Abstract
Monocarboxylate transporters (MCTs) are important cellular pH regulators in cancer cells; however, the value of MCT expression in cancer is still poorly understood. In the present study, we analysed MCT1, MCT2, and MCT4 protein expression in breast, colon, lung, and ovary neoplasms, as well as CD147 and CD44. MCT expression frequency was high and heterogeneous among the different tumours. Comparing with normal tissues, there was an increase in MCT1 and MCT4 expressions in breast carcinoma and a decrease in MCT4 plasma membrane expression in lung cancer. There were associations between CD147 and MCT1 expressions in ovarian cancer as well as between CD147 and MCT4 in both breast and lung cancers. CD44 was only associated with MCT1 plasma membrane expression in lung cancer. An important number of MCT1 positive cases are negative for both chaperones, suggesting that MCT plasma membrane expression in tumours may depend on a yet nonidentified regulatory protein.
1. Introduction
Uncontrolled tumour cell proliferation is a pivot mechanism in tumourigenesis, which consequently leads to significant metabolic changes. In tumour cells, the preference by anaerobic glycolysis, even in the presence of oxygen, phenomenon known as “the Warburg effect”, stimulates the conversion of pyruvate to lactic acid [1, 2]. To allow proliferation through continuous glycolysis and avoid acid-induced apoptosis, cells must develop mechanisms to oppose the increased generation of lactic acid. Thus, several plasma membrane transporters and exchangers have been implicated in the maintenance of the intracellular pH of cancer cells, by exporting the accumulating acid, leading to acidification of the extracellular milieu [3]. Currently, it is acknowledged that this acidic tumour microenvironment is associated with tumour aggressiveness features, such as growth advantage, increased survival, migration, invasion, and angiogenesis [1, 4].
Monocarboxylate transporters (MCTs) are among the most important cellular pH regulators likely involved in cancer pH homeostasis [3, 5]. The MCT family comprises fourteen members, being the isoforms 1, 2, 3, and 4 responsible for the H+-linked transport of monocarboxylates such as lactic acid across the plasma membrane [6]. The underlying molecular events involved in MCT regulation are poorly understood; however, it was recently demonstrated that proper plasma membrane expression and activity of MCTs, particularly MCT1 and MCT4, require the presence of a chaperone, CD147 [7–9], also known as EMMPRIN and basigin. Interestingly, CD147 expression seems to be also dependent on MCT1 and MCT4 expressions [10, 11]. Most recently, it was suggested that constitutive interactions between hyaluronan and CD44 also contribute to regulation of MCT localization and function [12]. In the past few years, some studies reported abnormal expression of MCTs, particularly MCT1, 2, 3, and 4 in distinct solid tumours, however, with contradictory conclusions [13–24]. Besides acting as MCT chaperone, CD147 plays many other roles, including production of matrix metalloproteinases and vascular endothelial growth factor, being upregulated in a variety of human cancers [25–28]. Additionally, activation of CD44 has been described as important in various aspects of cancer progression including cell growth control, adhesion, migration, invasion, and chemoresistance [29, 30].
The aim of the present study was to perform a comprehensive analysis of MCT1, MCT2, and MCT4 protein expression in a variety of tumours, namely, breast, colon, lung, and ovary neoplasms in order to elucidate their pattern of expression and their role in the development of these tumours. In addition, CD147 and CD44 expressions were analysed to infer the contribution of these chaperones to MCT expression in these different tumours.
2. Materials and Methods
2.1. Cases
A commercial human multitumour tissue microarray (TARP) (NCI Tumour Repository MTA, MD, USA), containing 200 tumour samples, was used to perform the immunohistochemical reactions, corresponding to 50 breast carcinomas (42 ductal, 5 lobular, and 3 not classified), 50 colon adenocarcinomas, 50 nonsmall cell lung cancers, and 50 ovarian adenocarcinomas (32 serous, 8 clear cell, 4 mucinous, 4 endometrioid, and 2 not classified). Additionally, to allow comparison between nonneoplastic and malignant tissues, 15 normal breast samples and 11 normal lung samples were included in the analysis. Since ovarian normal tissues are not readily available, they were not included in this study. MCT expression in nonneoplastic colon epithelia was already described by our group [19].
