Abstract
Gliotransmitters are chemicals released from glial cells fulfilling a following set of criteria: i) they are synthesized by and/or stored in glia; ii) their regulated release is triggered by physiological and/or pathological stimuli; iii) they activate rapid (milliseconds to seconds) responses in neighboring cells; and iv) they play a role in (patho)physiological processes. Astrocytes can release a variety of gliotransmitters into the extracellular space using several different mechanisms. In this review, we focus on exocytotic mechanism(s) underlying the release of three classes of gliotransmitters: (i) amino acids, such as, glutamate and D-serine; (ii) nucleotides, like adenosine 5'-triphosphate; and (iii) peptides, such as, atrial natriuretic peptide and brain-derived neurotrophic factor. It is becoming clear that astrocytes are endowed with elements that qualify them as cells communicating with neurons and other cells within the central nervous system by employing regulated exocytosis.
Keywords: astrocytes, exocytosis, glutamate, D-serine, ATP, atrial natriuretic peptide, brain-derived neurotrophic factor
Introduction
The criteria for a chemical released from neurons to be classified as a neurotransmitter have been defined and frequently modified [6,15]. Since transmitter release from glia was demonstrated at a much latter time than that from neurons [16], only recently has a similar set of criteria been put forth [27,52,91] to establish what compounds qualify as “gliotransmitters”: i) synthesis by and/or storage in glia; ii) regulated release triggered by physiological and/or pathological stimuli; iii) activation of rapid (milliseconds to seconds) responses in neighboring cells; and iv) a role in (patho)physiological processes.
Astrocytes and other glial cells can release a variety of transmitters into the extracellular space using several different mechanisms: (i) through channels like anion channel opening, induced by cell swelling [67], release through functional unpaired connexons/pannexons, “hemichannels”, on the cell surface [23,39] and ionotropic purinergic receptors [29]; (ii) through transporters, such as, reversal of uptake by plasma membrane excitatory amino acid transporters [87], exchange via the cystine-glutamate antiporter [93] or organic anion transporters [77]; and (iii) through Ca2+-dependent exocytosis [62].
In this review we focus on the exocytotic mechanism(s) underlying the release of three classes of gliotransmitters: (i) amino acids, such as glutamate and D-serine; (ii) nucleotides, like adenosine 5'-triphosphate (ATP); and (iii) peptides, such as, atrial natriuretic peptide (ANP) and brain-derived neurotrophic factor (BDNF). We only disclose the consequences of transmitter release from astrocytes onto neighboring cells when the effect of transmitter release from astrocytes is used as an assay for release.
Amino acids as astrocytic transmitters
Glutamate is synthesized within astrocytes as a by-product of the tricarboxylic acid (TCA) cycle. Since astrocytes possess the enzyme pyruvate carboxylase, they can synthesize glutamate de novo [35]. Glutamate is converted from the TCA cycle intermediate, α-ketoglutarate, usually via transamination of another amino acid, such as, aspartate [94](Figure 1). D-serine is converted from L-serine by the action of serine racemase, an enzyme found predominately in astrocytes [97].
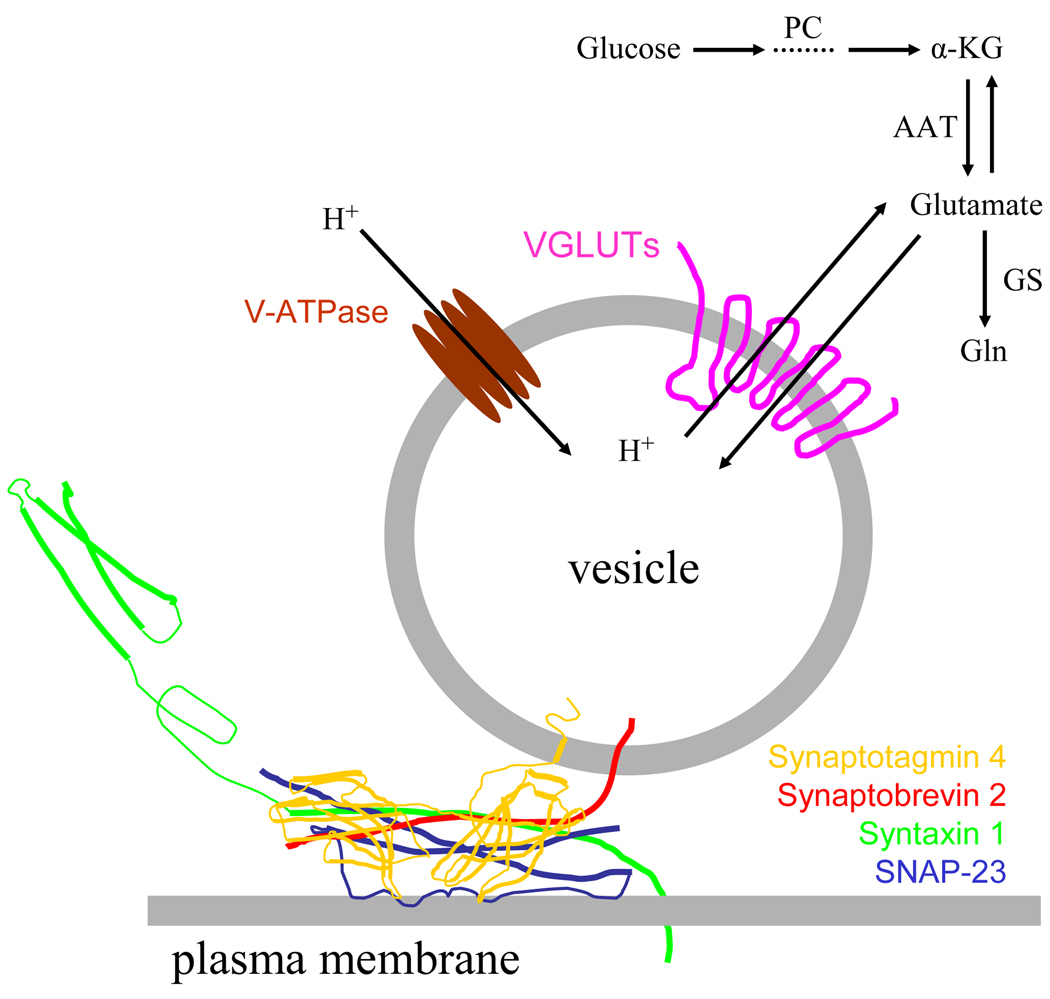
Figure 1.
