Abstract
Lentiviral vectors are established as efficient and convenient vehicles for gene transfer. They are almost always pseudotyped with the envelope glycoprotein of vesicular stomatitis virus (VSV-G) due to the high titers that can be achieved, their stability, and broad tropism. We generated a novel cocal vesiculovirus envelope glycoprotein plasmid and compared the properties of lentiviral vectors pseudotyped with cocal, VSV-G, and a modified feline endogenous retrovirus envelope glycoprotein (RD114/TR). Cocal-pseudotyped lentiviral vectors can be produced at titers as high as with VSV-G, have a broad tropism, and are stable, allowing for efficient concentration by centrifugation. Additionally, cocal vectors are more resistant to inactivation by human serum than VSV-G-pseudotyped vectors, and efficiently transduce human CD34+ nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse–repopulating cells (SRCs), and long-term primate hematopoietic repopulating cells. These studies establish the potential of cocal-pseudotyped lentiviral vectors for a variety of scientific and therapeutic gene transfer applications, including in vivo gene delivery and hematopoietic stem cell (HSC) gene therapy.
Introduction
Lentiviral vectors are routinely used for stable gene transfer and have shown great promise for gene therapy in preclinical animal models and more recently also in clinical studies. They have several advantages over γ-retroviral vectors including the ability to more efficiently transduce quiescent cells.1 This is thought to be due to their ability to enter the nucleus independently of mitosis, but other differences may also be involved.2 Lentiviral vectors can transduce a variety of nondividing target cells,3,4 and they do not integrate very close to promoter regions as frequently as γ-retroviral vectors,5 an important consideration for gene therapy.6 Additionally, self-inactivating (SIN) lentiviral vectors are likely to have an improved safety profile7,8 and can be produced at high titer.
Lentiviral vectors are commonly pseudotyped with vesicular stomatitis virus envelope glycoprotein (VSV-G) which confers a broad tropism and allows for efficient concentration by centrifugation.9 VSV-G-pseudotyped lentiviral vectors are effective for gene transfer to a wide variety of tissues including liver, muscle, brain, kidney, retina, and hematopoietic cells and efficiently transduce hematopoietic repopulating cells assayed in large animal models.10,11 However, there are some disadvantages to using VSV-G. Toxicity is associated with constitutive expression of VSV-G, which has made generation of stable packaging cell lines difficult,12 and vectors pseudotyped with the VSV-G envelope glycoprotein are inactivated by human serum complement,13 which may limit their use for in vivo delivery, although PEGylation may improve their effectiveness.14 Also, humans may develop potent immune responses against VSV-G after the administration of VSV-G vector–transduced cells which could limit the efficacy of future infusions of VSV-G-pseudotyped vectors.
Thus, alternate pseudotypes have been evaluated including the feline endogenous virus (RD114) glycoprotein. RD114-pseudotyped vectors are resistant to human serum complement,15,16 and RD114-pseudotyped γ-retroviral vectors have been used for in vivo delivery in the canine X–SCID model.17 Sandrin et al.16 developed an RD114-based envelope glycoprotein with a modified transmembrane region (RD114/TR) that allows for efficient pseudotyping and concentration of lentiviral vectors and efficient gene transfer into hematopoietic repopulating cells.18,19,20,21
Cocal virus is in the Vesiculovirus genus and is a causative agent of vesicular stomatitis in mammals. Cocal virus was originally isolated from mites in Trinidad,22 but infections have since been identified in Trinidad, Brazil, and Argentina from insects, cattle and horses. Antibodies to vesiculoviruses are common among people living in rural areas where the viruses are endemic and infections in humans usually result in influenza-like symptoms. The cocal virus envelope glycoprotein shares 71.5% identity at the amino acid level with VSV-G Indiana, and phylogenetic comparison of the envelope gene shows that cocal virus is distinct among the vesiculoviruses, but is most closely related to VSV-G Indiana strains. Cocal virus is serologically distinct from VSV-G Indiana.22,23
Here we evaluated HIV-1-based lentiviral vectors pseudotyped with cocal virus envelope glycoprotein and compared them to VSV-G and RD114/TR pseudotypes. We also compared the efficiency of transduction of hematopoietic repopulating cells in a clinically relevant nonhuman primate model.
Results
Construction of a cocal expression plasmid and efficient pseudotyping of lentiviral vectors
A human codon–optimized version of cocal envelope glycoprotein DNA based on the amino acid sequence reported by Bhella et al.24 was inserted into an expression plasmid (Figure 1a). SIN lentiviral vectors that express enhanced green fluorescent protein (EGFP) pseudotyped with cocal, VSV-G, or the RD114/TR envelope were produced by transient transfection. Protamine sulfate and polybrene are cationic polymers commonly used to enhance infection with retroviruses25 and transduction with retroviral vectors.26 We found that the addition of protamine sulfate at a concentration of 8 µg/ml enhanced transduction of HT1080 cells approximately sevenfold, so it was included for all subsequent experiments. For all three pseudotypes, the titers varied with the amount of envelope glycoprotein used in the transient transfection. We were able to routinely achieve high titers using 3 µg of cocal, 6 µg of VSV-G, or 9 µg of the RD114/TR envelope plasmid in our standard transient transfection protocol. The cocal envelope plasmid has a human codon–optimized open reading frame which may explain in part the lower amount of cocal envelope plasmid required.
Figure 1.
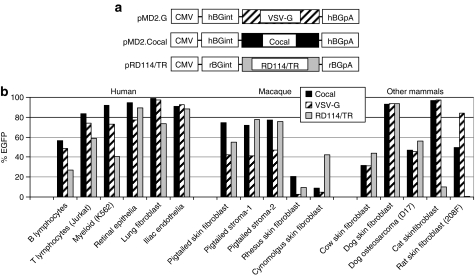
Envelope constructs and cell tropism. (a) Envelope plasmids. All envelopes were expressed from a cytomegalovirus (CMV) promoter with a human or rabbit β-globin intron (hBGint, rBGint) 5′ to the open reading frame (ORF) and a human or rabbit β-globin poly A sequence (hBGpA, rBGpA). The cocal ORF was codon-optimized for human cells. (b) Tropism of cocal envelope relative to vesicular stomatitis virus envelope glycoprotein (VSV-G) and RD114/TR envelopes. The indicated cell lines and primary cell cultures were transduced at a multiplicity of infection of 5 and the percentage of enhanced green fluorescent protein (EGFP)-expressing cells was determined 6 days after vector exposure.