2.2. Immunohistochemistry
2.2.1. MCT Detection
Immunohistochemistry was performed according to the avidin-biotin-peroxidase complex principle (R.T.U. VECTASTAIN Elite ABC Kit (Universal), Vector Laboratories, Burlingame, CA, USA), with the primary antibodies for MCT1 (AB3538P, Chemicon International, Temecula, CA, USA), MCT2 (sc-14926, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and MCT4 (AB3316P, Chemicon International, Temecula, CA, USA), diluted 1 : 200 for both MCT1 and MCT2 and 1 : 100 for MCT4, as previously described by our group [18, 19].
2.2.2. CD147 Detection
Immunohistochemistry was performed according to the avidin-biotin-peroxidase complex principle (Ultravision Detection System Anti-polyvalent, HRP, Lab Vision Corporation, Fremont, CA, USA), using a primary antibody raised against CD147 (18-7344, ZYMED Laboratories Inc., South San Francisco, CA, USA) diluted 1 : 750, as previously described by our group [31].
2.2.3. CD44 Detection
Immunohistochemistry was performed according to the avidin-biotin-peroxidase complex principle (Ultravision Detection System Anti-polyvalent, HRP, Lab Vision Corporation, Fremont, CA, USA), using a primary antibody raised against total CD44 (clone 156-3C11, Cell Signalling Technology, Beverly, MA, USA), diluted 1 : 100. Briefly, deparaffinised and rehydrated sections were immersed in 0.01 M citrate-buffered solution (pH 6.0), heated up to 98°C in a water bath for 15 minutes, and washed in PBS. Endogenous peroxidases were inactivated with 3% hydrogen peroxide in methanol for 10 minutes, followed by washing in PBS. Tissue sections were incubated with blocking solution for 10 minutes and incubated at room temperature with the primary antibody for 30 minutes. Sections were then sequentially washed in PBS, incubated with biotinylated goat anti-polyvalent antibody for 10 minutes, streptavidin peroxidase for 10 minutes, and developed with 3,3′-diamino-benzidine (DAB+ Substrate System, Dako, Carpinteria, CA, USA) for 10 minutes. Negative controls were performed by using the adequate serum control (N1698, Dako, Carpinteria, CA, USA) and tonsil was used as positive control. Tissue sections were counterstained with haematoxylin and permanently mounted.
The specificity of CD147 and CD44 antibodies was further demonstrated by Western-blot, as shown in Figure 1. Antibodies for the MCT isoforms have been previously validated by our group by Western-blot [19].
Figure 1.
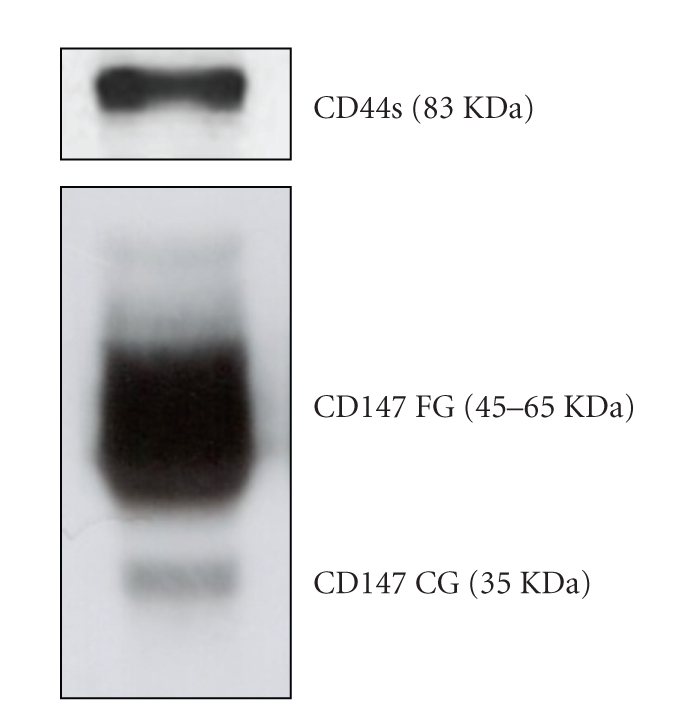
Western-blot for CD44 (breast cancer cell line MDA MB 231) and CD147 (human colon tissue). The protein molecular weights observed are in accordance with the predicted for these proteins. FG: fully glycosylated, CG: core glycosylated.