Glutamate release by Ca2+-dependent exocytosis. Glutamate packaged in vesicles is released from the astrocyte when the vesicle fuses with the plasma membrane. This fusion process is mediated by synaptotagmin 4 and SNARE proteins: syntaxin 1, synaptobrevin 2 and synaptosome-associated protein of 23 kDa (SNAP-23). Glutamate can be synthesized in astrocytes de novo from glucose entry to the tricarboxylic acid cycle via pyruvate carboxylase (PC). Glutamate is converted from the cycle intermediate, α-ketoglutarate (α-KG), usually by transamination of aspartate via aspartate amino transferase (AAT). The synthesized glutamate once in the cytosol can then be converted to glutamine (Gln) by glutamine synthetase (GS), or transported into vesicles via proton-dependent vesicular glutamate transporters (VGLUTs), especially isoform 3. The proton gradient is generated by vacuolar type H+-ATPases (V-ATPase).
Evidence for Ca2+-dependent glutamate release from astrocytes was first shown using high performance liquid chromatography to monitor the release of this transmitter from cultured astrocytes [62]. Astrocytes were equilibrated for prolonged periods of time (40–60 minutes) either in a solution containing normal external free Ca2+ (2.4 mM), or in a solution depleted of external free Ca2+ (24 nM); the latter solution caused a depletion of internal Ca2+ stores and prevented Ca2+ entry from the extracellular space. Addition of the Ca2+ ionophore, ionomycin, in the presence of normal external Ca2+, caused an increase in the release of glutamate from astrocytes. Stimulation of astrocytes, bathed in a solution depleted of free Ca2+, failed to cause an increase in glutamate release. These data indicate that elevated intracellular Ca2+ concentration ([Ca2+]i) is sufficient and necessary to stimulate glutamate release. Consistent with the former finding, other stimuli that directly increased astrocytic [Ca2+]i, including mechanical stimulation [4,5,36,57,62], photostimulation [62], and photolysis of Ca2+ cages [5,64,100], all caused release of glutamate. The notion that elevated [Ca2+]i is necessary for glutamate release from astrocytes is further supported by the reduction of the evoked glutamate release from astrocytes when the astrocytic buffering capacity for cytosolic Ca2+ was augmented using the Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), or when [Ca2+]i increases where dampened by the depletion of internal Ca2+ stores due to pre-incubation of these cells with thapsigargin, a blocker of store specific Ca2+-ATPase [4,12,36,40,57].
The majority of intracellular Ca2+ necessary for glutamate release originates from endoplasmic reticulum (ER) internal stores (Figure 2), since Ca2+-dependent glutamate release from astrocytes is most prominently reduced in the presence of thapsigargin [36]. Diphenylboric acid 2-aminoethyl ester (2-APB) solution, a cell-permeant inositol 1,4,5-trisphosphate (IP3) receptor antagonist, greatly reduced exocytotic glutamate release from astrocytes, implicating the role of IP3- sensitive internal stores in mediating Ca2+-dependent glutamate release from astrocytes. Similarly, ryanodine/caffeine-sensitive ER stores play a role, as well, since the treatment of astrocytes with ryanodine, at concentrations that blocked the release of Ca2+ from the ryanodine/caffeine- sensitive stores, also attenuated mechanically-induced glutamate release. Furthermore, the sustained presence of caffeine, that depleted ryanodine/caffeine stores, also reduced mechanically-induced glutamate release. Thus, Ca2+-dependent glutamate release from astrocytes requires co-activation of IP3- and ryanodine/caffeine-sensitive internal Ca2+ stores, which operate jointly [36]. It should be noted, however, that the functionality of ryanodine receptors in astrocytes is still debated, since the lack of their activity in astrocytes in situ had been reported [9]. Nonetheless, increase in Ca2+ within subplama membrane cytosolic microdomains, delimited by ER and containing glutamatergic vesicles, controls exocytotic vesicular fusions [51].
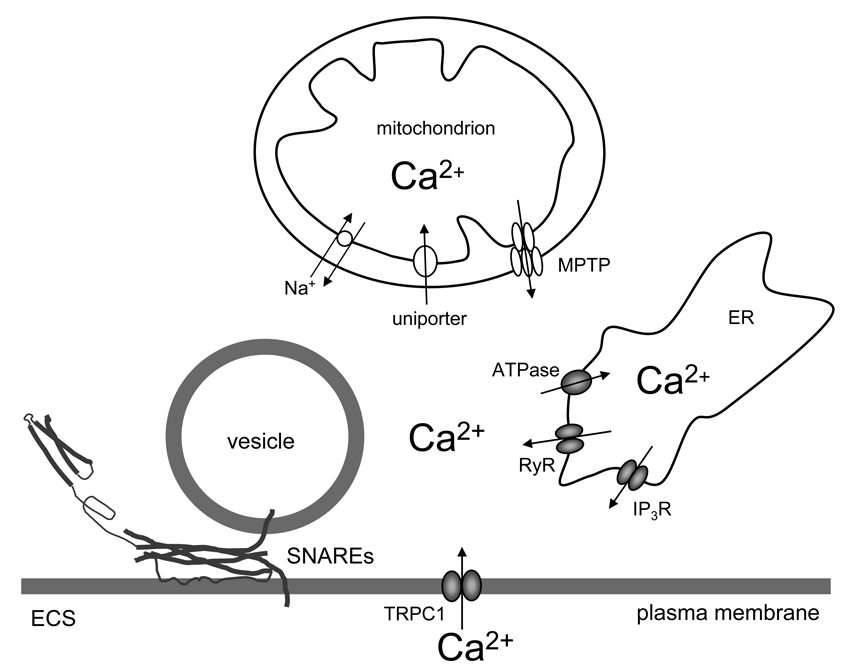
Figure 2.
Sources of cytosolic Ca2+ in vesicular release from astrocytes. Increase of cytosolic Ca2+ is sufficient and necessary to cause vesicular fusions and release of gliotransmitters. This process of regulated exocytosis requires the action of the ternary SNARE complex. Cytosolic Ca2+ accumulation could be caused by the entry of Ca2+ from the ER internal stores via IP3 and ryanodine receptors (IP3 and RyR). Store specific Ca2+-ATPase fills these stores, although ultimately this action requires Ca2+ entry from the extracellular space (ECS) through canonical type 1 transient receptor potential (TRPC1). Mitochondrial Ca2+ uptake is mediated by the uniporter, while free Ca2+within the mitochondrial matrix exits through the Na+/ Ca2+ exchanger and the mitochondrial permeability transition pore (MPTP). Drawing is not to scale.
Ca2+ entry from the extracellular space across the astrocytic plasma membrane is ultimately required for the (re)filling of ER Ca2+ stores (Figure 2). This occurs via so-called store-operated Ca2+ (SOC) channels [33,88]. Astrocytes express canonical transient receptor potential (TRPC) channels, implicated in SOC entry, which play a role in the regulation of Ca2+ homeostasis in these cells [33,34,69]. Specifically, TRPC1 functionally contributes to Ca2+-dependent glutamate release from astrocytes [50], since an antibody against TRPC1, that was designed to bind to the pore forming region of TRPC1 and that blocks the functioning of the channel [92], caused a significant decrease in the measured SOC entry and mechanically-induced glutamate release from these cells. This is consistent with the finding that the presence of extracellular Cd2+, a blocker of Ca2+ entry from the extracellular space, reduces mechanically-induced Ca2+-dependent glutamate release from astrocytes [36]. Voltage-gated Ca2+ channels (Parri et al., 2001) might mediate additional entry of Ca2+ from the extracellular space that is utilized to trigger exocytotic glutamate release from astrocytes of ventrobasal thalamus. The role of the ionotropic transmitter receptors, which represent an additional entry of Ca2+ in astrocytes [reviewed in [90]], in exocytotic glutamate release from astrocytes is intangible at the moment [reviewed in [75]].
