Abstract
Biotrophic fungal and oomycete pathogens alter carbohydrate metabolism in infected host tissues. Symptoms such as elevated soluble carbohydrate concentrations and increased invertase activity suggest that a pathogen-induced carbohydrate sink is established. To identify pathogen-induced regulators of carbohydrate sink strength, quantitative real-time polymerase chain reaction was used to measure transcript levels of invertase and hexose transporter genes in biotrophic pathogen-infected grapevine (Vitis vinifera) leaves. The hexose transporter VvHT5 was highly induced in coordination with the cell wall invertase gene VvcwINV by powdery and downy mildew infection. However, similar responses were also observed in response to wounding, suggesting that this is a generalized response to stress. Analysis of the VvHT5 promoter region indicated the presence of multiple abscisic acid (ABA) response elements, suggesting a role for ABA in the transition from source to sink under stress conditions. ABA treatment of grape leaves was found to reproduce the same gene-specific transcriptional changes as observed under biotic and abiotic stress conditions. Furthermore, the key regulatory ABA biosynthetic gene, VvNCED1, was activated under these same stress conditions. VvHT5 promoter::β-glucuronidase-directed expression in transgenic Arabidopsis (Arabidopsis thaliana) was activated by infection with powdery mildew and by ABA treatment, and the expression was closely associated with vascular tissue adjacent to infected regions. Unlike VvHT1 and VvHT3, which appear to be predominantly involved in hexose transport in developing leaves and berries, VvHT5 appears to have a specific role in enhancing sink strength under stress conditions, and this is controlled through ABA. Our data suggest a central role for ABA in the regulation of VvcwINV and VvHT5 expression during the transition from source to sink in response to infection by biotrophic pathogens.
The wine grape species Vitis vinifera is highly susceptible to a number of pathogens that can cause economically devastating diseases, including powdery mildew caused by the ascomycete fungus Erysiphe necator (syn. Uncinula necator) and downy mildew caused by the oomycete Plasmopora viticola. Both powdery mildew and downy mildew are obligate biotrophs that acquire nutrients via specialized feeding structures called haustoria, which are formed within host cells after penetration through the cell wall. In the case of powdery mildew, this involves the penetration of cells within the epidermal layer, whereas downy mildew initially invades via the stomates and forms haustoria within mesophyll cells (O'Connell and Panstruga, 2006).
Although some of the early research on nutrient transfer from the plant to the fungus was carried out on powdery mildew (Hall and Williams, 2000), much of our current understanding of the role of haustoria in nutrient uptake comes from research on the bean rust fungus Uromyces fabae. Voegele et al. (2001) identified a fungal hexose transporter (HT), HXT1, that was highly expressed in rust haustoria and was exclusively localized to the haustorial plasma membrane. Amino acid transporter genes were also found to be highly expressed within rust haustoria. Finally, a novel fungal invertase gene, Uf-INV1, has been shown to be secreted from the rust haustoria into the extrahaustorial matrix, where it is proposed to play a role in the supply of hexose substrates for the HXT1 transporter (Voegele et al., 2006).
The growing fungal mass competes for photoassimilates from within the tissues of its host, where it acts as an additional nutrient sink and alters normal patterns of carbohydrate partitioning. Decreased assimilation, increased invertase activity, and elevated Glc and Fru concentrations are characteristic of biotrophic pathogen-infected leaves of several different plant species, including grapevine (Brem et al., 1986; Moriondo et al., 2005), cereals (Scholes et al., 1994; Sutton et al., 2007), and Arabidopsis (Arabidopsis thaliana; Chou et al., 2000; Fotopoulos et al., 2003). As assimilation is typically inhibited, additional sugars may arise by increased importation from adjacent noninfected and otherwise healthy leaves, and this may be caused by elevated cell wall invertase (cwINV) activity (Fotopoulos et al., 2003; Sutton et al., 2007). Increased phloem unloading and reduced phloem loading are expected outcomes from elevated cwINV activity in the apoplast (von Schaewen et al., 1990), resulting in leaves switching from source organs to carbohydrate sinks. Additionally, starch reserves may also be catabolized to soluble sugars (Chou et al., 2000), further demonstrating the high demand for carbohydrate and the transformed metabolic environment associated with infection.
In plant-pathogen interactions where a host defense response is mounted, cwINV is rapidly induced and provides carbohydrate to power these energetically expensive counteractions. Indeed, RNA interference knockdown of cwINV in tobacco (Nicotiana tabacum ‘Samsun NN’) leaves inhibited defense responses such as callose deposition, induction of pathogenesis-related proteins, and hydrogen peroxide-mediated cell death against the biotrophic oomycete Phytophthora nicotianae, allowing colonization of this normally resistant plant cultivar (Essmann et al., 2008). However, in the case of compatible interactions, such as grapevine powdery mildew disease where defense responses are mounted (Jacobs et al., 1999; Godfrey et al., 2007; Fung et al., 2008) but are insufficient to stop disease progression, it is conceivable that the induction of sink metabolism may actually aid disease progression. For this reason, the signaling pathways that mediate these responses are of interest when considering strategies to improve the resistance of crop plants to fungal pathogens.
Fotopoulos et al. (2003) reported the up-regulation of a cwINV (AtcwINV1; syn. AtβFRUCT1) and the HT (AtSTP4) in Erysiphe cichoracearum-infected Arabidopsis leaves and postulated that there may be coordinated induction of specific host HTs and cwINV in response to powdery mildew infection. We have carried out an extended quantitative analysis of the expression of five different HTs and three different invertase genes from grapevine in response to infection with the grapevine biotrophic pathogens E. necator and P. viticola and compared this with the response following wounding. Our results indicate that while there are some specific differences in the response of these carbohydrate biosynthesis pathway genes to the different biotrophic pathogens, the underlying transcriptional response of grapevine tissues to these different abiotic/biotic stresses is the same. Furthermore, we provide evidence for a possible role of the plant hormone abscisic acid (ABA) in the molecular response of carbohydrate pathway genes to stress.
RESULTS
Changes in Expression of Invertase and HT Genes in Grapevine Leaves in Response to Infection by Biotrophic Pathogens
Brem et al. (1986) previously reported enhanced acid invertase activity and elevated hexose concentrations and a simultaneous decline in net photosynthetic activity in powdery mildew-infected grapevine leaves relative to uninfected control samples. Considering the above observation, we decided to focus on the expression of host invertase and HT genes during powdery mildew infection because they are key determinants of carbohydrate sink strength.
Expression of both cwINV and vacuolar invertase have been shown to be modulated by biotrophic pathogen infection in other plant species (Scholes et al., 1994; Chou et al., 2000; Fotopoulos et al., 2003; Sutton et al., 2007). We have previously reported on the expression of an apoplastic cwINV, VvcwINV (AAT09980), in different grapevine carbohydrate source and sink tissues (Hayes et al., 2007). Bioinformatic analysis of the grapevine genotype PN40024 genome sequence (Jaillon et al., 2007) indicates the presence of a second putative VvcwINV gene (CAO15886) based on both sequence homology with other known cwINV genes and its translation product having a predicted basic pI value of 9.05 (De Conninck et al., 2005). However, a search of the National Center for Biotechnology Information EST database did not reveal any expressed sequences for this gene. Genes encoding grapevine vacuolar invertases VvGIN1 and VvGIN2 were previously reported by Davies and Robinson (1996) and, based on the PN40024 genome sequence, are the only two predicted vacuolar invertase sequences in the grapevine genome. The grapevine genome contains 16 putative VvHT gene sequences (Supplemental Table S1) belonging to the STP subfamily of plasma membrane H+-monosaccharide symporters (Johnson et al., 2006; Büttner, 2007), but only five of these genes (VvHT1 to -5) have been functionally characterized to date (Fillion et al., 1999; Hayes et al., 2007).