2.2.4. Immunohistochemical Evaluation
MCTs, CD147, and CD44 immunoreactions were evaluated semiquantitatively using the criteria previously described [19, 31]. Immunoreaction extent was scored semiquantitatively as follows: 0: 0% of immunoreactive cells, 1: <5% of immunoreactive cell, 2: 5%–50% of immunoreactive cells, and 3: >50% of immunoreactive cells. Also, intensity of staining was scored semi-qualitatively as 0: negative, 1: weak, 2: intermediate, and 3: strong. Immunoreaction final score was defined as the sum of both parameters (extent and intensity), and grouped as negative (scores 0 and 2) and positive (3–6). Finally, since plasma membrane location is essential for MCT1 and MCT4 membrane localization and activity, we also analysed the plasma membrane positive cases separately. Evaluation was performed blindly by two independent observers (AL, FS). Discordant results were discussed in a double-head microscope and a final score was agreed.
2.3. Statistical Analysis
Data were stored and analysed using the SPSS statistical software (version 16.0, SPSS Inc., Chicago, IL, USA). All comparisons were examined for statistical significance using Pearson's Chi-square (χ2) test and Fisher's exact test (when n < 5), being threshold for significance P values <.05.
3. Results
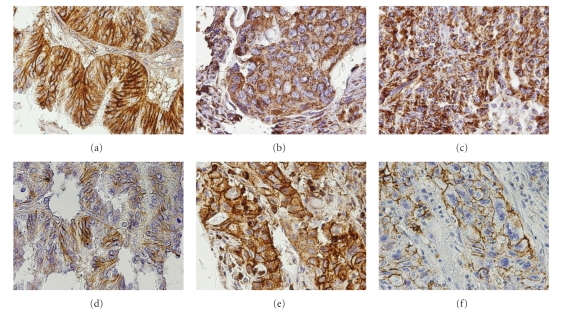
From the 200 tumour samples, only approximately 60% were suitable for analysis. The remaining tumour spots were missing, dropped out during immunohistochemistry protocol, or did not contain representative tissue, with some heterogeneity between TARP slides. Tumour samples were assessed for MCT1, MCT2, MCT4, CD147, and CD44 immunohistochemical expressions and the results are summarised in Table 1. Positive MCT1 expression was observed in both plasma membrane and cytoplasm (Figures 2(a) and 2(e)), while MCT2 expression was only observed in the cytoplasm (Figure 2(b)) and MCT4 was commonly found in the cytoplasm (Figure 2(c)) and rarely in the plasma membrane. Regarding CD147 and CD44, expression was always present in the plasma membrane (Figures 2(d) and 2(f), resp.), with some cytoplasmic staining. There was a strong tendency for differences in MCT1 and MCT4 expression frequencies among the different tumours (P = .056 and P = .061, resp.); however, differences were only significant when considering MCT1 plasma membrane staining (P = .012), being highest for colon, followed by ovary. MCT2 and CD147 expression frequencies were not significantly different among the tumour types studied. CD44 expression was also significantly different among tumours (P = .004), being more frequent in lung, followed by breast. Overall, and looking at each tumour entity, we observed that MCT2 was the most frequently expressed MCT isoform, followed by MCT1 and 4. MCT1 exhibited the highest frequency of staining at the plasma membrane, while MCT4 was barely present and MCT2 was absent.
Table 1.