Mitochondria can modulate intracellular Ca2+ handling and affect exocytosis in astrocytes [74]. These organelles possess a mitochondrial Ca2+ uniporter that can transport Ca2+ into the mitochondrial matrix and it operates at cytosolic [Ca2+] greater than ~ 0.5 µM [55,80]. Blocking this uniporter with ruthenium 360 increased mechanically-induced cytosolic Ca2+ accumulation and glutamate release in cortical astrocytes. Conversely, decreasing mitochondrial Ca2+ efflux by blocking the mitochondrial Na+/Ca2+ exchanger with 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157), or increasing mitochondrial Ca2+ load by inhibiting formation of the mitochondrial permeability transition pore with cyclosporin A, decreased cytosolic Ca2+ accumulation and glutamate release in cortical astrocytes. Taken together, these data suggest that mitochondria have the capacity to modulate the magnitude of Ca2+-dependent glutamate release from astrocytes (Figure 2).
Ca2+-dependent release of transmitters depends on the presence of exocytotic secretory machinery. Indeed, astrocytes express the proteins of the soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptor (SNARE) complex: synaptobrevin 2 [(Sb2), also referred to as vesicle–associated membrane protein 2 (VAMP 2)], syntaxin 1, synaptosome-associated protein of 23 kDa (SNAP-23), as well as several ancillary proteins to this complex, including synaptotagmin 4 [reviewed in [56]] (Figure 1). The use of Clostridial, tetanus, and various types of botulium toxins, which cleave SNARE proteins necessary for exocytosis, caused a reduction in the level of Ca2+-dependent glutamate release in astrocytes [reviewed in [56]]. Additionally, the use of tetanus toxin, which cleaves astrocytic Sb2 and cellubrevin [63], caused a reduction in plasma membrane capacitance (Cm) increase [46], and a reduction in the number of amperometric spikes [21], both reporting on exocytosis from astrocytes. The experimental manipulation of the SNARE complex by expressing the cytoplasmic domain of Sb2, that acted as a dominant negative SNARE, resulted in the inhibition of glutamate release from astrocytes [100]. It should be noted that Sb2 cytosolic domain contains the SNARE domain, but lacks the ability to anchor to the vesicular membrane. Similarly, the expression of mutated synaptotagmin 4, acting in a dominant-negative manner, caused the reduction of Ca2+-dependent glutamate release from astrocytes [99]. Furthermore, α-latrotoxin, an active component of black widow spider venom, which binds to neurexins/latrophilins to induce release of transmitter by stimulating the secretory machinery [reviewed in [86]], has been shown to cause glutamate release from astrocytes [42,65].
Proteins utilized for sequestering glutamate into vesicles have also been found in astrocytes (Figure 1). Hence, the vacuolar type of proton ATPase (V-ATPase), which drives protons into the vesicular lumen creating the proton concentration gradient necessary for glutamate transport into vesicles, has been detected in astrocytes [95]. Its blockage with bafilomycin A1 reduces glutamate release from astrocytes caused by various stimuli [3,13,24,57,68]. The three known isoforms of vesicular glutamate transporters (VGLUTs) 1, 2 and 3, which use the proton gradient created by V-ATPases to package glutamate into vesicles, have been detected in astrocytes [2,14,24,32,46,57,100]. These transporters are functional within astrocytes since Rose Bengal, an allosteric modulator of VGLUTs, greatly reduced glutamate release [57]. VGLUT3 and the cytosolic concentration of glutamate appear to be key limiting factors in regulating the Ca 2+-dependent release of glutamate from astrocytes [60] (Figure 1). Selective over-expression of individual VGLUT proteins in astrocytes showed that VGLUT3, but neither VGLUT1 nor VGLUT2, enhanced mechanically-induced Ca 2+-dependent glutamate release. Similarly, inhibition of glutamine synthetase activity by L-methionine sulfoximine in astrocytes, which leads to increased cytosolic glutamate concentration, greatly increased their mechanically-induced Ca 2+-dependent glutamate release without affecting intracellular Ca2+ dynamics [60].
Secretory vesicles are the essential morphological elements for regulated Ca2+-dependent exocytosis. Hence, based on immunoelectron microscopy (IEM), Sb2 can be found located in vicinity of electron-lucent (clear) vesicular structures [49], while VGLUTs 1 or 2 in astrocytes in situ were found associated with small, clear vesicles with a mean diameter of ~30 nm [14]. Similarly, immunoisolated Sb2-containing vesicles that originated from cultured astrocytes [24] were heterogeneous in size, ranging from 30 to over 100 nm, and predominately appeared as electron-lucent. Recycling glutamatergic vesicles which can capture the extracellular antibody against VGLUT1 in a Ca2+-dependent manner are electron-lucent and have a diameter of ~ 50 nm [83]. Furthermore, gliosomes [85], pinched off astrocytic processes, expressing Sb2 and VGLUT 1, contained clear vesicles with diameters of ~30 nm; some of them were clathrin-coated vesicles. Much larger vesicles, over 1 µm in diameter, have been observed to form within minutes of repeated stimulation with pharmacological dosages (5–50 mM) of glutamate [44,98]; these vesicles can release glutamate, although it is highly likely that they represent a pharmacologically-induced phenomenon or may play a role in pathological processes [see also the discussion in [11]]
The recycling of secretory vesicles at the plasma membrane has been investigated in astrocytes using fluorescence microscopy. Application of ionomycin in the presence of extracellular Ca2+, but not in its absence, caused uptake of the membrane recycling dye, FM 4–64 [47]. Similarly, using a pre-loading technique that stimulated membrane recycling and the trapping of styryl dyes (FM 1–43 or FM 2–10) in secretory organelles, astrocytes displayed a punctate pattern of FM fluorescence [21]. Trafficking of glutamatergic vesicles in astrocytes was assessed using an immunological approach. Hence, after increasing cytoplasmic Ca2+ levels in astrocytes in the presence of antibodies against VGLUT1 in extracellular space, presumably binding to luminal/intravesicluar epitope of this transporter, there was an increase in intracellular fluorescent puncta [83]. The delivery of secretory vesicles and fusion to the plasma membrane was also studied in astrocytes. Crippa et al. [24] expressed a chimeric protein, where enhanced green fluorescent protein (EGFP) was fused to the C-terminus of Sb2 (Sb2-EGFP), in astrocytes. Since the C-termimus of Sb2 is located in the vesicular lumen, EGFP was targeted intravesicularly. When astrocytes were stimulated with Ca2+ ionophore, many fluorescent Sb2-EGFP puncta vanished with a simultaneous increase in plasma membrane fluorescence, consistent with regulated exocytosis and fusion of labeled vesicles to the plasma membrane. Net addition of vesicular membrane to the plasma membrane can be directly measured by monitoring changes in Cm. Indeed, an agonist-induced rise in astrocytic [Ca2+]i,, causing regulated exocytosis, resulted in an increase of Cm, while concomitant measurements recorded a release of glutamate [100]. Further evidence for vesicular exocytosis from astrocytes was provided by total internal reflection fluorescence (TIRF) microscopy [14,17,28,51], where exocytosis of VGLUT1-,VGLUT2- or Sb2- positive vesicles were reported. As a consequence of vesicular fusions, quantal events of glutamate release, representing an exocytotic hallmark [26], have been recorded from astrocytes. Such events were detected using reporter cells expressing N-methyl-D-aspartate (NMDA) receptors [68], or by amperometric measurements used to detect the release of dopamine, acting as a “surrogate” transmitter for glutamate, from glutamatergic vesicles [21].
