Abstract
Immune responses can be compartmentalized into innate versus adaptive components. This relatively recent dichotomy positioned the innate immune system at the interface between the host and the external environment and provided a new conceptual framework with which to view allergic diseases, including asthma. Airway epithelial cells and dendritic cells are key components of the innate immune system in the nose and lung and are now known to be intimately involved in allergen recognition and in modulating allergic immune responses. Here we review current thinking about how these two key cell types sense and respond to inhaled allergens, and emphasize how an understanding of “allergic innate immunity” can translate into new thinking about mechanisms of allergen sensitization and potentially lead to new therapeutic targets.
Keywords: Innate immunity, Allergy, Asthma, Dendritic cells
Introduction
The compartmentalization of immune responses into innate versus adaptive components was an important recent advance in immunology [1]. As opposed to adaptive immunity, which is the major provenance of B and T lymphocytes, innate immune responses theoretically can encompass many other cell types that respond to infections or tissue injury. Epithelial cells and dendritic cells (DCs) are crucial components of the innate immune system in the airway because they sense and respond to inhaled allergens and particles. Key distinguishing features between the innate and adaptive arms of the immune system include the patterns or antigens recognized, kinetics of activation, and capacity for memory. In contrast to adaptive immune cells, which recognize an extremely broad repertoire of antigens due to genetic recombination of antigen receptor genes, innate immune cells respond to a more limited set of targets via a conserved set of pattern-recognition receptors (PRRs). Similarly, innate immune cells generally respond quickly to activating signals and have a limited capacity for long-term memory.
PRRs tend to be ancient and conserved, and likely evolved to recognize invading pathogens. For example, the Toll-like receptor (TLR) family recognizes a diverse family of pathogen-encoded patterns and evolved at least 100 million years before the rearranged antigen receptors that underlie adaptive immunity. In recent years, genetic epidemiology studies have associated single nucleotide polymorphisms in TLRs with different allergic phenotypes [2–4]. Furthermore, animal models and in vitro studies have firmly implicated TLRs in allergen sensitization and regulation of allergic immune responses [5, 6]. The mechanisms by which TLRs regulate allergic sensitization and inflammation are complex and likely allergen and disease specific [7]. Most studies to date have investigated asthma and atopic dermatitis, whereas less is known about TLRs in allergic rhinitis. One recent study found that a single nucleotide polymorphism in TLR4, which is part of the lipopolysaccharide signaling complex, conferred significant protection from hay fever [8]. In addition to their potential role in disease pathogenesis, natural TLR ligands (as well as synthetic analogues) are being explored as novel adjuvants in allergen immunotherapy. Some TLR/ligand pairs potentially have protective effects in allergen immunotherapy (eg, TLR9 and CpG oligonucleotides [9]). In contrast, other TLR ligands have been linked to allergen sensitization, although the dose and timing of exposure are critical modulating factors (eg, TLR4 and lipopolysaccharide).
In addition to recognizing cell wall components, nucleic acids, and carbohydrates derived from microbes, innate immune receptors respond to an array of endogenous danger signals that are produced or released extracellularly during tissue injury. There has been an explosion of research in this area in recent years, with new PRR-ligand interactions being discovered at a rapid pace [10–13]. Endogenous ligands produced during inflammation or released by dead and injured cells include heat shock proteins, extracellular matrix components (eg, low molecular weight hyaluronic acid), extracellular adenosine triphosphate, high mobility group box 1, modified lipoproteins (eg, oxidized low-density lipoproteins), complement factors, and uric acid crystals that can also be sensed as dangerous [14].
In addition to TLRs, other PRRs include the Dectin family of cell-surface C-type lectins that recognize fungal cell wall components [15], as well as the intracellular Nod-like receptor (NLR) NLRP3. Upon activation, NLRP3 forms a complex with the adaptor protein ASC and pro-caspase-1, which is referred to as the inflammasome [12]. Recent studies have linked the NLRP3 inflammasome with exposure to various pathogens or toxins with caspase-1-dependent processing of cytokines, including interleukin (IL)-1β, IL-18, and IL-33 [16]. Interestingly, the inflammasome was recently demonstrated to be required for activity of the proallergic adjuvant alum [17], suggesting that it may be involved in generation of T-helper type 2 (Th2)-dependent immune responses, although alum can also act in an inflammasome-independent manner [18]. As these cytokines contribute to allergic inflammation [19, 20], targeting components of the inflammasome may hold therapeutic promise in allergic diseases.