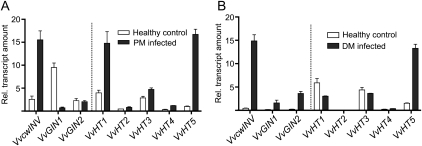
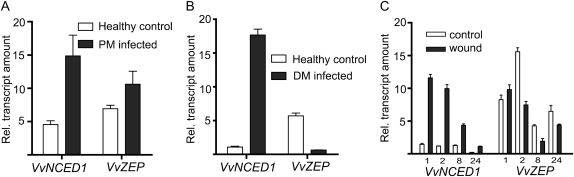
Grapevine leaves were inoculated with powdery mildew spores and sampled after 8 d to analyze changes in invertase and HT gene expression relative to uninoculated control leaves (Fig. 1A). A 4-fold increase in VvcwINV expression was observed in powdery mildew-infected leaves relative to expression in control leaves. In contrast, VvGIN1 expression was reduced approximately 10-fold and there was no significant change in VvGIN2 expression (Fig. 1A). The observed changes in invertase transcript levels in response to powdery mildew infection were also reflected in changes in the extractable invertase enzyme activity, with an increase in insoluble (cell wall) acid invertase activity and a reduction in soluble (vacuolar) acid invertase activity (Supplemental Fig. S1). All of the VvHT genes analyzed showed some increase in expression in response to powdery mildew infection, but the largest increases observed were for VvHT5 (approximately 15-fold) and VvHT1 (approximately 4-fold; Fig. 1A).
Figure 1.
Effect of biotrophic pathogen infection on invertase and HT expression in grapevine. Leaves were inoculated with powdery mildew (PM [E. necator]; A) or downy mildew (DM [P. viticola]; B) and sampled after 8 or 10 d, respectively, for quantitative RT-PCR analysis. All values were normalized to the expression of actin, and each data point is the average of triplicate testing of two biological replicates independently extracted for RNA and independently analyzed. Error bars represent se.
To investigate if infection by a different biotrophic fungal pathogen would elicit similar transcriptional responses to those observed with powdery mildew, leaves were inoculated with sporangia of P. viticola, the causative agent of grapevine downy mildew (Fig. 1B). Comparison of Figure 1, A and B, shows that there are some common responses of genes associated with host carbohydrate metabolism to infection by the two biotrophic pathogens. Both VvcwINV and VvHT5 were significantly induced in response to infection by both pathogens. However, there are also some interesting differences in transcriptional response. Whereas VvHT1 was induced in powdery mildew-infected leaves (Fig. 1A), it was found to be slightly repressed in response to downy mildew infection (Fig. 1B). Conversely, whereas the expression of the vacuolar invertase genes was unchanged (VvGIN2) or significantly repressed (VvGIN1) in powdery mildew-infected leaves, both genes were markedly up-regulated (VvGIN1 approximately 26-fold; VvGIN2 approximately 16-fold) in response to downy mildew infection.
Also interesting is the markedly reduced level of GIN1 expression in healthy leaves in the downy mildew experiment (Fig. 1B) compared with the control leaves used in the powdery mildew inoculation experiment (Fig. 1A). This is a reflection of the use of more mature leaves for the downy mildew inoculation assay than for the powdery mildew assay and the fact that GIN1 transcript levels decline significantly during leaf development (Davies and Robinson, 1996).
Analysis of Invertase and HT Expression in Wounded Grapevine Leaves
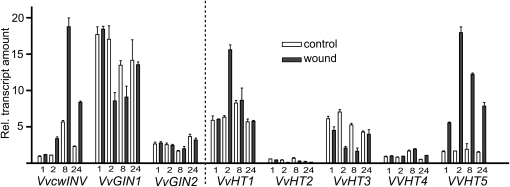
To investigate if an abiotic stimulus would produce similar changes in grapevine invertase and HT gene expression, leaves were wounded by abrasion of the upper surfaces with sandpaper and collected at 1, 2, 8, and 24 h after wounding. Quantitative real-time PCR (RT-PCR) analysis revealed that the transcriptional responses of grapevine invertases and HTs to wounding were very similar to those observed in response to powdery mildew infection (Fig. 2). Thus, transcript levels of VvcwINV were elevated in leaves within 2 h of wounding, peaked at 8 h (approximately 3-fold increase), and had decreased again by 24 h. VvGIN1 expression represented a mirror image of this pattern, with expression down-regulated after 2 h but increasing again after 24 h. VvGIN2 expression remained constant over this period. As with powdery mildew infection, wounding had little or no effect on the transcription of VvHT2, VvHT3, or VvHT4. VvHT1 showed a minor transient increase in transcription after wounding, peaking after 2 h (approximately 2.5-fold increase). As with the biotic stress treatments, VvHT5 displayed the most dramatic transcriptional response to wounding, with expression significantly induced within 1 h of wounding, peaking at 2 h (approximately 11-fold increase) and remaining elevated 24 h after the application of the abiotic stress.
Figure 2.
Effect of wounding on invertase and HT expression in grapevine. Leaves at node positions 5 and 6 were wounded by abrasion of the upper surface with sandpaper and collected at 1, 2, 8, and 24 h afterward for quantitative RT-PCR analysis. All values were normalized to the expression of actin, and each data point is the average of triplicate testing of two biological replicates independently extracted for RNA and independently analyzed. Error bars represent se.
Identification of ABA Regulatory Motifs in the VvHT5 Promoter
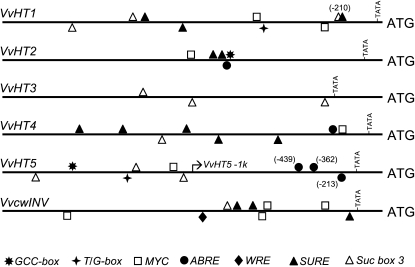
Having established that VvHT5 transcription is rapidly and markedly induced by both biotrophic pathogen infection and wounding, we decided to analyze the promoter of this gene in an attempt to identify any specific regulatory elements that may mediate its response to stress. Two kilobases of sequence upstream of the predicted start codon of each of the VvHT1 to -5 and VvcwINV genes was obtained from the published grapevine PN40024 genome sequence (Jaillon et al., 2007) and analyzed for putative regulatory motifs associated with stress-induced transcription using the PLACE database (Higo et al., 1999). A number of motifs were identified: GCC box (Brown et al., 2003), MYC recognition (Abe et al., 1997), T/G box (Boter et al., 2004), wound response element (Palm et al., 1990), and abscisic acid response elements (ABREs; Choi et al., 2000; Hattori et al., 2002). Figure 3 also shows the presence of Suc-responsive motifs (SURE1, Suc box 3) shown previously by Cakir et al. (2003) to be involved in the transcriptional regulation of VvHT1.
Figure 3.