Astrocytes can also release the amino acid D-serine [79], a ligand to the glycine modulatory binding site of the NMDA receptor. Mothet at al. [58] investigated the mechanism of this release using an enzyme-linked assay to measure extracellular D-serine concentration. Following glutamate receptor stimulation, astrocytes released D-serine in a Ca2+-dependent manner; the release was augmented by Ca2+ and inhibited by application of thapsigargin or removal of extracellular Ca2+. Furthermore, this release of D-serine was reduced by concanamycin A, a V-ATPase inhibitor, and tetanus toxin, implicating the involvement of a vesicular mechanism. Consistent with this notion, D-serine was found to co-localize with Sb2 based on immunocytochemistry and fluorescence microscopy. The investigation of the mechanism underlying a Ca2+-dependent release of D-serine from astrocytes was expanded in a subsequent study using confocal fluorescence microscopy [53]. Using pharmacological inhibition of vesicular budding indicated that D-serine was packaged in vesicles down stream of the Golgi apparatus. The molecular identity of the vesicular transporter for D-serine, however, remains undetermined. Nonetheless, the delivery of secretory vesicles and fusion to the plasma membrane showed the recruitment of Sb2 to the plasma membrane with related disappearance of intracellular D-serine punctate stain. Taken together, D-serine appears to be secreted from astrocytes using a regulated exocytosis/vesicular pathway.
ATP as an astrocytic transmitter
ATP is produced via glycolysis and oxidative phosphorylation. Intracellular ATP provides energy for a variety of processes, including vesicular recycling. Once released into extracellular space, ATP can be used in intercellular signaling acting directly onto purinergic receptors. Alternatively, upon its hydrolysis by membrane-bound ecto-nucleotidases, the extracellular degradation products, ADP and adenosine, can activate different plasma membrane receptors [reviewed in [31]]
As already outlined, astrocytes possess secretory vesicles and a variety of exocytotic proteins. Cultured astrocytes investigated under EM displayed large dense core granules with diameters of ~115 nm, containing the secretory peptide secretogranin II [18] and ATP [22]. Based on immunoblotting, subcellular fractions containing secretogranin II were mainly distinct from fractions containing Sb2 [18]. Consistent with this finding, dense core vesicles represented ~2 % of the total number of immuno-isolated Sb2-containing vesicles [24]. Similarly, using IEM, it was demonstrated that Sb2 can be associated with some dense core vesicular structures, with diameters ranging from 100–700 nm [49]. Following subcellular fractionations, immunoblotting for several exocytotic proteins, Sb2, syntaxin 1, cellubrevin and synaptotagmin 1, were found to co-localize with ATP containing organelles [49]. It should be noted, however, that the presence of synaptotagmin 1 was not detected in astrocytes by others [24,62,99]. The protein responsible for the ATP accumulation in secretroy vesicles has recently been identified as SLC17A9 [78]. This vesicular nucleotide transporter (VNUT) was found present in astrocytes based on immunocytochemistry.
Morphological and biochemical evidence suggests that ATP as an astrocytic transmitter may be released by Ca2+-dependent exocytosis. The first evidence in support of such a notion comes from experiments in which astrocytes exposed to nitric oxide responded with an increase in cytoplasmic Ca2+ and the release of ATP to the extracellular space [8]. Buffering of intracellular Ca2+ with BAPTA or preventing vesicular release with botulinum toxin C greatly reduced the release of ATP. Furthermore, Coco et al. [22] demonstrated that mechanically stimulated astrocytes released ATP which could be inhibited by application of bafilomycin A1 or tetanus toxin. Interestingly, the reduction of ATP release caused by tetanus toxin was less pronounced than the reduction in release of glutamate, indicating that ATP and glutamate release may be regulated in a different manner, perhaps through distinct vesicular pools.
ATP release from cultured astrocytes could be evoked by uridine 5'-triphosphate (UTP) via the likely activation of P2Y2 receptors [1]. This release was reduced by thapsigargin and lithium ions that can block the intracellular generation of IP3. Further pharmacology on vesicular trafficking implicates that the exocytotic pathway is involved in UTP-induced ATP release from astrocytes: a blocker of transport vesicles budding off the Golgi apparatus, brefeldin A, a disruptor of actin microfilaments, cytochalasin D, and the exocytosis inhibitor, botulinum toxin A, all blocked ATP release. However, the pre-incubation with a cell permeable form of BAPTA showed a trend in the reduction of release, although the effect was insignificant; this may be ascribed to an insufficient concentration of BAPTA within the cell.
To study the quantal nature of ATP release from astrocytes, Pangrsic et al. [61] incubated astrocytes with quinacrine, a compound that fluorescently labels ATP containing structures. Using TIRF microscopy, quinacrine showed punctate stain. The rapid loss of these puncta was evident upon receptor stimulation using glutamate or ATP and stimuli that directly raise intracellular Ca2+ levels, ionomycin or flash photolysis of caged Ca2+ [73]. Expressing a dominant negative SNARE in astrocytes resulted in the inhibition of the Ca2+-induced reduction in the quinacrine fluorescent puncta representing ATP-containing vesicle exocytosis [61]. Glutamate stimulation of astrocytes showed quantal release of ATP as recorded by ATP reporter cells [61], human embryonic kidney cells expressing a mutated P2X3 receptor with reduced desensitization [30].
