Abstract
Summary: Infection of the airways remains the primary cause of morbidity and mortality in persons with cystic fibrosis (CF). This review describes salient features of the epidemiologies of microbial species that are involved in respiratory tract infection in CF. The apparently expanding spectrum of species causing infection in CF and recent changes in the incidences and prevalences of infection due to specific bacterial, fungal, and viral species are described. The challenges inherent in tracking and interpreting rates of infection in this patient population are discussed.
INTRODUCTION
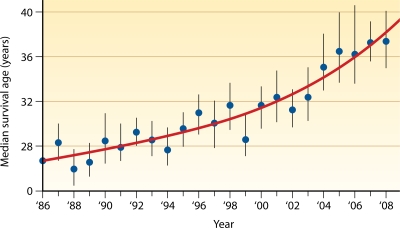
It is estimated that approximately 30,000 persons in the United States, and an equal number elsewhere, have cystic fibrosis (CF), making it the most common lethal genetic disorder in Caucasians. CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) protein, which normally functions to regulate the transepithelial ion flow critical to maintaining the proper ionic composition and volume of airway surface fluid (105). For reasons that are incompletely understood, the alterations of airway surface liquid resulting from dysfunctional or absent CFTR render CF patients susceptible to chronic endobronchial infections. The associated neutrophilic inflammatory response leads to progressive lung disease and, ultimately, pulmonary failure, the primary cause of death in CF. Despite impressive advances in life expectancy in CF during the last 3 decades, the median predicted survival is approximately 37 years (72).
There is a perception that the epidemiology of respiratory tract infection in persons with CF has changed in recent years. The available data seem to support this perception, and there are several possible reasons to explain the changes observed in the rates of infection due to specific microbial species and the apparent emergence of additional species that previously have not been associated with CF. However, there are also several caveats that confound interpretations of these observations.
This review will provide an update of the salient features of the epidemiology of specific opportunistic bacterial species that infect the airways of persons with CF, highlighting recent apparent changes in this epidemiology. Updates on the epidemiology of select fungal and viral species will also be provided. Factors that challenge our ability to more precisely interpret currently available data for tracking CF microbial epidemiology will be described. Reviews of the pathogenesis, microbiology, treatment, and clinical outcomes of respiratory tract infection in CF, as well as infection control in CF, can be found elsewhere (76, 105, 174, 180, 187, 190, 264, 266, 283, 297).
BACTERIA
The bacterial species most commonly associated with respiratory tract infection in CF include common human pathogens such as Staphylococcus aureus and Haemophilus influenzae as well as several opportunistic pathogens, the most important of which is Pseudomonas aeruginosa. Other opportunists include nosocomial pathogens such as Stenotrophomonas maltophilia and Achromobacter xylosoxidans and species that are only occasionally associated with human infections apart from CF, such as the Burkholderia cepacia complex, Burkholderia gladioli, Ralstonia species, Cupriavidus species, and Pandoraea species. Although some of these species have been associated with poor outcomes in CF, the role that other species may play in contributing to disease progression remains uncertain. The following sections will highlight important features of the epidemiology of these species in CF.
Pseudomonas aeruginosa
Incidence and prevalence.
P. aeruginosa is the most common bacterial species involved in respiratory tract infection in CF. In the United States, 52.5% of patients included in the Cystic Fibrosis Foundation (CFF) Patient Registry were reported to have had P. aeruginosa recovered from sputum culture specimens in 2008 (72). The prevalence of infection varied significantly by age, ranging from approximately 25% for children 5 years of age and younger to 80% for adults 25 to 34 years of age (72). However, it appears that the prevalence of P. aeruginosa infection in CF may be decreasing. In a recent analysis of data from the CFF Patient Registry, Razvi and colleagues (249) reported that the prevalence of P. aeruginosa infection in U.S. CF patients decreased from 60.4% in 1995 to 56.1% in 2005. The overall incidence of infection did not change during this interval; however, significant increases in the incidences of P. aeruginosa infection were observed for infants 1 year of age and younger and for children 2 to 5 years of age. In contrast, another recent analysis did not show a significant decrease in the prevalence of P. aeruginosa infection in adult CF patients receiving care at the Royal Brompton Hospital, London, United Kingdom, between 1985 and 2005 (203).
The deleterious impact that chronic P. aeruginosa infection has on lung function in CF has been well described (131, 155, 165, 175, 230). Initially, infection can be intermittent and involve multiple strains. Eventually, a single strain dominates and establishes chronic infection, which is most often marked by a shift in the infecting strain to a mucoid phenotype (117). Eradication of pulmonary infection after this point is generally not possible.
Epidemic strains.
Several studies demonstrated that people with CF who are infected with P. aeruginosa typically harbor distinct strains, presumably acquired from the natural environment (188, 260, 277). Although the transmission of strains between siblings has been well documented (120, 311, 352), the risk of patient-to-patient spread of P. aeruginosa had been considered to be low. However, in 1996, genotyping analysis of isolates recovered during an antibiotic trial identified a β-lactam-resistant strain infecting 55 children receiving care in a Liverpool, United Kingdom, treatment center (45). This was followed by reports describing other shared or “epidemic” P. aeruginosa strains in CF care centers in the United Kingdom and Australia (7, 10, 147, 224). In 2004, Scott and Pitt (277) reported the results of a genotyping analysis of 1,225 P. aeruginosa isolates recovered from CF patients receiving care in 31 treatment centers in the United Kingdom and demonstrated that 28% of infected patients harbored a strain that was shared with another patient. Several small clusters of patients with common strains were identified and were found in all but three centers. The two most prevalent strains alone accounted for more than 20% of the patient isolates examined. Some strains, including the Liverpool and Midlands 1 epidemic strains, were widely distributed, being identified in 48% and 29% of CF treatment centers, respectively.
The impact that epidemic P. aeruginosa strains have on clinical outcomes has been debated. Most strains are multidrug resistant, and some have been associated with increased morbidity in infected patients (5, 10, 146, 221). Superinfection of CF patients already chronically infected with P. aeruginosa and transmission from an infected CF patient to non-CF family members have been reported for the Liverpool epidemic strain (199, 200); however, it is not clear if all epidemic P. aeruginosa strains have comparable capacities for spread.
Acquisition and transmission.