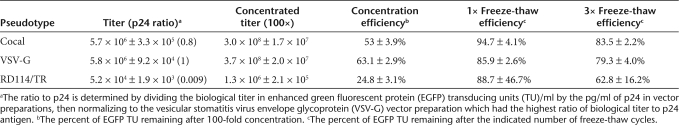
Vector stocks were prepared in triplicate, concentrated 100-fold by centrifugation, and the titers were compared before and after a single freeze-thaw or after three freeze-thaw cycles (Table 1). Lentiviral vectors prepared with either the cocal or the VSV-G envelope reproducibly resulted in concentrated titers of ~108 EGFP transducing units (TU)/ml, whereas pseudotyping with the RD114/TR envelope yielded lower titers and was more variable with a mean titer just over 106 TU/ml. The differences in titer of VSV-G- and cocal-pseudotyped vectors were not statistically significant for unconcentrated (P = 0.67) or concentrated vector preparations (P = 0.12). Analysis of the HIV-1 p24 antigen in vector preparations showed that all pseudotypes contained a similar amount of p24. Thus the ratio of the biological titer in TU/ml to p24 content was much lower for RD114/TR than for cocal or VSV-G which were similar. It is unclear whether this difference reflects an inability of the RD114/TR to efficiently mediate entry relative to VSV-G and cocal, or instead reflects a higher percentage of nonfunctional vector particles generated when using RD114/TR, because the p24 assay only measures HIV-1 Gag protein present, not functional vector particles. During concentration by centrifugation, the cocal envelope retained titer at similar efficiency to VSV-G (P = 0.22) and was as stable after three freeze-thaw cycles (P = 0.41).
Table 1.
Efficiency of concentration and freeze-thaw stability of lentiviral pseudotypes
Cocal-pseudotyped lentiviral vectors have a broad tropism
The high titers and stability of cocal-pseudotyped vectors suggested that they may be effective for many gene transfer applications. We thus compared transduction efficiency in a panel of cell lines and primary cells derived from several tissues that are important therapeutic targets. Transduction was evaluated in human cells and other species commonly used as preclinical models for gene therapy. Figure 1b shows the relative transduction efficiency of transformed cell lines or primary cells derived from blood, retinal epithelia, lung fibroblasts, bone endothelia, skin fibroblasts, and stroma using a multiplicity of infection (MOI) of 5. The cocal envelope mediated efficient transduction of cells from all tissues and most species tested, with the exception of rhesus macaque (Macaca mulatta) and cynomolgus (Macaca fascicularis) cells, where the transduction efficiency was low for all pseudotypes. This is expected due to host cell restriction of the HIV-based lentiviral vector transduction by TRIM5α.27 In pigtailed macaque (Macaca nemestrina) cells, defective TRIM5α isoforms28 allow for infection with HIV-129 and also for efficient gene transfer with lentiviral vectors.10 Overall, gene transfer was similar using cocal and VSV-G in all tissues and species tested with the exception of macaques, where cocal envelope mediated higher gene transfer efficiency relative to VSV-G in all five primary cell types tested. The transduction efficiency using cocal-pseudotyped vectors was also similar to RD114/TR-pseudotyped vector except in the cynomolgus macaque, where RD114/TR mediated the most efficient gene transfer, and in cat and rat fibroblasts where both cocal- and VSV-G-pseudotyped vectors mediated much higher transduction efficiencies than the RD114/TR pseudotype.
Cocal-pseudotyped lentiviral vectors are resistant to human serum
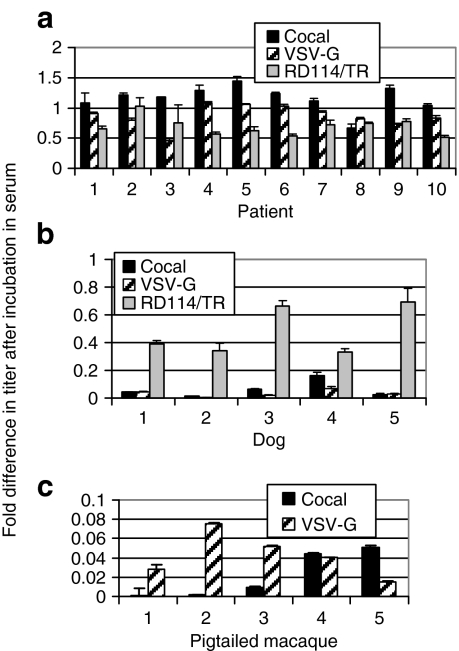
One limitation of VSV-G-pseudotyped lentiviral vectors is that in humans, serum neutralization of VSV-G-pseudotyped vectors limits their effectiveness in vivo.13 VSV-G and cocal envelope glycoproteins have only 71.5% identity at the amino acid level, and VSV-G and cocal vesiculoviruses are distinct serologically,22,23 so we compared the ability of serum from 10 human donors to inactivate each pseudotype (Figure 2a). The level of serum inactivation varied significantly between human individuals (P < 0.0001) for VSV-G and cocal. The level of serum resistance was not significantly different between individuals for RD114/TR (P = 0.12). In humans, cocal-pseudotyped vector virions were overall relatively resistant to serum neutralization. In 7/10 individuals, VSV-G-pseudotyped vectors were neutralized significantly more than cocal-pseudotyped vectors (Pts. #2, 3, 5, 6, 9, P < 0.01, Pts #7, 10 P < 0.05). The RD114/TR-pseudotyped vector was inactivated more than cocal-pseudotyped vector in 6/10 individuals (Pts. #4, 5, 6, 7, 9, 10). One individual's serum (Pt. #8) had a higher neutralizing activity to cocal than VSV-G or RD114/TR but the difference was not significant (P = 0.95 and P = 0.31, respectively). Overall, these data suggest that cocal-pseudotyped vectors may be more effective than VSV-G- or RD114/TR-pseudotyped vectors for in vivo delivery, as they are more resistant to inactivation when incubated in human serum. The ability to produce cocal-pseudotyped vectors at ~100-fold higher titers than RD114/TR pseudotypes (Table 1) should also be taken into consideration for in vivo applications.
Figure 2.
Serum neutralization of lentiviral pseudotypes. Serum from (a) 10 individuals, (b) 5 dogs, and (c) 5 macaques was incubated with vector at 37 °C for 30 minutes then added to HT1080 cells to evaluate the number of enhanced green fluorescent protein (EGFP) transducing units. EGFP expression was evaluated by flow cytometry 3 days after vector exposure, and the percentage of EGFP-expressing cells after incubation in the serum was determined relative to the percentage of EGFP-expressing cells in the vector-only control to determine the fold increase or decrease in titer after exposure to serum. VSV-G, vesicular stomatitis virus envelope glycoprotein.
