.6% and 25.2% of the men (Table 3B) and 5.1%, 40.2%, 38.4% and 24.9% of the women (Table 3C) in the same risk groups. Overall, more individuals were reclassified to a lower risk group (~12.4%) than to a higher risk group (~10.8%), and nobody was reclassified from the low risk group (<5% estimated 10-year CHD risk) to the high risk (>20%, 10-year estimated CHD risk) or vice versa.
| Table 3A: Number and percent reclassified in CHD risk categories and observed CHD risk* when C-IMT and plaque information are added to traditional risk prediction models (overall sample) | |||||
|---|---|---|---|---|---|
| CHD risk by TRF only | CHD risk by TRF + C-IMT + plaque |
||||
| <5% | 5–10% | 10–20% | >20% | All | |
| Number % KM 10-year risk(%) |
Number % KM 10- year risk (%) |
Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
|
| ≤5%, Low risk, n | 5585 | 523 | 0 | 0 | 6108 |
| 91.4 | 8.6 | 0.00 | 0.00 | 46.5 | |
| 2 | 5 | . | . | 2 | |
| 5–10%, Low-intermediate risk, n | 839 | 2340 | 563 | 0 | 3742 |
| 22.4 | 62.5 | 15.1 | 0.00 | 28.5 | |
| 5 | 7 | 17 | . | 8 | |
| 10–20% High-intermediate risk, n | 0 | 627 | 1560 | 340 | 2527 |
| 0.00 | 24.8 | 61.7 | 13.5 | 19.2 | |
| . | 11 | 15 | 24 | 15 | |
| >20% High risk, n | 0 | 0 | 165 | 603 | 768 |
| 0.00 | 0.00 | 21.5 | 78.5 | 5.8 | |
| . | . | 14 | 31 | 27 | |
| All, n | 6264 | 3490 | 2288 | 943 | 13145 |
| 48.9 | 26.6 | 17.4 | 7.2 | 100.0 | |
| 2 | 7 | 15 | 28 | 7 | |
| Table 3B: Number and percent reclassified in CHD risk categories and observed CHD risk* when C-IMT and plaque information are added to traditional risk prediction models (men) | |||||
|---|---|---|---|---|---|
| CHD risk by TRF only | CHD risk by TRF + C-IMT + plaque |
||||
| <5% | 5–10% | 10–20% | >20% | All | |
| Number % KM 10 year risk(%) |
Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
|
| ≤5%, Low risk, n | 563 | 119 | 0 | 0 | 682 |
| 82.6 | 17.5 | 0.00 | 0.00 | 12.0 | |
| 2 | 2 | . | . | 2 | |
| 5–10%, Low-intermediate risk, n | 338 | 1419 | 355 | 0 | 2112 |
| 16.0 | 67.2 | 16.8 | 0.00 | 37.2 | |
| 5 | 7 | 13 | . | 8 | |
| 10–20% High-intermediate risk, n | 0 | 526 | 1413 | 290 | 2229 |
| 0.00 | 23.6 | 63.4 | 13.0 | 39.2 | |
| . | 11 | 14 | 24 | 15 | |
| >20% High risk, n | 0 | 0 | 166 | 493 | 659 |
| 0.00 | 0.00 | 25.2 | 74.8 | 11.6 | |
| . | . | 16 | 31 | 27 | |
| All, n | 901 | 2064 | 1934 | 783 | 5682 |
| 15.9 | 36.3 | 34.0 | 13.8 | 100.0 | |
| 3 | 8 | 14 | 28 | 12 | |
| Table 3C: Number and percent reclassified in CHD risk categories and observed CHD risk* when C-IMT and plaque information are added to traditional risk prediction models (women) | |||||
|---|---|---|---|---|---|
| CHD risk by TRF only | CHD risk by TRF + C-IMT + plaque |
||||
| <5% | 5–10% | 10–20% | >20% | All | |
| Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
Number % KM 10-year risk (%) |
|
| ≤5%, Low risk, n | 5305 | 287 | 0 | 0 | 5592 |
| 94.9 | 5.1 | 0.00 | 0.00 | 74.9 | |
| 2 | 6 | . | . | 2 | |
| 5–10%, Low-intermediate risk, n | 316 | 704 | 157 | 0 | 1177 |
| 26.9 | 59.8 | 13.3 | 0.00 | 15.8 | |