The epidemiological and microbiological basis for epidemic P. aeruginosa strains remains poorly understood. The finding of multiple, relatively small clusters of patients within the same treatment center who are infected with a shared strain (224, 277, 287) suggests interpatient transmission, possibly resulting from inadequate infection control measures. Acquisition from a common nosocomial source is also a possibility; however, surveillances of hospital inpatient and outpatient environments have typically not revealed a reservoir for shared strains (45, 147). Strains that are more widely distributed (i.e., found in multiple care centers) suggest contact among and cross-infection between patients receiving care in different centers or an independent acquisition of a strain that is particularly common in the natural environment. Romling and colleagues described a P. aeruginosa strain (designated clone C) that was recovered from infected CF patients as well as from environmental samples in geographically diverse areas of Germany (261). This same strain was subsequently identified in cultures from CF patients in diverse regions of the United Kingdom (277), suggesting that it is widely distributed in the natural environment. Other epidemic strains have not been identified in environmental samples, although genotyping surveys of large numbers of strains recovered from the environment are lacking. In any event, the finding that some strains are shared by many CF patients suggests that certain strains are particularly fit for human infection.
Strains that are common to large numbers of CF patients have not been described in North America. Speert and colleagues analyzed isolates recovered between 1981 and 1999 from 174 CF patients in Vancouver, British Columbia, and found 157 distinct strains, 123 of which were unique to individual patients (287). Several distinct strains were shared by clusters of two, three, or four patients. Although two strains were shared by larger numbers of patients (21 patients and 18 patients), epidemiological investigations found little evidence for direct contact between patients in either cluster. The presence of epidemic P. aeruginosa strains in the United States remains uncertain in the absence of genotyping studies of isolates recovered from large numbers of CF patients. Of note, most epidemic strains in Europe and Australia described have had a multidrug-resistant phenotype, making their occurrence in patients with relatively little antibiotic exposure the subject of further investigation. The presence in a patient population of epidemic strains that do not have an unusual or noteworthy phenotype might not be so easily detected. Active surveillance of sufficiently large numbers of patient isolates is required to rule out the presence and ongoing spread of shared strains. Unfortunately, such surveillance is not always a component of routine CF care.
Burkholderia Species
When the genus Burkholderia was named in 1992, it was composed of seven species that were formerly classified in rRNA homology group II of the genus Pseudomonas (357). These species included B. cepacia, B. gladioli, Burkholderia pickettii, Burkholderia mallei, Burkholderia pseudomallei, Burkholderia caryophylli, and Burkholderia solanacearum. Since then, further taxonomic studies identified numerous additional Burkholderia species. The genus currently consists of more than 60 species, most of which are found in the natural environment and are not pathogenic for healthy humans (see http://www.bacterio.cict.fr/ for an updated list of species). Several species, however, are capable of causing chronic and often severe respiratory tract infections in persons with CF. Most of these species are within the B. cepacia complex, a group currently consisting of 17 closely related species. An important exception is B. gladioli, which, although not a member of the B. cepacia complex, now accounts for a significant proportion of Burkholderia infection in CF. Among the remaining Burkholderia species, only Burkholderia fungorum and B. pseudomallei have been identified as causing infection of CF patients (58). The latter species has most often involved individuals who have traveled to regions of endemicity (70, 73, 223, 276, 337) and is notable as being listed, together with B. mallei, as a “select agent” by the U.S. Department of Health and Human Services and the Department of Agriculture (http://www.selectagents.gov) owing to its potential use as an agent of bioterrorism.
B. cepacia complex and B. gladioli. (i) Taxonomy and nomenclature.
Pseudomonas cepacia was initially described as being a pathogen of onions (34) and for many years was identified only sporadically in association with human infections, typically involving debilitated patients with underlying diseases. The first reports of B. cepacia infection in CF patients appeared in the late 1970s and early 1980s (26, 112, 171, 222, 263). In 1984 Isles and colleagues (140) documented the increasing prevalence of B. cepacia infection among patients receiving care in a Toronto CF center and described the occurrence of a rapidly progressive deterioration in respiratory function characterized by necrotizing pneumonia, bacteremia, and sepsis. This “cepacia syndrome” was observed for as many as 20% of infected patients. Similar increases in incidences of B. cepacia infection were noted subsequently in other CF treatment centers in North America (302, 303, 308).
During the mid-1990s, Vandamme and colleagues (322) performed comprehensive taxonomic studies to demonstrate that bacteria being identified as B. cepacia strains actually comprised several distinct species. These species were found to be unusually closely related, forming a distinct phylogenetic group within the genus. By taxonomic convention these species were referred to as “genomovars” until distinguishing phenotypic characteristics were identified, which then allowed each new species to receive a formal binomial designation. Currently, there are 17 species in what is collectively referred to as the B. cepacia complex (Table 1). B. gladioli, although phenotypically quite similar to these species, is phylogenetically not a member of the B. cepacia complex (349).
TABLE 1.
The Burkholderia cepacia complex
| Species | Former genomovar designation | Yr identified and/or named | Reference(s) |
|---|---|---|---|
| B. cepacia | I | 1950, 1997 | 34, 322 |
| B. multivorans | II | 1997 | 322 |
| B. cenocepacia | III | 1997, 2003 | 321, 322 |
| B. stabilis | IV | 1997, 2000 | 322, 323 |
| B. vietnamiensis | V | 1995, 1997 | 107, 322 |
| B. dolosa | VI | 2001, 2004 | 58, 335 |
| B. ambifaria | VII | 2001 | 63 |
| B. anthina | VIII | 2002 | 319 |
| B. pyrrocinia | IX | 2002 | 319 |
| B. ubonensis | 2000, 2008 | 333, 355 | |
| B. latens | 2008 | 333 | |
| B. diffusa | 2008 | 333 | |
| B. arboris | 2008 | 333 | |
| B. seminalis | 2008 | 333 | |
| B. metallica | 2008 | 333 | |
| B. contaminans | 2009 | 332 | |
| B. lata | 2009 | 332 |
(ii) Incidence and prevalence.
The prevalence of B. cepacia complex infection in CF patients in the United States has ranged between 3% and 4% for many years. As is the case with P. aeruginosa, significant differences in age-specific prevalence rates are found, with rates as high as 8% being reported for adults. Razvi and colleagues (249) reported that the incidence of infection, based on CFF Patient Registry data, decreased from 1.3% to 0.8% between 1995 and 2005. The overall prevalence of infection also decreased from 3.6% to 3.1% during this period. An analysis of microbiological data from the Royal Brompton Hospital indicates that while the prevalence of B. cepacia complex infection increased significantly in adult CF patients between 1985 and 1990, it decreased from 9% to 4% between 1990 and 2005 (203).
(iii) Species distribution in CF.