Location of putative regulatory elements in the promoters of VvHT1 to -5 and VvcwINV. The presence of regulatory motifs associated with stress-induced transcription in a 2-kb promoter region from the published grapevine PN40024 genome sequence is shown. Recognition sequences are as follows: GCC box (GCCGCC; Brown et al., 2003), T/G box (AACGTG; Boter et al., 2004), MYC (CACATG; Abe et al., 1997), ABRE (ACGTGG/TC; Hattori et al., 2002), and wound response element (WRE; AAA/TGTATCC/GA; Palm et al., 1990). Also shown are the Suc-responsive motifs SURE (ATAGAAA) and Suc box 3 (AAATCA------AA), identified by Cakir et al. (2003) to be involved in the transcriptional regulation of VvHT1. The start position of the VvHT5-1K promoter fragment is also indicated.
The most striking difference between the cis-acting regulatory elements detected in the VvHT5 promoter region and comparable regions in VvHT1 to -4 and VvcwINV is the presence of a cluster of three ABREs at nucleotide positions −213 (− strand), −362 (+ strand), and −439 (+ strand) within the VvHT5 promoter, whereas these were absent or predicted only once in the promoters of VvHT2 and VvHT4. ABREs bind basic Leu zipper-type proteins known as ABRE binding factors, which are induced under stress conditions (Choi et al., 2000; Uno et al., 2000). Significantly, the VvHT5 promoter region also appears to lack the SUREs found in the promoter regions of VvHT1 and VvcwINV, which are also induced in response to powdery mildew (Fig. 1) and wounding (Fig. 2).
Effect of ABA on VvHT5 Expression
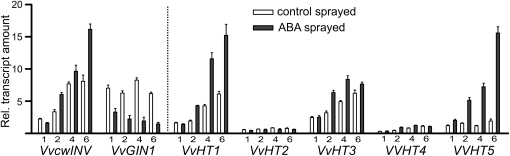
Having identified the presence of a unique cluster of ABREs in the VvHT5 promoter region, we investigated the effect of ABA application on the expression of VvHT5 in comparison with the other VvHT and invertase genes. Grapevine leaves were sprayed with a solution containing 200 mg L−1 ABA and 0.05% (v/v) Tween 20 or 0.05% (v/v) Tween 20 alone and sampled at 1, 2, 4, and 6 h post treatment. The pattern of ABA-induced changes in VvHT and invertase gene expression (Fig. 4) showed a marked similarity to those observed in powdery mildew-infected (Fig. 1A) and wounded (Fig. 2) leaves. That is, VvHT5 expression was increased rapidly, showing an approximately 10-fold increase in 6 h, whereas VvcwINV and VvHT1 transcript levels showed minor increases after 4 to 6 h and VvGIN1 transcription declined. Interestingly, transcript levels of VvcwINV, VvHT1, and VvHT3 were found to be significantly elevated in control leaves across the treatment period, suggesting that these genes may be diurnally regulated. Diurnal regulation of a cwINV from tomato (Solanum lycopersicum; Proels and Roitsch, 2009) and a HT from Arabidopsis (Stadler et al., 2003) have been reported previously.
Figure 4.
Effect of ABA on invertase and HT expression in grapevine. Leaves were sprayed with a solution containing 200 mg L−1 ABA and 0.05% (v/v) Tween 20 or with 0.05% (v/v) Tween 20 alone and sampled at 1, 2, 4, and 6 h post treatment for quantitative RT-PCR analysis. All values were normalized to the expression of actin, and each data point is the average of triplicate testing of two biological replicates independently extracted for RNA and independently analyzed. Error bars represent se.
Biotrophic Pathogen Infection and Wounding Induce the ABA Biosynthetic Pathway in Grapevine
As ABA treatment of grapevine leaves was observed to result in changes in transcription of VvHT5, VvHT1, and VvcwINV that were similar to those observed in response to powdery mildew infection and wounding, we examined the effect of these stress treatments on the transcription of zeaxanthin epoxidase (ZEP) and 9-cis-epoxycarotenoid dioxygenase (NCED), which are key steps in the regulation of ABA biosynthesis (Liotenberg et al., 1999). In grapevine, a single VvZEP gene and two VvNCED genes (VvNCED1 and -2) have been identified, but only VvNCED1 transcription appears to be linked to ABA biosynthesis in leaves under stress conditions; VvNCED2 appears to be developmentally regulated (Soar et al., 2004). Leaves infected with powdery mildew or downy mildew were found to have VvNCED1 transcript levels 3- or 12-fold higher, respectively, than healthy leaves (Fig. 5, A and B). Similarly, VvNCED1 was rapidly up-regulated in response to wounding (Fig. 5C), with a 7-fold increase in transcript levels 1 h after wounding that had declined back to control levels by 24 h. In contrast, no clear trend in VvZEP transcription was observed in response to these biotic and abiotic stresses, with expression increasing in response to powdery mildew infection (Fig. 5A) but decreasing in response to downy mildew infection (Fig. 5B) and wounding (Fig. 5C).
Figure 5.
Effect of biotic and abiotic stress on ABA biosynthetic pathway genes in grapevine. VvNCED1 and VvZEP transcript levels were determined by quantitative RT-PCR in powdery mildew (PM)-infected (A), downy mildew (DM)-infected (B), and wounded (C) grapevine leaves. Analysis was conducted on the same cDNA samples used in Figures 1 and 2.
Functional Analysis of the VvHT5 Promoter
Vignault et al. (2005) have previously shown VvHT1 expression to be localized to the phloem region of the vascular bundles in grapevine leaves and berries. In order to determine the localization of VvHT5 induction under stress conditions, a VvHT5-2K promoter::GUS construct was transformed into Arabidopsis using the VvHT5 promoter sequence amplified from grapevine cv Cabernet Sauvignon genomic DNA (GenBank accession no. EF122147). The Cabernet Sauvignon VvHT5 promoter sequence was confirmed to contain the three ABREs motifs predicted in the PN40024 genome sequence (Fig. 3).
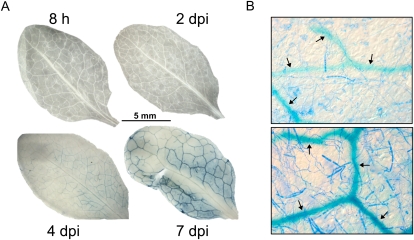
VvHT5-2K::GUS Arabidopsis F3 lines were infected with E. cichoracearum, the causative agent of Arabidopsis powdery mildew. Powdery mildew spores normally take approximately 12 to 16 h to germinate and establish feeding structures (haustoria) within epidermal cells, after which time the fungal hyphae rapidly expand across the leaf surface, establishing new feeding structures. No GUS staining was visible in leaves 2 d post inoculation, but GUS expression began to appear in leaves 4 d after infection and was highly visible after 7 d (Fig. 6A). With the exception of the central vein area, GUS staining in powdery mildew-infected leaves was exclusively associated with the vascular bundles, and the intensity of GUS expression was directly related to the density of the powdery mildew infection of the adjacent tissue (Fig. 6B).
Figure 6.
Induction of the VvHT5-2K promoter in transgenic Arabidopsis in response to powdery mildew infection. A, Homozygous transgenic F3 Arabidopsis plants containing VvHT5-2K::GUS fusion constructs were inoculated with E. cichoracearum and sampled 8 h, 2 d, 4 d, and 7 d post inoculation (dpi). Leaves were fixed and stained for both GUS (5-bromo-4-chloro-3-indolyl-β-glucuronic acid) and fungal structures (Coomassie Brilliant Blue) as described in “Materials and Methods.” B, Higher magnification of leaves at 4 and 7 d post inoculation. Arrows indicate blue GUS staining associated with the vascular bundles. Images are representative of four lines analyzed.
