Abstract
Dietary restriction (DR) delays or prevents age-related diseases and extends lifespan in species ranging from yeast to primates. Although the applicability of this regimen to humans remains uncertain, a proportional response would add more healthy years to the average life than even a cure for cancer or heart disease. Because it is unlikely that many would be willing or able to maintain a DR lifestyle, there has been intense interest in mimicking its beneficial effects on health, and potentially longevity, with drugs. To date, such efforts have been hindered primarily by our lack of mechanistic understanding of how DR works. Sirtuins, NAD+-dependent deacetylases and ADP-ribosyltransferases that influence lifespan in lower organisms, have been proposed to be key mediators of DR, and based on this model, the sirtuin activator resveratrol has been proposed as a candidate DRmimetic. Indeed, resveratrol extends lifespan in yeast, worms, flies, and a short-lived species of fish. In rodents, resveratrol improves health, and prevents the early mortality associated with obesity, but its precise mechanism of action remains a subject of debate, and extension of normal lifespan has not been observed. This review summarizes recent work on resveratrol, sirtuins, and their potential to mimic beneficial effects of DR.
Keywords: Resveratrol, Sirtuins, Dietary Restriction, Longevity, Mimetic
Introduction
Although genetic manipulations that extend rodent lifespan are being reported with increasing frequency, none have yet exceeded the benefit of dietary restriction (DR), a simple reduction in caloric intake in the absence of malnutrition. This regimen was first described as a means to extend lifespan by McCay and colleagues in 1935 (McCay et al., 1935). By way of comparison, single-gene mutations were first shown to extend lifespan in worms in 1988 (Friedman and Johnson, 1988), and in rodents in 1996 (Brown-Borg et al., 1996). Whereas most genetic manipulations in rodents have been tested only once, with limited phenotypic assessment, the effects of DR have been tested in hundreds of labs, and its effects on nearly every age-related change have been documented (Baur, 2009; Masoro, 2005; Weindruch and Walford, 1988). The overwhelming conclusion is that DR affects something very fundamental to the aging process, as determined by its effects on disparate age-related diseases, as well as detailed analyses of mortality rates (Yen et al., 2008). Among DR's beneficial effects are the delay or prevention of major causes of morbidity and mortality such as cancer, heart disease, neurodegenerative diseases, sarcopenia (age-related loss of muscle mass), and diabetes. While there are some initial tradeoffs in terms of fertility and bone density, and nagging questions about the ultimate effects on the immune system (Ritz et al., 2008), the available evidence suggests that there may be some long-term benefits even on these parameters (Dixit, 2008; McShane and Wise, 1996; Tatsumi et al., 2008). In contrast, there is little information available, and much reason to be concerned about potential trade-offs in long-lived genetically manipulated mice. For example, Snell dwarf mice, which carry a recessive mutation in the Pit-1 gene that affects anterior pituitary development, are long-lived. However, these mice also develop increased adiposity (Flurkey et al., 2001) that might be harmful in humans and are quite frail, to the point where lifespan is shortened if special care is not taken to provide a protective environment (Flurkey et al., 2002). Similarly, telomerase-overexpressing mice are long-lived on a tumor-suppressing background (Tomas-Loba et al., 2008), but in wild type mice this manipulation increases early cancer-related deaths (Gonzalez-Suarez et al., 2005). Because of examples like this, the study of DR remains the most promising approach to discovering a safe and effective way to slow age-related decline in humans.
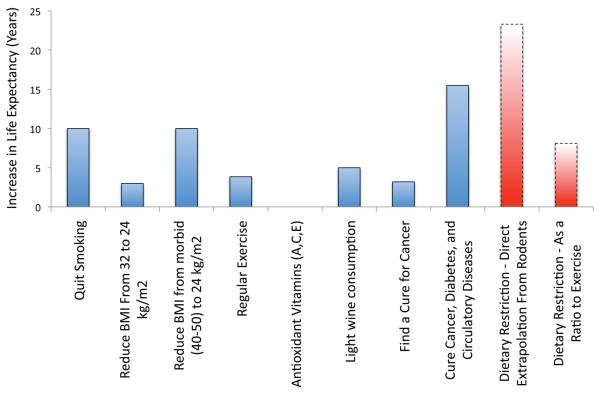
Studies in monkeys and humans have shown that DR improves parameters relevant to health (Anderson and Weindruch, 2006; Lefevre et al., 2009), and in monkeys this translates into a delay in age-related mortality (Colman et al., 2009). Although there are theoretical arguments to suggest that effects on human longevity will be smaller based on evolutionary forces (Phelan and Rose, 2005), or on comparisons between populations with different caloric intakes (Everitt and Le Couteur, 2007), the potential effect of DR is enormous as compared to other factors that can influence lifespan (Figure 1). Moreover, the effects on health that have already been demonstrated could significantly improve the quality of life for many individuals (Lefevre et al., 2009; Weiss et al., 2006). Because it is viewed as unlikely that a large proportion of the population would be willing or able to maintain a DR lifestyle, there has been growing interest in mimicking the effects of DR with drugs (Ingram et al., 2006). These efforts raise important questions about the nature of the DR effect and highlight our ignorance of the mechanisms involved. For example, if one proposes the simple passive hypothesis that caloric input drives metabolic rate, which determines the amount of damage, which is the cause of aging, then it would seem that reproducing the effect without manipulating caloric intake would not be possible. However, this model is clearly not correct, since a number of mutations block the ability of caloric intake to influence lifespan (Anderson et al., 2003; Bonkowski et al., 2006; Panowski et al., 2007), indicating the involvement of an active signaling mechanism. Moreover, the assertion that long-term DR decreases metabolic rate (e.g. (Sohal et al., 2009)) has been called into question (Hempenstall et al., 2010), and some interventions, such as fat-specific deletion of the insulin receptor (Bluher et al., 2003), appear to simultaneously increase longevity and metabolism. To side-step many of these issues, Lane et al selected 2-deoxyglucose, a glycolysis inhibitor, as the first candidate DR mimetic (Lane et al., 1998). Impressively, this relatively non-specific approach to mimicking energy stress recapitulates the increase in insulin sensitivity and decrease in core body temperature observed in DR animals. However, toxicity near the therapeutic doses has prevented studies on longevity, or further development of the molecule for use in humans. A second approach was based on the ability of metformin to promote insulin sensitivity, which is a hallmark of DR (Roth et al., 2001). Strikingly, metformin mimicks a large proportion of the transcriptional changes induced by DR in liver (Dhahbi et al., 2005), and inhibits tumor development (Anisimov et al., 2005). Although the effects of metformin on oxidative metabolism are complex and incompletely understood (Leverve et al., 2003), activation of AMP-activated protein kinase (AMPK), a sensor of energy stress, is thought to play a central role (Zhou et al., 2001). In addition, a related compound, phenformin, was shown in 1980 to extend mouse lifespan, albeit in a tumor-prone strain (Dilman and Anisimov, 1980). However, the precise role of AMPK in DR has been debated (Gonzalez et al., 2004), and phenformin has been pulled off the market as a human drug due to fatal cases of lactic acidosis (Kwong and Brubacher, 1998). While metformin remains an interesting candidate DR mimetic (and as an anti-diabetic, remains one of the most widely prescribed drugs), it is clear that the rational design of interventions to mimic DR will require a better understanding of the underlying mechanisms.