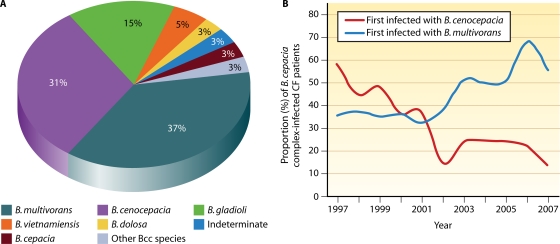
With the exception of Burkholderia ubonensis, all species in the B. cepacia complex have been isolated from CF sputum cultures; however, some are much more commonly encountered in CF than are others. The approximate distribution of B. gladioli and B. cepacia complex species among Burkholderia-infected CF patients in the United States is provided in Fig. 1A (184; my unpublished observations). Burkholderia multivorans and Burkholderia cenocepacia are most frequently recovered, together accounting for approximately 70% of infected patients. In Canada and some European countries, the proportion of CF patients infected with B. cenocepacia is higher (3, 288). Of note, B. gladioli is much more frequently recovered in CF than are most species of the B. cepacia complex, and B. cepacia is relatively infrequently encountered. Furthermore, some CF patients are infected with bacteria that, based on genetic analyses, are most appropriately included in the B. cepacia complex but cannot be assigned to one of the currently named species. These strains most likely represent additional novel species within the complex. Finally, the incidences of B. multivorans and B. cenocepacia infection in CF appear to have shifted during the last few years (115, 251). Whereas B. cenocepacia previously accounted for the majority of Burkholderia infection in CF (251), currently, approximately three times as many CF patients in the United States become infected with B. multivorans as with B. cenocepacia (Fig. 1B). In the United Kingdom, the recovery of B. multivorans from CF patients now also exceeds that of B. cenocepacia (115).
FIG. 1.
(A) Distribution of Burkholderia species among U.S. CF patients. The proportions of CF patients infected with various Burkholderia species are shown. The data are based on 2,024 CF patients who were infected with Burkholderia species and whose isolates were referred to the Burkholderia cepacia Research Laboratory and Repository (University of Michigan) between 1997 and 2007. “Other Bcc species” indicates patients infected with B. cepacia complex species other than those specified in the chart. “Indeterminate” refers to patients infected with strains that phylogenetically are members of the B. cepacia complex species but that cannot be definitively placed into one of the 17 defined species in this group. (B) Incidence of B. cenocepacia and B. multivorans infection in U.S. CF patients. The proportions of B. cepacia complex-infected CF patients who were first infected with either B. cenocepacia (red line) or B. multivorans (blue line) in the years indicated are shown.
(iv) Epidemic strains.
A variety of methods have been used to genotype Burkholderia to assess the epidemiology of infection in CF (14, 59, 75, 179, 182, 189, 290). Genotyping studies in the late 1980s identified strains that were common to multiple patients receiving care in the same CF centers (182), suggesting interpatient spread. More compelling evidence of patient-to-patient transmission of Burkholderia soon followed (116, 181). Further studies identified strains common to CF patients in wider geographic regions. Among such so-called “epidemic” strains, the ET12 (for electrophoretic type 12) strain was found to be prevalent in eastern Canada and the United Kingdom (145, 239). The Midwest clone and strain PHDC were subsequently identified in CF patients in the Midwest and mid-Atlantic regions of the United States, respectively (43, 60, 168, 293). A strain referred to as ST04 (or randomly amplified polymorphic DNA [RAPD] type 04) was identified in samples from several patients in western Canada, and strain CZ1 was identified in the majority of isolates from Burkholderia-infected CF patients in a Prague, Czech Republic, CF center (89, 191, 288). Interestingly, these five strains are representatives of B. cenocepacia. Additional B. cenocepacia strains were found to be shared by multiple patients attending various Italian CF centers (39, 194). Common or shared strains from other B. cepacia complex species have been reported, but these have generally involved fewer CF patients (22, 39, 88, 278). Notable exceptions include Burkholderia dolosa strain SLC6, which was identified in an outbreak involving a large proportion of patients receiving care in the same U.S. CF center, and the “Glasgow strain” of B. multivorans, which was identified from an outbreak of infection in CF patients in that city in the early 1990s (22, 151, 350).
(v) Acquisition and transmission.
The identification of epidemic strains suggests that some Burkholderia strains are particularly well adapted to human infection, have an enhanced capacity for interpatient transmission, and/or are widely distributed in the natural environment. Compelling evidence for the interpatient transmission of B. cenocepacia strains ET12 and PHDC and B. dolosa strain SLC6 has been provided by epidemiological investigations of outbreaks of infection in select patient populations (43, 116, 181). The decreases in incidences of infection by these strains following the institution of strict infection control measures confirm the importance of interpatient transmission in their epidemiology (43, 213). In fact, the acquisition of strains ET12 and SLC6 by CF patients in care centers wherein stringent infection control policies have been maintained has been essentially eliminated. The situation with respect to PHDC and the Midwest clone is less clear. While the incidence of infection due to these strains has decreased with improved infection control (my unpublished observations), it has not been eliminated, and the recovery of these strains in the absence of apparent contact with other infected patients suggests acquisition from nonpatient sources. Strain PHDC has been recovered from agricultural soil in the United States and from CF patients in diverse locations in the United States and Europe, strongly suggesting that this strain is widely distributed in the natural environment, which serves as a source of ongoing acquisition (39, 65, 100, 183). In contrast, the Midwest strain has not been found in surveys of strains recovered from the natural environment in regions where this strain is found in patients (244, 293). Thus, at present, it appears that infection control (or a lack thereof), distribution in the natural environment, and possibly some innate, as-yet-uncharacterized bacterial factors have contributed to the capacity of epidemic strains to become prevalent in the CF population.
Despite the importance of epidemic strains in the epidemiology of Burkholderia, however, it must be emphasized that the majority of infected CF patients harbor genotypically distinct strains. In particular, although B. multivorans and B. gladioli together account for greater than half of the Burkholderia infections in the CF population, at least in the United States, strains belonging to these two species that are shared by multiple CF patients are uncommon. These observations indicate that the majority of new Burkholderia infection in CF patients currently involves the acquisition of strains from independent sources, most likely in the natural environment (115, 210). Indeed, a recent analysis employing multilocus sequence typing (MLST) demonstrated that more than 20% of 381 CF isolates were indistinguishable from strains recovered from the environment (13). Interestingly, although B. gladioli and B. cepacia are well-recognized plant pathogens and other species, particularly Burkholderia ambifaria, can be readily recovered from the rhizosphere of certain plants, B. multivorans has been only infrequently recovered from the environment, and its preferred natural habitat remains elusive (47, 99, 204, 244, 335).