| 5 | 9 | 12 | . | 8 | |
| 10–20% High-intermediate risk, n | 0 | 132 | 321 | 68 | 521 |
| 0.00 | 25.3 | 61.6 | 13.1 | 7.0 | |
| . | 6 | 14 | 32 | 14 | |
| >20% High risk, n | 0 | 0 | 43 | 130 | 173 |
| 0.00 | 0.00 | 24.9 | 75.1 | 2.3 | |
| . | . | 8 | 37 | 30 | |
| All, n | 5621 | 1123 | 521 | 198 | 7463 |
| 75.3 | 15.1 | 7.0 | 2.7 | 100.0 | |
| 2 | 8 | 3 | 35 | 4 | |
All observed risks have been interpolated to 10-year event rates by Kaplan Meier (K-M) risk estimates using the actual observed events over a mean follow up of 15.1 years
All observed risks have been interpolated to 10-year event rates by Kaplan Meier (K-M) risk estimates using the actual observed events over a mean follow up of 14.4 years
All observed risks have been interpolated to 10-year event rates by Kaplan Meier (K-M) risk estimates using the actual observed events over a mean follow up of 15.7 years
We then examined the goodness-of-fit of the various models using the Grønnesby-Borgan statistic. When the overall population was considered, although model fit improved with the addition of C-IMT and/or plaque, none of the models had a good fit with the Chi-square statistic (p-value) being 30.0 (p=0.0004), 23.7 (p=0.005) and 24.3 (p=0.004) for the TRF only model, TRF + C-IMT model and TRF + C-IMT + plaque model, respectively. When men and women were considered separately, the model fit improved. In men, the C-IMT + TRF model was the best fit (Chi-square statistic=14.12, p=0.11), while the C-IMT+TRF+plaque model and TRF-only model were not as good fits (Chi-square statistic [p-values] = 17.9 [p=0.04] and 18.7 [p=0.028], respectively). On the other hand, in women, the Chi-square test statistic (p values) were 15.0 (p=0.09), 9.1 (p=0.43) and 8.7 (p=0.47) for the TRF only, TRF + C-IMT and TRF + C-IMT + plaque models, respectively, which suggested that the TRF+C-IMT+plaque model had the best model fit.
Finally, we examined the NRI and the clinical NRI (NRI in the intermediate groups). We compared several models (Table 4) and found that the TRF+C-IMT+plaque model was better than the TRF-only model in the overall sample, in men, and in women. However, adding plaque data minimally affected the TRF+C-IMT model in men, while adding C-IMT information minimally affected the TRF+plaque model in women. Overall, the TRF+C-IMT+plaque model when compared to the TRF-only model was associated with significant NRI’s of 9.9% (clinical NRI 21.7%) in the overall sample, 8.9% (clinical NRI 16.4%) in men and 9.8% (clinical NRI 25.4%) in women. In the overall sample, adding C-IMT or plaque individually to TRF was associated with a significant NRI of ~7.1–7.7% while adding the second variable (i.e. plaque to a TRF+C-IMT model or C-IMT to a TRF+plaque model) non-significantly increased the NRI by about 2–3%. The IDI showed that the model predictivity was significantly improved by adding C-IMT and plaque to TRF: in the overall population the IDI was 0.011, while in women, it was 0.009; and, in men, 0.013 (Supplemental Table).
Table 4.
