Abstract
Background
Basic science studies at the neuronal systems level have indicated that gamma-range (30–50 Hz) neural synchronization may be a key mechanism of information processing in neural networks, reflecting integration of various features of an object. Furthermore, gamma-range synchronization is thought to depend on the glutamatergically mediated interplay between excitatory projection neurons and inhibitory neurons utilizing g-aminobutyric acid (GABA), which postmortem studies suggest may be abnormal in schizophrenia. We therefore tested whether auditory neural networks in patients with schizophrenia could support gamma-range synchronization.
Methods
Synchronization of the electroencephalogram (EEG) to different rates (20–40 Hz) of auditory stimulation was recorded from 15 patients with schizophrenia and 15 sex-, age-, and handedness-matched control subjects. The EEG power at each stimulation frequency was compared between groups. The time course of the phase relationship between each stimulus and EEG peak was also evaluated for gamma-range (40 Hz) stimulation.
Results
Schizophrenic patients showed reduced EEG power at 40 Hz, but not at lower frequencies of stimulation. In addition, schizophrenic patients showed delayed onset of phase synchronization and delayed desynchronization to the click train.
Conclusions
These data provide new information on selective deficits in early-stage sensory processing in schizophrenia, a failure to support the entrainment of intrinsic gamma-frequency oscillators. The reduced EEG power at 40 Hz in schizophrenic patients may reflect a dysfunction of the recurrent inhibitory drive on auditory neural networks.
GAMMA-FREQUENCY band neural activity (30–50 Hz or broader, centered on 40 Hz) has been hypothesized to reflect the synchronization of neural assemblies involved in binding or integration of various features of an object within a single sensory modality, across modalities, and across time.1–9 For example, gamma synchronization may be involved in the perception of a complex object, such as a cat, which requires integration of many features, such as luminance, color, texture, contours, and position, which are analyzed by discrete neurons or neural systems in the brain.6 Although the precise mechanisms of gamma synchronization are unknown, there is evidence of participation of γ-aminobutyric acid (GABA)ergic interneuronal circuits.5,10 There is also considerable evidence suggestive of abnormalities in GABAergic circuitry in schizophrenia.11,12 Failure or abnormality in gamma synchronization could result in a variety of perceptual and cognitive abnormalities, including abnormal perceptions, aberrant semantic association, hallucinations, and discontinuities in thinking, that occur in schizophrenia. Clementz et al13 recently proposed that transient disturbances of gamma-band response contribute to poor P50 evoked potential auditory response suppression in schizophrenia.
To test whether neural circuits in schizophrenic patients could support normal gamma synchronization at specific frequencies, we evaluated entrainment of the electroencephalogram (EEG) to trains of clicks presented at varying frequencies, including gamma range (40 Hz). When receiving periodic auditory input, neural networks behave like a tuned oscillator with a preferred resonance around 40 Hz,7,14,15 ie, the EEG synchronizes to the frequency of the periodic stimulus. Anatomically, the primary auditory cortex is probably one of the major sources of the auditory “steady-state” or entrainment response to periodic stimuli, although interconnected temporal lobe and thalamic regions may also be involved. Testing the capacity of this neural circuit to support gamma-range entrainment provides a method to determine the relationship of the power or phase of the output (EEG) to the characteristics of the input (periodic auditory stimuli).