Finally, numerous health care-associated outbreaks of Burkholderia infection due to contaminated medical devices and products, including mouthwashes, ultrasound gels, skin antiseptics, and medications, have been described (15, 21, 32, 96, 126, 139, 170, 186, 206, 219, 231, 233, 245, 250, 259, 304, 344, 345). These outbreaks have generally involved hospitalized non-CF patients, but the potential for the acquisition of Burkholderia infection among CF patients from such products must be considered in any outbreak investigation.
(vi) Epidemiology of chronic infection.
Although a transient infection of the respiratory tract may occur for some patients, the acquisition of Burkholderia most typically results in chronic infection. Most often, chronic infection involves a single strain (359); however, prolonged coinfection with two distinct strains can occur (my unpublished observations). The replacement of an initial infecting strain with another strain during the course of chronic infection has been described. Superinfection due to ET12, wherein this strain replaced another infecting strain, most likely occurred as a consequence of cross-infection from other ET12-infected patients (116, 173). The replacement of B. multivorans by various B. cenocepacia strains occurred in six patients described by Mahenthiralingam and colleagues (191). In only one of these cases was the superinfecting B. cenocepacia strain from the ET12 lineage. Conversely, the replacement of B. cenocepacia by strains belonging to other B. cepacia complex species was not observed. In an analysis of 1,095 Burkholderia isolates recovered from 379 CF patients, Bernhardt and colleagues (20) found a change in the infecting strain for 24 patients (6.9%) infected with B. cepacia complex strains and for 3 patients (9%) infected with B. gladioli. In most cases (63%), a B. cenocepacia strain replaced a non-B. cenocepacia strain; none of the superinfecting B. cenocepacia strains were of the ET12 lineage.
Staphylococcus aureus
Incidence and prevalence.
S. aureus is often the first and is among the most common bacterial pathogens recovered from the respiratory tract of persons with CF (9). In 2008, S. aureus was isolated from respiratory tract cultures from 50.9% of CF patients included in the CFF Patient Registry (72). The prevalence was greatest, exceeding 60%, among children aged 6 to 10 years and adolescents aged 11 to 17 years. Analysis of the CFF Patient Registry also indicates that the prevalence of S. aureus infection in CF patients in the United States increased steadily during the last 14 years. In 1995, S. aureus was isolated from the respiratory tracts of only approximately 37% of patients.
MRSA.
As dramatic as the overall increase in S. aureus infection in CF is the increase in the prevalence of methicillin-resistant S. aureus (MRSA) in this patient population. In 2001, 7.3% of patients reported to the CFF Patient Registry had a MRSA strain recovered in culture (72). The prevalence increased to 22.6% in 2008. Significant increases in the incidence and prevalence of MRSA infection occurred for all ages, but prevalence was greatest for adolescents aged 11 to 17 years (249). However, MRSA incidence and prevalence rates vary significantly among CF treatment centers. Elizur and colleagues (92) reported a steady increase in the rate of MRSA infection in a large U.S. CF center for several years leading up to 2004, at which time incidence and prevalence rates of 10% and 27%, respectively, were observed.
The increase in MRSA infection in CF parallels the increase in the incidence of community-acquired MRSA (CA-MRSA), in general, in the United States and elsewhere (71, 135, 160, 208, 242, 298, 324). CA-MRSA strains are characterized by the presence of the mecA gene, which mediates methicillin resistance, on mobile genetic elements designated staphylococcal cassette chromosome mec (SCCmec) type IV or SCCmec type V. This feature along with the presence of the lukS-PV and lukF-PV genes that encode the Panton-Valentine leukocidin (PVL) distinguish CA-MRSA from so-called health care-associated MRSA (HA-MRSA) (102, 196, 209, 218, 307). Whether the increase in the incidence of infection due to CA-MRSA in the general population accounts for the increase in MRSA infection in CF is not entirely clear. Glikman and colleagues (110) analyzed 34 MRSA isolates recovered from 34 CF patients receiving care at two large treatment centers between 2004 and 2005. Although HA-MRSA isolates were more than twice as common as CA-MRSA isolates, the latter predominated in patients who were newly infected. More recently, Goodrich and colleagues (114) assessed the prevalence of CA-MRSA among CF patients receiving care in another large treatment center between 2005 and 2007. A total of 140 (19.8%) of the 707 CF patients in this center were infected with MRSA, a rate comparable to that observed for CF patients nationally. Among the MRSA-infected patients, 19 (14%) harbored CA-MRSA, while the remainder were infected with HA-MRSA.
Acquisition and transmission.
S. aureus is a frequent colonizer of the anterior nares, being present in approximately 37% of children aged 1 to 19 years (167). This colonization, although most often benign for the host, is an important reservoir for the acquisition of S. aureus by others. Colonization of the nares also presents a risk factor for subsequent infection of particularly vulnerable individuals, including residents of chronic care facilities, patients undergoing cardiothoracic surgery, and newborn infants in intensive care units (119, 122, 162). Goerke and colleagues (111) cultured the anterior nares of 72 individuals with CF aged 1 to 25 years and 72 age-matched non-CF controls. They also investigated the S. aureus colonization status of 128 family members of 38 children with CF and 79 members of 23 non-CF families. They found a significantly greater prevalence of nasal carriage of S. aureus among CF patients who had not been treated with antistaphylococcal antibiotics during the 4 weeks preceding culture (66%) than among CF patients who had not been recently treated (29%) or individuals without CF (32%). Genotyping analyses indicated that colonized individuals within the same family most often shared the same S. aureus strain, suggesting that family members are a source of acquisition of S. aureus. More recently, Elizur and colleagues (93) reported two episodes of transmission of CA-MRSA between siblings with CF. The transmission of S. aureus between nonsibling CF patients as well as the spread of MRSA from hospitalized patients without CF to patients with CF were also reported (109, 273). In a study by Nadesalingam and colleagues (215), MRSA-infected CF patients spent more time in the hospital, received more treatment days of ciprofloxacin or cephalosporin antibiotics, and were more likely to be infected with Aspergillus fumigatus than age- and sex-matched, uninfected, control CF patients.
Stenotrophomonas maltophilia
Taxonomy.
As with Burkholderia species, S. maltophilia was initially classified as a Pseudomonas species. It was subsequently placed into the genus Xanthomonas (301), and in 1993, it was transferred into the new genus Stenotrophomonas (229). Although phylogenetically not as closely related to Burkholderia as the other species causing infection in CF, including Achromobacter and Ralstonia species, S. maltophilia can also be misidentified as a member of the B. cepacia complex (33, 201). The use of rRNA-targeted PCR assays for species identification avoids this diagnostic pitfall (348).