Consequences of Innate Immune Activation: Dendritic Cell Recruitment and Migration to Lymph Nodes
An important consequence of innate immune activation is the recruitment and activation of DCs in affected tissues. Respiratory tract DCs are interdigitated throughout the epithelium of the nose and lung and are uniquely poised to respond to tissue injury or infection and alert adaptive immune cells to the presence of “danger” [21••]. Current thinking is that after activation by appropriate danger signals, DCs undergo a complex maturation process involving simultaneous downregulation of endocytosis, enhanced antigen processing and presentation, and secretion of proinflammatory and immunoregulatory cytokines. DC maturation also results in changes in chemokine receptor expression that facilitate homing to regional lymph nodes, where they present peptide antigen in the context of major histocompatibility complex (MHC) (signal 1) as well as costimulatory molecules (signal 2) to naïve T cells. Epithelial and other stromal cells are thought to influence DC maturation and their subsequent ability to activate T cells. DCs then integrate these stromal signals in a way that influences their ability to instruct T-cell homing and T-cell lineage commitment (ie, signal 3). This is a new and exciting area of immunology about which very little is known in human allergic diseases. Experiments in mice clearly show that signals imprinted in DCs during their maturation induce the expression of tissue-specific homing receptors in responding T cells [22]. In the case of skin Langerhans cells, this involves upregulation of chemokine receptors and adhesion molecules on T cells (eg, C-C chemokine receptor [CCR] 10 and cutaneous lymphocyte antigen) that bind to their respective ligands expressed in inflamed skin (eg, C-C chemokine ligand [CCL] 27 and E- and P-selectin) [23]. In the case of gut-educated DCs, this involves upregulation of chemokine receptors and adhesion molecules on T cells (eg, CCR9 and the integrin α4β7) that bind to their respective ligands expressed in inflamed skin (eg, CCL25 and MadCAM-1 [mucosal vascular addressin cell adhesion molecule-1]) [24]. Although it seems likely that similar receptor/ligand pairs will govern T-cell homing to the respiratory tract, to date there is little firm evidence in support of this notion. Precisely when and where naïve allergen-specific T cells are educated in humans is not known. In the case of ubiquitous allergens such as house dust mites and pollens, sensitization likely occurs early in life. Sensitization to some allergens may occur epicutaneously, especially in susceptible children who have defects in the epidermal barrier, whereas other aeroallergens preferentially deposit in the nose and lung. Future studies investigating the precise mechanisms by which allergens activate DCs at mucosal sites to initiate and sustain allergen-specific immune responses will likely prove valuable.
Dendritic Cells Influence Th-Cell Differentiation
Subtle differences during DC maturation can profoundly impact the development of subsequent adaptive immune responses by regulating Th-cell differentiation. In contrast to Th1-promoting DCs, which induce interferon (IFN)-γ-producing Th1 cells via secretion of high amounts of IL-12 family members, the molecular mechanisms by which DCs induce differentiation of other T-cell lineages (eg, Th2 and Th17) are not as well understood. DCs do not produce IL-4, the critical Th2-promoting cytokine. One early hypothesis was that Th2-promoting DCs arose by “default” in the absence of strong TLR signals. This was thought to reflect low-grade DC activation, sufficient to upregulate costimulatory molecules but not enough to induce robust secretion of Th1-promoting cytokines such as IL-12 or IL-23. This provided a cellular and molecular correlate of the hygiene hypothesis, although it is now clear that this default model is an oversimplification and that DCs can actively influence Th2 lineage differentiation. DCs can promote Th2 responses through contact-dependent or soluble signals, and it seems likely that multiple mechanisms are involved, depending on the precise mechanisms of DC activation [25]. Unfortunately, no single cell-surface marker or cytokine discovered to date can reliably identify a “pro-Th2” DC, which must be defined operationally in co-culture with naïve T cells. These assays are cumbersome and not well standardized, which makes it challenging to compare results from different research groups. In a recent and exciting field of study, cytokines produced by airway epithelial cells or epidermal keratinocytes have been shown to induce the maturation of Th2-promoting DCs. These proallergic innate cytokines include thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 [26]. The precise mechanisms by which these cytokines induce the maturation of Th2-promoting DCs include, in part, suppression of IL-12 production, but also induction of cell-surface molecules that preferentially induce IL-4 gene transcription in responding T cells. TSLP-exposed DCs can also perpetuate Th2 responses by promoting survival of memory Th2 cells [27].