RESULTS
EEG POWER
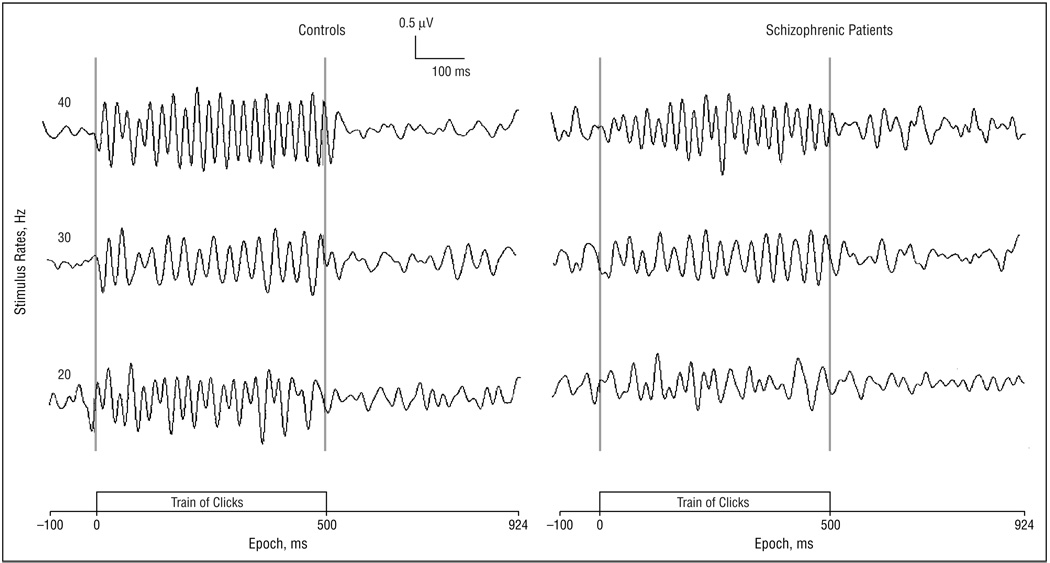
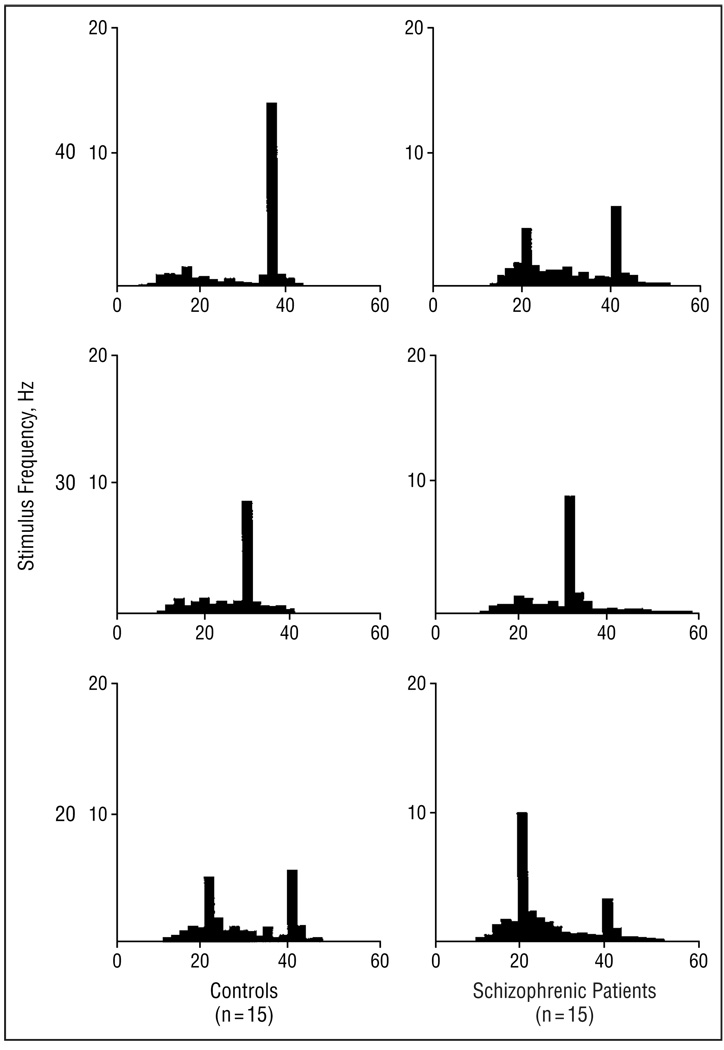
Figure 2 presents the averaged EEG waveforms to click trains with frequencies at 20, 30, and 40 Hz in controls and schizophrenic subjects. Subjects in both groups entrained to the stimulus frequency, as shown by graphs of spectral power at each stimulation rate in Figure 3. The log10 of the EEG power to the stimulating frequency was compared between groups at the 3 frontal electrode sites. Analysis of variance revealed a main effect of stimulus (F2,56, 8.78; P<.001), with 40-Hz stimulation producing greater power than 20 or 30 Hz. There was also an interaction of group by stimulus (F2,56, 3.24; P = .05). The same main effect and interaction were present when a direct measure of power was used in the ANOVA. To characterize the nature of the group by stimulus interaction, individual post hoc ANOVAs were calculated for each stimulus condition. Each post hoc ANOVA had the factors group (n = 2) and electrode site (n = 3). The mean square within value from the overall repeated-measures ANOVA was used as the denominator of the F test to calculate a significance value of the group effect for each stimulus condition. The group effect for power was significant for the 40-Hz stimulus condition (F1,28, 5.29; P = .03), but not for the 20-Hz (F1,28, 0.07; P = .79) or the 30-Hz condition (F1,28, 0.05; P = .83).