Under particular experimental conditions, the exocytotic release of ATP stored in astrocytic lysosomes could be detected [101]. Hence, prolonged (more than 1 hour) incubation with FM recycling dyes stained astrocytic lysosomes based on a co-localization of FM and various lysosomal markers under fluorescence microscopy. Agonist stimulation or metabolic blockade of astrocytes revealed regulated exocytosis of these lysosomes under TIRF microscopy that was blocked by intracellular Ca2+ buffering with BAPTA. These lysosomes could readily load with the fluorescent ATP analogue MANT-ATP, that was also released upon stimulation. Indeed, the astrocytic lysosomal fraction in density gradient centrifugation contained abundant ATP. Two subsequent studies confirmed that in astrocytes lysosome-like organelles can assume the role of secretory vesicles and undergo Ca2+-depenent exocytosis [41,48]. Thus, it appears that ATP, and perhaps other gliotransmittes, in astrocytes could be stored in at least two distinct organelles, secretroy vesicles and lysosomes, from which it can be released by regulated exocytosis. It will be necessary to determine under which conditions these two distinct pools of organelles would be recruited. For example, do the same organelles deliver transmitter for release under physiological and pathological conditions or are there specific organelles that operate under particular conditions?
Peptides as astrocytic transmitters
In contrast to amino acids and ATP, which are loaded into vesicles by membrane transporters, peptidergic gliotransmitters enter vesicles via the synthetic secretory pathway (Figure 3). Pro-peptides are made in the ER, transit Golgi compartments where they get concentrated and sorted into organelles; then, they are processed to their final form before release [25]. Vesicles carrying peptidergic transmitters appear to have a distinct morphological appearance under EM; they exhibit electron dense cores and are termed dense-core vesicle, large dense-core vesicles or secretory granules. Their diameter is somewhat larger in comparison with the small synaptic-like clear-core vesicles and appear to contain secretogranins [96]. Therefore, in the first studies where the presence of dense-core vesicles in astrocytes was considered, subcellular distribution and the secretory pathway of secretogranin II was studied [18]. The EM results of this study have shown that, in astrocytes, dense-core vesicles are present near the Golgi complex, typically have a diameter of approximately 100 nm, and that secretogranin II is released upon stimulation by different secretagogues, including bradykinin, adenosine 3’:5’ cyclic monophosphate (cAMP), ionomycin, and phorbol 12-myristate 13-acetate (PMA). In Ca2+-containing media, the Ca2+ ionophore ionomycin in combination with PMA produced large increases in cytosolic Ca2+ activity and appeared to be the most effective stimulus for secretogranin II release [18]. This study also reported that astrocytes contain fewer smaller and less dense secretory granules containing secretogranin II, indicating that peptidergic granules in astrocytes are not uniform in morphological appearance.
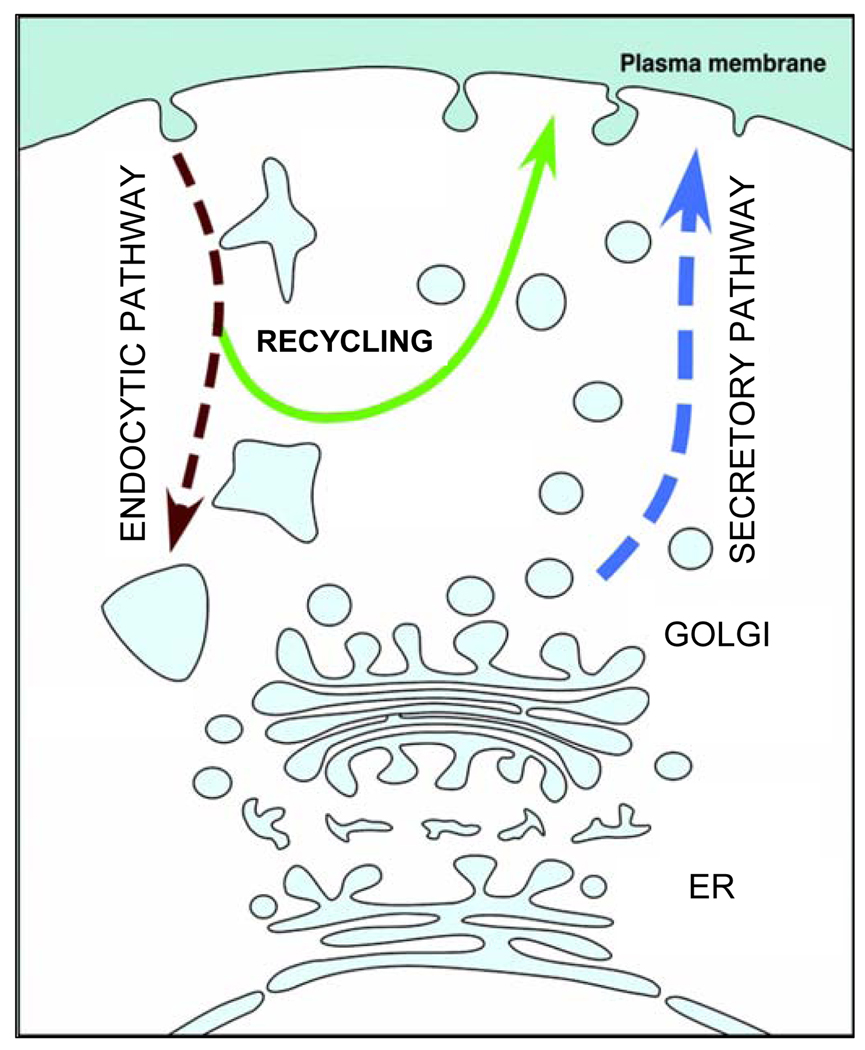
Figure 3.
Secretory pathway of peptidergic transmitters The pro-peptides are synthetized in the endoplasmic reticulum (ER). They then enter the Golgi compartments from which vesicles bud off, containing concentrated and sorted peptides. Secretory vesicles traffic away from the Golgi compartment along the secretory pathway to the plasma membrane where they dock and fuse with the plasma membrane upon a stimulus delivery, typically an increase in cytosolic Ca2+ levels/activity. Vesicles pinching off the plasma membrane via the endocytic pathway may be rerouted to the recycling pathway, where the substances captured from the extracellular space may be returned to the surface plasma membrane/extracellular space by entering regulated exocytosis of the secretory pathway.
One of the first peptides studied for exocytotic release from astrocytes was ANP. This peptide is a diuretic vasorelaxant hormone typically stored in specific secretory vesicles and is secreted from the heart atrial myocytes in response to overload and oxygen deficiency [7,43]. The function of ANP release from astrocytes, however, may play a role in cerebral blood regulation [54]. To study the discharge of ANP, Krzan et al. [47] transfected astrocytes with a construct to express pro-ANP fused with the emerald green fluorescent protein (ANP.emd). Transfection of astrocytes resulted in fluorescent puncta, representing vesicles. The number of puncta was reduced upon stimulating the cells by the Ca2+ ionophore ionomycin, which strictly depended on the extracellular Ca2+. Concomitant with the Ca2+-dependent decrease in fluorescent puncta, the fluorescence intensity of the FM 4–64 dye, a reporter of cumulative exocytosis, increased in a Ca2+-dependent manner as well. Together these data strongly indicated that regulated exocytosis mediates the release of ANP from astrocytes. Interestingly, vesicles containing ANP also appear to contain ATP [61], which is consistent with the report that ATP is stored in secretorgranin II-containing vesicles [22].