Figure 1.
Estimates of the effects of various interventions on human life expectancy. Note that data from unrelated studies on different populations are combined in this figure. The indicated effect for smoking is based on quitting at the age of 30, although there is a significant benefit to quitting even at advanced ages (Doll et al., 2004; Taylor et al., 2002). The estimated effects of body mass index (BMI) are based on observational studies, and may not accurately reflect the consequences of deliberate weight loss (Whitlock et al., 2009). The effect of exercise compares the highest tertile of physical activity to the lowest based on assessments conducted after the age of 50 years (Franco et al., 2005; Jonker et al., 2006). Vitamins A and E may actually be associated with increased mortality (Bjelakovic et al., 2008). Light wine consumption was defined as less than half a glass per day (Streppel et al., 2009). Only males were included in the study, and life expectancy was calculated at age 50. Of the 5-year increase, 2 years were attributed to alcohol per se, while 3 years were attributed to other components of wine, such as polyphenols (including resveratrol). Effects of disease cures are the estimates of Olshansky et al (Olshansky et al., 1990). Extrapolation of the effect of dietary restriction was based on a 30% increase in mean lifespan, which is typical of rodent studies (Weindruch et al., 1986), and the current Centers for Disease Control (CDC) estimate of life expectancy in the US, 77.7 years. The influence of exercise appears to be greater in rodents than in humans, and this could reflect an inherent difference in the plasticity of lifespan between species. Therefore an alternate approach to estimating the effect of DR in humans is to assume it will be ~2.1 times as effective as exercise, as was the case for rats, albeit at a sub-optimal level of DR (Holloszy et al., 1985). Note that lifestyle changes, particularly the effects of smoking and obesity, are relevant to only a subset of the population. Thus, the changes in individual life expectancy presented here overestimate the potential impact of these interventions on average human lifespan.
Sirtuins as Mediators of DR in Lower Organisms
In 2000, Lin et al proposed that sirtuins, homologues of the yeast Sir2 protein, are critical mediators of the effects of DR (Lin et al., 2000). This hypothesis was based in part on the observation that SIR2 copy number determines yeast lifespan, but was prompted most directly by the discovery that the enzymatic activity of all sirtuins is dependent on the co-substrate nicotinamide adenine dinucleotide (NAD+) (Imai et al., 2000; Smith et al., 2000). Since the reduced form of the molecule, NADH, is a competitive inhibitor of sirtuins, it was suggested that an increase in the NAD+:NADH ratio, driven by a shift to oxidative metabolism, could account for increased sirtuin activity, and subsequently lifespan extension, in yeast subjected to DR (Lin et al., 2004). In support of this hypothesis, deleting the SIR2 gene in wild type yeast completely blocks the ability of glucose restriction (i.e. yeast DR) to extend lifespan (Lin et al., 2000). Further, lifespan extension in eat-2 mutant worms, considered a model of DR due to a defect in pharangeal pumping, is dependent on the Sir2 homolog in that organism (Wang and Tissenbaum, 2006), and lifespan extension by DR has been shown to require a Sir2 homolog in flies (Rogina and Helfand, 2004).
As new data have emerged, it has become clear that the Sir2 story is more complex than it at first appeared. It has been suggested that the concentration of the product inhibitor nicotinamide, rather than NAD+:NADH ratio, is the primary determinant of sirtuin activity (Anderson et al., 2003), and the relative contributions of these two factors have never been fully resolved. In addition, it has been shown that DR can extend yeast lifespan even in respiration-deficient yeast, raising additional questions about how Sir2 activity is linked to DR (Kaeberlein et al., 2005a). More alarming, however, is the observation that a second mutation (fob1) can be introduced into sir2 null yeast to correct the genomic instability that limits lifespan, and the resulting strain is able to respond to DR (Kaeberlein et al., 2004). Whether the basis of this response is an unrelated mechanism, or compensation by Sir2 homologues has been debated (Lamming et al., 2005; Tsuchiya et al., 2006). Further, it has become clear that multiple protocols for “DR” in worms extend lifespan through independent signaling pathways, and several of these work in the absence of the closest Sir2 homolog (Greer et al., 2007). These findings make it clear that the response to DR is more complicated than was initially appreciated, even in lower organisms.