The relative sensitivity to serum neutralization of VSV-G-, cocal-, and RD114/TR-pseudotyped lentiviral vectors was also compared in dogs, a commonly used large animal model for preclinical gene therapy studies. For all pseudotypes, the level of inactivation varied significantly between dogs (P < 0.001 for RD114/TR and VSV-G and P < 0.05 for cocal). Canine serum potently inactivated both cocal- and VSV-G-pseudotyped vectors, and RD114/TR was significantly more resistant than either cocal or VSV-G (P < 0.01) to inactivation in all five dogs (Figure 2b). In five macaques (Figure 2c), the level of inactivation varied significantly between animals for cocal (P < 0.05), but not for VSV-G (P = 0.95). In 4/5 animals, there was no significant difference in the level of inactivation for cocal or VSV-G pseudotypes, and in one monkey VSV-G-pseudotyped lentiviral vectors were more resistant to neutralization than cocal-pseudotyped vectors.
Efficient transduction of hematopoietic progenitors with cocal-pseudotyped lentiviral vectors
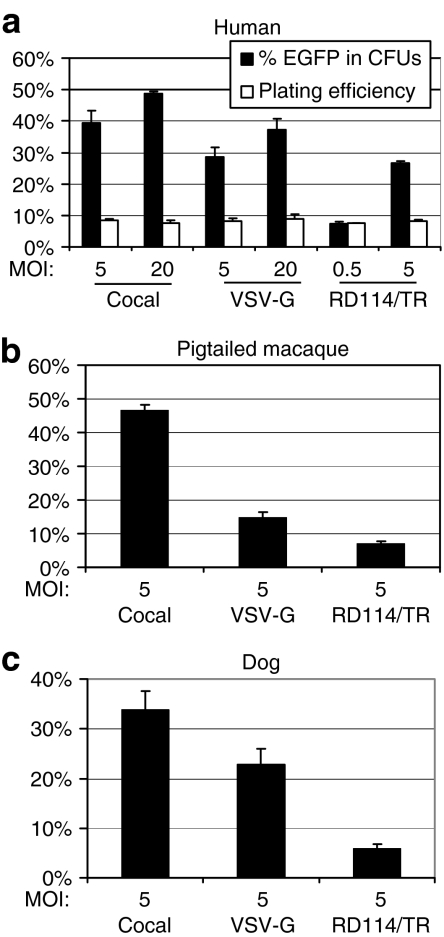
Hematopoietic stem cell (HSCs) are an attractive therapeutic target because these cells can be easily harvested and transduced ex vivo, then reintroduced into patients to reconstitute the entire hematopoietic system. We compared the efficiency of transduction of human CD34+ hematopoietic progenitors using the three envelope pseudotypes at an MOI of 5 or 20 (Figure 3a). The cocal envelope mediated efficient transduction of human hematopoietic progenitors. Analysis of the plating efficiency for all three pseudotypes showed that there was no significant difference in the relative ability of hematopoietic progenitors to form colonies after exposure to either vector preparation (P = 0.12). Thus, in our hands, there was no difference in toxicity to hematopoietic progenitors between vector preparations of the different pseudotypes.
Figure 3.
Comparison of pseudotypes for gene transfer efficiency to hematopoietic progenitors. (a) Human CD34+ cells were exposed to vector for 20 hours and then added to each well of a 24-well plate for colony forming unit (CFU) analysis. The percentage of enhanced green fluorescent protein (EGFP)-expressing CFUs was determined by fluorescent microscopy 14 days after vector exposure. (b) Pigtailed macaque CD34+ cells from three donors were exposed to vector at a multiplicity of infection (MOI) of 5 and the percentage of EGFP-expressing cells was determined 10 days after vector exposure. (c) Dog CD34+ cells from three donors were exposed to vector at an MOI of 5 and the percentage of EGFP-expressing cells was determined 10 days after vector exposure. The mean and the SE from three replicates are shown. VSV-G, vesicular stomatitis virus envelope glycoprotein.
We also compared the transduction efficiency of the three pseudotypes in pigtailed macaque and dog CD34+ cells at an MOI of 5 (Figure 3b,c). Cocal-pseudotyped vectors transduced pigtailed macaque CD34+ cells 3.2- and 6.7-fold more efficiently than VSV-G- and RD114/TR-pseudotyped vectors, respectively. The transduction frequency in pigtailed macaque cells was similar to the transduction efficiency of human CD34+ cells. In dog CD34+ cells, the transduction efficiency was similar for cocal and VSV-G pseudotypes, and the percentage of EGFP-expressing cells was 4.3-fold higher for cocal compared to RD114/TR-pseudotyped vectors at an MOI of 5.
Efficient transduction of NOD-SCID IL-2Rγnull–repopulating cells with cocal-pseudotyped lentiviral vectors
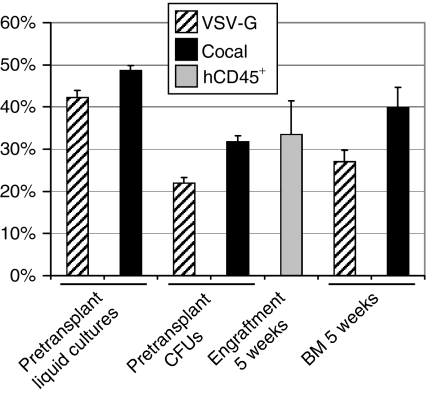
To assess the ability of cocal-pseudotyped vectors to transduce human hematopoietic repopulating cells, we performed a competitive repopulation experiment in the xenogeneic NOD-SCID IL-2Rγnull mouse model. In this approach, engraftment of human CD34+ cells transduced with VSV-G- or cocal-pseudotyped lentiviral vectors is assessed in the same animal eliminating interanimal variability. A lentiviral EGFP vector was pseudotyped with cocal envelope, and an enhanced yellow fluorescent protein (EYFP) vector was pseudotyped with VSV-G envelope. The green and yellow proteins differ only by five amino acids and are thus immunologically highly similar. Transduced repopulating cells can be easily distinguished and accurately quantitated in vivo by flow cytometry. We performed this competitive repopulation transplant in a cohort of five mice at an MOI of 20 using human mobilized peripheral blood CD34+ cells. Engraftment was high, and the marking levels in vivo were very high, especially when taking into account that overall marking was divided between two arms (Figure 4). Marking was significantly higher for the cocal experimental arm in bone marrow (BM) SCID-repopulating cells (SRCs) at 5 weeks after transplantation (P < 0.05).
Figure 4.
Comparison of cocal and vesicular stomatitis virus envelope glycoprotein (VSV-G)-mediated gene transfer to human CD34+ severe combined immunodeficient–repopulating cells (SRCs). The mean marking for the cocal-pseudotyped enhanced green fluorescent protein (EGFP)-expressing vector and the VSV-G-pseudotyped EYFP-expressing vector is shown in pretransplant liquid cultures, progenitor cultures, and in human SRCs from mouse bone marrow (BM) 5 weeks after transplantation as determined by flow cytometry. The mean engraftment of human CD45+ cells as determined by flow cytometry is also shown in mouse BM at 5 weeks. The SE from five transplanted mice is shown. CFU, colony forming unit.