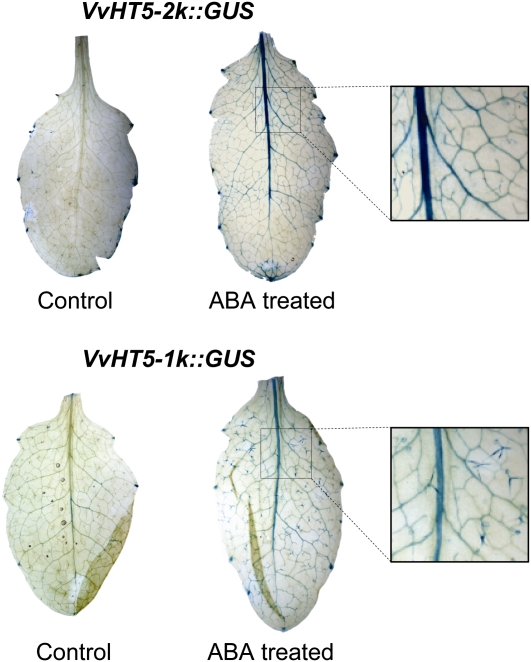
To further analyze the possible involvement of the predicted ABRE motifs located at nucleotide positions −213, −362, and −439 (Fig. 3) in the ABA responsiveness of this promoter, transgenic Arabidopsis lines containing a VvHT5-1K promoter::GUS construct (Fig. 3) were also generated. Leaves of both the VvHT5-2K::GUS and VvHT5-1K::GUS transgenic lines were sprayed with a solution containing 200 mg L−1 ABA and 0.05% (v/v) Tween 20 or 0.05% (v/v) Tween 20 alone. ABA-induced GUS expression was observed in both VvHT5-2K::GUS and VvHT5-1K::GUS Arabidopsis lines and, as with powdery mildew-induced expression (Fig. 6), was localized exclusively to the vascular tissue (Fig. 7). Although the intensity of GUS expression appeared to be lower in the lines containing the VvHT5-1K promoter compared with the VvHT5-2K promoter, the response to ABA supports the hypothesis that ABA-induced VvHT5 gene expression is mediated via the ABRE motifs.
Figure 7.
ABA-induced VvHT5 promoter activity in transgenic Arabidopsis. Transgenic Arabidopsis plants containing either the VvHT5-2K::GUS or VvHT5-1K::GUS fusion construct were sprayed with a solution containing 200 mg L−1 ABA and 0.05% (v/v) Tween 20 or with 0.05% (v/v) Tween 20 alone and sampled at 8 h post treatment for staining for GUS expression. Images are representative of three lines analyzed.
DISCUSSION
Changes to plant carbohydrate metabolism in biotrophic pathogen-infected tissues are well documented, with enhanced acid invertase activity and elevated monosaccharide concentrations consistently observed that are characteristic of stress-induced changes in sink metabolism (Brem et al., 1986; Scholes et al., 1994; Wright et al., 1995; Clark and Hall, 1998; Chou et al., 2000; Fotopoulos et al., 2003; Sutton et al., 2007). To date, all of these studies have focused on single pathosystems. In this study, we examined the responses of key genes regulating carbohydrate transport and partitioning in grapevine to two different biotrophic pathogens and compared these with an abiotic stress imposed through wounding.
Transcription of VvcwINV was consistently induced in grapevine leaves in response to biotic (Fig. 1) and abiotic (Fig. 2) stress. In contrast, the response of the vacuolar invertases VvGIN1 and VvGIN2 was much more variable. The relative changes in invertase transcription in response to powdery mildew were also supported by measurement of invertase enzymic activity (Supplemental Fig. S1), which confirmed the increase in insoluble (cell wall) invertase activity and the reduction in soluble (cytoplasmic/vacuolar) invertase in powdery mildew-infected grape leaves. A similar decrease in vacuolar invertase transcription was observed in Vicia faba leaves in response to infection by the biotrophic rust fungus U. fabae, and this was suggested to result from the decrease in available Suc for vacuolar storage under these conditions (Voegele et al., 2006).
Like VvcwINV, VvHT5 was also found to be up-regulated in response to both biotic (Fig. 1) and abiotic (Fig. 2) stress, but the rate and magnitude of the increased transcription were significantly greater than for VvcwINV. This is particularly evident from the wounding experiment, which showed that VvHT5 transcription was increased 4-fold within 1 h and 10-fold within 2 h of the wounding treatment, whereas VvcwINV transcription was unchanged after 1 h and had increased only 3-fold within 2 h of wounding (Fig. 3). Of the other VvHT genes examined, only VvHT1 responded significantly to powdery mildew infection and wounding, but the magnitude of the response was much lower than that observed for VvHT5 (Figs. 1 and 2). Interestingly, VvHT1 expression appears to be repressed in downy mildew-infected grape leaves.
In contrast to the other VvHT genes examined, VvHT5 was found to be strongly induced in response to infection by both biotrophic pathogens and wounding, suggesting a specific role in the provision of hexoses to cells under stress conditions. We reasoned, therefore, that the VvHT5 promoter region might contain unique cis-acting regulatory DNA elements regulating this stress induction. Sequence analysis of a 2-kb region of the VvHT5 promoter identified three ABREs within the first 450 nucleotides upstream of the VvHT5 start codon. Promoter analyses of ABA-regulated genes have previously revealed that many ABA-regulated genes are controlled by cis-regulatory elements sharing the ABRE consensus sequence ACGTG(G/T)C (Busk and Pages, 1998; Hattori et al., 2002), which interact with ABA-, drought-, and salinity-induced basic Leu zipper ABRE binding factors (Choi et al., 2000; Rock, 2000; Uno et al., 2000). Furthermore, the clustered arrangement of three putative ABREs in the VvHT5 promoter is highly significant in terms of transcriptional regulation, because it has been established that a single copy of an ABRE is not sufficient for ABA-mediated induction of transcription and that ABRE-ABRE pairs commonly form the functional ABA-responsive complex (Gómez-Porras et al., 2007). The predicted locations of the ABREs in VvHT5 (Fig. 3) at nucleotide positions −213 (+ strand), −362 (− strand), and −439 (− strand) are also in good agreement with the locations of ABREs in ABA-responsive genes in Arabidopsis in terms of the mean distance from the proximal end of the complex to the ATG codon (354 nucleotides) and the mean gap length between the ABREs (236 nucleotides).
The possible role of ABA in stress-induced transition from source to sink is also supported by expression analysis of ABA-treated grapevine leaves. ABA application induced changes in expression of invertase and HT genes (Fig. 4), which mirrored the responses observed in powdery mildew-infected (Fig. 1A) and wounded (Fig. 2) leaves. In particular, the rapid increase in VvHT5 expression and the differential responses of VvcwINV and VvGIN1 strongly suggest that the stress-induced transcriptional changes observed in Figures 1 and 2 were mediated through changes in ABA levels.