Resveratrol in Lower Organisms and In Vitro
Based on the hypothesis that sirtuins are critical mediators of DR, Howitz et al performed an in vitro screen for small molecule activators of the closest mammalian Sir2 homolog, SIRT1 (Howitz et al., 2003). Their most potent hit was resveratrol, a small polyphenol that was already suspected to be cardioprotective and have cancer chemopreventive activity, supporting the hope that compounds identified in this manner would mimic beneficial aspects of DR. Subsequently, resveratrol was shown to extend yeast, worm, and fly lifespan in a sirtuin-dependent manner (Howitz et al., 2003; Wood et al., 2004). Notably, the benefits of resveratrol have been disputed (Bass et al., 2007; Kaeberlein et al., 2005b), but also reproduced (Bauer et al., 2004; Greer et al., 2007; Jarolim et al., 2004; Viswanathan et al., 2005; Yang et al., 2007), in all three organisms. In addition, resveratrol extends lifespan and delays cognitive decline in N. furzeri, a short-lived species of fish (Valenzano et al., 2006), however, it has not yet been possible to assess the dependence of its effect on sirtuins in this organism.
In addition to the effects discussed above, resveratrol has proven to be a valuable tool for mammalian cell culture, where it has consistently been shown to produce SIRT1-dependent effects (for example, see (Csiszar et al., 2009; Picard et al., 2004)). It therefore created considerable confusion when two groups reported that in vitro activation of SIRT1 by resveratrol is dependent on the use of a fluorescent substrate (Borra et al., 2005; Kaeberlein et al., 2005b). This has alternately been interpreted to mean that resveratrol does not activate sirtuins at all, that activation of sirtuins by resveratrol proceeds through an indirect mechanism, or that the fluorescent assay recapitulates an important aspect of in vivo biology more accurately than the non-fluorescent version. The most obvious way in which this last possibility might occur is that the addition of a bulky, hydrophobic molecule to the peptide substrate could better mimic the steric constraints experienced by endogenous proteins that SIRT1 targets. In support of this possibility, SIRT1 has a much lower Km for unconjugated peptide substrates (Borra et al., 2005; Kaeberlein et al., 2005b). However, Pacholec et al have recently challenged this argument by showing that resveratrol does not activate SIRT1 even against full-length protein substrates in the absence of a fluorophore (Pacholec et al., 2010). There is now abundant evidence that resveratrol has SIRT1-dependent effects in mammalian cells, but resolving whether this occurs through a direct or indirect mechanism is likely to require a structural explanation, or identification of the putative upstream pathway. In the meantime, testing the hypothesis that resveratrol will ultimately mimic beneficial aspects of DR in mammals requires the consideration of additional factors, such as off-target effects (discussed in more detail below) and pharmacokinetic parameters, which must be examined in vivo.
Does Resveratrol Mimic Dietary Restriction in Rodents?
At the phenotypic level the assertion that resveratrol mimics DR in lower organisms relies heavily on lifespan extension. This raises an important question about what constitutes a DR mimetic. In the broadest sense, a DR mimetic could be considered any compound that produces a beneficial effect of DR through the same mechanism without the need to restrict energy intake. By this definition, lifespan extension in lower organisms makes resveratrol a DR mimetic, as long as activation of Sir2 (or any other common process) is the mechanism in both cases. In mammals, DR has many well-documented effects, affording an opportunity to ask first whether resveratrol has similar benefits, and second, whether these effects are mechanistically related to DR.
A significant body of work on resveratrol existed even prior to the suggestion that it might be a DR mimetic, largely because of a 1997 report showing that it inhibits carcinogenesis in rodents (Jang et al., 1997). Following that report, resveratrol was convincingly shown to protect against additional tumor types, various aspects of heart disease and stroke, and inflammatory conditions (for a review, see (Baur and Sinclair, 2006)). These findings are all consistent with the suggestion that resveratrol might act as a DR mimetic, and have inspired further efforts to test parameters more directly relevant to DR. We and others have since shown that resveratrol prevents insulin resistance, enhances mitochondrial biogenesis, and restores normal longevity in obese mice, while inducing transcriptional profiles that resemble those of animals consuming fewer calories (Barger et al., 2008; Baur et al., 2006; Lagouge et al., 2006; Pearson et al., 2008). At the same time, it is clear that resveratrol fails to mimic other aspects of DR, such as slowing heart rate and decreasing core body temperature (Mayers et al., 2009), or thus far, extending lifespan in non-obese animals (Pearson et al., 2008).
Interestingly, the effects of resveratrol on insulin sensitivity and longevity have thus far been observed only in the context of obese, insulin resistant animals. While the effect of a DR mimetic in the context of obesity is complicated to predict, improved glucose tolerance and longer survival are generally encouraging, and consistent with previous evidence that the positive effects of DR can trump detrimental effects of adiposity (Harrison et al., 1984). However, DR further enhances insulin sensitivity and longevity in normal mice, and resveratrol has not been shown to possess this property, suggesting an important difference between the two. In terms of longevity, this comparison may not be entirely fair, since resveratrol has been tested only beginning at one year of age, a point at which the ability of DR to extend life is significantly diminished, for reasons that are not yet clear (Weindruch and Walford, 1982). Nevertheless, taking the existing data at face value, it is tempting to suggest that resveratrol confers a subset of the beneficial effects of DR, most directly related to fat metabolism and cardiovascular health. In fact, necropsy data from our studies supports the conclusion that lifespan extension in the obese groups was almost entirely due to the prevention of deaths associated with “fatty changes in the liver, with severe congestion and edema in the lungs”, a syndrome that does not occur in lean mice (Pearson et al., 2008). It is also worth noting that while in our studies, cardiovascular complications are not a significant cause of mortality in C57Bl/6 mice fed a standard diet, they are a very significant cause of mortality in humans.