Efficient transduction of pigtailed macaque repopulating cells with cocal-pseudotyped lentiviral vectors
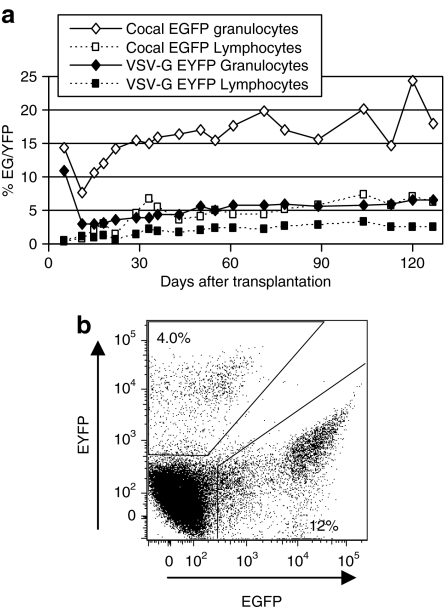
We then compared the relative ability of cocal- and VSV-G-pseudotyped vectors to transduce long-term repopulating cells in a clinically relevant nonhuman primate model. We again employed a competitive repopulation strategy to directly compare engraftment of CD34+ cells transduced with cocal- or VSV-G-pseudotyped lentiviral vectors in the same animal using an MOI of 20. Analysis of pretransplant marking showed ~4.5-fold higher marking in the cocal arm in liquid cultures at day-11 postvector exposure. Marking in colony forming units (CFUs) was similar to these results in liquid cultures (Supplementary Table S1). Engraftment was rapid with 14 days to an absolute neutrophil count >500/µl and 25 days to a platelet count >50,000/µl. The percentage of transgene-expressing cells in the peripheral blood was determined by flow cytometry (Figure 5). The cocal-pseudotyped vector allowed for highly efficient gene transfer with up to 24% marking in granulocytes as assessed by transgene expression and up to 7.3% in lymphocytes. Marking in the cocal arm relative to the VSV-G arm was ~2.8-fold higher in granulocytes and 2.2-fold higher in lymphocytes.
Figure 5.
Transgene expression from cocal and vesicular stomatitis virus envelope glycoprotein (VSV-G)-pseudotyped vectors in macaque peripheral blood cells. (a) The percentages of enhanced green fluorescent protein (EGFP) (cocal) and enhanced yellow fluorescent protein (EYFP) (VSV-G)-expressing leukocytes in peripheral blood detected by flow cytometry are shown. (b) A representative example of the gating used to distinguish EGFP- and EYFP-expressing leukocytes.
No difference in lineage specificity for cocal and VSV-G pseudotypes
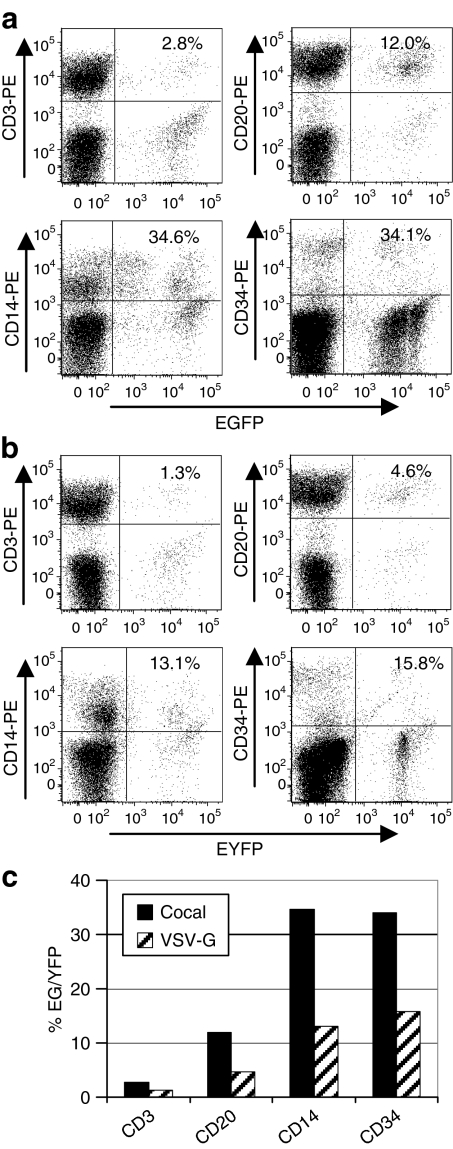
To determine whether cocal and VSV-G pseudotypes transduce myeloid and lymphoid repopulating cells at similar relative efficiencies, the marking in CD3+ T lymphocytes, CD20+ B lymphocytes, and CD14+ myeloid cells was compared to the marking in CD34+ BM cells for cocal (Figure 6a) and VSV-G (Figure 6b). The cocal pseudotype was more efficient than VSV-G in all lineages examined as expected based on transgene expression data in total lymphoid cells and granulocytes in Figure 5. There were no obvious differences in the relative transduction frequency for any lineage relative to BM CD34+ cells (Figure 6c). The transduction frequency for CD14+ cells relative to CD34+ cells was similar for both VSV-G and cocal. For both pseudotypes, transgene expression was lower in CD20+ B lymphocytes and lowest in CD3+ T lymphocytes relative to CD34+ cells.
Figure 6.
Comparison of cocal and vesicular stomatitis virus envelope glycoprotein (VSV-G)-mediated gene transfer in peripheral blood leukocyte subpopulations and bone marrow (BM) CD34+ cells. The percentage of leukocytes that (a) express enhanced green fluorescent protein (EGFP) (cocal) or (b) express enhanced yellow fluorescent protein (EYFP) (VSV-G) in different leukocyte subpopulations was determined using phycoerythrin (PE)-labeled lineage-specific antibodies. PB CD3+ T lymphocytes, CD20+ B lymphocytes, and CD14+ myeloid cells are shown at day 104 after transplantation. For each subset either EYFP- (a), or EGFP- (b) positive cells in this subset are excluded by gating and the percentage of cocal EGFP-positive cells (a) or VSV-G EYFP-positive cells (b) are shown. (c) The percentage of EYFP/EGFP cells in each lineage is graphed for each experimental arm. For both pseudotypes, transgene-expressing cells were found in all lineages examined at similar frequencies relative to BM CD34+ cells.