In addition to higher plants, some phytopathogenic fungi also have the potential to produce ABA (Asselbergh et al., 2008). In order to determine whether these stress treatments were activating ABA biosynthesis in the plant tissues, we examined the key rate-limiting steps in the plant ABA biosynthetic pathway catalyzed by ZEP and NCED (Liotenberg et al., 1999). Previous studies on grapevine leaves have showed that water stress specifically induced VvNCED1 but not VvZEP, and this is associated with an increase in bulk leaf ABA concentrations (Soar et al., 2004, 2006). Our analysis shows that powdery and downy mildew infection and wounding also led to rapid and significant increases in VvNCED1 expression, but no consistent pattern in VvZEP expression was observed (Fig. 5). This appears to support the theory that ZEP gene expression does not regulate ABA biosynthesis in leaves (Liotenberg et al., 1999), where its expression is more crucial to the control of carotenoid synthesis (Finkelstein and Rock, 2002). In Arabidopsis, three members of the six-member NCED gene family were also induced by infection with the hemibiotrophic bacterial pathogen Pseudomonas syringae, resulting in a significant elevation of ABA level in infected leaves (Fan et al., 2009).
Transgenic Arabidopsis plants transformed with a VvHT5-2K::GUS construct showed no detectable GUS activity in healthy source leaves (Fig. 6A). However, as the powdery mildew pathogen established feeding sites in epidermal cells across the leaf surface, activation of the VvHT5 promoter was observed within the vascular tissue adjacent to the infected regions (Fig. 6). This indicates that the role of VvHT5 is not to specifically increase hexose transport across the plasma membrane of powdery mildew-infected epidermal cells but rather to enhance the supply of sugar from the phloem to the surrounding tissues during the transition from source to sink. Vignault et al. (2005) previously showed VvHT1 to be localized to the plasma membrane of the sieve element/companion cell interface in developing grape berries, and the location of VvHT5 promoter-directed expression in Arabidopsis appears to be similar. However, further work will be required to confirm the exact cellular location within the vascular complex of grapevine leaves. A similar GUS staining pattern was also observed with VvHT5-2K::GUS and VvHT5-1K::GUS transgenic lines following ABA treatment (Fig. 7). This result supports a role for ABA in powdery mildew-induced VvHT5 expression and suggests that stress-induced VvHT5 transcription may be mediated through the ABRE motifs located within the first 450 nucleotides of the VvHT5 promoter (Fig. 3).
This is not the first report of a plant HT regulated by the plant stress hormone ABA. VvHT1 has been shown to be transcriptionally regulated by VvMSA, a member of the ASR (for ABA, stress, and ripening induced) family (Atanassova et al., 2003; Cakir et al., 2003), which binds to a sugar response domain comprising overlapping SURE1 + Suc box 3 elements located approximately 210 nucleotides upstream of the VvHT1 coding start site (Fig. 3). VvMSA expression is induced by ABA, but only in the presence of Suc (Cakir et al., 2003). Thus, VvHT1 expression appears to be regulated primarily by sugar levels, but this may be enhanced by ABA, as shown in Figure 4. It is well established that cwINVs are also transcriptionally regulated by hexoses (Roitsch and González, 2004); therefore, it is not surprising to find multiple SUREs within the VvcwINV promoter (Fig. 3). However, also interesting is the presence in this promoter of multiple recognition motifs identified for ABA-induced MYC transcription factors in Arabidopsis (Abe et al., 1997). This might suggest synergistic roles for sugar and ABA in the transcriptional regulation of VvcwINV as well. In contrast, SUREs are absent from the VvHT5 promoter region and VvHT5 transcription has been shown to be insensitive to sugar regulation in grape suspension cells (Conde et al., 2006). This further reinforces the hypothesis that, unlike VvHT1 and VvHT3, which appear to be predominantly involved in hexose transport in developing leaves and berries (Vignault et al., 2005; Hayes et al., 2007), VvHT5 appears to have a specific role in enhancing sink strength under stress conditions and that this is controlled through ABA.
One characteristic of VvHT5 that may be central to its role in the rapid supply of sugar under stress conditions is its substrate specificity. Uptake studies with VvHT1 and VvHT5 reveal that while there is little difference in their affinity for Glc, VvHT5 is capable of binding Fru whereas VvHT1 is not (Vignault et al., 2005; Hayes et al., 2007). Interestingly, VvHT5 is most closely related to the Arabidopsis HT AtSTP13 (83% amino acid identity compared with only 48%–58% identity for the other 13 AtSTPs), which has also been demonstrated to facilitate Fru uptake into Xenopus oocytes (Norholm et al., 2006). The ability to transport Fru is not a feature commonly demonstrated by monosaccharide transporters (Schofield et al., 2009), and as such, VvHT5 would have the capacity to provide more invertase-generated hexose to stressed cells than other VvHT proteins.
Our data clearly show a high degree of similarity in the responses of VvcwINV and VvHT5 to the imposition of both biotic and abiotic stresses (Figs. 1 and 2). These results tend to support the concept that the biotrophic pathogen-induced transition from source to sink, through the coordinated induction of cwINV and HT genes, is part of a generalized ABA-mediated stress response. Fotopoulos et al. (2003) previously reported the coordinated expression of a cwINV (AtcwINV1; syn. AtβFRUCT1) and a HT (AtSTP4) in response to powdery mildew infection of Arabidopsis leaves. Given that AtSTP4 has also been shown to be induced by wounding (Truernit et al., 1996), they suggested that the aim of this coordinated response was to try and retain carbon within the infected leaf to support defense or repair purposes. cwINV-induced sink metabolism would increase local sugar concentrations through enhanced Suc cleavage and/or reduced Suc export to provide the additional energy required by host cells under these conditions. This was well demonstrated by Essmann et al. (2008), who showed that defense responses against the oomycete pathogen P. nicotianae were reduced in tobacco plants in which cwINV had been silenced. Similarly, silencing of the cwINV Lin8 led to the abolition of defense-related gene induction in tomato during the compatible interaction with Xanthomonas campestris pv vesicatoria (Kocal et al., 2008).
However, if the coordinated induction of cwINV and HT genes in response to either biotrophic pathogen infection or wounding is part of a generalized ABA-mediated stress response in grapevine, what is the common link between these biotic and abiotic treatments that triggers the ABA response? It is possible that wounding may result in water loss at the cut surface, causing localized osmotic stress that, in turn, activates ABA signaling. However, biotrophic pathogens minimize wounding damage to plant cells during invasion to ensure that the penetrated cell remains viable. Therefore, biotrophic infection would not be expected to result in a similar ABA-mediated stress response. One explanation may be that changes in sugar flux driven by carbon uptake by the biotrophic pathogen may also result in changes in osmotic status and, thus, ABA induction. The ABA produced would, in turn, activate the retention and transport of sugar to the infection site through induction of VvcwINV and VvHT5, thereby further amplifying the response.
An alternative hypothesis that must also be considered is that these biotrophic fungal pathogens hijack the abiotic stress-induced ABA signaling pathway to promote their own infection by providing increased carbon for fungal growth and/or suppressing host defense responses. The ABA-induced increase in cwINV would aid fungal nutrition, as biotrophic fungi preferentially take up hexose sugars rather than Suc (O'Connell and Panstruga, 2006), although certain biotrophic pathogens may secrete their own invertase to achieve this goal (Voegele et al., 2006). ABA has also been demonstrated to have a suppressive effect on the salicylic acid-dependent defense pathway that normally promotes resistance against biotrophic pathogens (Mohr and Cahill, 2007). Biotrophic pathogens may manipulate the host ABA signaling pathway through the secretion of effectors. In the case of the bacterial pathogen P. syringae, there is convincing evidence to suggest that type III-secreted effectors such as AvrPtoB are able to induce ABA biosynthesis in Arabidopsis through activation of NCED3 (de Torres-Zabala et al., 2007). Whether effectors secreted by E. necator or P. viticola are also able to suppress defense responses in grapevine remains to be determined.