In atrial myocytes, EM indicates that pro-ANP is condensed in the trans-Golgi network and, because pro-ANP is cleaved only on release, secretory vesicles budding off the trans-Golgi network are already mature. Their shape and size (120 to 175 nm) appears to be determined by the aggregation of the pro-ANP in vesicles [7]. In astrocytes the size of ANP recycling vesicles was studied by IEM after exposing live astrocytes to extracellular anti-ANP antibody, which sequestred within vesicles with diameters averaging 50 nm and ranging between 30 to 100 nm [72]. The mobility of these recycling ANP-containing vesicles was one order of magnitude smaller than that of ANP-containing vesicles trafficking to the plasma membrane vesicle docking site [70,71]. The clear-core nature and the smaller size of anti-ANP capturing vesicles in astrocytes relative to the values reported in atrial myocytes [7] is consistent with the observation that the vesicular ANP content determines the size and the shape of ANP-containing secretory vesicles [7].
The mobility of anti-ANP antibody capturing vesicles is dramatically reduced upon the stimulation of cells [72], which differs from the stimulation-increased mobility of anti-VGLUT1 antibody capturing vesicles in astrocytes [83]. The functional significance of these observations is not clear, but the results clearly show that retrieving vesicle mobility is subject to the physiological state of the astrocyte [72]. This may play a role in the regulation of the vesicle cycle and vesicle cargo discharge [72]. It is possible to envision that arrested mobility of retrieving vesicles may affect the vesicle cargo discharge, at least by prolonging the interaction between the plasma membrane and the vesicle membrane. It was shown that the main mode of peptidergic vesicle exocytosis is the transient fusion (kiss-and-run), and that stimulation increases the frequency of occurrence of vesicle fusion as well as the dwell time of the established fusion pore and vesicle content discharge [84,89]. This mode of vesicle fusion would be facilitated, if the vesicle interaction with the cytoskeleton during retrieval is prevented or attenuated. The stimulation-induced vesicle mobility arrest is consistent with this view of vesicle cycle regulation; it increases the probability of peptide hormone discharge. In contrast, while such a mechanism may be related to peptidergic vesicles, in glutamatergic vesicles capturing the anti-VGLUT1 antibody, stimulation-induced enhanced post-fusion vesicle mobility may have a different function [83]. In this case, where the diffusional mobility of glutamate transmitter is orders of magnitude more mobile than the peptidergic hormones, the stimulation-increased vesicle mobility may terminate the glutamate discharge upon the reduction of interaction time between the vesicle and the plasma membrane. Furthermore, recycling vesicles may not only carry lumenal cargo but, also, membrane associated signalling molecules which participate in contact cell-to-cell interactions [45,81] or in determining the density of plasma membrane transporters [76], such as, the glutamate transporter EAAT2 [82].
Astrocytes also contain recycling vesicles, specialized endocytic compartments, which may serve bidirectional communication between neurons and glia. On one hand, these vesicles may take-up extracellular peptides, process them, and recycle them back into the extracellular space via secretory pathway and regulated exocytosis (Figure 3). When studying the activity-dependent secretion of BDNF and its extracellular availability, Bergami et al. [10] conducted very interesting experiments and provided evidence that BDNF, which is de novo synthetized in neurons, gets secreted after theta-burst stimulation in its pro-form into the extracellular medium. Then the pro-BDNF is rapidly internalized via the the pan-neurotrophin receptor p75NTR in perineuronal astrocytes via endocytosis, thereby restricting the availability of this neurotrophin at neuron-astrocyte contacts. After internalization, the pro-BDNF can undergo a recycling process, endowing astrocytes with the ability to resecrete this neurotrophin upon their stimulation. Ultrastructural IEM characterization revealed that BDNF fluorescently tagged with yellow fluorescent protein (BDNF-YFP) and gold particles labelled clear-core vesicles with 125 nm in diameter. Subsequently, the fluoresently labelled vesicles exposed their lumen to extracellular solution, presumably via a fusion pore, since the lumenal pH increased, detected as an increase in pH-dependent YFP fluorescence. To further investigate the entry of BDNF into the secretory pathway, astrocytes were preincubated with BDNF-YFP and their secretion was studied by stimulating cells with glutamate. The glutamate-evoked secretion of BDNF-YFP was inhibited, if cells were pretreated with tetanus neurotoxin, which cleaves Sb2. Co-localization between pro-BDNF and Sb2 confirmed that endocytosed pro-BDNF was routed into Sb2-containing vesicles. Taken together, this study shows that endocytic vesicles expressing p75NTR represent the main storage/recycling compartment for endocytosed pro-BDNF before routing it to the SNARE-dependent secretory pathway [10].
Concluding remarks
It is becoming clear that astrocytes are endowed with elements that qualify them as cells communicating with neurons and other cells in brain by employing regulated exocytosis. Astrocytes can synthesize and store gliotransmitters, i.e., amino acids, ATP and peptides, in SNARE-associated vesicles. The vesicular cargo discharge from these cells via regulated release occurs upon a delivery of physiological/pathological stimulus. It should be noted that neighboring neurons and other cells rapidly sense and respond to the released gliotransmitters, albeit this subject was out of scope of the present review [reviewed in [59]]. Consequently, astrocytes appear as key players in central nervous system (patho)physiological processes. Further understanding of vesicular traffic to and from the plasma membrane via secretory pathway and within endocytic routes/recycling, as well as determining the location of exocytotic sites on astrocytes is of importance for astrocyte-neuron signaling. While astrocytic processes appear to be the ideal site for the location of vesicular fusions, exocytotic release can also occur on their bodies (reviewed in [56]). Although studies addressing these specific issues are only at the very beginning, there is palpable evidence that they will provide new insights into the understanding of how astrocytic membrane dynamics shape the signaling within the complex network of the brain tissue.
Acknowledgments
We thank J. Robert Grammer for comments on a previous version of this manuscript. The authors’ work is supported by grants from the National Institute of Mental Health (R01 MH 069791 to VP), the National Science Foundation (CBET 0943343 to VP) and from the Slovenian Research Agency (P3 310 381; J3 -0133; J3-0031, J3-9417 to RZ). We dedicate this work to the late Glenn I. Hatton, whose energy and creativity inspired new views of astrocyte-neuronal interactions.