Discussion
We describe here efficient pseudotyping of lentiviral vectors using a novel human codon–optimized envelope of cocal vesiculovirus glycoprotein.24 Cocal-pseudotyped vectors can be produced at high titer, are stable, have a broad tropism, and efficiently transduce human SRCs and also long-term repopulating cells in a nonhuman primate model. They are also resistant to human serum, and may be highly effective for in vivo delivery of transgenes for many applications including gene therapies and vaccines.
Cocal envelope glycoprotein mediated efficient transduction of cell types from several important therapeutic target cell types including skin fibroblasts, stroma, and hematopoietic progenitors. One interesting observation was that at a relatively low MOI (5) lentiviral vectors pseudotyped with cocal envelope consistently transduced pigtailed macaque cells more efficiently than VSV-G-pseudotyped vectors, but had similar transduction efficiency in human cells. It is currently unclear why we observed more efficient transduction of macaque cells with the cocal envelope. Cocal envelope may be a better pseudotype than VSV-G for studies in the pigtailed macaque model, as transduction efficiency was similar to human cells, suggesting gene transfer efficiency in patients may be better predicted.
Vector virions pseudotyped with cocal envelope were resistant to inactivation when incubated with human serum in 7/10 individuals tested. We found that cocal-pseudotyped vectors may be more effective for in vivo gene delivery in individuals with high levels of pre-existing serum neutralizing activity to VSV-G. Additionally, because different individuals have different pre-existing serum resistance to cocal and VSV-G pseudotypes, the choice of pseudotype for in vivo delivery might be tailored for a specific individual by performing a simple serum neutralization test prior to vector administration. Also for in vivo applications such as vaccines where two vector administrations are necessary, using cocal and VSV-G sequentially may be beneficial. In future preclinical animal studies it will be interesting to evaluate in vivo delivery with cocal, and to determine whether animals injected with VSV-G-pseudotyped vectors that develop specific antibody-mediated serum resistance to VSV-G are more permissive for a second vector dose of cocal-pseudotyped vectors, or vice versa.
Transduction of HSCs is limited by several factors which include the quiescence of the target long-term repopulating cell, and also the availability of the host envelope receptor that mediates efficient entry of viral vectors.30,31 We observed efficient transduction of human mobilized peripheral blood human SRCs with cocal-pseudotyped vector. Transduction efficiency was modestly but significantly improved over VSV-G in a cohort of five mice in this model. Early SRCs may not model the most primitive human repopulating cells,32,33 so we also used a more stringent assay, long-term repopulation in the macaque nonhuman primate model, to compare cocal and VSV-G envelopes. We also observed higher marking in macaque repopulating cells using cocal-pseudotyped vectors than with VSV-G-pseudotyped vectors in long-term repopulating cells (up to 127 days post-transplant). For both pseudotypes, we observed a similar efficiency of transgene expression in myeloid and lymphoid lineages relative to transgene expression in CD34+ BM cells. Also, for both pseudotypes, marking up to 127 days post-transplant was higher in short-lived granulocytes than in long-lived lymphocytes, which is similar to what we have observed in previous studies with VSV-G where the marking in lymphocytes gradually rises at later time points.10 It will be of interest to monitor marking in this animal longer term to determine whether obvious differences in lineage marking occur in longer-term repopulating cells. Additional studies will be needed to confirm our observation that cocal is more efficient than VSV-G for HSC gene transfer in the nonhuman primate model. However, we have demonstrated that cocal envelope can mediate efficient HSC gene transfer in this clinically relevant model. It is important to note that in both in vivo models, that only one of two experimental arms was performed using cocal. Although we report here very efficient transduction, the percentage of gene-modified repopulating cells should be even higher if only one experimental arm with cocal is used without a competing VSV-G arm.
In summary, we have developed a novel and effective pseudotype for lentiviral vectors and demonstrated efficient transduction of several cell types important for gene therapy. Cocal-pseudotyped lentiviral vectors will be useful for many scientific and therapeutic gene transfer applications, and may be particularly useful for in vivo delivery in humans due to their resistance to inactivation by human serum.
Materials and Methods
Construction of the cocal envelope pseudotype plasmid. The deduced open reading frame from the published sequence of cocal envelope24 was used to generate a human codon–optimized nucleotide sequence that was synthesized by Blue Heron Biotechnology (Bothell, WA) using GeneMaker technology according to the sequence specified with ClaI and StuI flanking restriction sites. The optimized cocal open reading frame was subcloned by standard techniques into pMD2.G kindly provided by Didier Trono, Lausanne, Switzerland (Addgene plasmid #12259), replacing the nucleotide sequence of the VSV-G open reading frame to create pMD2.CocalG. The sequence is available upon request.
Production of pseudotyped lentiviral vector preparations and determination of titer. The SIN HIV vectors used were pRRLSIN.cPPT.PGK-GFP.WPRE (Addgene #12252) and pRRLSIN.cPPT.PGK-YFP.WPRE (kindly provided by Luigi Naldini, San Raffaele Telethon Institute for Gene Therapy, Italy) which contain a central polypurine tract, a woodchuck post-transcriptional regulatory element, and an internal phosphoglycerate kinase promoter driving expression of EGFP or EYFP. HIV-based vectors were produced by transient transfection of human embryonic kidney (HEK) 293 cells34 by calcium phosphate method as previously described11 or by polyethylenimine method35 with 27 µg of vector plasmid, 17.5 µg of the lentiviral helper plasmid pCMVΔR8.74,36 and 3 µg (cocal), 6 µg (VSV-G), or 9 µg (RD114/TR) of each envelope plasmid. For polyethylenimine-mediated transfection, the plasmid DNA was added to 2 ml of serum-free Dulbecco's modified Eagle's medium, and 3 µl of 1 µg/µl polyethylenimine (25 kd; Linear Cat. #23966 from Polysciences, Warrington, PA) per µg of DNA was added. The solution was immediately mixed by vortexing for 10 seconds and allowed to stand at room temperature for 15 minutes. The solution was then added dropwise to the HEK 293 cells and all subsequent steps were the same as for calcium phosphate–mediated transfection. Vectors were concentrated 100-fold by centrifugation for 20–22 hours at 6,300g at 4 °C. For freeze-thaw experiments, the vectors were frozen at −70 °C for 1 hour, placed in a 37 °C water bath until completely thawed, and then removed to room temperature. The titer of vector preparations was determined by adding vector preparations to HEK 293 cells or human HT1080 fibrosarcoma cells37 plated at 1 × 105 cells/ml the day before vector addition. Protamine sulfate was added immediately before addition of vector at a final concentration of 8 µg/ml. Transduced cells were assayed by flow cytometry 3–4 days after vector exposure, and the percentage of EGFP-expressing cells was used to calculate the number of EGFP TU/ml of vector preparation. The p24 content of vector preparations was determined using an Alliance HIV-1 p24 antigen ELISA kit (Perkin Elmer, Waltham, MA) following the manufacturer's instructions.