Dangerous Allergens: Mechanisms and Consequences of Allergen Recognition by the Innate Immune System
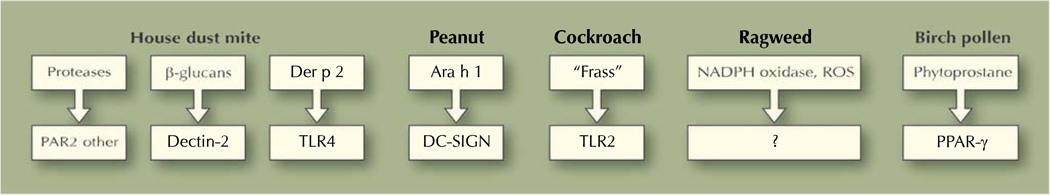
The remainder of this review focuses on allergen-associated danger signals and how they are recognized by innate immune cells and receptors to initiate allergen-specific immune responses. Two general categories of allergen-encoded pro-Th2 signals can be considered, namely protease dependent and protease independent (Fig. 1). It is worth remembering that many allergens will likely activate multiple pathways simultaneously and that in the real world, allergens will be “contaminated” to some degree with lipopolysaccharide and other microbial products that activate TLRs. Thus, understanding how allergen-encoded signals interact with TLR (and other PRR) signaling pathways will be important in future studies. The house dust mite Dermatophagoides pteronyssinus (Der p) is probably the best studied in this regard and is now known to contain discrete allergens, many of which have enzymatic activity. These include the class 1 cysteine protease Der p 1 and class 3 serine protease Der p 3 [28]. A seminal early paper reported that Der p disrupted tight junctions of airway epithelium in a protease-dependent manner [29•], suggesting a mechanism by which inhaled allergen would gain access to innate immune cells in the airway. The cysteine protease activity of Der p 1 seems to be essential for its proallergic properties. For example, Kikuchi et al. [30] recently showed that mice immunized with “protease-dead” Der p 1 (induced with the irreversible inhibitor E-64 or by heat denaturation) did not mount IgE responses when immunized by intraperitoneal injection with alum. Immunization with E-64 did not affect ova-driven IgE responses, suggesting that it was specifically targeting Der p [30]. One possibility is that protease activity of Der p 1 was required for “pro-Th2” DC activation, but this has not been formally investigated to date. Studies with Der p were reminiscent of fungal-associated allergenic proteases, which were found to be potent inducers of Th2-type inflammation even in the absence of T cells [31•].
Fig. 1.