Frequency of MCTs, CD147, and CD44 expressions in tumour samples.
| Tumour type | Expression | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCT1 | MCT2 | MCT4 | CD147 | CD44 | |||||||||
| Positive (%) | Positive (%) | Positive (%) | Positive (%) | Positive (%) | |||||||||
| n | Cyta | PM | n | Cyta | PM | n | Cyta | PM | n | Cyta/PM | n | Cyta/PM | |
| Breast carcinoma | 22 | 21 (95.5) | 5 (22.7) | 24 | 34 (100.0) | 0 (0.0) | 24 | 19 (79.2) | 1 (4.2) | 28 | 13 (46.4) | 27 | 8 (29.6) |
| Colon adenocarcinoma | 32 | 23 (71.9) | 19 (59.4) | 29 | 29 (100.0) | 0 (0.0) | 26 | 19 (73.1) | 0 (0.0) | 34 | 13 (38.2) | 31 | 4 (12.9) |
| Nonsmall cell lung cancer | 23 | 14 (60.9) | 5 (21.7) | 21 | 21 (100.0) | 0 (0.0) | 23 | 16 (69.6) | 0 (0.0) | 28 | 13 (46.4) | 27 | 12 (44.4) |
| Ovarian adenocarcinoma | 24 | 18 (75.0) | 10 (41.7) | 30 | 18 (93.3) | 0 (0.0) | 28 | 13 (46.4) | 1 (3.6) | 26 | 11 (42.3) | 28 | 2 (7.1) |
Cyt: cytoplasm; PM: plasma membrane; awith or without plasma membrane staining.
Note: for CD147 and CD44, frequency results are the same for cytoplasm and plasma membrane expression.
Figure 2.
Representative immunohistochemical expression of MCT1 in ovarian carcinoma (a), MCT2 in breast carcinoma (b), MCT4 in ovarian carcinoma (c), CD147 in ovarian carcinoma (d), MCT1 in lung cancer (e), and CD44 in lung cancer (f). Plasma membrane staining for both MCT1 (a) and CD147 (d) is shown in the same tumour area of an ovary cancer case and for both MCT1 (e) and CD44 (f) in the same area of lung cancer case.
Comparison of MCT expression frequencies in breast and lung malignant tissues with the corresponding normal tissues is depicted in Table 2. In breast carcinomas, there was a significant increase in MCT1 and MCT4 staining (P = .001 and P = .004, resp.), while in lung cancer, there was a significant decrease in MCT4 plasma membrane expression (P = .001).
Table 2.
Frequency of MCTs expression in nonneoplastic and malignant breast and lung tissues.
| Breast tissues | Lung tissues | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cyta | PM | Cyta | PM | |||||||
| n | Positive (%) | P | Positive (%) | P | n | Positive (%) | P | Positive (%) | P | |
| MCT1 | .001 | .067 | 1 | .612 | ||||||
| Normal | 15 | 7 (46.7) | 0 (0.0) | 6 | 4 (66.7) | 2 (33.3) | ||||
| Tumour | 22 | 21 (95.5) | 5 (22.7) | 23 | 14 (60.9) | 5 (21.7) | ||||
| MCT2 | .142 | .300 | ||||||||
| Normal | 15 | 13 (86.7) | 9 | 8 (88.9) | ||||||
| Tumour | 24 | 24 (100.0) | 21 | 21 (100.0) | ||||||
| MCT4 | .004 | 1 | .289 | .001 | ||||||
| Normal | 15 | 5 (33.3) | 0 (0.0) | 6 | 6 (100.0) | 4 (66.7) | ||||
| Tumour | 24 | 19 (79.2) | 1 (4.2) | 23 | 16 (69.6) | 0 (0.0) | ||||
Cyt: cytoplasm; PM: plasma membrane; awith or without plasma membrane staining.
In order to assess the association of CD147 and CD44 to MCT expression in tumours, we searched for associations among expressions of these proteins (Tables 3 and 4). Overall, considering all tumour entities, both MCT1 and MCT4 immunoreactions correlated with CD147 (P = .001 and P < .001, resp.), while, for plasma membrane localisation, this association was only observed for MCT1 (P = .025), as illustrated in Figures 2(a) and 2(d). No association was observed between CD147 and MCT2. Considering the different tumours individually, associations between CD147 and MCT4 immunoreaction were only observed in breast and lung cancers (P = .024 and P = .023, resp.); however, other values showed a tendency for significance but the low number of cases limited statistical power. Regarding CD44, the only significant association was with MCT1 plasma membrane expression in lung cancer (P = .039), as illustrated in Figures 2(e) and 2(f). Importantly, among the 36 cases positive for MCT1 plasma membrane expression, 12 were negative for both CD147 and CD44 (data not shown).