Evaluation of cocal pseudotype tropism in cell lines and primary cell cultures. All cell cultures were supplemented with 10% fetal bovine serum (FBS) and penicillin and streptomycin unless otherwise stated. Epstein–Barr virus transformed human B lymphocytes (Cat. #AG09387), SV40-transformed fetus-derived human retinal epithelia (Cat. #AG06096), SV40-transformed human iliac artery–derived endothelial cells (Cat. #AG10427), untransformed M. nemestrina skin fibroblasts (Cat. #AG08426), untransformed M. mulatta skin fibroblasts (Cat. #AG06249), untransformed M. fascicularis skin fibroblasts (Cat #AG21329), untransformed Bos taurus skin fibroblasts (Cat. #AG08130), untransformed Canis familiaris skin fibroblasts, and untransformed Felis catus skin fibroblasts (Cat. #GM06207) were obtained from the Coriell Institute for Medical Research (Camden, NJ) and cultured as directed. Human IMR90 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA; Cat. #CCL-186) and cultured in Eagle's minimum essential medium with 15% FBS. D17 canine osteosarcoma cells (ATCC; Cat. #CRL-6248) and rat 208F embryo fibroblasts38 were cultured in Dulbecco's modified Eagle's medium. Human Jurkat cells were cultured in RPMI-1640,39 and K562 myelogenous leukemia cells40 were cultured in Iscove's modified Dulbecco's medium (IMDM). Macaque stromal cells were isolated from M. nemestrina BM aspirates that were depleted of red blood cells by two washes with hemolytic buffer (150 mmol/l ammonium chloride, 12 mmol/l sodium bicarbonate, 0.1 mmol/l EDTA), and nucleated cells were plated between 1 × 106 and 2 × 106 cells/ml in tissue culture–treated T-75 flasks and cultured for 3–4 weeks in α-minimum essential medium with 20% FBS and L-glutamine. For the first 3 days after plating, half of the media was replaced in each flask daily, and after the first 3 days, the medium was changed every third day. Cells were passaged once or twice per week upon reaching ~80% confluence. The absence of hematopoietic markers was confirmed after 3–4 weeks of culture by immunostaining and flow cytometry. For all cell types, cells were plated at 1 × 105 cells/ml the day before vector addition, and vector was added at an MOI of 5 based on the titer on HEK 293 cells. Gene transfer was evaluated by flow cytometry for EGFP expression 6 days after vector exposure.
Serum neutralization assays. Human serum samples were obtained under an institutional review board–approved protocol at Fred Hutchinson Cancer Research Center and stored frozen at −20 °C prior to use. Dogs were raised and housed at the Fred Hutchinson Cancer Research Center and macaques were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Serum (20 µl) was mixed with VSV-G, cocal, or RD114/TR vector preparations with 5 × 105 (cocal and VSV-G) or 5 × 104 (RD114/TR) EGFP TU in triplicate, incubated at 37 °C for 30 minutes, then added to 1 × 105 HT1080 cells. A vector-only control was also incubated at 37 °C for 30 minutes. Gene transfer was evaluated by flow cytometry for EGFP expression 3 days after vector exposure, and the percentage of EGFP-expressing cells after incubation in the serum was determined relative to the percentage of EGFP-expressing cells in the vector-only control to determine the fold increase or decrease in titer after exposure to serum.
Isolation and transduction of CD34+ hematopoietic progenitors. Human CD34+ cells were collected from volunteers under an institutional review board–approved protocol and isolated by magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions and stored in liquid nitrogen until use. CD34-enriched primate cells were isolated using the 12.8 IgM anti-CD34+ antibody and MACS IgM microbeads (Miltenyi Biotec) according to the manufacturer's instructions as previously described10 and stored in liquid nitrogen until use. Canine BM CD34+ cells were isolated as previously described41,42 using the biotinylated monoclonal antibody 1H6 (IgG1 anti-canine CD34) and used without cryopreservation.
Human CD34+ cells were thawed and cultured overnight in IMDM with 10% FBS, 100 ng/ml each of Flt-3 ligand, stem cell factor (SCF), megakaryocyte growth and development factor (MGDF), interleukin 3 (IL-3), interleukin 6 (IL-6), and granulocyte colony–stimulating factor (G-CSF). 1 × 105 CD34+ cells were then added to each well of a 24-well plate pretreated with CH-296 fibronectin fragment (Takara, New York, NY) at 2 µg/ml and exposed to vector at an MOI of 5 for 20 hours with 8 µg/ml protamine sulfate. Cells were then washed, resuspended in medium, and plated for CFU analysis or cultured in tissue culture–treated 24-well plates for 10 days and analyzed by flow cytometry. For CFU assay, CD34-enriched cells (3,000 per 35-mm plate) were cultured in a double-layer agar culture system. Briefly, isolated cells were cultured in α-minimum essential medium (Invitrogen, Carlsbad, CA) supplemented with 25% FBS (HyClone, Logan, UT), 0.1% bovine serum albumin (fraction V; Sigma, St Louis, MO), 1 mmol/l L-glutamine, 50 U/ml penicillin, 50 µg/ml streptomycin, and 0.3% (wt/vol) SeaPlaque agarose (Cambrex, East Rutherford, NJ) overlaid on α-minimum essential medium with 0.5% SeaPlaque agarose (wt/vol) containing 100 ng/ml of SCF, MGDF, IL-3, IL-6, granulocyte–macrophage colony–stimulating factor, G-CSF, 4 U/ml erythropoietin, 25% FBS, 0.1% bovine serum albumin, 1 mmol/l L-glutamine, 50 U/ml penicillin, and 50 µg/ml streptomycin. Cultures were incubated at 37 °C in 5% CO2 in a humidified incubator. All CFU cultures were performed in triplicate. The total number, as well as the number of EGFP-positive colonies, was enumerated at day 14 of culture by fluorescence microscopy to determine the percentage of cells expressing the transgene.
CD34-enriched primate cells were thawed and cultured in IMDM with 10% FBS with 100 U/ml penicillin and 100 µg/ml streptomycin, supplemented with 100 ng/ml each of SCF, Flt3-ligand, IL-3, IL-6, MGDF, and G-CSF for 15–18 hours before transduction. For transduction, cells were supplemented with the same cytokine combination in flasks previously coated with the CH-296 fragment of fibronectin (RetroNectin; Takara, Shiga, Japan) at 2 µg/cm2 and with 8 µg/ml protamine sulfate. The cells were exposed to an initial dose of vector for 16 hours and then exposed to a second dose of vector overnight for 8 hours, then washed, resuspended in medium, and plated in 24-well plates. The cells were analyzed by flow cytometry for EGFP expression on day 10.