Diagram depicting potential mechanisms whereby allergen-encoded signals could directly activate immature dendritic cells (DCs) and induce their maturation into a T-helper type 2-promoting phenotype. Allergen-associated molecules are depicted in the top boxes, and potential DC receptors are depicted in the bottom boxes. PAR protease-activated receptor; PPAR peroxisome proliferator-activated receptor; ROS reactive oxygen species; SIGN specific intercellular adhesion molecule-3-grabbing nonintegrin; TLR Toll-like receptor
Two general mechanisms have emerged for the protease-dependent effects of Der p, and they are not mutually exclusive (Fig. 1, left side). First, Der p 1 can cleave several cell-surface molecules, including CD23, CD25, and CD40 [32]. This mechanism may be especially apparent after preactivating Der p in vitro with cysteine. Interestingly, dust mite allergen Der p 1 seemed to inhibit IL-12 secretion in lipopolysaccharide-stimulated DC by cleaving CD40 expression [32]. Second, Der p can activate the protease-activated receptor (PAR) family of G-protein-coupled cell-surface receptors [33]. PAR1, the first identified member of this family, was found to be the main thrombin receptor [34]. Different PAR family members have been associated with allergic diseases and asthma, and PAR agonists/antagonists are being developed therapeutically. Allergens seem to activate PAR family members in a cell type-specific manner, and there is currently a lack of consensus in this area. For example, Adam et al. [35] reported that Der p 1 activated A549 cells in a PAR2-independent manner and induced extracellular signal-related kinase 1/2− and nuclear factor κB-dependent IL-8 expression [35]. In contrast, another study suggested that Der p 1 activated PAR2, but not PAR1, in the same cell line [36]. Cockroach allergen was found to stimulate PAR2 on fibroblasts [37], whereas mold allergen pen C 13 activated epithelial cells via both PAR1 and PAR2 [38]. PAR2 may be particularly important for Th2 immune responses and allergic diseases [39]. For example, PAR2 is upregulated in human asthmatic epithelium [40], and PAR2 knockout mice are partially protected from asthma [41]in association with reduced IL-4 secretion [42]. Although it is expressed on keratinocytes and monocytes, a comprehensive understanding of the role of PAR2 and other related family members in allergic immune responses is lacking. Taken together, these studies suggest that strategies that counteract protease activity and signaling may have novel antiallergic effects, and that defective antiproteases may be novel susceptibility genes in allergic diseases.
Protease-Independent Effects of Allergens
Recent studies have uncovered protease-independent pathways by which house dust mites and other allergens activate their target cells. Two groups recently reported that β-glucans present in house dust mite extracts interact with Dectin receptors on epithelial cells and DCs [43, 44]. The consequences of these interactions were cell-type dependent, resulting in generation of cysteinyl leukotrienes by DCs [43] and secretion of the chemokine CCL20 by airway epithelial cells [44]. In contrast, Trompette et al. [45••] showed that Der p 2 is a structural mimic of MD2, a component of the TLR4 complex, and can reconstitute a TLR4 signaling complex. Thus, this groundbreaking article provides an example of allergenic mimicry in which Der p 2 co-opts other established signaling components to activate its target cells. In the case of cockroach allergen, Page et al. [46] recently found that cockroach “frass” (fecal matter) caused allergic airway inflammation and activated neutrophils in mice in a TLR2-dependent manner. Traidl-Hoffmann et al. [47] showed that phytoprostanes associated with birch pollen inhibited DC IL-12 production, resulting in a pro-Th2 DC phenotype, whereas Shreffler et al. [48] reported that the major peanut allergen Ara h 1 was a ligand for DC-SIGN (specific intercellular adhesion molecule-3-grabbing nonintegrin). Thus, different allergens may use different mechanisms to initiate and influence innate immune responses (Fig. 1).
In a seminal paper, Boldogh et al. [49•] reported that major pollen allergens, including ragweed, possess intrinsic NADPH oxidase activity and initiate airway inflammation in an oxidant-dependent manner. Subsequent studies documented the importance of ragweed-associated oxidants in inducing epithelial activation (and, at high concentrations, apoptosis) that could be reversed to differing degrees with antioxidants [50–52]. We recently reported that an identical short ragweed extract induced oxidative stress and activation of CD11c+ DC that was inhibited by the antioxidant N-acetyl cysteine but potentiated in the absence of the master antioxidant transcription factor Nrf2 [53]. Thus, ragweed provides a model allergen to study how oxidative stress is sensed as “dangerous” by the innate immune system.
Which Cell Type is Key: Epithelial Cells, Dendritic Cells, or Basophils?