Table 3.
Correlations between MCTs and CD147 expressions in tumours.
| CD147 expression | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast | Colon | Lung | Ovary | ||||||||||
| n | Positive (%) | P | n | Positive (%) | P | n | Positive (%) | P | n | Positive (%) | P | ||
| MCT1 | Cytoplasma | .381 | .184 | .074 | .245 | ||||||||
| Negative | 1 | 0 (0.0) | 7 | 1 (14.3) | 8 | 2 (25.0) | 3 | 0 (0.0) | |||||
| Positive | 20 | 13 (65.0) | 21 | 11 (52.4) | 14 | 10 (71.4) | 15 | 7 (46.7) | |||||
| Plasma membrane | .131 | .114 | 1 | .050 | |||||||||
| Negative | 17 | 9 (52.9) | 10 | 2 (20.0) | 17 | 9 (52.9) | 9 | 1 (11.1) | |||||
| Positive | 4 | 4 (100.0) | 18 | 10 (55.6) | 5 | 3 (60.0) | 9 | 6 (66.7) | |||||
| MCT4 | Cytoplasma | .024 | .118 | .023 | .214 | ||||||||
| Negative | 4 | 0 (0.0) | 5 | 0 (0.0) | 6 | 1 (16.7) | 12 | 3 (25.0) | |||||
| Positive | 19 | 13 (68.4) | 19 | 9 (47.4) | 16 | 12 (75.0) | 12 | 7 (58.3) | |||||
| Plasma membrane | 1 | — | — | 1 | |||||||||
| Negative | 22 | 12 (54.5) | 24 | 9 (37.5) | 22 | 13 (59.1) | 23 | 10 (43.5) | |||||
| Positive | 1 | 1 (100.0) | 0 | — | 0 | — | 1 | 0 (0.0) | |||||
awith or without plasma membrane staining.
Table 4.
Correlations between MCTs and CD44 expressions in tumours.
| CD44 expression | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast | Colon | Lung | Ovary | ||||||||||
| n | Positive (%) | P | n | Positive (%) | P | n | Positive (%) | P | n | Positive (%) | P | ||
| MCT1 | Cytoplasma | .364 | .218 | 1 | .250 | ||||||||
| Negative | 1 | 1 (100.0) | 6 | 2 (33.3) | 9 | 3 (33.3) | 5 | 1 (20.0) | |||||
| Positive | 21 | 7 (33.3) | 20 | 2 (10.0) | 13 | 5 (38.5) | 15 | 0 (0.0) | |||||
| Plasma membrane | .309 | .591 | .039 | 1 | |||||||||
| Negative | 17 | 5 (29.4) | 9 | 2 (22.2) | 17 | 4 (23.5) | 10 | 1 (10.0) | |||||
| Positive | 5 | 3 (60.0) | 17 | 2 (11.8) | 5 | 4 (80.0) | 10 | 0 (0.0) | |||||
| MCT4 | Cytoplasma | 1 | .539 | .613 | .482 | ||||||||
| Negative | 4 | 1 (25.0) | 5 | 1 (20.0) | 6 | 1 (16.7) | 13 | 2 (15.4) | |||||
| Positive | 18 | 6 (33.3) | 18 | 2 (11.1) | 15 | 6 (40.0) | 11 | 0 (0.0) | |||||
| Plasma membrane | .318 | — | — | 1 | |||||||||
| Negative | 21 | 6 (28.6) | 23 | 3 (13.0) | 21 | 7 (33.3) | 23 | 2 (8.7) | |||||
| Positive | 1 | 1 (100.0) | 0 | — | 0 | — | 1 | 0 (0.0) | |||||
awith or without plasma membrane staining.