Canine CD34-enriched cells were cultured in IMDM with 10% FBS, 10 U/ml penicillin, and 100 µg/ml streptomycin, supplemented with 50 ng/ml canine G-CSF, 50 ng/ml canine SCF, 50 ng/ml Flt3-ligand, and 50 ng/ml MGDF. 1 × 105 Freshly isolated CD34+ cells were plated in each well of a 12-well plate pretreated with CH-296 fibronectin fragment (Takara) at 2 µg/cm2 and supplemented with 8 µg/ml protamine sulfate. The cells were exposed to vector in triplicate at an MOI of 5 for 20 hours, then washed, resuspended in medium and plated in 24-well plates, and analyzed by flow cytometry on day 10.
Xenotransplantation studies in the NOD-SCID IL-2Rγnull mouse model using a competitive repopulation approach. Mice were housed at the Fred Hutchinson Cancer Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Study protocols were approved by the Institutional Animal Care and Use Committee. Human CD34+ cells were thawed and cultured in transduction medium (IMDM) with 10% heat-inactivated FBS, 100 ng/ml each of Flt-3 ligand, SCF, MGDF, IL-3, IL-6, and G-CSF overnight before vector addition. For each experimental arm of the competitive repopulation assay, 7.5 × 105 human CD34+ cells were added to one well of a 12-well plate pretreated with CH-296 fibronectin fragment (Takara) at 2 µg/cm2 and exposed overnight to vector at an MOI of 20 in transduction medium with 8 µg/ml protamine sulfate. The following morning, cells were washed with Dulbecco's phosphate-buffered saline (D-PBS), and cells from each well were resuspended in 200 µl of D-PBS and injected by tail vein into previously irradiated NOD-SCID IL-2Rγnull mice (Cat. #005557; The Jackson laboratory, Bar Harbor, ME). The mice were irradiated with 250 cGy from a 137Cs source at 89.5 cGy/min using a Mark I series 30 JL Shepherd irradiator the day before infusion. To determine the transduction efficiency, CD34+ cells were cultured in transduction medium, and the percentage of EGFP-positive cells was determined by flow cytometry on day 11 after vector exposure. BM aspirates were obtained from the femur using a 28-gauge needle. BM cells were incubated in hemolytic buffer, then washed with D-PBS with 2% FBS and incubated at 4 °C for 30 minutes with phycoerythrin-labeled mouse anti-human CD45-specific antibody (BD Pharmingen, San Jose, CA; Cat. #555483) or isotype control (BD Pharmingen; Cat. #555749), and then washed twice in D-PBS with 2% FBS and analyzed by flow cytometry.
Analysis of marking in the macaque model using a competitive repopulation assay. For macaque CD34+ transductions, CD34-enriched primate cells were cultured in IMDM with 10% FBS with 100 U/ml penicillin and 100 µg/ml streptomycin, supplemented with 100 ng/ml each of SCF, Flt3-ligand, IL-3, IL-6, MGDF, and G-CSF for ~20 hours before vector addition. For transduction, cells were supplemented with the same cytokine combination in flasks previously coated with the CH-296 fragment of fibronectin (RetroNectin; Takara) at 2 µg/cm2 and with 8 µg/ml protamine sulfate. The cells were exposed to an initial dose of vector for 6.5–8 hours and then exposed to a second dose of vector overnight for 17–18 hours at an MOI of 20 in the presence of 1 µg/ml cyclosporine. Following a total of ~44 hours ex vivo, cells were extensively washed and infused into the recipient after myeloablative total body irradiation conditioning. Using this approach, repopulating cells can be transduced by either the EGFP-expressing cocal-pseudotyped vector or the EYFP-expressing VSV-G-pseudotyped vector, but not both. The macaque received a myeloablative dose of 1,020 cGy total body irradiation divided into four fractionated doses of 255 cGy, from a single source linear X-ray accelerator (Linac Systems, Lakewood, NJ) at 7 cGy/min. After infusion of autologous gene-modified cells, the animal received recombinant G-CSF at 100 µg/kg daily until absolute neutrophil count was maintained at or >500/µl. To suppress a potential immune response to the EGFP and EYFP proteins, tacrolimus was orally administered daily at a concentration of 2.5 mg/kg 2 days prior to infusion of vector-exposed cells and continued for 10 days. The concentration was then increased to 3.5 mg/kg until 56 days post-transplant when it was decreased to 1.2 mg/kg and then stopped on day 79. The animal also received standard supportive care including intravenous hydration and broad spectrum antibiotics (ceftazidime, vancomycin, gentamicin), an antiviral agent (acyclovir), an antifungal agent (fluconazole), and transfusions with irradiated pigtailed macaque whole blood for treatment of post-transplant thrombocytopenia. Leukocytes isolated by ammonium chloride red cell lysis from heparinized peripheral blood and BM samples drawn at multiple time points after transplantation were analyzed for EGFP expression on a FACSVantage or FACSCanto (Becton Dickinson, San Jose, CA). Transgene expression in granulocyte, monocyte, and lymphocyte populations was determined by gating based on either forward and right-angle light scatter characteristics or expression of lineage-specific CD markers. The antibodies used for lineage-specific markers included CD3 (clone SP34-2), CD14 (clone M512), CD20 (clone 2H7), and CD34 (clone 563). All antibodies were supplied by Becton Dickinson (Franklin Lakes, NJ) and conjugated to phycoerythrin.
SUPPLEMENTARY MATERIALTable S1. Pre-infusion transduction efficiency and engraftment of pigtailed macaque CD34+ cells.
Supplementary Material
Pre-infusion transduction efficiency and engraftment of pigtailed macaque CD34+ cells.
Acknowledgments
This work was supported in part by grants DK47754, DK56465, DK077806, HL53750, AI061839, and AI063959, from the National Institutes of Health, Bethesda, MD. H.-P.K. is a Markey Molecular Medicine Investigator and recipient of the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research. We thank Brian Swanson, Megan Welsh, and Veronica Nelson for technical assistance. We thank the staff of the Primate Center of the University of Washington and the technicians of the hematology and pathology laboratories of the University of Washington. We thank François-Loïc Cosset, Luigi Naldini, and Didier Trono for supplying reagents. We also acknowledge the assistance of Bonnie Larson and Helen Crawford in preparing the manuscript. H.-P.K. and G.D.T. have filed a provisional patent application regarding technology described here; otherwise the authors have no conflicts of interests.