Many cells can potentially sense and respond to inhaled allergens, and it remains unclear if there is one “key” cell type in this regard in the initiation of proallergic Th2 immune responses. Current candidates include airway epithelial cells, respiratory tract DCs, and tissue basophils. Different cell types may be involved in the response to different allergens, depending on the precise makeup of the inhaled allergenic particles and location in the respiratory tract that they deposit. It is worth noting that many pollens are inhaled together with (and possibly complexed on) particulate matter, carbon-based pollution byproducts that have their own chemical constituents and effects on innate immune cell types [54]. Modeling the deposition of allergens and particulate matter in the respiratory tract is currently challenging. In an elegant recent study, Hammad et al. [55••] used bone marrow chimeras to uncover an essential role for epithelial TLR4 in sensing house dust mite extracts that were instilled intratracheally in mice. The use of chimeric mice allowed these authors to carefully distinguish between the role of TLR4 on hematopoietic cells (eg, DCs) compared with lung structural cells (eg, airway epithelium) in the response to house dust mites, and led to the surprising conclusion that TLR4 on hematopoietic cells was largely dispensable for allergen-induced DC activation. In contrast, a panel of epithelial-derived cytokines seemed to be involved in the response to house dust mite extracts, including thymic stromal lymphopoietin, IL-25, and IL-33 [55••]. It will be interesting to use this approach with other allergens and PRRs and determine its generalizability to other models. In addition to house dust mites, other allergens or allergen extracts have been shown to directly activate epithelial cells and other structural cells (eg, fibroblasts), including ragweed, cockroach, and mold allergen [38, 51]. Pichavant and colleagues [56] showed that Der p 1 stimulated epithelial monolayers to secrete chemokines, including CCL20, that induced DC chemotaxis using a transwell co-culture system. Nathan et al. [43] showed that house dust mite extract and ragweed extract (to a lesser extent) induced CCL20 secretion from epithelial cells in vitro. CCL20 (or macrophage inflammatory protein-3α) is a potent chemoattractant for immature DCs because immature DCs (like naïve T cells) express the CCL20 receptor CCR6. Thus, these studies suggest that in addition to epithelial cells, DCs will be rapidly recruited to the site of allergen deposition to endocytose soluble allergenic proteins. Interestingly, we recently reported that CCL20 is elevated in bronchoalveolar lavage fluids following segmental allergen challenge and correlated strongly with bronchoalveolar lavage lymphocytes, although we did not enumerate DCs in that report [57]. Thus, CCL20 may be a sentinel chemokine that alerts and recruits innate and adaptive immune cells to the lung, and is an attractive therapeutic target in allergic asthma.
In addition to epithelial cells and DCs, recent studies have suggested that basophils play a key role as antigen-presenting cells that induce Th2-type patent of cytokine production in responding T cells. For example, Sokol et al. [58] demonstrated that basophils were essential in eliciting Th2-driven immune responses in mice, using papain as a model allergenic protease. Future research investigating the precise receptors and antigen-presenting cell types activated by inhaled allergens using physiologic model systems and real world conditions will be important and should enhance our understanding of the precise molecular mechanisms involved in allergen sensitization. Future studies investigating allergic innate immune responses in humans will also be important. Although technically more challenging and more limited in scope, human studies will be needed to help determine the degree to which animal model data can be extrapolated to genetically diverse human cohorts.
Conclusions
The field of allergic innate immunity is rapidly growing and attracting the interest of many laboratories and research groups. It seems likely that new allergen-PRR interactions will be uncovered, and it may be possible to define an innate immune signature specific to allergic diseases. New therapeutic targets should be forthcoming as a result of research into the molecular mechanisms of innate immune receptor signal transduction, and it will be particularly interesting to follow research into negative regulation of innate immune receptors. These studies may uncover endogenous inhibitory compounds or pathways that can also be exploited therapeutically. DC-based therapeutics in allergic asthma, based on novel vaccination strategies or drug delivery approaches, are a real possibility in the next 5 years. Technical advances in multicolor flow cytometry should facilitate the identification of antigen-presenting cell subsets that may be particularly important in promoting sensitization to inhaled allergens. Although fundamental new discoveries will continue to be made using murine models, it will be important to remain alert to species-specific differences in innate immune cell lineage and function and expand human translational research in this field.
Footnotes
Disclosure Dr. Georas has served on the speakers’ bureau for Merck & Co.
Dr. Beck has served as a consultant for Regeneron Pharmaceuticals and GlycoMimetics and has received a research grant from Centocor. No other potential conflicts of interest relevant to this article were reported.