Abbreviations
- ANP
atrial natriuretic peptide
- ATP
adenosine 5'-triphosphate
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid
- BDNF
brain-derived neurotrophic factor
- EGFP
enhanced green fluorescent protein
- EM
electron microscopy
- ER
endoplasmic reticulum
- IP3
permeant inositol 1,4,5-trisphosphate
- IEM
immuno EM
- NMDA
N-methyl-D-aspartate
- PMA
phorbol 12-myristate 13-acetate
- Sb2
synaptobrevin 2
- SNAP-23
synaptosome-associated protein of 23 kDa
- SNARE
the soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptor
- SOC
called store-operated Ca2+
- TCA
tricarboxylic acid
- TIRF
total internal refection fluorescence
- TRPC
canonical transient receptor potential
- UTP
uridine 5'-triphosphate
- VGLUT
vesicular glutamate transporter
- V-ATPase
the vacuolar type of proton ATPase
- YFP
yellow fluorescent protein
- 2-APB
2-aminoethyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vladimir Parpura, Email: vlad@uab.edu.
Robert Zorec, Email: robert.zorec@mf.uni-lj.si.
References
- 1.Abdipranoto A, Liu GJ, Werry EL, Bennett MR. Mechanisms of secretion of ATP from cortical astrocytes triggered by uridine triphosphate. Neuroreport. 2003;14:2177–2181. doi: 10.1097/00001756-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 2.Anlauf E, Derouiche A. Astrocytic exocytosis vesicles and glutamate: a high-resolution immunofluorescence study. Glia. 2005;49:96–106. doi: 10.1002/glia.20094. [DOI] [PubMed] [Google Scholar]
- 3.Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 5.Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axelrod J. Neurotransmitters. Sci Am. 1974;230:59–71. [PubMed] [Google Scholar]
- 7.Baertschi AJ, Monnier D, Schmidt U, Levitan ES, Fakan S, Roatti A. Acid prohormone sequence determines size, shape, and docking of secretory vesicles in atrial myocytes. Circ Res. 2001;89:E23–E29. doi: 10.1161/hh1501.095715. [DOI] [PubMed] [Google Scholar]
- 8.Bal-Price A, Moneer Z, Brown GC. Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia. 2002;40:312–323. doi: 10.1002/glia.10124. [DOI] [PubMed] [Google Scholar]
- 9.Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium. 2004;35:47–58. doi: 10.1016/s0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 10.Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, Matteoli M, Canossa M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol. 2008;183:213–221. doi: 10.1083/jcb.200806137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergersen LH, Gundersen V. Morphological evidence for vesicular glutamate release from astrocytes. Neuroscience. 2009;158:260–265. doi: 10.1016/j.neuroscience.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 12.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 13.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 14.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 15.Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 16.Bowery NG, Brown DA, Collins GG, Galvan M, Marsh S, Yamini G. Indirect effects of amino-acids on sympathetic ganglion cells mediated through the release of gamma-aminobutyric acid from glial cells. Br J Pharmacol. 1976;57:73–91. doi: 10.1111/j.1476-5381.1976.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowser DN, Khakh BS. Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc Natl Acad Sci U S A. 2007;104:4212–4217. doi: 10.1073/pnas.0607625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calegari F, Coco S, Taverna E, Bassetti M, Verderio C, Corradi N, Matteoli M, Rosa P. A regulated secretory pathway in cultured hippocampal astrocytes. J Biol Chem. 1999;274:22539–22547. doi: 10.1074/jbc.274.32.22539. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Ewing AG. Multiple classes of catecholamine vesicles observed during exocytosis from the Planorbis cell body. Brain Res. 1995;701:167–174. doi: 10.1016/0006-8993(95)00989-9. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Gavin PF, Luo G, Ewing AG. Observation and quantitation of exocytosis from the cell body of a fully developed neuron in Planorbis corneus. J Neurosci. 1995;15:7747–7755. doi: 10.1523/JNEUROSCI.15-11-07747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Wang L, Zhou Y, Zheng LH, Zhou Z. "Kiss-and-run" glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 23.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M, Carmignoto G. Synaptobrevin2-expressing vesicles in rat astrocytes: insights into molecular characterization, dynamics and exocytosis. J Physiol. 2006;570:567–582. doi: 10.1113/jphysiol.2005.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- 26.Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Do KQ, Benz B, Sorg O, Pellerin L, Magistretti PJ. beta-Adrenergic stimulation promotes homocysteic acid release from astrocyte cultures: evidence for a role of astrocytes in the modulation of synaptic transmission. J Neurochem. 1997;68:2386–2394. doi: 10.1046/j.1471-4159.1997.68062386.x. [DOI] [PubMed] [Google Scholar]
- 28.Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem. 2006;281:30684–30696. doi: 10.1074/jbc.M606429200. [DOI] [PubMed] [Google Scholar]
- 29.Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabbretti E, Sokolova E, Masten L, D'Arco M, Fabbro A, Nistri A, Giniatullin R. Identification of negative residues in the P2X3 ATP receptor ectodomain as structural determinants for desensitization and the Ca2+-sensing modulatory sites. J Biol Chem. 2004;279:53109–53115. doi: 10.1074/jbc.M409772200. [DOI] [PubMed] [Google Scholar]
- 31.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca2+]I oscillations and is not involved in capacitative Ca2+ entry in glial cells. J Neurosci. 2003;23:4737–4745. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- 36.Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+- dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- 37.Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca(2+) stores in astrocytes. J Neurosci Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- 38.Huang LY, Neher E. Ca2+-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- 39.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte "hemichannels". J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaiswal JK, Fix M, Takano T, Nedergaard M, Simon SM. Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proc Natl Acad Sci U S A. 2007;104:14151–14156. doi: 10.1073/pnas.0704935104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeftinija SD, Jeftinija KV, Stefanovic G, Liu F. Neuroligand-evoked calcium-dependent release of excitatory amino acids from cultured astrocytes. J Neurochem. 1996;66:676–684. doi: 10.1046/j.1471-4159.1996.66020676.x. [DOI] [PubMed] [Google Scholar]
- 43.Jiao JH, Baertschi AJ. Neural control of the endocrine rat heart. Proc Natl Acad Sci U S A. 1993;90:7799–7803. doi: 10.1073/pnas.90.16.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94:4121–4130. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]
- 45.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon PG, Zorec R. Properties of Ca2+-dependent exocytosis in cultured astrocytes. Glia. 2004;46:437–445. doi: 10.1002/glia.20018. [DOI] [PubMed] [Google Scholar]