Net reclassification index using various comparison models in the overall sample, men and women
| Model | Overall | Men | Women | |||
|---|---|---|---|---|---|---|
| NRI (%) | Clinical NRI (%) |
NRI (%) |
Clinical NRI (%) |
NRI (%) |
Clinical NRI (%) |
|
| TRF vs. TRF+CIMT (95% CI) |
7.1 (2.2, 10.6) |
16.7 (9.3, 22.4) |
8.9 (3.4, 15.1) |
15.8 (8.6, 24.6) |
6.1 (−2.3, 9.4) |
15.9 (1, 23.3) |
| TRF vs. TRF+plaque (95% CI) |
7.7 (2.3, 11.4) |
17.7 (10.9, 24.7) |
4.2 (0.2, 12.2) |
10.5 (4.5, 20.5) |
10.2 (0.7, 15.4) |
25.6 (7.8, 37.6) |
| TRF vs. TRF+CIMT+plaque (95% CI) |
9.9 (3.8, 13.5) |
21.7 (13.4, 28.2) |
8.9 (4.1, 17.1) |
16.4 (9.5, 27) |
9.8 (1.1, 15.4) |
25.4 (9, 37) |
| TRF+CIMT vs. TRF+CIMT+ plaque (95% CI) |
2.8 (−1.2, 6.4) |
10.6 (3.8, 16.5) |
0.03 (−2.6, 6.3) |
5.1 (0.3, 13.2) |
3.6 (−1.7, 11.6) |
12.8 (2.5, 28.6) |
| TRF+plaque vs. TRF +CIMT+ plaque (95% CI) |
2.1 (−1.1, 5.3) |
7.9 (2.6, 13.3) |
4.8 (−0, 10) |
10.7 (4.3, 19) |
−0.3 (−3.7, 3.6) |
2.5 (−3.5, 10.3) |
When we added C-IMT and plaque information to a Framingham risk score (FRS)-based TRF model, the results were similar. The adjusted AUC in men and women using the FRS model alone were 0.661 and 0.741, respectively, and improved to 0.685 (95% confidence interval [CI] for the difference in adjusted AUC, 0.014, 0.032) and 0.751 (95% confidence interval [CI] for the difference in adjusted AUC, 0.003, 0.016) respectively by adding C-IMT and plaque. In men, 11.5%, 34%, 37.9% and 32% of those in the <5%, 5–10%, 10–20% and >20% FRS categories respectively were reclassified by adding C-IMT and plaque, resulting in a NRI of 12.7% and a clinical NRI of 18.9%. However, in women, 6.6%, 41%, 39.8% and 36.3% of those in the <5%, 5–10%, 10–20% and >20% FRS categories respectively were reclassified, resulting in a NRI of 7.7% and a clinical NRI of 21.2%. Finally, when the goodness-of-fit was tested using the Grønnesby-Borgan test statistic, the model with FRS+C-IMT+plaque was better than the FRS-only model in both men (Chi-square statistic for FRS only = 15.05, p=0.09, Chi-square statistic for FRS+C-IMT+plaque =10.18, p=0.34) and women (Chi-square statistic for FRS only = 8.63, p=0.47, Chi-square statistic for FRS+C-IMT+plaque =4.97, p=0.84).
DISCUSSION
Although CHD risk prediction models based on “traditional risk factors” have formed the basis for the clinical practice of CHD prevention they are far from optimal (26). Several efforts have looked at adding biomarkers to improve cardiovascular risk prediction (1,2), and other recent efforts have examined the use of genetic markers as well (5). Of these, hs-CRP has shown the most promise.
Imaging tests such as carotid artery ultrasound and coronary calcium score offer another marker that could be used in improving CHD risk prediction by directly visualizing atherosclerosis. Although several efforts have examined the use of these imaging modalities, there is limited data using contemporary statistical methodology that have evaluated whether the addition of imaging markers to risk models can improve risk prediction. Furthermore, most of the studies examining C-IMT have had limited CHD events in follow up and did not utilize information about plaque presence or absence.
We now show that, in the 13,145 ARIC participants followed for ~15 years, using C-IMT and plaque information can improve CHD risk prediction. Adding C-IMT and plaque information resulted in the reclassification of ~23% of the individuals with a net reclassification improvement of ~9.9%. However, it must be noted that more individuals were reclassified to a lower risk group than to a higher risk group. Almost 61.9% of those reclassified from the intermediate risk group (5–20% estimated 10 year CHD risk) were reclassified to lower risk. Furthermore, nobody from the low-risk group was reclassified to a high-risk group, and nobody from the high-risk group was reclassified to the low-risk group.
Plaque presence seemed to have a more profound effect in improving risk prediction in women than men and it is not completely clear why. There are likely several possible explanations: One possible explanation is that perhaps, since middle-aged women have a relatively low prevalence of atherosclerosis, plaque presence, which reflects a definite area of atherosclerosis, was more powerful than using a sex-specific percentile “thickness” (C-IMT). Similarly, given the overall lower prevalence of atherosclerosis in women, it is possible that a C-IMT >75th percentile misclassifies individuals without atherosclerosis as higher risk, and a specific C-IMT cutpoint may be better in women. However, it is clear that when one considers the intermediate risk groups, the groups in which one would advocate further risk stratification, adding plaque and C-IMT data best improved risk prediction in men and women.
Overall, the NRI and clinical NRI (9.9% and 21.7% respectively, in the overall sample population when the TRF+C-IMT+plaque model was compared to the TRF-only model) was similar to other recent strategies that have been used in improving risk prediction (2,25).