References
Papers of particular interests, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 2.Fageras Bottcher M, Hmani-Aifa M, Lindstrom A, et al. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12(p70) responses in Swedish children. J Allergy Clin Immunol. 2004;114:561–567. doi: 10.1016/j.jaci.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 3.Eder W, Klimecki W, Yu L, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113:482–488. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- 4.Kormann MS, Depner M, Hartl D, et al. Toll-like receptor heterodimer variants protect from childhood asthma. J Allergy Clin Immunol. 2008;122:86–92. 92e1–92e8. doi: 10.1016/j.jaci.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RC, Richmond P, Upham JW. Toll-like receptor 2 ligands inhibit Th2 responses to mite allergen. J Allergy Clin Immunol. 2006;117:1148–1154. doi: 10.1016/j.jaci.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder JT, Chichester KL, Bieneman AP. Toll-like receptor 9 suppression in plasmacytoid dendritic cells after IgE-dependent activation is mediated by autocrine TNF-alpha. J Allergy Clin Immunol. 2008;121:486–491. doi: 10.1016/j.jaci.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Horner AA. Toll-like receptor ligands and atopy: a coin with at least two sides. J Allergy Clin Immunol. 2006;117:1133–1140. doi: 10.1016/j.jaci.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 8.Senthilselvan A, Rennie D, Chenard L, et al. Association of polymorphisms of Toll-like receptor 4 with a reduced prevalence of hay fever and atopy. Ann Allergy Asthma Immunol. 2008;100:463–468. doi: 10.1016/S1081-1206(10)60472-3. [DOI] [PubMed] [Google Scholar]
- 9.Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed-Toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 13.Ye Z, Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol. 2008;20:3–9. doi: 10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 15.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 16.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee AS, Munks MW, MacLeod MK, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa Y, Yoshimoto T, Nakanishi K. Contribution of IL-18-induced innate T cell activation to airway inflammation with mucus hypersecretion and airway hyperresponsiveness. Int Immunol. 2006;18:847–855. doi: 10.1093/intimm/dxl021. [DOI] [PubMed] [Google Scholar]
- 20.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 21. Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008.. This was a comprehensive and thoughtful review from two leaders in the field.
- 22.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Homey B, Alenius H, Muller A, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 24.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 25.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YH, Ito T, Homey B, et al. Maintenance and polarization of human Th2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Platts-Mills TA, Vervloet D, Thomas WR, et al. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S2–S24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 29. Herbert CA, King CM, Ring PC, et al. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995;12:369–378. doi: 10.1165/ajrcmb.12.4.7695916.. One of the first studies to demonstrate that an allergen protease increased bronchial epithelial permeability, providing one potential mechanism whereby inhaled allergens could act as “auto-adjuvants” by gaining access to immune and inflammatory cells in the subepithelial space.
- 30.Kikuchi Y, Takai T, Kuhara T, et al. Crucial commitment of proteolytic activity of a purified recombinant major house dust mite allergen Der p1 to sensitization toward IgE and IgG responses. J Immunol. 2006;177:1609–1617. doi: 10.4049/jimmunol.177.3.1609. [DOI] [PubMed] [Google Scholar]
- 31. Kiss A, Montes M, Susarla S, et al. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–342. doi: 10.1016/j.jaci.2007.04.025.. This report provides strong support for the notion that fungal allergenic proteases induce Th2-type allergic inflammation in mice in an innate way, independently of adaptive immune cells and signaling via the TLR adaptor Myd88 or complement proteins.