Figure 2.
Averaged electroencephalographic frequency across subjects to the train of clicks at the following 3 stimulus rates: 40 Hz (upper), 30 Hz (middle), and 20 Hz (lower). The recordings were obtained from the Fz electrode site.
Figure 3.
Mean power (in microvolts squared) spectra (y-axis) for electroencephalograms recorded to trains of clicks at the following 3 stimulus rates: 40 Hz (upper), 30 Hz (middle), and 20 Hz (lower). The schizophrenic patients (n = 15) show decreased power at 40-Hz stimulation compared with control subjects (n = 15), but there was no difference between groups at lower frequencies of stimulation.
PHASE RELATIONSHIPS AT 40 Hz
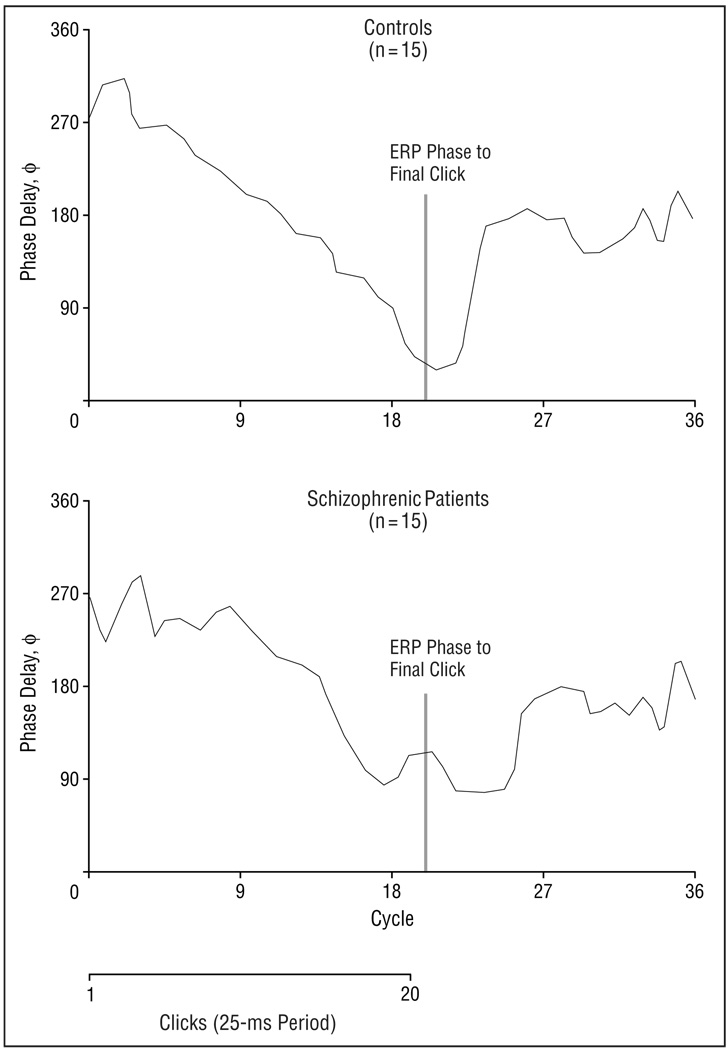
Visual inspection of the 40-Hz waveforms (Figure 2) suggested that schizophrenic patients may show delayed onset of entrainment and delayed return to desynchronization of the EEG, as well as reduced power. To test these effects statistically, we evaluated peak by peak phase of the EEG waveform. Figure 4 shows the relationship of phase delay to time in cycles (period, 25 milliseconds) in each group at the midline frontal electrode site. The response of the first click is at cycle 1 and to the last click is at cycle 20. We also evaluated phase delay after offset of the click train (cycles 21–36) to test whether phase remained entrained to the 40-Hz stimulus frequency after click offset.
Figure 4.