Incidence and prevalence.
The role of S. maltophilia as a nosocomial opportunistic pathogen, particularly among debilitated and immunocompromised patients and patients requiring ventilatory support in intensive care units, is well established (6, 127, 156, 212, 336). The incidence of infection in non-CF patients has increased steadily in recent years (83, 270, 289), and a variety of clinical syndromes have been described, including bacteremia, pneumonia, urinary tract infection, ocular infection, endocarditis, meningitis, soft tissue and wound infection, mastoiditis, cholangitis, osteochondritis, bursitis, and peritonitis (270).
Several studies reported that the incidence of S. maltophilia infection in CF has also increased in recent years (16, 85, 195, 305). Data from the CFF Patient Registry support this, indicating that the prevalence of S. maltophilia increased from 4.0% in 1996 to 12.4% in 2005, with the highest rates of infection (15.8% in 2005) occurring for adolescents 11 to 17 years old (72, 249). Data from the Royal Brompton Hospital CF database indicate that the prevalence of S. maltophilia in adult CF patients increased from 1% in 1985 to 4% in 2005, with the highest rate (7%) in 2005 being found among patients 16 to 25 years of age (203). However, the unreliability of historical data regarding the prevalence of this species in CF limits firm conclusions. Furthermore, in contrast to Burkholderia and P. aeruginosa infection in CF, the infection of the respiratory tract by S. maltophilia tends to be transient and recurrent (82, 195, 197, 313). Thus, as described in more detail below, estimates of the incidence and prevalence of S. maltophilia infection in CF are significantly affected by the frequency with which sputum cultures are obtained.
Acquisition and transmission.
S. maltophilia is commonly found in soil, especially around the roots of certain crops (129). In fact, it is among the most common causes of wound infection due to trauma involving agricultural machinery (2). It has also been identified in well and river water, stream sediment, raw milk, frozen fruit, and sewage (129, 289). However, the sources of acquisition in nosocomial infection are poorly understood. The inappropriate use of hand moisturizing lotion instead of soap by a health care worker was associated with infection among bone marrow transplant recipients in one center (159), while in another center, contamination of faucet aerators in intensive care unit sinks was implicated as the source of S. maltophilia infection (343). Other studies also reported the recovery of this species from hospital sink drains, faucets, and potable water (84, 85). However, genotyping analyses typically have not provided a link between isolates obtained from such hospital environmental sources and those recovered from patients (84, 197). Furthermore, genotyping analyses of several dozen strains recovered from non-CF patients in a large hospital demonstrated that the great majority were genetically distinct, indicating a lack of a common source of acquisition (314).
Denton and colleagues (85) found that 33 of 41 S. maltophilia-infected CF patients in one treatment center harbored genetically distinct strains; four pairs of patients shared strains, suggesting either interpatient spread or acquisition from a common environmental site. Krzewinski and colleagues (166) analyzed S. maltophilia isolates from 183 infected CF patients from multiple centers and found only three instances of shared strains. More recently, Marzuillo and colleagues (197) analyzed 110 isolates recovered from 50 CF patients at a large care center and identified five distinct strains that were each shared by groups of two or three patients. None of 24 isolates recovered from water, taps, and sinks in patient rooms matched any of the strains recovered from infected patients. Thus, although evidence for occasional interpatient transmission can be found, it appears that the majority of S. maltophilia infections in CF results from independent acquisition, most likely from environmental sources.
The risk factors for infection with S. maltophilia by persons with CF were reviewed previously (178). Broad-spectrum antibiotic therapy, resulting in selective pressure that could promote the emergence of multiresistant bacteria, has long been implicated as a risk factor for S. maltophilia acquisition. Among non-CF patients, the use of carbapenem antibiotics, especially imipenem, to which S. maltophilia strains are uniformly resistant, has been widely considered to confer an increased risk of infection (94, 269, 271). However, similarly high rates of S. maltophilia acquisition have been found between persons receiving imipenem and those treated with the β-lactam ceftazidime (41). In CF, the use of steroids or antipseudomonal agents, including quinolone antibiotics and inhaled aminoglycosides, has been implicated as a risk factor (86). A multivariate analysis of data generated in clinical trials of tobramycin solution for inhalation (TSI) demonstrated that the only significant predictor of S. maltophilia isolation during the last month of the trial was the concomitant use of oral quinolones and S. maltophilia isolation prior to treatment (118). The use of TSI, systemic or inhaled steroids, or β-lactam antibiotics did not predict subsequent S. maltophilia infection. In another study, oral steroid use, multiple courses of intravenous antibiotics, and the isolation of A. fumigatus from sputum samples were significantly associated with the isolation of S. maltophilia (195). The positive association between the isolation of Aspergillus fumigatus and the isolation of S. maltophilia from sputum culture specimens were reported again recently (252).
Achromobacter (Alcaligenes) xylosoxidans
Taxonomy and nomenclature.
Although Achromobacter xylosoxidans has been recognized for many years as being capable of causing infection in persons with CF (161), the proper nomenclature of this species has presented a challenge. The species has been consecutively named Achromobacter xylosoxidans, Alcaligenes denitrificans subsp. xylosoxidans, and Alcaligenes xylosoxidans subsp. xylosoxidans (320). The name Achromobacter xylosoxidans was again proposed in 1998 (356), but this has not been uniformly accepted. Thus, the species currently is correctly called either Achromobacter xylosoxidans or Alcaligenes xylosoxidans. Ongoing taxonomic evaluation of the genus Achromobacter is suggesting that multiple distinct species are likely included among isolates currently identified as being “A. xylosoxidans-like” or “Achromobacter species” (my unpublished observations).
Incidence and prevalence.
As with the B. cepacia complex and S. maltophilia, the true frequency of infection due to A. xylosoxidans in CF is difficult to ascertain. Data from the CFF Patient Registry indicated a prevalence (defined as having had at least a single positive culture) of 1.9% among patients reported to the registry in 1995 (72). The reported prevalence increased to 6.0% in 2005, with the highest rate (8.1%) being seen among persons aged 18 to 25 years (249). Studies elsewhere showed widely differing rates of infection, ranging from less than 2% to nearly 30% depending upon how infection was defined, the period of observation used in the study, and the frequency with which surveillance cultures were obtained (78, 152, 247, 262, 295, 306).
Acquisition and transmission.