In summary, the role of ABA in biotic stress is complex and varies depending on the pathogen and the development stage of the invaded tissue (Robert-Seilaniantz et al., 2007; Asselbergh et al., 2008; Ton et al., 2009). It has been shown to act as a negative regulator of disease resistance by interfering with biotic stress signaling, but it can also promote plant defense through ABA-dependent stomatal closure and callose deposition. Our data now suggest that, apart from its role in regulating defense responses, ABA may also have an important role in regulating the carbohydrate supply to tissues under biotic stress. In grapevine leaves, this appears to be mediated through the coordinated increase in VvcwINV and VvHT5 transcription, leading to increased cleavage of Suc derived from the phloem sieve elements by apoplastic VvcwINV and increased transport of hexoses from the apoplast into surrounding companion cell/phloem parenchyma (Turgeon and Wolf, 2009) via VvHT5.
MATERIALS AND METHODS
Plant and Fungal Materials
Potted grapevine (Vitis vinifera ‘Chardonnay’ and ‘Cabernet Sauvignon’) was grown in temperature-controlled glasshouses at Waite Campus (Adelaide, South Australia) maintained between 23°C and 25°C. Erysiphe necator (isolate APC1; kindly provided by Eileen Scott, University of Adelaide) was maintained on detached leaves of Cabernet Sauvignon using an 8- to 10-d rotation as described previously (Donald et al., 2002). Leaves of potted vines of grapevine cv Cabernet Sauvignon and cv Chardonnay were inoculated with E. necator using a fine paintbrush and sampled 8 d after inoculation. Healthy and powdery mildew-infected leaves were of similar developmental ages and always sampled at the same time of day.
Arabidopsis (Arabidopsis thaliana ecotype Columbia) plants were grown in chambers with a 14-h-light/10-h-dark cycle and maintained at 24°C. Erysiphe cichoracearum was maintained on cucumber (Cucumis sativus) plants grown in chambers with a 16-h-light/8-h-dark cycle and maintained at 22°C. Arabidopsis VvHT5 promoter::GUS homozygous F3 lines were infected with E. cichoracearum using a fine paintbrush.
Downy mildew (Plasmopora viticola) inoculum was collected from infected grapevines in the field and maintained on detached leaves incubated upside down on moist filter paper in large petri dishes at 22°C to facilitate sporulation. P. viticola sporangia were collected by placing sporulating leaves in a 50-mL Falcon tube containing 5 mL of water and agitated to displace the spores. The spore solution was diluted to a concentration of 1 × 106 spores mL−1 before being sprayed on the abaxial surface of fully expanded leaves on glasshouse-grown Cabernet Sauvignon potted vines. Control leaves were sprayed with water alone. Sprayed leaves were enclosed in plastic bags overnight to maintain humidity. Inoculated leaves and water-sprayed controls were sampled 10 d post inoculation and stored at −80°C before RNA was extracted.
ABA was applied as a solution containing 200 mg L−1 ABA and 0.05% (v/v) Tween 20 by spraying onto the upper and lower leaf surfaces. Control leaves were sprayed with a solution containing 0.05% (v/v) Tween 20 only. Duplicate ABA- and control-sprayed leaves were collected at 1, 2, 4, and 6 h after spray application, snap frozen, and stored at −80°C before RNA was extracted from each leaf independently.
For wounding experiments, the adaxial surface of glasshouse-grown Cabernet Sauvignon leaves was gently rubbed with fine sandpaper, and two control and two wounded leaves were sampled at 1, 2, 8, and 24 h after the wound event (9:00 am; GMT + 9.5 h). Leaves were immediately frozen in liquid nitrogen and stored at −80°C before RNA was extracted independently from each leaf.
Grapevine RNA Extraction and cDNA Synthesis
Total RNA was isolated from grape tissues using the sodium perchlorate method as described by Davies and Robinson (1996). Prior to reverse transcription, 100 μg of total RNA was DNase treated using an RNeasy Mini Kit (Qiagen) and an RNase-Free DNase Set (Qiagen) according to the manufacturer's instructions. DNase-treated RNA (2 μg) was reverse transcribed using the SuperScript III First-Strand Synthesis System (Invitrogen) using the oligo(dT)20 primer according to the manufacturer's instructions. Before use in RT-PCR experiments, cDNA reactions were diluted 10-fold to 200 μL with 10 mm Tris-HCl, pH 7.6.
Quantitative RT-PCR Analysis
Gene expression analysis was carried out by RT-PCR using a SYBR Green method on a Rotor-Gene 3000 thermal cycler (Corbett Research) as reported previously (Hayes et al., 2007). Each 15-μL PCR contained 330 nm of each primer (Supplemental Table S2), 3 μL of diluted cDNA, 1× ABsolute QPCR SYBR Green ROX Mix (Integrated Sciences), and water. The thermal cycling conditions used were 95°C for 15 min, followed by 40 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 30 s, followed by a melt cycle of 1°C increment per min from 65°C to 96°C. All primer pairs amplified a single product of the expected size and sequence, which was confirmed by melt-curve analysis, agarose gel electrophoresis, and DNA sequencing. After testing the suitability of actin (Vvi.7514), ubiquitin (Vvi.7131), and β-tubulin (Vvi.5732) for use as reference genes, actin was used for normalization in all experiments. The expression of each target gene was calculated relative to the expression of actin in each cDNA tested using Rotor-Gene 6.0 software (Corbett Research) to calculate cycle threshold values, observe melt profiles, and measure primer pair amplification efficiencies. Q-Gene software (Muller et al., 2002) was used to calculate the mean normalized expression level (and the se) of each gene in each cDNA tested relative to the reference gene using method 2 (Eq. 3 in Table II in Muller et al., 2002).
Invertase Enzyme Assay
Invertase was extracted from grape leaves and enzyme activity was measured using the method of Ruffner et al. (1995), with some modifications from Tang et al. (1996). Briefly, 0.5 g of tissue was ground under liquid nitrogen, 1.8 mL of extraction buffer was added (0.25 m MES, 20 mm Cys-HCl, 20 mm dithiothreitol, 3 mm EDTA, and 5% [w/v] polyethylene glycol 4000, pH 6.5) followed by further grinding. The semithawed slurry was centrifuged at 10,500 rpm for 10 min at 4°C. The supernatant was collected and stored on ice and then used as the soluble invertase extract. The pellet was washed three times by resuspending in 1.8 mL of ice-cold extraction buffer and subsequent centrifugation as above before finally resuspending in 1 mL of acetate buffer (0.2 m Na-acetate and 0.2 m acetic acid, pH 4). To measure invertase activity, 50 μL of cell wall pellet suspension or soluble extract was mixed with 200 μL of acetate buffer (pH 4) and 200 μL of 0.225 m Suc and incubated on a rotating wheel for 40 min at 30°C. To stop the reaction, 500 μL of DNSA reagent (1% [w/v] 3,5-dinitrosalicylic acid, 0.5 m KOH, and 1 m K/Na-tartrate) was added and this mixture was placed in a boiling-water bath for 10 min and then on ice for 5 min. Before the insoluble extract was boiled, tissue debris was removed by centrifugation at 13,000 rpm for 3 min and the supernatant was decanted to a new tube and then boiled. Absorbance was read at 560 nm. Protein concentration in extracts was determined using a DC protein assay kit (Bio-Rad) according to the manufacturer's instructions using bovine serum albumin standards (Fermentas) to make a standard curve.