Coronary calcium score is another imaging test used in clinical practice to identify higher risk individuals. A recent study reported that coronary calcium score was a better predictor of incident cardiovascular events, especially CHD events, when compared to C-IMT (6). However, this study did not consider plaque presence or absence. Furthermore, the overall number of incident cardiovascular disease events was only 222, included angina, and the follow-up was shorter. Other reports comparing the two modalities have yielded mixed results (27,28). Hence, a more long term comparison of coronary calcium scores with C-IMT+plaque in the prediction of cardiovascular risk will be instructive. In addition, several other factors including cost-effectiveness and safety and feasibility of testing will all need to be considered in identifying the role these imaging tests may have in risk stratification.
Finally, although current guidelines (29) suggest that individuals with a 0–10% predicted 10-year risk should be considered “low” in risk, reports suggest that there is a spectrum of risk in the 0–10% risk group, and therefore 5–20% 10-year estimated risk should be considered the “intermediate” risk group (1,30,31). Therefore, we divided the 0–10% risk group into 0–5% (low risk group) and 5–10% estimated (low-intermediate risk) risk groups. Plaque prevalence was almost ~10% higher in the 5–10% risk group (34% prevalence) when compared to the 0–5% predicted risk group (24% prevalence).
In summary, our data suggests that: 1. Adding C-IMT and/or plaque information individually and together to TRF improves CHD risk prediction; 2. Adding both C-IMT and plaque provides the most improvement but adding the second variable (i.e. C-IMT to a TRF+plaque model or plaque to a TRF+C-IMT model) results in minimal/modest improvements only; 3.As with other markers, the addition of C-IMT/plaque maybe most valuable in the intermediate-risk groups. Overall the improvement in risk prediction may be equivalent to other contemporary markers.
In the future, further improvement in our ability to stratify CHD risk may be possible through reliable quantification of plaque volume since the mere presence of plaque without any quantification helped improve overall CHD risk prediction in our analysis.
The strengths of our study include the use of contemporary statistical methodology (12), the long follow up and the number of incident CHD events accrued over the time period. Furthermore, we examined the ability of C-IMT and plaque to improve risk prediction when added to both the ACRS- and FRS-based TRF models. Finally, diabetes is included in the ACRS-based TRF model; although this is considered a CHD risk equivalent, we chose to include diabetes in the model in order for us to evaluate whether adding C-IMT and plaque can improve the best CHD prediction model in the ARIC study.
Limitations
We used data from the baseline ARIC visit for this analysis. We have not accounted for changes in the risk factors over the time period of this analysis or changes in the medications during this time period. However, this is similar to any risk prediction scheme that has been described. Recent data (32) suggests that persons with an increased lifetime risk may have a higher burden of sub-clinical atherosclerosis. We did not consider “lifetime risk,” but adding C-IMT and plaque data helped to better identify those at short term risk and hence, may have additional value over the estimation of lifetime risk. We did not account for the potential difference between plaque presence in one artery alone versus multiple arteries. It is possible that plaque presence in multiple carotid artery segments may be associated with a higher risk. Several individuals (n=909) had missing C-IMT data, and we do not know how their presence in the study would have impacted the results. Finally, at this time, there is no clinical study evidence that shows whether treating individuals by this strategy based on the identification of “higher” risk will prevent incident cardiovascular events; although, one would expect this to be the case.
Conclusion
Carotid ultrasound based–C-IMT measurement and identification of plaque presence or absence improves CHD risk prediction in the ARIC study and should be considered in the intermediate risk (5–20% estimated 10-year CHD risk) group. Ultrasound-based risk stratification strategies should be tested in clinical trials to evaluate whether improved prevention of cardiovascular events is possible.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions and Joanna Brooks, BA for editorial assistance.
Financial Support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022 from the National Heart, Lung, and Blood Institute (NHLBI), Bethesda, Md.
Abbreviations list
- CHD
coronary heart disease
- C-IMT
carotid intima-media thickness
- ARIC
Atherosclerosis Risk in Communities
- TRF
traditional risk factors
- MI
myocardial infarction
- ACRS
ARIC coronary risk score
- HDL-C
high-density lipoprotein cholesterol
- AUC
area under the receiver operator characteristic curve
- NRI
net reclassification index
- IDI
integrated discrimination improvement
Footnotes
Relationship with industry:
Vijay Nambi: Research collaboration with General Electric
Christie M. Ballantyne: Consultant for Abbott, Astra Zeneca, Atherogenics, Merck, Merck Schering Plough, Novartis, Pfizer, Reliant, Schering-Plough, Sanofi-Synthelabo, Takeda, GlaxoSmithKline; he has received grant/research support from: Abbott, ActivBiotics, Gene Logic, GlaxoSmithKline, Integrated Therapeutics, Merck, Pfizer, Schering-Plough, Sanofi-Synthelabo, Takeda; and he is on the speakers bureau for AstraZeneca, Merck, Pfizer, Reliant, Schering-Plough.