The improvements in physiology induced by resveratrol are accompanied by an increase in the mitochondrial content of liver, skeletal muscle, and other tissues (Baur et al., 2006; Lagouge et al., 2006), which is intriguing since an increase in mitochondrial biogenesis has been observed in liver, white and brown adipose, brain, and heart tissue from mice (Nisoli et al., 2005), and skeletal muscle from humans (Civitarese et al., 2007), during DR. The extra mitochondria induced by resveratrol treatment may have important roles in liver (Baur et al., 2006), endothelium (Csiszar et al., 2009), and brain (Robb et al., 2008), and appear to have dramatic consequences in skeletal muscle, since resveratrol-treated mice display an approximately 2-fold increase in endurance (Lagouge et al., 2006). Resveratrol increases the mitochondrial content of tissues even in lean mice, which do not show any benefit in terms of longevity. This might be taken to mean that the increase in mitochondrial mass observed during DR is not a critical determinant of lifespan; however, lifespan-extending DR has a more dramatic effect than resveratrol in brain (50-200% increase in various mitochondrial markers for DR vs. ~10% increase in citrate synthase activity following resveratrol treatement), and DR increases the mitochondrial content of heart and adipose tissue, while resveratrol does not (Pearson et al., 2008). In fact, resveratrol appears to repress mitochondrial biogenesis in adipose, raising the possibility that positive and negative effects on different tissues could offset each other. In support of the potential relevance of adipocyte mitochondria to longevity, increased transcription of mitochondrial genes in adipose has been reported as one of the most intriguing phenotypes of long-lived fat-specific insulin receptor knockout (FIRKO) mice (Katic et al., 2007).
At the behavioral level, resveratrol improves the ability of mice to balance on a rotating rod (rotarod), which is most often described as an assay of “motor coordination” (Baur et al., 2006; Lagouge et al., 2006). This may be related to the improvements in endurance and strength, but could also have a cognitive component. In support of this hypothesis, resveratrol has been shown to prevent cognitive decline in a number of disease models, and to reduce neurodegeneration in vivo (Kim et al., 2007; Sharma and Gupta, 2002). However, rigorous assessments of resveratrol's effects on normal cognitive function and age-related cognitive decline in rodents remain to be performed.
In addition to the results discussed above, resveratrol appears to have many other benefits, including improvements in vascular function in concert with a decrease in oxidative stress and apoptotic index, reduction of cataracts, improvements in kidney function, and the enhancement of bone maintenance (Pearson et al., 2008; Su et al., 2007). While all of these findings suggest that resveratrol can have a positive impact on health, the question of whether the effects are specifically the result of DR mimicry is more difficult to address. One approach to this has been the use of microarrays to detect global patterns of transcriptional changes in multiple tissues. Using this technique, Barger et al (Barger et al., 2008) and our group (Pearson et al., 2008) have independently come to the same conclusion that resveratrol broadly mimics the transcriptional changes associated with DR. A concern in the interpretation of these studies is that a large number of transcriptional changes could be secondary to a single common effect, such as weight loss. In this regard, it is important to note that no significant weight loss was observed in the resveratrol-treated group from either study. Since resveratrol did not extend life in non-obese mice, despite these transcriptional changes and improvements in health, these findings also raise the possibility that a small number of transcriptional pathways affected by DR, but not by resveratrol, account for changes in longevity. However, another strong possibility is that the response to resveratrol is “uncoordinated” in that many transcriptional changes go in the right direction, but to varying degrees, mimicking some of the beneficial aspects of DR, but not others. It should also be borne in mind that some of the key events in DR may simply have no obvious transcriptional signature in the tissues or metabolic state examined. Overall, the available data suggest that resveratrol influences many of the same signaling pathways as DR, and that these changes can be induced in the absence of a decrease in energy intake or body weight.
One of the more surprising aspects of this work, given the existing literature on resveratrol, is that deaths related to cancer were not significantly reduced in the treated groups. As noted above, the therapeutic value of resveratrol against a variety of induced and implanted tumors has been reproducibly demonstrated (Baur and Sinclair, 2006), although it is clear that some tumors are not susceptible or show discrepancies between in vitro and in vivo results (Bove et al., 2002; Gao et al., 2002). Most of the cancer-related deaths in our mice (~50% of all deaths) were due to spontaneous lymphomas, and the studies were severely underpowered to make conclusions about other tumor types. In fact, there is little evidence that resveratrol prevents leukemias or lymphomas in any in vivo setting, with the exception of one study in which a lymphoma cell line was implanted subcutaneously (Li et al., 2007). In irradiated p53+/− mice, resveratrol at a dose sufficient to decrease the incidence of characteristic thymic lymphomas had no effect on other lymphomas or leukemias (Oberdoerffer et al., 2008). Therefore, it remains unclear whether our results indicate a lack of effect on most lymphoid tumors, or a lack of effect on spontaneous tumors in general. In either case, DR clearly does prevent spontaneous lymphomas in C57Bl/6 mice (Volk et al., 1994), and reproducing this effect will be an important goal in the future development of DR mimetic drugs.
Is SIRT1 the Critical Target of Resveratrol In Vivo?