4. Discussion
Upregulation of glycolysis and adaptation to acidosis are key events in the transition from in situ to invasive cancer [1] and MCTs may play an important role through their involvement in exporting lactate [6]. MCTs have been described to be up-regulated in several cancers [13–19]; however, there are still some controversies [22, 23]. Moreover, their regulation in cancer is starting to be unravelled.
In the present study, we observed a high and heterogeneous frequency of MCT expression among the different tumour entities. Importantly, only MCT1 presented a relevant expression at the plasma membrane, a fact which is essential for lactate transporter activity. It appears that the pair MCT1/CD147 is the most relevant in the tumours studied, likely by promoting lactate efflux from cancer cells. Lactate efflux allows continuous proliferation, avoiding apoptosis by intracellular acidification, thus conferring a proliferative advantage to cancer cells [1, 4]. The high frequencies of MCT2 and MCT4 in the cytoplasm might mean that they are involved in other functions in the cell, such as in lactate/pyruvate transport through the mitochondrial/peroxisomal membrane. Actually, MCT2 and MCT4, as well as MCT1, have been described to be present in the mitochondrial membrane [32–34].
In the present study, we showed that MCT1 is upregulated in breast carcinomas, which does not corroborate a previous report in breast cancer, pointing to a possible silencing of MCT1 expression by gene promoter hypermethylation [10, 22]. However, this study only presented the results of MCT1 promoter methylation and was not supported by MCT1 protein expression. Taking into consideration that MCT2 is not present in the plasma membrane and MCT4 is expressed at low levels, it is reasonable to hypothesise that MCT1 is the main isoform responsible for lactate plasma membrane transport in breast carcinoma. To confirm this hypothesis, we are currently evaluating MCT expression in a larger series of breast carcinomas. The important role of MCTs in substrate transport in colonic epithelium has been vastly studied [23, 24, 35–38]. However, data on MCT expression in colorectal carcinoma are still contradictory [17, 19, 23, 24]. Our findings on MCT expression are in agreement with our previous report [19] in which we described MCT upregulation, especially MCT1 and MCT4. Comparative studies on lung cancer showed an absent MCT expression in normal lung but a high MCT plasma membrane expression in cancer cells, especially MCT1 [16]. Although at a significantly lower level (20% versus 100%) we also detected MCT1 in the membrane of cancer cells, the same was not observed for MCT2 and MCT4. Therefore, it appears that MCT1 is the most important MCT isoform likely involved in lactate efflux from lung cancer cells. To the best of our knowledge, there are no reports on MCT expression in ovarian cancer. Thus, the results here presented are novel and may shed some light onto the metabolic alterations occurring in this type of cancer. Indeed, the upregulation of glycolytic enzymes in ovarian cancer [39–41] points to an increased production of lactate and, consequently, to the need for upregulation of lactate transport. In harmony with this line of evidence, we found a relevant MCT1 expression in ovarian carcinoma, which warrants further studies on MCT expression in this type of carcinoma.
Importantly, we assessed the association between MCTs and the glycoproteins CD147 and CD44 in human tumour samples. We observed a close association between CD147 expression and MCT1 and MCT4 isoforms, which corroborates the data from the literature stating that CD147 is essential for MCT1 and MCT4 regulation [7, 9, 10] and that CD147 maturation and cell surface expression is dependent on MCT1 and MCT4 [10, 11]. It is important to note that these associations were not homogeneous among tumour entities, which might indicate that regulation of these proteins diverges from tumour to tumour. Thus, additional studies are necessary to unveil the possible synergism between MCT and CD147 in malignant progression of tumours from different origins. Since it was recently described that CD44 was also involved in MCT regulation in breast cancer [12], we analysed MCT and CD44 coexpression. However, we only found an association between CD44 and MCT1 plasma membrane expression in lung cancer. Notably, we observed that there are still an important number of cases positive for MCT1, which are negative for both CD147 and CD44, suggesting that MCT1 plasma membrane expression in tumours may depend on a yet nonidentified regulatory protein.