Remaining authors have nothing to disclose.
References
- 1.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2451. doi: 10.1161/CIRCULATIONAHA.108.814251. 4p following 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.del Sol AI, Moons KG, Hollander M, et al. Is carotid intima-media thickness useful in cardiovascular disease risk assessment? The Rotterdam Study Stroke. 2001;32:1532–1538. doi: 10.1161/01.str.32.7.1532. [DOI] [PubMed] [Google Scholar]
- 4.Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 5.Brautbar A, Ballantyne CM, Lawson K, et al. Impact of adding a single allele in the 9p21 locus to traditional risk factors on reclassification of coronary heart disease risk and implications for lipid-modifying therapy in the Atherosclerosis Risk in Communities study Circulation. Cardiovascular Genetics. 2009;2:279–285. doi: 10.1161/CIRCGENETICS.108.817338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106:2055–2060. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Tian L. Predicting cardiovascular risk: so what do we do now? Arch Intern Med. 2006;166:1342–1344. doi: 10.1001/archinte.166.13.1342. [DOI] [PubMed] [Google Scholar]
- 11.Hunt KJ, Sharrett AR, Chambless LE, Folsom AR, Evans GW, Heiss G. Acoustic shadowing on B-mode ultrasound of the carotid artery predicts CHD. Ultrasound Med Biol. 2001;27:357–365. doi: 10.1016/s0301-5629(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 12.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Li R, Cai J, Tegeler C, Sorlie P, Metcalf PA, Heiss G. Reproducibility of extracranial carotid atherosclerotic lesions assessed by B-mode ultrasound: the Atherosclerosis Risk in Communities Study. Ultrasound Med Biol. 1996;22:791–799. doi: 10.1016/0301-5629(96)00084-1. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Duncan BB, Metcalf PA, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–2383. doi: 10.1161/01.str.25.12.2377. [DOI] [PubMed] [Google Scholar]
- 16.High-resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities Study (ARIC). The ARIC Study Group. J Neuroimaging. 1991;1:68–73. [PubMed] [Google Scholar]
- 17.National Heart L, and Blood Institute. Atherosclerosis Risk in Communities (ARIC) Study. Operations manual, no. 7: blood collection and processing. Version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 18.Chambless LE, Zhong MM, Arnett D, Folsom AR, Riley WA, Heiss G. Variability in B-mode ultrasound measurements in the atherosclerosis risk in communities (ARIC) study. Ultrasound Med Biol. 1996;22:545–554. doi: 10.1016/0301-5629(96)00039-7. [DOI] [PubMed] [Google Scholar]
- 19.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 20.Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25:3474–3486. doi: 10.1002/sim.2299. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York Chapman & Hall; 1993. [Google Scholar]
- 23.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 24.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998;4:109–120. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
- 25.Pencina MJ, D'Agostino RB, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 26.Schlendorf KH, Nasir K, Blumenthal RS. Limitations of the Framingham risk score are now much clearer. Prev Med. 2009;48:115–116. doi: 10.1016/j.ypmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Naydeck BL, Ives DG, et al. Coronary artery calcium, carotid artery wall thickness, and cardiovascular disease outcomes in adults 70 to 99 years old. Am J Cardiol. 2008;101:186–192. doi: 10.1016/j.amjcard.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lester SJ, Eleid MF, Khandheria BK, Hurst RT. Carotid intima-media thickness and coronary artery calcium score as indications of subclinical atherosclerosis. Mayo Clin Proc. 2009;84:229–233. doi: 10.4065/84.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Pasternak RC, Abrams J, Greenland P, Smaha LA, Wilson PW, Houston-Miller N. 34th Bethesda Conference: Task force #1--Identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol. 2003;41:1863–1874. doi: 10.1016/s0735-1097(03)00358-9. [DOI] [PubMed] [Google Scholar]
- 31.Nambi V, Ballantyne CM. "Risky business": ten years is not a lifetime. Circulation. 2009;119:362–364. doi: 10.1161/CIRCULATIONAHA.108.830281. [DOI] [PubMed] [Google Scholar]
- 32.Berry JD, Liu K, Folsom AR, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation. 2009;119:382–389. doi: 10.1161/CIRCULATIONAHA.108.800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.