Resveratrol treatment leads to deacetylation of SIRT1 targets in vivo, supporting its proposed mechanism of action (Baur et al., 2006; Lagouge et al., 2006). In some cases, these effects have the potential to directly explain observed changes in physiology. For example, SIRT1 deacetylates PGC-1α (Rodgers et al., 2005), which directly activates mitochondrial biogenesis and most likely explains the increase in mitochondria observed in resveratrol-treated animals. However, resveratrol has many other effects in mammalian cells, including direct inhibition of many kinases (Shakibaei et al., 2009), cyclooxygenases (Jang et al., 1997), and quinone reductase 2 (Buryanovskyy et al., 2004), signaling through the estrogen receptor (Gehm et al., 1997) and the aryl hydrocarbon receptor (Ciolino et al., 1998), and activation of AMP-activated protein kinase (AMPK) in cells and in vivo through an unknown mechanism (Baur et al., 2006; Zang et al., 2006). Although SIRT1 can contribute to AMPK activation through deacetylation of the upstream kinase, LKB1 (Lan et al., 2008), this pathway does not appear to be required for resveratrol to have an effect (Dasgupta and Milbrandt, 2007). AMPK activation by resveratrol is intriguing since it could independently account for an increase in mitochondrial biogenesis and fatty acid oxidation, and potentially SIRT1 activation as a downstream consequence (Fulco et al., 2008). In fact, Um et al have recently suggested that this is the case, based on their observation that resveratrol fails to increase glucose tolerance, mitochondrial biogenesis, or endurance in AMPK null mice (Um et al., 2009). However, it is difficult to formally exclude the possibility that SIRT1 might act upstream of AMPK, or to be confident that the metabolic disruption in these animals is not simply too severe to be rescued. The existing data on resveratrol treatment can therefore be said to be broadly consistent with the proposal that SIRT1 is the key mediator of its beneficial effects, but other plausible mechanisms remain, and it is almost certain that multiple targets contribute to some degree.
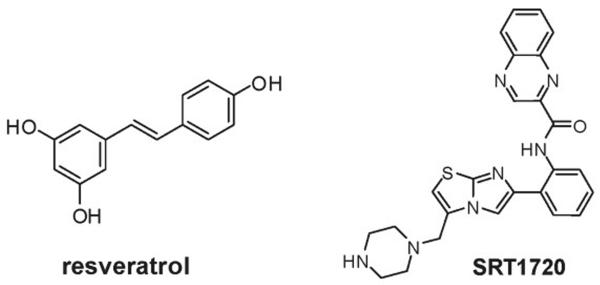
The specific effects of increased SIRT1 activity have been studied both by overexpression of the enzyme and by treatment of animals with a novel activator, designated SRT1720 (Figure 2). It should be borne in mind that despite the lack of a structural relationship between the two, SRT1720 suffers from the same caveat as resveratrol, in that it only activates SIRT1 against fluorescent substrates (Pacholec et al., 2010). Interestingly, both SRT1720 and SIRT1 overexpression improve glucose homeostasis, decrease body weight (which occurs at high doses of resveratrol), prevent fatty liver, and improve rotarod performance, providing support for the assertion that SIRT1 mediates salient effects of resveratrol (Bordone et al., 2007; Feige et al., 2008; Milne et al., 2007; Pfluger et al., 2008). Notably, Pacholec et al have reported that SRT1720 does not lower glucose and may exhibit toxicity in leptin-deficient (ob/ob) mice (Pacholec et al., 2010). This contradicts a previous report (Milne et al., 2007), but is intriguing in light of the fact that resveratrol fails to activate mitochondrial biogenesis in the muscles of ob/ob mice (Mayers et al., 2009), and SIRT1 overexpression tends to increase, rather than decrease, body weight in leptin receptor deficient (db/db) mice (Banks et al., 2008), suggesting a potential interaction between SIRT1 and leptin signaling. Like resveratrol, SRT1720 increases mitochondrial capacity and endurance in wild type mice, although in skeletal muscle this may be due almost exclusively to fiber type switching, without the more dramatic increase in mitochondrial density that is observed after resveratrol treatment (Feige et al., 2008). In microarray experiments, SRT1720 has been shown to mimic many of the effects of DR in liver, and to produce a transcriptional profile that closely resembles the effect of resveratrol treatment (Smith et al., 2009). Importantly, SRT1720 is devoid of the ability to acutely activate AMPK, suggesting that direct action on SIRT1 is sufficient to initiate the changes in physiology that are observed (Feige et al., 2008). Data on mitochondrial content and global transcriptional effects following SIRT1 overexpression have not been reported, but will provide important insight into the specificity of small molecule activators.
Figure 2.
Structures of resveratrol and SRT1720. Resveratrol is a natural product found in grapes and medicinal plants that activates SIRT1 (Howitz et al., 2003) and influences a number of other mammalian enzymes. SRT1720 is a novel SIRT1 activator described by Sirtris Pharmaceuticals (Milne et al., 2007), which is not thought to share off-target effects with resveratrol, with the possible exception of the norepinephrine transporter (Pacholec et al., 2010).