In summary, we analysed the expression of MCT1, MCT2, MCT4, CD147, and CD44 in different primary tumours. Importantly, we evaluated for the first time MCT expression in ovarian carcinoma and the association between MCT and both CD147 and CD44 expressions in four different types of tumours. The results herein presented can contribute to understanding of MCTs' regulation and role in human tumours. Nevertheless, additional studies, including larger and well-characterized tumour series, especially of breast and ovary, will definitely complement this work and pave the way to the possible exploitation of MCTs as targets for cancer therapy.
Competing Interests
These authors declare no competing interests.
Acknowledgments
Céline Pinheiro received a Ph.D. fellowship from the Portuguese Science and Technology Foundation (SFRH/BD/27465/2006). The authors acknowledge NCI (National Cancer Institute) Tumour Repository MTA, MD, USA for the multitumour tissue microarray (TARP).
References
- 1.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Izumi H, Torigoe T, Ishiguchi H, et al. Cellular pH regulators: potentially promising molecular targets for cancer chemotherapy. Cancer Treatment Reviews. 2003;29(6):541–549. doi: 10.1016/s0305-7372(03)00106-3. [DOI] [PubMed] [Google Scholar]
- 4.Fang JS, Gillies RD, Gatenby RA. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Seminars in Cancer Biology. 2008;18(5):330–337. doi: 10.1016/j.semcancer.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahl ML, Owen JA, Burd R, et al. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Molecular Cancer Therapeutics. 2002;1(8):617–628. [PubMed] [Google Scholar]
- 6.Halestrap AP, Meredith D. The SLC16 gene family—from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Archiv European Journal of Physiology. 2004;447(5):619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 7.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. The EMBO Journal. 2000;19(15):3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson MC, Meredith D, Fox JEM, Manoharan C, Davies AJ, Halestrap AP. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is embigin (gp70) Journal of Biological Chemistry. 2005;280(29):27213–27221. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 9.Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Investigative Ophthalmology and Visual Science. 2003;44(3):1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Research. 2007;67(9):4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 11.Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(45):16245–16250. doi: 10.1073/pnas.0504419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slomiany MG, Grass GD, Robertson AD, et al. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Research. 2009;69(4):1293–1301. doi: 10.1158/0008-5472.CAN-08-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froberg MK, Gerhart DZ, Enerson BE, et al. Expression of monocarboxylate transporter MCT1 in normal and neoplastic human CNS tissues. NeuroReport. 2001;12(4):761–765. doi: 10.1097/00001756-200103260-00030. [DOI] [PubMed] [Google Scholar]
- 14.Mathupala SP, Parajuli P, Sloan AE. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55(6):1410–1419. doi: 10.1227/01.neu.0000143034.62913.59. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Quinones QJ, Holman TL, et al. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Molecular Pharmacology. 2006;70(6):2108–2115. doi: 10.1124/mol.106.026245. [DOI] [PubMed] [Google Scholar]
- 16.Koukourakis MI, Giatromanolaki A, Bougioukas G, Sivridis E. Lung cancer: a comparative study of metabolism related protein expression in cancer cells and tumor associated stroma. Cancer Biology and Therapy. 2007;6(9):1476–1479. doi: 10.4161/cbt.6.9.4635. [DOI] [PubMed] [Google Scholar]
- 17.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Research. 2006;66(2):632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro C, Longatto-Filho A, Ferreira L, et al. Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. International Journal of Gynecological Pathology. 2008;27(4):568–574. doi: 10.1097/PGP.0b013e31817b5b40. [DOI] [PubMed] [Google Scholar]
- 19.Pinheiro C, Longatto-Filho A, Scapulatempo C, et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Archiv. 2008;452(2):139–146. doi: 10.1007/s00428-007-0558-5. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro C, Albergaria A, Paredes J, et al. Monocarboxylate transporter 1 is upregulated in basal-like breast carcinoma. doi: 10.1111/j.1365-2559.2010.03560.x. Histopathology. In press. [DOI] [PubMed] [Google Scholar]