Phase delay of 40-Hz response in schizophrenic patients (n = 15) compared with healthy control subjects (n = 15). Patients with schizophrenia were slower than controls to synchronize to clicks initially, as measured by decreasing phase delay after onset of the click train. Schizophrenic patients were then slower to desynchronize following click offset. ERP indicates event-related potential.
These effects were statistically analyzed using a group (n = 2) by cycle (n = 36) by electrode site (n = 3) ANOVA on phase delay across the 900-millisecond epoch. There was no main effect for group (F2,28, 0.06; P = .80). However, the ANOVA revealed an effect of cycle on phase delay (F35,980, 20.61; P<.001), indicating that phase delay decreased over the stimulus period, then increased after stimulus offset. There was also a group by cycle interaction (F35,980, 1.86; P = .002), indicating that the phase delay of patients differed from that of controls, and that this difference changed with time. The significance of the main effect for cycle and the group by cycle interaction remained significant (P = .03) after applying the Huynh-Feldt epsilon correction. To clarify the nature of this interaction, between-group t tests were computed for phase delay at each sample point at the midline frontal electrode site. These tests revealed that patients showed increased phase delay while clicks were presented (P≤.05 at cycles 8, 9, 10, 13, 14, 20, and 21), but shorter phase delays after stimulus offset (P≤.05 at cycles 24 and 25), ie, 40-Hz activity in patients remained synchronized after the stimulus ceased.
SUBJECTS AND METHODS
SUBJECTS
Fifteen right-handed male schizophrenic patients were recruited from the Brockton Veterans Affairs Medical Center, Brockton, Mass. The patients met DSM-IV criteria16 for schizophrenia on the basis of interviews conducted using the Structured Clinical Interview for DSM-IV and medical chart review. Six patients were receiving conventional neuroleptics; 8 patients, novel antipsychotics; and 1 patient, no antipsychotics. Daily mean chlorpromazine hydrochloride equivalent dose17 was 440.1 ± 261.6 mg. (Unless otherwise indicated, data are given as mean ± SD.) All patients satisfied the age criterion of 20 to 55 years. No patient had had electroconvulsive therapy, neurologic illness, major head trauma, or alcohol or other drug abuse within the previous 5 years (DSM-IV criteria). Mean duration of illness was 21.1 ± 7.1 years, with near continuous administration of antipsychotics during that period. The healthy control group consisted of 15 right-handed male subjects who were recruited from newspaper advertisements. Controls received a structured interview regarding neurologic and psychiatric disorders, alcohol and other drug use, occupational and educational history, and psychiatric disorders in first-degree relatives. None of the controls had a history of alcohol or other drug abuse (DSM-IV criteria); neither they nor their first-degree relatives had a history of psychiatric or neurologic illness. The mean age of the control group (44.6 ± 8.6 years) did not differ from that of the patient group (43.3 ± 6.7 years; Mann-Whitney test, P = .59). Parental socioeconomic status, as measured by the Hollingshead Two-Factor Index of Social Position,18 did not differ between the control (2.8 ± 0.9) and patient groups (2.5 ± 1.2; Mann-Whitney test, P = .60). The Mini-Mental State Examination score19 was slightly depressed in the patient (28.1 ± 1.4) compared with the control group (29.3 ± 0.8; Mann-Whitney test, P = .02). All subjects received detailed information about the study protocol and gave written informed consent. Psychiatric symptoms were rated using a structured interview for the Positive and Negative Syndrome Scale (PANSS).20 These chronically ill patients had a mean positive symptom score of 17.1 ± 6.9, a mean negative symptom score of 18.1 ± 7.0, and a mean general psychopathology score of 34.5 ± 10.2 on the PANSS.
ELECTROPHYSIOLOGIC ASSESSMENT
Stimulus Procedure
During the evaluation, subjects were asked to relax with eyes open and listen to trains of clicks presented through insert earphones (Etymotic Research, Elk Grove Village, Ill). The stimuli were 1-millisecond duration clicks, presented as trains of clicks that varied in rate of presentation (20, 30, and 40 Hz) in each of 3 blocks. The duration of the click train was 450 milliseconds for 20-Hz, 467 milliseconds for 30-Hz, and 475 milliseconds for 40-Hz stimuli. Each block had 150 trains of clicks with 700-millisecond intertrain intervals.