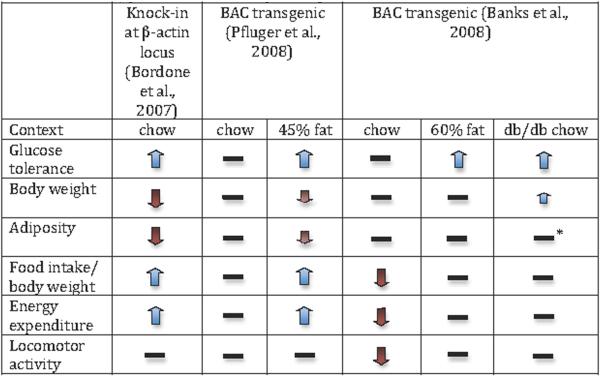
The metabolic effects of SIRT1 overexpression have been studied in a number of independently-derived strains, in some cases with conflicting results (Table 1). Beta cell-specific overexpression of SIRT1 (the BESTO mouse) was reported to enhance glucose-stimulated insulin secretion and improve glucose tolerance (Moynihan et al., 2005), although the effect was lost at advanced ages (Ramsey et al., 2008). Bordone et al reported that overexpression of SIRT1 from the β-Actin locus resulted in increased protein expression in white and brown adipose tissue, as well as brain, but not liver or skeletal muscle (Bordone et al., 2007). These mice had decreased body weight and increased insulin sensitivity relative to wild type littermates, even on a standard diet, and manifested additional phenotypes resembling DR. However, a number of questions remained unanswered since liver and skeletal muscle are thought to play critical roles during DR, and adenoviral overexpression of SIRT1 in the liver decreases glucose tolerance (Rodgers and Puigserver, 2007), implying a potentially detrimental consequence of overexpression in hepatocytes. To better assess the effects of increased SIRT1 activity in the whole body, two groups independently generated bacterial artificial chromosome (BAC) transgenics expressing an extra copy of SIRT1 under the control of its native promoter (Banks et al., 2008; Pfluger et al., 2008). In both cases, glucose tolerance was not affected under standard diet conditions, but was dramatically improved in the context of obesity. Surprisingly, the improvement was due primarily to an increase in hepatic insulin sensitivity and the resulting suppression of hepatic glucose output. Despite the agreement on this critical finding, the two groups analyzing BAC-transgenics found disparate results on body weight, food intake, and energy expenditure, and attributed the improvement in hepatic insulin sensitivity to different mechanisms. Banks et al showed an increase in the circulating level of adiponectin (Banks et al., 2008), which has been shown to promote insulin sensitivity, while Pfluger et al reported a decrease in inflammation related to NFκB-dependent pathways and no effect on adiponectin (Pfluger et al., 2008).
Table 1.
Phenotypes of SIRT1 overexpressing mice.
|
Fat percentages are based on energy content of the diet. Dashes indicate that the parameter was measured, but no significant effect was reported. Faded arrows indicate trends that were not statistically significant. The smaller blue arrow indicates that body weight was increased in db/db transgenics only at the latest timepoints measured.
Adiposity was assessed prior to the emergence of a difference in body weight.
Overall, the effects reported in mice overexpressing SIRT1 generally support the idea that it could mediate key effects of resveratrol, however much remains to be clarified. For example, both resveratrol and SRT1720 improve peripheral glucose uptake, while SIRT1 BAC-transgenics show only an improvement in hepatic insulin sensitivity. On the other hand, the transgenic animals created by Bordone et al, which do not overexpress hepatic SIRT1 at all, still show improved glucose tolerance, suggesting that peripheral glucose uptake is increased, hepatic insulin sensitivity is improved indirectly, or both. One factor that may contribute to discrepancies in results between various experiments is the apparent sensitivity of SIRT1 to dosage effects. For example, moderate (up to 7.5-fold) overexpression of SIRT1 in the heart is protective, while higher (12.5-fold) overexpression leads to cardiomyopathy (Alcendor et al., 2007). In addition, a low dose of resveratrol can actually increase body weight (Pearson et al., 2008), while a higher dose has no effect, and a still higher dose decreases body weight. Mice lacking SIRT1 or overexpressing SIRT1 are born at submendelian ratios (Bordone et al., 2007; McBurney et al., 2003), and increasing the gene dosage further does not result in viable pups, suggesting an optimal window for SIRT1 activity during development. Although expression of SIRT1 from its native promoter might be hypothesized to produce similar effects to systemic treatment with an activating drug, resveratrol is not likely to penetrate all tissues, or even subcellular compartments, equally. In fact, concentrations up to 30-fold higher than serum levels have already been reported in the small intestine (Sale et al., 2004), as well as significant enrichments in other tissues. The susceptibility of SIRT1 to activation by resveratrol could also depend on many other factors that vary between tissues and, at least in worms, it appears that resveratrol may actually change the substrate specificity of sirtuins (Viswanathan et al., 2005), a possibility that has not been addressed in mammals. All of these factors contribute to the complexity of comparing the effects of drugs and protein overexpression in vivo.
Perhaps the most important question that must be addressed experimentally is what resveratrol does in the absence of SIRT1 in mice. Unfortunately, the developmental defects, poor viability, and altered metabolism of constitutive knockout animals make such experiments technically challenging. These limitations have been overcome to show that DR does not induce an increase in activity level, and may not affect longevity in SIRT1 null mice (Chen et al., 2005; Li et al., 2008), and recently, it was shown that the chemopreventive effect of resveratrol is also compromised in SIRT1 null animals (Boily et al., 2009). However, the interpretation of such experiments remains controversial (Pani et al., 2006), highlighting the need for better models. Liver-specific SIRT1 knockout mice have been described, and are grossly normal, but were reported to display a surprising protection from high fat diet that would pre-empt the demonstration of protection by resveratrol (Chen et al., 2008). More recently, another group reported diet-induced hepatic steatosis in liver-specific SIRT1 knockout animals, reviving the possibility of testing for protective effects of resveratrol in this system (Purushotham et al., 2009). However, even if resveratrol improves metabolism in the absence of hepatic SIRT1, the effects could be attributed to activation of SIRT1 in the brain or other tissues. Ideally, it will be possible to delete SIRT1 in the whole animal following normal development, allowing a more definitive test of its involvement in the protective effects of resveratrol and DR.