- 32.Ghaemmaghami AM, Gough L, Sewell HF, Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy. 2002;32:1468–1475. doi: 10.1046/j.1365-2745.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- 33.Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. 2006;4:5. doi: 10.1186/1476-7961-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coughlin SR. How the protease thrombin talks to cells. Proc Natl Acad Sci U S A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adam E, Hansen KK, Astudillo Fernandez O, et al. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006;281:6910–6923. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 36.Asokananthan N, Graham PT, Stewart DJ, et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 37.Kondo S, Helin H, Shichijo M, Bacon KB. Cockroach allergen extract stimulates protease-activated receptor-2 (PAR-2) expressed in mouse lung fibroblast. Inflamm Res. 2004;53:489–496. doi: 10.1007/s00011-004-1287-8. [DOI] [PubMed] [Google Scholar]
- 38.Chiu LL, Perng DW, Yu CH, et al. Mold allergen, pen C 13, induces IL-8 expression in human airway epithelial cells by activating protease-activated receptor 1 and 2. J Immunol. 2007;178:5237–5244. doi: 10.4049/jimmunol.178.8.5237. [DOI] [PubMed] [Google Scholar]
- 39.Henry PJ. The protease-activated receptor2 (PAR2)-prostaglandin E2-prostanoid EP receptor axis: a potential bronchoprotective unit in the respiratory tract? Eur J Pharmacol. 2006;533:156–170. doi: 10.1016/j.ejphar.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 40.Knight DA, Lim S, Scaffidi AK, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 41.Schmidlin F, Amadesi S, Dabbagh K, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 42.Shichijo M, Kondo S, Ishimori M, et al. PAR-2 deficient CD4+ T cells exhibit downregulation of IL-4 and upregulation of IFN-gamma after antigen challenge in mice. Allergol Int. 2006;55:271–278. doi: 10.2332/allergolint.55.271. [DOI] [PubMed] [Google Scholar]
- 43.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol. 2009;123:612–618. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrett NA, Maekawa A, Rahman OM, et al. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trompette A, Divanovic S, Visintin A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548.. An innovative study demonstrating that the house dust mite allergen Der p 2 is structurally similar to MD-2, a key component of the LPS/TLR4 signaling complex. This study suggests that lipid-binding activity may be a shared feature of common allergens enhancing their “auto-adjuvant” activity.
- 46.Page K, Lierl KM, Hughes VS, et al. TLR2-mediated activation of neutrophils in response to German cockroach frass. J Immunol. 2008;180:6317–6324. doi: 10.4049/jimmunol.180.9.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traidl-Hoffmann C, Mariani V, Hochrein H, et al. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med. 2005;201:627–636. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shreffler WG, Castro RR, Kucuk ZY, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 49. Boldogh I, Bacsi A, Choudhury BK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422.. This groundbreaking study established a new mechanism by which allergens may be sensed as dangerous, namely via induction of reactive oxygen intermediates via NADPH oxidase activity, which was detected in most common aeroallergens.
- 50.Bacsi A, Choudhury BK, Dharajiya N, et al. Subpollen particles: carriers of allergenic proteins and oxidases. J Allergy Clin Immunol. 2006;118:844–850. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dharajiya N, Choudhury BK, Bacsi A, et al. Inhibiting pollen reduced nicotinamide adenine dinucleotide phosphate oxidase-induced signal by intrapulmonary administration of antioxidants blocks allergic airway inflammation. J Allergy Clin Immunol. 2007;119:646–653. doi: 10.1016/j.jaci.2006.11.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav UC, Ramana KV, Aguilera-Aguirre L, et al. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PLoS One. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rangasamy T, Williams MA, Bauer S, et al. Nrf2 inhibits the maturation of murine dendritic cells by ragweed extract. Am J Respir Cell Mol Biol. 2009 Oct 5; doi: 10.1165/rcmb.2008-0438OC. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams MA, Rangasamy T, Bauer SM, et al. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–4559. doi: 10.4049/jimmunol.181.7.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hammad H, Chieppa M, Perros F, et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946.. This study demonstrates the power of bone marrow chimeric mice to unravel complex signaling pathways using in vivo models of allergen exposure. The authors conclude that TLR4 expressed on structural cells (and not hematopoietic cells) is crucial for sensing inhaled house dust mite extracts, as well as LPS. It remains to be seen whether this applies to other model allergens or models of exposure.
- 56.Pichavant M, Charbonnier AS, Taront S, et al. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J Allergy Clin Immunol. 2005;115:771–778. doi: 10.1016/j.jaci.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 57.Georas SN, Berdyshev E, Hubbard W, et al. Lysophosphatidic acid is detectable xin human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37:311–322. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 58.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]