EEG Recording and Power Analysis
The EEG was recorded continuously (band pass, 0.1-100 Hz; sampling rate, 500 Hz) from the scalp, using a 64-channel Geodesic Sensor Net (Electrical Geodesics, Eugene, Ore). The Sensor Net consisted of a network of silver–silver chloride sponge sensors.21 Recordings were vertex referenced and later digitally rereferenced to the right mastoid. Electrode impedances were maintained below 30 kΩ. The EEG was segmented into epochs, which included the EEG from 100 milliseconds before to 924 milliseconds after the onset of each click train. After baseline correction, epochs that contained voltage exceeding ±100 µV at any site were excluded. Averages were computed at each block and digitally filtered using a band-pass Butterworth filter (band pass, 12–50 Hz; slope, 24 dB/octave) using commercially available software (NeuroScan; Neuro Scan Inc, El Paso, Tex). The averaged epoch across subjects collected during the click train (0–512 milliseconds) was transformed into power spectra by means of fast-Fourier transform. Power spectra graphed the power (in microvolts squared) for each EEG frequency under consideration using a bin width of 1 Hz.
Phase Analysis
Peak-by-peak phase of the EEG waveform (the difference, in degrees, between a click presentation and the negative peak in the EEG) was evaluated. Phase delay, or ϕ (phi), of the EEG was measured following response during and after the stimulus train after isolating 40-Hz activity by using a narrow band-pass digital Butterworth filter (35–45 Hz; 48 dB/octave roll-off). Phase delay was defined in the following equation:
where tr is the time corresponding to the negative peak in the EEG, and ti corresponds to the time of the ith stimulus presentation. Phase delay was then calculated by multiplying ϕ by 360°. Thus, phase delay may vary between 0° and 360°. Since the interval beween 40-Hz clicks is 25 milliseconds, 360° represents a phase delay of 25 milliseconds; 180°, a phase delay of 12.5 milliseconds; etc.
STATISTICAL ANALYSIS
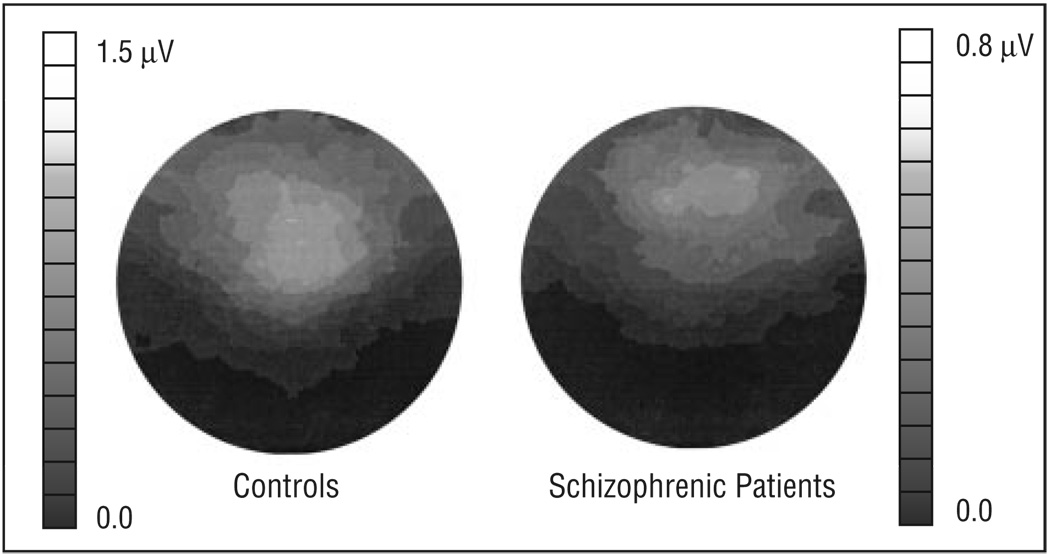
Power at the frequency of stimulation was measured for each condition at the midline frontal electrode site (anterior to the 10–20 system electrode site of Fz) and at 2 adjacent lateral sites where the 40-Hz entrained response was largest, as shown by the averaged maps of the power topography over the head in the 40-Hz stimulation condition (Figure 1). Repeated measures analysis of variance (ANOVA) was used to evaluate the effects of group (n = 2), rate of stimulation (n = 3), and electrode site (n = 3) on power. Phase delay was evaluated statistically for the 20 cycles (clicks) during the stimulus train and for 16 cycles after stimulus offset. Repeated-measures ANOVA was used to evaluate the effects of group and cycle on phase delay at the same frontal electrode sites that were used in the power analysis. A criterion of P≤.05 was used to determine statistical significance.