The acquisition and transmission of Achromobacter species by persons with CF were reviewed previously (178). Similar to S. maltophilia, A. xylosoxidans is an opportunistic pathogen associated with a variety of human infections, including bacteremia, meningitis, pneumonia, endocarditis, peritonitis, osteomyelitis, urinary tract infection, and endophthalmitis (90). Most infections are nosocomially acquired, with neonates, burn victims, and other immunocompromised patients being at the greatest risk (338, 346). Outbreaks attributed to contaminated disinfectant solutions, dialysis fluids, saline solution, and deionized water were described previously (185). The other species in this genus, Achromobacter piechaudii, Achromobacter ruhlandii, and Achromobacter denitrificans, are soil inhabitants that rarely cause human infection (154).
There is little evidence that A. xylosoxidans is transmitted between persons with CF. In two small single-center studies, all infected patients harbored genotypically distinct strains (91, 339). In a multicenter analysis by Krzewinski and colleagues (166), isolates from 92 infected patients were analyzed, and five instances of shared strains were detected. A study of a CF care center in Athens, Greece, reported that five of nine A. xylosoxidans-infected patients, who were close social contacts, shared a common strain (152), while a multicenter study in Belgium identified two patient clusters (one of 4 patients and the other of 10 patients), each with a shared strain (316). Although infection with A. xylosoxidans is frequently transient, chronic infection for several years can occur and is most often due to the persistence of the same strain (166, 205, 235, 262).
Mycobacteria
Taxonomy and nomenclature.
There are currently some 175 species in the genus Mycobacterium. The great majority of these are free-living inhabitants of the natural environment, found in water and soil. Most of these species are not pathogenic for healthy humans. Other species are obligate parasites, with species in the Mycobacterium tuberculosis complex (M. tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium caprae, Mycobacterium canetti, Mycobacterium microti, and Mycobacterium pinnipedii), the causative agents of tuberculosis, and Mycobacterium leprae and Mycobacterium lepromatosis, which cause leprosy, being the most notable. Among the remaining nontuberculous mycobacteria (NTM) are several opportunistic human pathogens (97, 149). These include slow-growing species such as Mycobacterium kansasii, Mycobacterium marinum, Mycobacterium scrofulaceum, Mycobacterium gordonae, Mycobacterium xenopi, and Mycobacterium terrae as well as species in the Mycobacterium avium complex (M. avium subsp. avium, M. avium subsp. hominissuis, M. avium subsp. paratuberculosis, and Mycobacterium intracellulare) (153). The rapid-growing NTM species include Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, and Mycobacterium smegmatis (31).
Incidence and prevalence.
The isolation of NTM from respiratory specimens from CF patients has been reported since the 1970s (134). Since then, estimates of the incidence and prevalence of these species in CF have varied widely, with reported prevalence rates ranging between 2% and 28% (4, 17, 95, 98, 101, 108, 133, 176, 214, 227, 279, 310). In 2003, a prospective multicenter study of 986 CF patients aged 10 years to 51 years (mean, 23 years) receiving care at 21 centers in the United States found a prevalence rate of 13% (226). M. avium complex isolates accounted for 72% of infected patients; M. abscessus was the next most common, occurring in 16% of patients. A subsequent study by Pierre-Audigier and colleagues (237) of 385 CF patients younger than 24 years (mean, 12 years) receiving care in three CF centers in Paris, France, identified NTM infection in 8% of patients. In contrast to the U.S. study, M. abscessus was most commonly found (39%), followed by M. avium complex (21%) and M. gordonae (18%) isolates. Of note, the prevalence of NTM was significantly lower in patients younger than 15 years of age than in older patients, and although M. abscessus was isolated at all ages, M. avium complex isolates were not recovered in patients younger than 15 years. A more recent study by Levy and colleagues (177) found a prevalence of NTM infection of 22% among 186 CF patients (mean age, 24 years) receiving care in seven centers in Israel. Mycobacterium simiae was the most common species recovered, accounting for 40% of NTM strains isolated. This species, which is otherwise rarely associated with human infection, is the most common NTM type isolated from clinical specimens in Israel (268). As in other studies, M. abscessus and M. avium complex strains were the next most common species found, representing 31% and 14% of NTM strains isolated, respectively.
The numbers of patients reported to the CFF Patient Registry as being infected with NTM are relatively low; nevertheless, the reported prevalence has increased steadily in each of the last 10 years, ranging from 0.85 in 1999 to 2.18 in 2008 (72). Until culture for mycobacteria becomes a routine element in the microbiological processing of CF sputum samples, these data will underestimate the true incidence and prevalence of NTM infection in U.S. CF patients.
Acquisition and transmission.
The risk factors for the acquisition of NTM by persons with CF remain incompletely understood. A multicenter study by Olivier and colleagues (226), in which M. avium complex isolates accounted for the majority of NTM infection, found that compared with patients without NTM infection, patients who were NTM culture positive were older, had better lung function, and had a higher frequency of S. aureus infection and a lower frequency of P. aeruginosa infection. Studies by others identified the presence of Aspergillus species in sputum samples and steroid treatment of allergic bronchopulmonary aspergillosis as being possible risk factors for NTM acquisition (177, 214). The striking differences in the distributions of NTM species recovered from CF patients in different age groups noted by Pierre-Audigier and colleagues (237), as described above, indicate that NTM species vary significantly with respect to their epidemiology and, most likely, virulence in CF.
There is little evidence for the interpatient transmission of these species (17, 226). Jönsson and colleagues (148) found that among 14 CF patients with NTM infection, a shared strain was found only in a pair of siblings.
Other Bacterial Species
Haemophilus influenzae.
Nontypeable H. influenzae isolates are common commensals of the human upper respiratory tract that are capable of causing a variety of infections, including otitis media, sinusitis, bronchitis, and community-acquired pneumonia. Chronic infection with H. influenzae can occur in persons with lower airway diseases, such as chronic obstructive pulmonary disease (COPD) and bronchiectasis. In CF, H. influenzae has long been recognized as a frequent colonizer of the respiratory tracts of infants and children; this species is recovered from approximately 20% of children with CF under the age of 1 year (72). The prevalence peaks at approximately 32% for children aged 2 to 5 years and decreases thereafter, falling to fewer than 10% among adults with CF. In 1995, the overall prevalence of nontypeable H. influenzae isolates for patients reported to the CFF Patient Registry was 10.3% (72). This increased to 16.3% in 2008, with the greatest increases being observed for children less than 5 years old. It is not clear if this increase has resulted from the evaluation of a greater number of young children with mild disease who have been identified through newborn screening programs. Similar to the general population, colonization of the upper respiratory tract with H. influenzae in persons with CF appears to be quite dynamic. Román and colleagues (258) assessed isolates recovered from 27 CF patients over the course of 7 years (the median period of observation per patient was 41 months). The majority (90%) of patients was cocolonized with two or more distinct strains during the study period, and for only five patients was the same strain repeatedly isolated in serial cultures during the observation period.