In Silico Analysis
Putative VvHT sequences were identified using the BLASTp tool at the National Center for Biotechnology Information using each of the AtSTP1 to -14 sequences as protein query sequences. The chromosomal locations of putative VvHT protein sequences within the recently sequenced grapevine genotype PN40024 (Jaillon et al., 2007) were identified with the BLAT-Search tool of the Grape Genome Browser (http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/). Promoter sequences were submitted to the PLACE database (Higo et al., 1999) to identify and map potential regulatory motifs. The PLACE database is served at http://www.dna.affrc.go.jp/PLACE/signalscan.html.
VvHT5 Promoter Isolation
A 1,988-bp fragment corresponding to bases −3 to −1,990 upstream of the VvHT5 open reading frame start codon and a 997-bp fragment corresponding to bases −3 to −999 were amplified from Cabernet Sauvignon genomic DNA with Platinum Taq HiFi (Invitrogen) using the forward primers HT5-2k and HT5-1k (incorporating a 5′ HindIII site) in combination with HT5-R (incorporating a 5′ NcoI site; Supplemental Table S1). The PCR fragments were cloned into pGEM T-Easy Vector System I (Promega) and verified by sequencing. The pCAMBIA1301 binary vector was digested with HindIII and NcoI (partial digest) to remove the 35S promoter that drives GUS expression and replaced with HindIII/NcoI fragments of the 1,988- and 997-bp VvHT5 promoter region to generate the constructs VvHT5-2K promoter::GUS and VvHT5-1K promoter::GUS. Constructs were transformed into Agrobacterium tumefaciens strain AGL1 for Arabidopsis transformation.
Arabidopsis Transformation and GUS Histochemical Staining
VvHT5 promoter::GUS constructs were transformed into Arabidopsis ecotype Columbia using the floral dip method (Clough and Bent, 1998). Transformant Arabidopsis plants were selected on half-strength Murashige and Skoog plates supplemented with 15 μg mL−1 hygromycin B (Clontech). Localization of GUS activity in homozygous transgenic F3 Arabidopsis plants containing VvHT5::GUS fusion constructs followed the method of Jefferson (1987) using 2 mm 5-bromo-4-chloro-3-indolyl-β-glucuronic acid (Gold Biotechnology) and incubation at 37°C for 16 h. Chlorophyll was cleared by incubation of stained tissue in 90% ethanol. To visualize E. cichoracearum fungal structures following GUS staining, leaves were stained with Coomassie Brilliant Blue according to Schweizer et al. (1993). Imaging was performed using a Zeiss Axioplan 2 microscope and a digital camera.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number EF122147.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Acid invertase activity assays on soluble (vINV) and insoluble (cwINV) extracts from healthy and powdery mildew-infected leaves.
Supplemental Table S1. Monosaccharide-H+ symporter (STP subfamily) genes in grapevine.
Supplemental Table S2. Oligonucleotide primers used in this study.
Supplementary Material
Acknowledgments
We are grateful to Dr. Jim Speirs (Commonwealth Scientific and Industrial Research Organization Plant Industry, Adelaide) for providing VvZEP and VvNCED1 gene-specific primers. We acknowledge the excellent technical assistance of Nicole Kempster (Commonwealth Scientific and Industrial Research Organization Plant Industry).
References
- Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. (1997) Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 9: 1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergh B, De Vleesschauwer D, Hofte M. (2008) Global switches and fine-tuning: ABA modulates plant pathogen defense. Mol Plant Microbe Interact 21: 709–719 [DOI] [PubMed] [Google Scholar]
- Atanassova R, Leterrier M, Gaillard C, Agasse A, Sagot E, Coutos-Thevenot P, Delrot S. (2003) Sugar-regulated expression of a putative hexose transport gene in grape (Vitis vinifera). Plant Physiol 131: 326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boter M, Ruiz-Rivero O, Abdeen A, Prat S. (2004) Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev 18: 1577–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Rast DM, Ruffner HP. (1986) Partitioning of photosynthate in leaves of Vitis vinifera infected with Uncinula necator or Plasmopora viticola. Physiol Mol Plant Pathol 29: 285–291 [Google Scholar]
- Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM. (2003) A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol 132: 1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busk PK, Pages M. (1998) Regulation of abscisic acid-induced transcription. Plant Mol Biol 37: 425–435 [DOI] [PubMed] [Google Scholar]
- Büttner M. (2007) The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett 581: 2318–2324 [DOI] [PubMed] [Google Scholar]
- Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R. (2003) A grape ASR protein involved in sugar and ABA signaling. Plant Cell 15: 2165–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HI, Hong JH, Ha JO, Kang JY, Kim SY. (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275: 1723–1730 [DOI] [PubMed] [Google Scholar]
- Chou HM, Bundock N, Rolfe SA, Scholes JD. (2000) Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol Plant Pathol 1: 99–113 [DOI] [PubMed] [Google Scholar]
- Clark J, Hall JL. (1998) Solute transport into healthy and powdery mildew-infected leaves of pea and uptake by powdery mildew mycelium. New Phytol 140: 261–269 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conde C, Agasse A, Glissant D, Tavares R, Geros H, Delrot S. (2006) Pathways of glucose regulation of monosaccharide transport in grape cells. Plant Physiol 141: 1563–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Robinson SP. (1996) Sugar accumulation in grape berries: cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues. Plant Physiol 111: 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Conninck B, Le Roy K, Francis I, Clerens S, Vergauwen R, Halliday A, Smith S, Van Laere A, Van Den Ende W. (2005) Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant Cell Environ 28: 432–443 [Google Scholar]
- de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Egea PR, Bögre L, Grant M. (2007) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J 26: 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald TM, Pellerone F, Adam-Blondon A-F, Bouquet A, Thomas MR, Dry IB. (2002) Identification of resistance gene analogs linked to a powdery mildew resistance locus in grapevine. Theor Appl Genet 104: 610–618 [DOI] [PubMed] [Google Scholar]
- Essmann J, Schmitz-Thom I, Schon H, Sonnewald S, Weis E, Scharte J. (2008) RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol 147: 1288–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hill L, Crooks C, Doerner P, Lamb C. (2009) Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol 150: 1750–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillion L, Ageorges A, Picaud S, Coutos-Thevenot P, Lemoine R, Romieu C, Delrot S. (1999) Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiol 120: 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Rock CD. (2002) Abscisic acid biosynthesis and response. Somerville CR, Meyerowitz EM, , The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0058, http://www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanon AJ, Sauer N, Hall JL, Williams LE. (2003) The monosaccharide transporter gene, AtSTP4, and the cell wall invertase, AtBfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol 132: 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung RWM, Gonzalo M, Fekete C, Kovacs LG, He Y, Marsh E, McIntyre LM, Schachtman DP, Qiu W. (2008) Powdery mildew induces defense-oriented reprogramming of the transcriptome in a susceptible but not in a resistant grapevine. Plant Physiol 146: 236–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D, Able AJ, Dry IB. (2007) Induction of a grapevine germin-like protein (VvGLP3) gene is closely linked to the site of Erysiphe necator infection: a possible role in defense? Mol Plant Microbe Interact 20: 1112–1125 [DOI] [PubMed] [Google Scholar]