- 47.Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J Neurosci. 2003;23:1580–1583. doi: 10.1523/JNEUROSCI.23-05-01580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, Ropert N, Koulakoff A, Giaume C, Oheim M. Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J Neurosci. 2008;28:7648–7658. doi: 10.1523/JNEUROSCI.0744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maienschein V, Marxen M, Volknandt W, Zimmermann H. A plethora of presynaptic proteins associated with ATP-storing organelles in cultured astrocytes. Glia. 1999;26:233–244. doi: 10.1002/(sici)1098-1136(199905)26:3<233::aid-glia5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 50.Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008 doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- 51.Marchaland J, Cali C, Voglmaier SM, Li H, Regazzi R, Edwards RH, Bezzi P. Fast subplasma membrane Ca2+ transients control exo-endocytosis of synaptic-like microvesicles in astrocytes. J Neurosci. 2008;28:9122–9132. doi: 10.1523/JNEUROSCI.0040-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- 53.Martineau M, Galli T, Baux G, Mothet JP. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia. 2008;56:1271–1284. doi: 10.1002/glia.20696. [DOI] [PubMed] [Google Scholar]
- 54.McKenzie JC, Juan YW, Thomas CR, Berman NE, Klein RM. Atrial natriuretic peptide-like immunoreactivity in neurons and astrocytes of human cerebellum and inferior olivary complex. J Histochem Cytochem. 2001;49:1453–1467. doi: 10.1177/002215540104901113. [DOI] [PubMed] [Google Scholar]
- 55.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 56.Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- 57.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- 60.Ni Y, Parpura V. Dual regulation of Ca2+-dependent glutamate release from astrocytes: Vesicular glutamate transporters and cytosolic glutamate levels. Glia. 2009;57:1296–1305. doi: 10.1002/glia.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- 62.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 63.Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- 64.Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parpura V, Liu F, Brethorst S, Jeftinija K, Jeftinija S, Haydon PG. Alpha-latrotoxin stimulates glutamate release from cortical astrocytes in cell culture. FEBS Lett. 1995;360:266–270. doi: 10.1016/0014-5793(95)00121-o. [DOI] [PubMed] [Google Scholar]
- 66.Parpura V, Tong W, Yeung ES, Haydon PG. Laser-induced native fluorescence (LINF) imaging of serotonin depletion in depolarized neurons. J Neurosci Methods. 1998;82:151–158. doi: 10.1016/s0165-0270(98)00056-9. [DOI] [PubMed] [Google Scholar]
- 67.Pasantes Morales H, Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. J Neurosci Res. 1988;20:503–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- 68.Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 70.Potokar M, Kreft M, Li L, Daniel Andersson J, Pangrsic T, Chowdhury HH, Pekny M, Zorec R. Cytoskeleton and vesicle mobility in astrocytes. Traffic. 2007;8:12–20. doi: 10.1111/j.1600-0854.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 71.Potokar M, Kreft M, Pangrsic T, Zorec R. Vesicle mobility studied in cultured astrocytes. Biochem Biophys Res Commun. 2005;329:678–683. doi: 10.1016/j.bbrc.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 72.Potokar M, Stenovec M, Kreft M, Kreft ME, Zorec R. Stimulation inhibits the mobility of recycling peptidergic vesicles in astrocytes. Glia. 2008;56:135–144. doi: 10.1002/glia.20597. [DOI] [PubMed] [Google Scholar]
- 73.Pryazhnikov E, Khiroug L. Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia. 2008;56:38–49. doi: 10.1002/glia.20590. [DOI] [PubMed] [Google Scholar]
- 74.Reyes RC, Parpura V. Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci. 2008;28:9682–9691. doi: 10.1523/JNEUROSCI.3484-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reyes RC, Parpura V. The trinity of Ca2+ sources for the exocytotic glutamate release from astrocytes. Neurochem Int. 2009;55:2–8. doi: 10.1016/j.neuint.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- 77.Rosenberg PA, Knowles R, Knowles KP, Li Y. Beta-adrenergic receptor-mediated regulation of extracellular adenosine in cerebral cortex in culture. J Neurosci. 1994;14:2953–2965. doi: 10.1523/JNEUROSCI.14-05-02953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simpson PB, Russell JT. Role of mitochondrial Ca2+ regulation in neuronal and glial cell signalling. Brain Res Brain Res Rev. 1998;26:72–81. doi: 10.1016/s0165-0173(97)00056-8. [DOI] [PubMed] [Google Scholar]
- 81.Soos JM, Morrow J, Ashley TA, Szente BE, Bikoff EK, Zamvil SS. Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol. 1998;161:5959–5966. [PubMed] [Google Scholar]
- 82.Stenovec M, Kreft M, Grilc S, Pangrsic T, Zorec R. EAAT2 density at the astrocyte plasma membrane and Ca2+-regulated exocytosis. Mol Membr Biol. 2008;25:203–215. doi: 10.1080/09687680701790925. [DOI] [PubMed] [Google Scholar]
- 83.Stenovec M, Kreft M, Grilc S, Potokar M, Kreft ME, Pangrsic T, Zorec R. Ca2+-dependent mobility of vesicles capturing anti-VGLUT1 antibodies. Exp Cell Res. 2007;313:3809–3818. doi: 10.1016/j.yexcr.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 84.Stenovec M, Kreft M, Poberaj I, Betz WJ, Zorec R. Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. Faseb J. 2004;18:1270–1272. doi: 10.1096/fj.03-1397fje. [DOI] [PubMed] [Google Scholar]
- 85.Stigliani S, Zappettini S, Raiteri L, Passalacqua M, Melloni E, Venturi C, Tacchetti C, Diaspro A, Usai C, Bonanno G. Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. J Neurochem. 2006;96:656–668. doi: 10.1111/j.1471-4159.2005.03631.x. [DOI] [PubMed] [Google Scholar]
- 86.Südhof TC, Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991;6:665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- 87.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 88.Takemura H, Putney JW., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989;258:409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–4746. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verkhratsky A. Neurotransmitter receptors in astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2009. pp. 50–67. [Google Scholar]
- 91.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 92.Wang W, O'Connell B, Dykeman R, Sakai T, Delporte C, Swaim W, Zhu X, Birnbaumer L, Ambudkar IS. Cloning of Trp1beta isoform from rat brain: immunodetection and localization of the endogenous Trp1 protein. Am J Physiol. 1999;276:C969–C979. doi: 10.1152/ajpcell.1999.276.4.C969. [DOI] [PubMed] [Google Scholar]
- 93.Warr O, Takahashi M, Attwell D. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J Physiol. 1999;514(Pt 3):783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Westergaard N, Drejer J, Schousboe A, Sonnewald U. Evaluation of the importance of transamination versus deamination in astrocytic metabolism of [U-13C]glutamate. Glia. 1996;17:160–168. doi: 10.1002/(SICI)1098-1136(199606)17:2<160::AID-GLIA7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 95.Wilhelm A, Volknandt W, Langer D, Nolte C, Kettenmann H, Zimmermann H. Localization of SNARE proteins and secretory organelle proteins in astrocytes in vitro and in situ. Neurosci Res. 2004;48:249–257. doi: 10.1016/j.neures.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 96.Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO, Jr, Ferris CD, Snyder SH. Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci U S A. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J. Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem. 2007;282:24185–24197. doi: 10.1074/jbc.M700452200. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci U S A. 2004;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]