Ralstonia and Cupriavidus species.
The genus Ralstonia was named in 1995, with Ralstonia pickettii (formerly named Pseudomonas pickettii and then Burkholderia pickettii) being designated the type species (358). This species, although an infrequent human pathogen, was identified in nosocomial outbreaks and pseudoepidemics caused by contaminated diagnostic and patient care solutions (28, 66). The genus Ralstonia subsequently expanded to include several more species, some of which, specifically, Ralstonia mannitolilytica, Ralstonia paucula, and Ralstonia gilardi, were also identified in human clinical specimens and hospital-associated outbreaks (55, 77, 318, 325, 342). In 2003, two new Ralstonia species, Ralstonia insidiosa and Ralstonia respiraculi, were identified from among “R. pickettii-like” isolates recovered from CF sputum cultures (56, 67). Shortly thereafter, comparative 16S rRNA gene sequence analysis, along with more detailed phenotypic analyses, indicated that two distinct phylogenetic subgroups existed within the genus Ralstonia. The name Wautersia was proposed for one group, while the name Ralstonia was preserved for species in the group that included R. pickettii (326). It soon became clear, however, that Wautersia eutropha, the type species of the genus Wautersia, was a junior synonym of Cupriavidus necator, the type species of the genus Cupriavidus, which was named in 1987 (193). Thus, by taxonomic convention, the name Wautersia had to be replaced by Cupriavidus, and all species of the genus Wautersia became species of the genus Cupriavidus.
There are now five species in the genus Ralstonia. The species known to cause human infection, including infection in CF patients, are R. pickettii, R. mannitolilytica (previously known as R. pickettii biovar 3/“thomasii”) (77), and R. insidiosa (56). Among the 11 species now included in the genus Cupriavidus (317) are Cupriavidus pauculus (previously known as Centers for Disease Control and Prevention [CDC] group IVc-2) (318), Cupriavidus gilardii (55), Cupriavidus respiraculi (67), and Cupriavidus taiwanensis (44), all of which have also been recovered from respiratory cultures from CF patients (64).
The frequency and clinical impact of Ralstonia and Cupriavidus species infection in CF have not been systematically studied (178). Burns and colleagues (35) found R. pickettii in only 2 of 559 CF patients participating in TSI clinical trials. A study in 2002 by Coenye and colleagues (66) identified 38 individuals infected with Ralstonia species from among several hundred CF patients in the United States. Two-thirds (n = 25) of these patients were infected with R. mannitolilytica: nine patients were infected with R. pickettii, two harbored R. gilardii, one harbored R. taiwanensis, and the remaining patient was infected with a Ralstonia species that could not be classified into one of the currently known species. No evidence of shared strains, which is suggestive of interpatient transmission, was noted, but genotyping analyses indicated chronic infection of several patients. A more recent analysis of CF sputum isolates referred to the Burkholderia cepacia Research Laboratory and Repository (BcRLR) (University of Michigan) confirmed the recovery of Ralstonia and Cupriavidus species from 72 and 73 CF patients, respectively, in the United States during the 5 years between 2004 and 2008 (Table 2). Again, R. mannitolilytica accounted for the majority (60%) of Ralstonia infections, while C. respiraculi was the most common (53%) Cupriavidus species identified. It is important to note that these numbers are based on isolates referred for analysis and so likely underestimate the true frequency of infection by these species in this patient population.
TABLE 2.
CF patients infected from 2004 to 2008
| Genus | No. of patientsa |
|---|---|
| Acinetobacterb | 53 |
| Bordetellac | 31 |
| Cupriavidusd | 73 |
| Herbaspirillume | 29 |
| Inquilinusf | 20 |
| Pandoraeag | 74 |
| Ralstoniah | 72 |
Number of U.S. CF patients from whom species within the genera indicated were recovered from sputum cultures and referred to the Burkholderia cepacia Research Laboratory and Repository (University of Michigan) for analysis between 1 January 2004 and 31 December 2008 (my unpublished data).
Numbers of isolates are as follows: A. baumannii, 5; A. calcoaceticus,1; A. haemolyticus, 1; A. johnsonii, 2; A. junii, 6; A. ursingii, 3; Acinetobacter species, 35.
Numbers of isolates are as follows: B. avium, 2; B. bronchiseptica/B. parapertusis 9; B. hinzii, 5; B. holmesii, 1; B. petrii, 3; Bordetella species, 11.
Numbers of isolates are as follows: C. gilardii, 9; C. metallidurans, 2; C. pauculus, 9; C respiraculi, 39; C. taiwanensis, 2; Cupriavidus species, 12.
Numbers of isolates are as follows: H. frisingense, 3; H. putei, 1; H. seropedicae, 2; Herbaspirillum species, 23.
All isolates were Inquilinus limosus.
Numbers of isolates are as follows: P. apista, 22; P. pulmonicola, 6; P. pnomenusa, 21; P. sputorum, 20; Pandoraea species, 5.
Numbers of isolates are as follows: R. pickettii, 25; R. mannitolilytica, 43; R. insidiosa, 4.
Pandoraea species.
The genus Pandoraea was described in 2000 following a comprehensive taxonomic evaluation of bacterial isolates recovered primarily from CF sputum cultures (54, 178). These isolates were initially identified as being either Burkholderia or Ralstonia species based on routine phenotypic testing but could not be confirmed as such by genotypic assays. Four species were named within this new genus, including Pandoraea apista, Pandoraea pulmonicola, Pandoraea pnomenusa, and Pandoraea sputorum. A taxonomic study also indicated that the species Burkholderia norimbergensis should be reclassified as a member of this genus as well. An additional Pandoraea genomospecies (i.e., species represented by too few isolates to receive a formal binomial designation) was also defined. Subsequently, taxonomic study by the Centers for Disease Control and Prevention demonstrated that bacteria previously categorized as CDC weak oxidizer group 2 (WO-2) isolates were P. apista, P. pnomenusa, or one of three additional Pandoraea genomospecies (74).