- Gómez-Porras JL, Riaño-Pachón DM, Dreyer I, Mayer JE, Mueller-Roeber B. (2007) Genome-wide analysis of ABA-responsive elements ABRE and CE3 reveals divergent patterns in Arabidopsis and rice. BMC Genomics 8: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL, Williams LE. (2000) Assimilate transport and partitioning in fungal biotrophic interactions. Aust J Plant Physiol 27: 549–560 [Google Scholar]
- Hattori T, Totsuka M, Hobo T, Kagaya Y, Yamamoto-Toyoda A. (2002) Experimentally determined sequence requirement of ACGT-containing abscisic acid response element. Plant Cell Physiol 43: 136–140 [DOI] [PubMed] [Google Scholar]
- Hayes MA, Davies C, Dry IB. (2007) Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. J Exp Bot 58: 1985–1997 [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AK, Dry IB, Robinson SP. (1999) Induction of different pathogenesis-related cDNAs in grapevine infected with powdery mildew and treated with ethephon. Plant Pathol 48: 325–336 [Google Scholar]
- Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, et al. (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449: 463–467 [DOI] [PubMed] [Google Scholar]
- Jefferson R. (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 [Google Scholar]
- Johnson DA, Hill JP, Thomas MA. (2006) The monosaccharide transporter gene family in land plants is ancient and shows differential subfamily expression and expansion across lineages. BMC Evol Biol 6: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocal N, Sonnewald U, Sonnewald S. (2008) Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv. vesicatoria. Plant Physiol 148: 1523–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotenberg S, North H, Marion-Poll A. (1999) Molecular biology and regulation of abscisic acid biosynthesis in plants. Plant Physiol Biochem 37: 341–350 [Google Scholar]
- Mohr PG, Cahill DM. (2007) Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato. Funct Integr Genomics 7: 181–191 [DOI] [PubMed] [Google Scholar]
- Moriondo M, Orlandini S, Giuntoli A, Bindi M. (2005) The effect of downy and powdery mildew on grapevine (Vitis vinifera L.) leaf gas exchange. J Phytopathol 153: 350–357 [Google Scholar]
- Muller PY, Jarovjak H, Miserez AR, Dobbie Z. (2002) Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32: 1372–1379 [PubMed] [Google Scholar]
- Norholm MH, Nour-Eldin HH, Brodersen P, Mundy J, Halkier BA. (2006) Expression of the Arabidopsis high-affinity hexose transporter STP13 correlates with programmed cell death. FEBS Lett 580: 2381–2387 [DOI] [PubMed] [Google Scholar]
- O'Connell RJ, Panstruga R. (2006) Tête à tête inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol 171: 699–718 [DOI] [PubMed] [Google Scholar]
- Palm CJ, Costa MA, An G, Ryan CA. (1990) Wound-inducible nuclear protein binds DNA fragments that regulate a proteinase inhibitor II gene from potato. Proc Natl Acad Sci USA 87: 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proels RK, Roitsch T. (2009) Extracellular invertase LIN6 of tomato: A pivotal enzyme for integration of metabolic, hormonal, and stress signals is regulated by a diurnal rhythm. J Exp Bot 60: 1555–1567 [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG. (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10: 372–379 [DOI] [PubMed] [Google Scholar]
- Rock C. (2000) Pathways to abscisic acid-regulated gene expression. New Phytol 148: 357–396 [DOI] [PubMed] [Google Scholar]
- Roitsch T, González MC. (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9: 606–613 [DOI] [PubMed] [Google Scholar]
- Ruffner HP, Hurlimann M, Skrivan R. (1995) Soluble invertase from grape berries: purification, deglycosylation and antibody specificity. Plant Physiol Biochem (Paris) 33: 25–31 [Google Scholar]
- Schofield RA, Bi YM, Kant S, Rothstein SJ. (2009) Over-expression of STP13, a hexose transporter, improves plant growth and nitrogen use in Arabidopsis thaliana seedlings. Plant Cell Environ 32: 271–285 [DOI] [PubMed] [Google Scholar]
- Scholes JD, Lee PJ, Horton P, Lewis DH. (1994) Invertase: understanding changes in the photosynthetic and carbohydrate metabolism of barley leaves infected with powdery mildew. New Phytol 126: 213–222 [Google Scholar]
- Schweizer P, Gees R, Mosinger E. (1993) Effect of jasmonic acid on the interaction of barley (Hordeum vulgare L.) with the powdery mildew Erysiphe graminis f. sp. hordei. Plant Physiol 102: 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soar CJ, Speirs J, Maffei SM, Loveys BR. (2004) Gradients in stomatal conductance, xylem sap ABA and bulk leaf ABA along canes of Vitis vinifera cv. Shiraz: molecular and physiological studies investigating their source. Funct Plant Biol 31: 659–669 [DOI] [PubMed] [Google Scholar]
- Soar CJ, Speirs J, Maffei SM, Penrose AB, McCarthy MG, Loveys BR. (2006) Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Aust J Grape Wine Res 12: 2–12 [Google Scholar]
- Stadler R, Büttner M, Ache P, Hedrich R, Ivashikina N, Melzer M, Shearson SM, Smith SM, Sauer N. (2003) Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol 133: 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton PN, Gilbert MJ, Williams LE, Hall JL. (2007) Powdery mildew infection of wheat leaves changes host solute transport and invertase activity. Physiol Plant 129: 787–795 [Google Scholar]
- Tang X, Rolfe SA, Scholes JD. (1996) The effect of Albugo candida (white blister rust) on the photosynthetic and carbohydrate metabolism of leaves of Arabidopsis thaliana. Plant Cell Environ 19: 967–975 [Google Scholar]
- Ton J, Flors V, Mauch-Mani B. (2009) The multifaceted role of ABA in disease resistance. Trends Plant Sci 14: 310–317 [DOI] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N. (1996) The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors and pathogen challenge. Plant Cell 8: 2169–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. (2009) Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignault C, Vachaud M, Cakir B, Glissant D, Dedaldechamp F, Buttner M, Atanassova R, Fleurat-Lessard P, Lemoine R, Delrot S. (2005) VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J Exp Bot 56: 1409–1418 [DOI] [PubMed] [Google Scholar]
- Voegele RT, Struck C, Hahn M, Mendgen K. (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc Natl Acad Sci USA 98: 8133–8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegele RT, Wirsel S, Möll U, Lechner M, Mendgen K. (2006) Cloning and characterization of a novel invertase from the obligate biotroph Uromyces fabae and analysis of expression patterns of host and pathogen invertases in the course of infection. Mol Plant Microbe Interact 19: 625–634 [DOI] [PubMed] [Google Scholar]
- von Schaewen A, Stitt M, Schmidt R, Sonnewald U, Willmitzer L. (1990) Expression of a yeast-derived invertase in the cell wall in tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J 9: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DP, Baldwin BC, Shephard MC, Scholes JD. (1995) Source-sink relationships in wheat leaves infected with powdery mildew. 1. Alterations in carbohydrate metabolism. Physiol Mol Plant Pathol 47: 237–253 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.