REFERENCES
- Lewis P, Hensel M., and , Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., and , Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006;344:88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Case SS, Price MA, Jordan CT, Yu XJ, Wang L, Bauer G, et al. Stable transduction of quiescent CD34(+)CD38(-) human hematopoietic cells by HIV-1 based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Harmison G, Kluepfel-Stahl S, Brady RO, Karlsson S., and , Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder AR, Shinn P, Chen H, Berry C, Ecker JR., and , Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E, et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M., and , Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Beard BC, Gooch C, Wohlfahrt M, Olsen P, Fletcher J, et al. Efficient transduction of pigtailed macaque hematopoietic repopulating cells with HIV-based lentiviral vectors. Blood. 2008;111:5537–5543. doi: 10.1182/blood-2007-09-115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn PA, Keyser KA, Peterson LJ, Neff T, Thomasson BM, Thompson J, et al. Efficient lentiviral gene transfer to canine repopulating cells using an overnight transduction protocol. Blood. 2004;103:3710–3716. doi: 10.1182/blood-2003-07-2414. [DOI] [PubMed] [Google Scholar]
- Ory DS, Neugeboren BA., and , Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ, et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther. 2000;2:218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Callahan SM, Auricchio A, Schumer G, Linse KD, Wilson JM, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004;78:912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosset FL, Takeuchi Y, Battini JL, Weiss RA., and , Collins MK. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrin V, Boson B, Salmon P, Gay W, Nègre D, Le Grand R, et al. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100:823–832. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- Ting-De Ravin SS, Kennedy DR, Naumann N, Kennedy JS, Choi U, Hartnett BJ, et al. Correction of canine X-linked severe combined immunodeficiency by in vivo retroviral gene therapy. Blood. 2006;107:3091–3097. doi: 10.1182/blood-2005-10-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nunzio F, Piovani B, Cosset FL, Mavilio F., and , Stornaiuolo A. Transduction of human hematopoietic stem cells by lentiviral vectors pseudotyped with the RD114-TR chimeric envelope glycoprotein. Hum Gene Ther. 2007;18:811–820. doi: 10.1089/hum.2006.138. [DOI] [PubMed] [Google Scholar]
- Neff T, Peterson LJ, Morris JC, Thompson J, Zhang X, Horn PA, et al. Efficient gene transfer to hematopoietic repopulating cells using concentrated RD114-pseudotype vectors produced by human packaging cells. Mol Ther. 2004;9:157–159. doi: 10.1016/j.ymthe.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Hu J, Kelly P, Bonifacino A, Agricola B, Donahue R, Vanin E, et al. Direct comparison of RD114-pseudotyped versus amphotropic-pseudotyped retroviral vectors for transduction of rhesus macaque long-term repopulating cells. Mol Ther. 2003;8:611–617. doi: 10.1016/s1525-0016(03)00239-9. [DOI] [PubMed] [Google Scholar]
- Kelly PF, Donahue RE, Vandergriff JA, Takatoku M, Bonifacino AC, Agricola BA, et al. Prolonged multilineage clonal hematopoiesis in a rhesus recipient of CD34 positive cells marked with a RD114 pseudotyped oncoretroviral vector. Blood Cells Mol Dis. 2003;30:132–143. doi: 10.1016/s1079-9796(03)00005-6. [DOI] [PubMed] [Google Scholar]
- Jonkers AH, Shope RE, Aitken TH., and , Spence L. Cocal virus, a new agent in Trinidad related to vesicular stomatitis virus, type Indiana. Am J Vet Res. 1964;25:236–242. [PubMed] [Google Scholar]
- Travassos da Rosa AP, Tesh RB, Travassos da Rosa JF, Herve JP., and , Main AJ., Jr Carajas and Maraba viruses, two new vesiculoviruses isolated from phlebotomine sand flies in Brazil. Am J Trop Med Hyg. 1984;33:999–1006. doi: 10.4269/ajtmh.1984.33.999. [DOI] [PubMed] [Google Scholar]
- Bhella RS, Nichol ST, Wanas E., and , Ghosh HP. Structure, expression and phylogenetic analysis of the glycoprotein gene of Cocal virus. Virus Res. 1998;54:197–205. doi: 10.1016/s0168-1702(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Manning JS, Hackett AJ., and , Darby NB., Jr Effect of polycations on sensitivity of BALD-3T3 cells to murine leukemia and sarcoma virus infectivity. Appl Microbiol. 1971;22:1162–1163. doi: 10.1128/am.22.6.1162-1163.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornetta K., and , Anderson WF. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. J Virol Methods. 1989;23:187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P., and , Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Brennan G, Kozyrev Y, Kodama T., and , Hu SL. Novel TRIM5 isoforms expressed by Macaca nemestrina. J Virol. 2007;81:12210–12217. doi: 10.1128/JVI.02499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agy MB, Frumkin LR, Corey L, Coombs RW, Wolinsky SM, Koehler J, et al. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science. 1992;257:103–106. doi: 10.1126/science.1621083. [DOI] [PubMed] [Google Scholar]
- Sabatino DE, Do BQ, Pyle LC, Seidel NE, Girard LJ, Spratt SK, et al. Amphotropic or gibbon ape leukemia virus retrovirus binding and transduction correlates with the level of receptor mRNA in human hematopoietic cell lines. Blood Cells Mol Dis. 1997;23:422–433. doi: 10.1006/bcmd.1997.0161. [DOI] [PubMed] [Google Scholar]
- Kurre P, Kiem HP, Morris J, Heyward S, Battini JL., and , Miller AD. Efficient transduction by an amphotropic retrovirus vector is dependent on high-level expression of the cell surface virus receptor. J Virol. 1999;73:495–500. doi: 10.1128/jvi.73.1.495-500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenechea G, Gan OI, Dorrell C., and , Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- Hogan CJ, Shpall EJ., and , Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci USA. 2002;99:413–418. doi: 10.1073/pnas.012336799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC., and , Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P., and , Gardner MB. Characterization of a newly derived human sarcoma cell line (HT-1080) Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Quade K. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology. 1979;98:461–465. doi: 10.1016/0042-6822(79)90569-5. [DOI] [PubMed] [Google Scholar]
- Gillis S., and , Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980;152:1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio CB., and , Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- Goerner M, Horn PA, Peterson L, Kurre P, Storb R, Rasko JE, et al. Sustained multilineage gene persistence and expression in dogs transplanted with CD34(+) marrow cells transduced by RD114-pseudotype oncoretrovirus vectors. Blood. 2001;98:2065–2070. doi: 10.1182/blood.v98.7.2065. [DOI] [PubMed] [Google Scholar]
- Neff T, Horn PA, Valli VE, Gown AM, Wardwell S, Wood BL, et al. Pharmacologically regulated in vivo selection in a large animal. Blood. 2002;100:2026–2031. doi: 10.1182/blood-2002-03-0792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pre-infusion transduction efficiency and engraftment of pigtailed macaque CD34+ cells.