COMMENT
These findings suggest a decrease in the ability of auditory neural networks to support synchronous neural activity at 40 Hz, but not at lower frequencies, in schizophrenia. Since N-methyl-d-aspartate (NMDA)–modulated GABAergic activity may play a critical role in gamma-frequency synchronization, these results may provide a neurophysiological link to cellular models of schizophrenia that posit NMDA dysregulation.11 An important role for NMDA receptors in the clinical manifestations of schizophrenia is supported by the finding that NMDA antagonists, such as phencyclidine hydrochloride, mimic the positive and negative symptoms of the disorder.22 Our laboratory12 has shown in the rat hippocampus in vitro that the NMDA receptor mediating recurrent collaterals of projection neurons onto GABAergic interneurons is some 10-fold more sensitive to blockade by NMDA receptor blockers than the NMDA receptor mediating excitatory (Schaffer collateral) input onto the projection neurons. The net result of NMDA blockade is thus reduced inhibition. Also, in postmortem studies in the temporal lobes of schizophrenic patients, Tsai and coworkers23 have demonstrated elevated concentrations of N-acetyl-aspartyl-glutamate, a compound that in vitro has been demonstrated to block preferentially the NMDA receptor mediating excitatory input on inhibitory interneurons,12 and that thus may be an endogenous NMDA channel blocker.
Recent basic science investigations using in vitro24 and in vivo25 preparations suggest the critical involvement of excitatory drive on interneurons in the generation of synchronized gamma activity, or at least in the ability of networks to resonate in the gamma range. A reduction of this recurrent drive might result in lessened recurrent inhibition, and a lessened ability of the network to entrain faithfully to a gamma frequency, as shown in schizophrenic patients. Intrinsic abnormalities of GABAergic neurons, such as have been reported in postmortem tissue of schizophrenic patients, also might be a mechanism contributing to a failure of recurrent inhibition.26
Failure of gamma-range inhibition also has been reported to contribute to sensory gating deficits as measured by the P50 paradigm.13 Dysregulation in the circuits producing the gamma rhythm might interfere with transmission of transient or high temporal frequency information, and thus contribute to behavioral deficits noted for tasks that require rapid temporal integration, such as motion perception27 and backward masking.28 More speculatively, an inability to support 40-Hz oscillations could lead to abnormalities in perceptual and temporal binding, and consequently might play a role in the reality distortions and disordered trains of thought seen in schizophrenia. However, further investigations of driven and transient gamma activity will be required to establish this relationship.
There are important limitations in the interpretation of our study, which will require further investigation. First, the relationship between synchronized auditory gamma activity to specific anatomic regions remains controversial, as does whether the response is entirely evoked or reflects intrinsic resonance, as is suggested by our data (eg, Pantev et al3; Picton et al7; Galambos14; Bressler and Freeman29; and Hari et al30). Second, the roles of antipsychotics and anticholinergic medication and the EEG abnormalities reported herein remain to be clarified. Since these medications likely influence neurotransmission within glutamatergic circuits, studies with larger sample sizes, and including subjects not receiving medication, will be required for definitive clarification of these relationships. An evaluation of our data showed gamma power was not significantly correlated with chlorpromazine equivalent dosage of typical and atypical neuroleptics (Spearman ρ). There were no statistically significant group differences using Mann-Whitney tests in gamma power in a comparison of subjects receiving typical vs atypical neuroleptics or in a comparison of subjects receiving vs those not receiving anticholinergics. Third, the specificity of these abnormalities to schizophrenia has not been tested. Finally, the subjects in this study were chronically ill patients who were relatively refractory to treatment. It remains to be determined whether gamma-range abnormalities would be present in patients at the first episode of the illness, as well.
Figure 1.
Averaged 40-Hz power across the head to 40-Hz stimulation in the control (n = 15) and schizophrenic groups (n = 15). The anterior of the head is upward, and viewer left is subject left. Maximal 40-Hz power in controls was recorded over the frontal scalp, near the 10–20 system electrode site of Fz. Patients showed reduced 40-Hz power compared with controls. Scales for 2 topographic maps are different.