Other Sirtuins
Another important question is whether other mammalian sirtuins (SIRT2-7) are critical mediators of DR. While SIRT1 is the closest mammalian homolog to the enzymes associated with lifespan in lower organisms, and has been the focus of drug discovery efforts, other sirtuins also use NAD+ as a co-substrate and may respond to DR. Resveratrol is thought to act mainly on SIRT1, but may also activate SIRT7 (Vakhrusheva et al., 2008), while SRT1720 is currently considered to be specific for SIRT1, based on its failure to activate the closest homologues, SIRT2 and SIRT3 (Milne et al., 2007). However, either one could conceivably influence other sirtuins as well, given the correct substrates and conditions. A complete discussion of all seven sirtuins is beyond the scope of this review, and has been adequately covered elsewhere (for example, see Dali-Youcef et al (Dali-Youcef et al., 2007)), however some specific observations have been reported that pertain to DR and longevity. SIRT6 null mice are extremely short-lived, have a defect in base excision repair, and show phenotypes resembling premature aging (Mostoslavsky et al., 2006). Suppression of NFκB signaling partially restores lifespan (Kawahara et al., 2009), implying that a normal function of SIRT6 is to limit inflammation, and broad suppression of inappropriate age-related inflammatory responses, including those mediated by NFκB, is an important feature of DR (Chung et al., 2002). SIRT4 and SIRT5 have been shown to regulate amino acid stimulated insulin secretion (Haigis et al., 2006) and ammonia disposal (Nakagawa et al., 2009), respectively, during DR, suggesting additional changes in physiology that would not be recapitulated by activation of SIRT1 alone. SIRT7 null mice are short-lived, with a degenerative phenotype in cardiomyocytes, indicating that it may promote stress-resistance in that organ (Vakhrusheva et al., 2008). Although SIRT2 and SIRT3 have not been clearly linked to specific functions during DR, or to mouse longevity, an allele of SIRT3 is associated with longevity in a human population (Rose et al., 2003), hinting that much about these enzymes remains to be discovered. It is therefore likely that multiple sirtuins, in addition to other factors that remain to be identified, contribute to the range of beneficial effects induced by DR.
Conclusion
There is compelling evidence that resveratrol can ameliorate many of the negative health consequences associated with obesity in rodents, and provides some benefit even in lean animals. Overexpression studies, and novel small molecule activators support a role for SIRT1 in many of these effects, however the poor phenotype of SIRT1 null mice has thus far precluded a more definitive experiment. Based on transcriptional profiling, resveratrol mimics a striking number of the changes induced by dietary restriction, arguing that the effort to mimic salient effects of DR with a small molecule has been at least partially successful. However a number of critical aspects of the normal DR response, such as the prevention of spontaneous lymphomas, and ultimately extension of lifespan in normal mice, have not yet been achieved. These observations highlight the need for continued efforts to elucidate the signaling pathways that mediate the response to DR in mammals, in order to fully harness their potential to improve human health and delay or prevent age-related diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–5. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Calorie restriction: progress during mid-2005-mid-2006. Exp Gerontol. 2006;41:1247–9. doi: 10.1016/j.exger.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–93. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–41. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–52. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980–5. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA. Encyclopedia of Life Sciences. John Wiley & Sons, Ltd.; 2009. Metabolic Effects of Caloric Restriction. [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2008:CD007176. doi: 10.1002/14651858.CD007176. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–4. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009 doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–5. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–67. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–95. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;291:1001–5. doi: 10.1006/bbrc.2002.6554. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, Zhang Z. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry. 2004;43:11417–26. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–7. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim KW, Choi JS, Yu BP. Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc Res Tech. 2002;59:264–72. doi: 10.1002/jemt.10203. [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–12. [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39:335–45. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–22. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005;23:343–50. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- Dilman VM, Anisimov VN. Effect of treatment with phenformin, diphenylhydantoin or L-dopa on life span and tumour incidence in C3H/Sn mice. Gerontology. 1980;26:241–6. doi: 10.1159/000212423. [DOI] [PubMed] [Google Scholar]
- Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008;84:882–92. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt AV, Le Couteur DG. Life extension by calorie restriction in humans. Ann N Y Acad Sci. 2007;1114:428–33. doi: 10.1196/annals.1396.005. [DOI] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–58. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002;123:121–30. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–41. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165:2355–60. doi: 10.1001/archinte.165.20.2355. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–73. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–81. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A. 1997;94:14138–43. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab. 2004;287:E1032–7. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Geserick C, Flores JM, Blasco MA. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene. 2005;24:2256–70. doi: 10.1038/sj.onc.1208413. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–56. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–54. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci U S A. 1984;81:1835–8. doi: 10.1073/pnas.81.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempenstall S, Picchio L, Mitchell SE, Speakman JR, Selman C. The impact of acute caloric restriction on the metabolic phenotype in male C57BL/6 and DBA/2 mice. Mech Ageing Dev. 2010 doi: 10.1016/j.mad.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59:826–31. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jarolim S, Millen J, Heeren G, Laun P, Goldfarb DS, Breitenbach M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004;5:169–77. doi: 10.1016/j.femsyr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Jonker JT, De Laet C, Franco OH, Peeters A, Mackenbach J, Nusselder WJ. Physical activity and life expectancy with and without diabetes: life table analysis of the Framingham Heart Study. Diabetes Care. 2006;29:38–43. doi: 10.2337/diacare.29.01.06.dc05-0985. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Hu D, Kerr EO, Tsuchiya M, Westman EA, Dang N, Fields S, Kennedy BK. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2005a;1:e69. doi: 10.1371/journal.pgen.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell S, Napper A, Curtis R, Distefano PS, Fields S, Bedalov A, Kennedy BK. Substrate specific activation fo sirtuins by resveratrol. J Biol Chem. 2005b doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos-Flier E, Kahn CR. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell. 2007;6:827–39. doi: 10.1111/j.1474-9726.2007.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–79. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong SC, Brubacher J. Phenformin and lactic acidosis: a case report and review. J Emerg Med. 1998;16:881–6. doi: 10.1016/s0736-4679(98)00103-6. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Latorre-Esteves M, Medvedik O, Wong SN, Tsang FA, Wang C, Lin SJ, Sinclair DA. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science. 2005;309:1861–4. doi: 10.1126/science.1113611. [DOI] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–35. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. 2-Deoxy-D-glucose feeding in rats mimics physiological effects of caloric restriction. J. Anti-Aging Med. 1998;1:327–337. [Google Scholar]
- Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–13. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverve XM, Guigas B, Detaille D, Batandier C, Koceir EA, Chauvin C, Fontaine E, Wiernsperger NF. Mitochondrial metabolism and type-2 diabetes: a specific target of metformin. Diabetes Metab. 2003;29:6S88–94. doi: 10.1016/s1262-3636(03)72792-x. [DOI] [PubMed] [Google Scholar]
- Li T, Fan GX, Wang W, Yuan YK. Resveratrol induces apoptosis, influences IL-6 and exerts immunomodulatory effect on mouse lymphocytic leukemia both in vitro and in vivo. Int Immunopharmacol. 2007;7:1221–31. doi: 10.1016/j.intimp.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGFI/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–8. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–6. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mayers JR, Iliff BW, Swoap SJ. Resveratrol treatment in mice does not elicit the bradycardia and hypothermia associated with calorie restriction. FASEB J. 2009;23:1032–40. doi: 10.1096/fj.08-115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. The Journal of Nutrition. 1935;10:63–79. [PubMed] [Google Scholar]
- McShane TM, Wise PM. Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biol Reprod. 1996;54:70–5. doi: 10.1095/biolreprod54.1.70. [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–29. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–17. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–70. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–18. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Carnes BA, Cassel C. In search of Methuselah: estimating the upper limits to human longevity. Science. 1990;250:634–40. doi: 10.1126/science.2237414. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Chrunyk BA, Cunningham D, Flynn D, Griffith DA, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010 doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani G, Fusco S, Galeotti T. Smaller, hungrier mice. Science. 2006;311:1553–4. doi: 10.1126/science.311.5767.1553. author reply 1553-4. [DOI] [PubMed] [Google Scholar]
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–5. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–8. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JP, Rose MR. Why dietary restriction substantially increases longevity in animal models but won't in humans. Ageing Res Rev. 2005;4:339–50. doi: 10.1016/j.arr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–6. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–38. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz BW, Aktan I, Nogusa S, Gardner EM. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J Nutr. 2008;138:2269–75. doi: 10.3945/jn.108.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb EL, Winkelmolen L, Visanji N, Brotchie J, Stuart JA. Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem Biophys Res Commun. 2008;372:254–9. doi: 10.1016/j.bbrc.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–8. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–6. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–6003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, BonaFe M, Franceschi C, Tan Q, Boiko S, Yashin AI, De Benedictis G. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–70. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann N Y Acad Sci. 2001;928:305–15. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- Sale S, Verschoyle RD, Boocock D, Jones DJ, Wilsher N, Ruparelia KC, Potter GA, Farmer PB, Steward WP, Gescher AJ. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br J Cancer. 2004;90:736–44. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–28. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002;71:2489–98. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A. 2000;97:6658–63. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Ferguson M, Sohal BH, Forster MJ. Life span extension in mice by food restriction depends on an energy imbalance. J Nutr. 2009;139:533–9. doi: 10.3945/jn.108.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streppel MT, Ocke MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term wine consumption is related tocardiovascular mortality and life expectancyindependently of moderate alcohol intake: theZutphen Study. J Epidemiol Community Health. 2009 doi: 10.1136/jech.2008.082198. [DOI] [PubMed] [Google Scholar]
- Su JL, Yang CY, Zhao M, Kuo ML, Yen ML. Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J Biol Chem. 2007;282:19385–98. doi: 10.1074/jbc.M702452200. [DOI] [PubMed] [Google Scholar]
- Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008;149:634–41. doi: 10.1210/en.2007-1089. [DOI] [PubMed] [Google Scholar]
- Taylor DH, Jr., Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92:990–6. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas-Loba A, Flores I, Fernandez-Marcos PJ, Cayuela ML, Maraver A, Tejera A, Borras C, Matheu A, Klatt P, Flores JM, Vina J, Serrano M, Blasco MA. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–22. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Dang N, Kerr EO, Hu D, Steffen KK, Oakes JA, Kennedy BK, Kaeberlein M. Sirtuin-independent effects of nicotinamide on lifespan extension from calorie restriction in yeast. Aging Cell. 2006;5:505–14. doi: 10.1111/j.1474-9726.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMPK-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2009 doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–10. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–15. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Volk MJ, Pugh TD, Kim M, Frith CH, Daynes RA, Ershler WB, Weindruch R. Dietary restriction from middle age attenuates age-associated lymphoma development and interleukin 6 dysregulation in C57BL/6 mice. Cancer Res. 1994;54:3054–61. [PubMed] [Google Scholar]
- Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–8. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. C.C. Thomas; Springfield, Ill., U.S.A.: 1988. p. xvii, 436. [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–54. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–42. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yang H, Baur JA, Chen A, Miller C, Adams JK, Kisielewski A, Howitz KT, Zipkin RE, Sinclair DA. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell. 2007;6:35–43. doi: 10.1111/j.1474-9726.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K, Steinsaltz D, Mobbs CV. Validated analysis of mortality rates demonstrates distinct genetic mechanisms that influence lifespan. Exp Gerontol. 2008;43:1044–51. doi: 10.1016/j.exger.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–91. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]