- 21.Pinheiro C, Longatto-Filho A, Simoes K, et al. The prognostic value of CD147/EMMPRIN is associated with monocarboxylate transporter 1 co-expression in gastric cancer. European Journal of Cancer. 2009;45(13):2418–2424. doi: 10.1016/j.ejca.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Asada K, Miyamoto K, Fukutomi T, et al. Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology. 2003;64(4):380–388. doi: 10.1159/000070297. [DOI] [PubMed] [Google Scholar]
- 23.Lambert DW, Wood IS, Ellis A, Shirazi-Beechey SP. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. British Journal of Cancer. 2002;86(8):1262–1269. doi: 10.1038/sj.bjc.6600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification of a monocarboxylate transporter isoform type l (MCT1) on the luminal membrane of human and pig colon. Biochemical Society Transactions. 1998;26(2, article S120) doi: 10.1042/bst026s120. [DOI] [PubMed] [Google Scholar]
- 25.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Experimental and Molecular Pathology. 2007;83(3):283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathology International. 2006;56(7):359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 27.Riethdorf S, Reimers N, Assmann V, et al. High incidence of EMMPRIN expression in human tumors. International Journal of Cancer. 2006;119(8):1800–1810. doi: 10.1002/ijc.22062. [DOI] [PubMed] [Google Scholar]
- 28.Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005;87(3-4):361–368. doi: 10.1016/j.biochi.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. Journal of Molecular Histology. 2004;35(3):211–231. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 30.Toole BP, Slomiany MG. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resistance Updates. 2008;11(3):110–121. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinheiro C, Longatto-Filho A, Pereira SMM, et al. Monocarboxylate transporters 1 and 4 are associated with CD147 in cervical carcinoma. Disease Markers. 2009;26(3):97–103. doi: 10.3233/DMA-2009-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benton CR, Campbell SE, Tonouchi M, Hatta H, Bonen A. Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochemical and Biophysical Research Communications. 2004;323(1):249–253. doi: 10.1016/j.bbrc.2004.08.084. [DOI] [PubMed] [Google Scholar]
- 33.Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. American Journal of Physiology. 2000;278(4):E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- 34.Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. Journal of Biological Chemistry. 1995;270(4):1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 35.Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-κB pathway. Journal of Cellular Biochemistry. 2008;103(5):1452–1463. doi: 10.1002/jcb.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill RK, Saksena S, Alrefai WA, et al. Expression and membrane localization of MCT isoforms along the length of the human intestine. American Journal of Physiology. 2005;289(4):C846–C852. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- 37.Iwanaga T, Takebe K, Kato I, Karaki S-I, Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomedical Research. 2006;27(5):243–254. doi: 10.2220/biomedres.27.243. [DOI] [PubMed] [Google Scholar]
- 38.Lecona E, Olmo N, Turnay J, et al. Kinetic analysis of butyrate transport in human colon adenocarcinoma cells reveals two different carrier-mediated mechanisms. Biochemical Journal. 2008;409(1):311–320. doi: 10.1042/BJ20070374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simaga S, Osmak M, Babic D, Sprem M, Vukelic B, Abramic M. Quantitative biochemical analysis of lactate dehydrogenase in human ovarian tissues: correlation with tumor grade. International Journal of Gynecological Cancer. 2005;15(3):438–444. doi: 10.1111/j.1525-1438.2005.14410.x. [DOI] [PubMed] [Google Scholar]
- 40.Cantuaria G, Fagotti A, Ferrandina G, et al. GLUT-1 expression in ovarian carcinoma: association with survival and response to chemotherapy. Cancer. 2001;92(5):1144–1150. doi: 10.1002/1097-0142(20010901)92:5<1144::aid-cncr1432>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 41.Shibata K, Kajiyama H, Mizokami Y, et al. Placental leucine aminopeptidase (P-LAP) and glucose transporter 4 (GLUT4) expression in benign, borderline, and malignant ovarian epithelia. Gynecologic Oncology. 2005;98(1):11–18. doi: 10.1016/j.ygyno.2005.03.043. [DOI] [PubMed] [Google Scholar]