Acknowledgments
Supported by grant 40799 (Dr McCarley) from the Department of Veterans Affairs Schizophrenia Center, Brockton, Mass; Research Scientist Development Award KO1-MH00746-04 (Dr Shenton),and First Award (Dr Shenton)from the National Institute of Mental Health, Rockville, Md; The Common wealth of Massachusetts Research Center, Boston (Dr McCarley);The National Alliance for Research on Schizophrenia and Depression, Chicago, Ill (Drs O’Donnell and McCarley); and the Stanley Foundation, Bethesda, Md(Dr Shenton).
We thank Sare J. Akdag and Joanna L. Gainski for technical assistance.
REFERENCES
- 1.Gray CM, DiPrisco GV. Stimulus-dependent neuronal oscillations and local synchronization in striate cortex of the alert cat. J Neurosci. 1997;17:3239–3253. doi: 10.1523/JNEUROSCI.17-09-03239.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joliot M, Ribary U, Llinas R. Human oscillatory brain activity near 40 Hz coexists with cognitive temporal binding. Proc Natl Acad Sci USA. 1994;91:11748–11751. doi: 10.1073/pnas.91.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C. Human auditory evoked gamma-band magnetic fields. Proc Natl Acad Sci U S A. 1991;88:8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer W, Gray CM. Visual figure integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 5.Traub RD, Whittington MA, Stanford IM, Jeffreys JGR. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- 6.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 7.Picton TW, Skinner CR, Champagne SC, Kellett AJC, Maiste AC. Potentials evoked by sinusoidal modulation of the amplitude or frequency of a tone. J Acoust Soc Am. 1987;82:165–178. doi: 10.1121/1.395560. [DOI] [PubMed] [Google Scholar]
- 8.Bressler S. The gamma wave: a cortical information carrier? Trends Neurosci. 1990;13:161–162. doi: 10.1016/0166-2236(90)90039-d. [DOI] [PubMed] [Google Scholar]
- 9.Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 10.Whittington M, Traub R, Jefferys J. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 11.McCarley RW, Hsiao J, Freedman R, Pfefferbaum A, Donchin E. Neuroimaging and the cognitive neuroscience of schizophrenia. Schizophr Bull. 1996;22:703–726. doi: 10.1093/schbul/22.4.703. [DOI] [PubMed] [Google Scholar]
- 12.Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- 14.Galambos R. Tactile and auditory stimuli presented at high rates (30–50 per sec) produce similar event related potentials. Ann N Y Acad Sci. 1982;388:722–728. doi: 10.1111/j.1749-6632.1982.tb50841.x. [DOI] [PubMed] [Google Scholar]
- 15.Basar E, Rosen B, Basar-Eroglu C, Greitschus F. The associations between 40 Hz–EEG and the middle latency response of the auditory evoked potential. Int J Neurosci. 1987;33:103–117. doi: 10.3109/00207458708985933. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 17.Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 5th rev ed. Seattle, Wash: Hogrefe & Huber Publishers; 1996. [Google Scholar]
- 18.Hollingshead AB. Two-Factor Index of Social Position. New Haven, Conn: Yale Station: 1965. [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale Manual. North Tanawanda, NY: Multi-Health Systems Inc; 1986. [Google Scholar]
- 21.Tucker DM. Spatial sampling of head electrical fields: the Geodesic Sensor Net. Electroencephalogr Clin Neurophysiol. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- 22.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 23.Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE, Coyle JT. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry. 1995;52:829–836. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- 24.Whittington MA, Traub RD, Faulkner HJ, Stanford IM, Jefferys JGR. Recurrent excitatory postsynaptic potentials induced by synchronized fast cortical oscillations. Proc Natl Acad Sci U S A. 1997;94:12198–12203. doi: 10.1073/pnas.94.22.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chrobak JJ, Buzsaki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small intereurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 28.Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients: evidence for a vulnerability indicator. Arch Gen Psychiatry. 1997;54:465–472. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- 29.Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr Clin Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- 30.Hari R, Hamalainen M, Joutsiniemi SL. Neuromagnetic steady-state responses to auditory stimuli. J Acoust Soc Am. 1989;86:1033–1039. doi: 10.1121/1.398093. [DOI] [PubMed] [Google Scholar]