Although many CF patients have been identified as being chronically infected with Pandoraea species (54; my unpublished observations), the prevalence of these species in CF is unknown. Analysis of CF sputum isolates sent to the BcRLR (University of Michigan) identified Pandoraea species in samples from 74 CF patients from the United States between 2004 and 2008 (Table 2). Among these patients, P. apista, P. pnomenusa, and P. sputorum accounted for approximately equal proportions of infections. Little is known regarding the pathogenic role of these species in CF. Bacteremia due to P. apista in an adolescent boy with CF as well as chronic respiratory tract infection with this species for several years in two adult CF patients have been described (11, 144). A report in 2003 described the spread of a P. apista strain among six CF patients attending a winter camp, most of whom experienced a significant deterioration in lung function thereafter (150). Another report that same year described sepsis and death due to P. pnomenusa in a non-CF patient after lung transplantation (299). Besides reports such as these, Pandoraea species are troublesome in CF insofar as they are generally misidentified as other species, particularly B. cepacia complex isolates, which has a profound psychosocial impact on CF patients and their families (61, 62, 207).
Inquilinus limosus.
The genus Inquilinus was described in 2002 after a comprehensive taxonomic evaluation of CF sputum isolates that defied assignment to a known bacterial species based on phenotypic analyses (57). The single species in the genus is Inquilinus limosus, which was named based on the Latin adjective for “full of slime” or “slimy,” in recognition of the highly mucoid phenotype of this species. A number of reports have since described the recovery of I. limosus from CF patients in North America and Europe (23, 46, 80, 130, 275, 347). An analysis of isolates referred to the BcRLR (University of Michigan) identified this species in isolates from 20 U.S. CF patients between 2004 and 2008 (Table 2). The genus Inquilinus also contains an as-yet-unnamed genospecies, representatives of which have also been recovered from CF specimens (57).
Although the significance of infection with I. limosus on the clinical course of persons with CF is unknown, it is clear that patients can remain chronically infected for prolonged periods (46, 347; my unpublished observations). The source of acquisition of this species is also unknown; however, a report of infection in a non-CF patient suggests nosocomial acquisition (157). Evidence for interpatient spread is lacking. In a report of six I. limosus-infected CF patients, Schmoldt and colleagues (275) found no shared strains, even among the three patients who received care at the same time in the same CF clinic.
Miscellaneous aerobic species.
Several other bacterial species have been reported as being recovered from CF sputum cultures. These include species that have been only rarely associated with human infection, such as Bordetella hinzii, Comamonas testosteroni, Moraxella osloensis, and Rhizobium radiobacter, as well as poorly defined Acinetobacter, Chryseobacterium, Herbaspirillum, and Xanthomonas species (57). Most of these taxa are widely distributed in the natural environment as saprophytes or pathogens of plants or animals. Their true frequency and role in pulmonary disease in CF are unknown.
An analysis of isolates referred to the BcRLR (University of Michigan) identified Acinetobacter species in specimens from 53 U.S. CF patients between 2004 and 2008 (Table 2). Only five patients were identified as being infected with Acinetobacter baumannii, a species that has received considerable attention as an emerging multidrug-resistant nosocomial pathogen (243). The majority of Acinetobacter-infected patients harbored strains that could not be confidently placed into one of the 24 species currently included in this genus.
A recent survey by Spilker and colleagues (291) identified several Bordetella species among isolates recovered from respiratory specimens from CF patients. Most were Bordetella bronchiseptica/Bordetella parapertussis, although Bordetella hinzii, Bordetella petrii, Bordetella avium, and several uncharacterized Bordetella species were also found. Chronic infection of an adult CF patient with B. hinzii was reported (103). Spilker and colleagues (292) also recently identified Herbaspirillum species in cultures from 28 CF patients in the United States. A more recent analysis of isolates referred to the BcRLR (University of Michigan) identified 31 CF patients infected with Bordetella species and 29 infected with Herbaspirillum species in the United States between 2004 and 2008 (Table 2). Although the role of these species in lung disease in CF remains unclear, they are notable as being frequently misidentified, most often as Burkholderia species, by routine phenotypic testing.
Recently, by using a combination of culture-independent and culture-based approaches, Sibley and colleagues (282) identified species within the Streptococcus milleri group (consisting of Streptococcus constellatus, Streptococcus intermedius, and Streptococcus anginosus) in several CF patients experiencing exacerbations of respiratory symptoms. These species have been detected in CF specimens by other investigators, again by using both culture-independent and culture-based techniques (38, 128, 312). That study by Sibley and colleagues as well as a retrospective analysis of 10 other S. milleri-infected CF patients suggest that species within this group may play a significant role in pulmonary disease in CF (232).
Several species of enteric Gram-negative bacteria, such as Escherichia coli, Morganella morganii, Klebsiella pneumoniae, and Serratia marcescens, can also be recovered from CF sputum cultures (35, 40). Among these bacteria, S. marcescens is most frequently recovered (35, 57). In general, these are believed to be transient colonizers that are not associated with severe disease.
Finally, a recent report described the recovery of Segniliparus rugosus from three patients with CF (37). This species along with Segniliparus rotundus constitute the recently described genus Segniliparus (36). These strongly acid-fast bacilli are phenotypically similar to, and may be easily confused with, rapidly growing mycobacteria. Whether or not these species will be identified with increasing frequency in CF as culture for NTM becomes routine remains to be seen.
Anaerobic species.
The role that anaerobic species may have in contributing to respiratory tract disease in CF has long been debated. A few reports from the 1980s and 1990s described the recovery of significant concentrations (>105 CFU/ml) of anaerobic species from transtracheal and thoracotomy specimens from CF patients, suggesting infection of the lower airways (30, 143, 309). More recently, the demonstration of steep oxygen gradients and fully anaerobic conditions in the lungs of CF patients (354, 360) and the identification of anaerobic species in CF sputum and bronchoalveolar lavage (BAL) specimens by using culture-independent microbial detection methods (24, 163, 254-256) have renewed interest in the significance of anaerobes in CF. Rogers and colleagues (254) used a 16S rRNA-based culture-independent analysis to detect several facultative and obligate anaerobic species in CF sputum samples, including Bacteroides sp., Porphyromonas sp., Prevotella sp., and Veillonella sp. Harris and colleagues (128) used a similar methodology to detect 65 different species in BAL fluid samples from 28 children with CF. Again, several anaerobic species, primarily Prevotella and anaerobic Streptococcus species, were detected. Studies employing anaerobic cultures of CF respiratory specimens also typically recover anaerobic species in significant quantities. Tunney and colleagues (312) recovered 14 genera of obligate anaerobes from cultures of 42 of 66 (64%) sputum samples from adults with CF as well as BAL fluid specimens from pediatric patients. The species were primarily within the genera Prevotella, Veillonella, Proprionibacterium, and Actinomyces and were isolated in concentrations comparable to those of P. aeruginosa (between 104 and 107 CFU/g of sputum). Infection with P. aeruginosa significantly increased the likelihood that anaerobic species would be recovered from these patients. More recently, Worlitzsch and colleagues (353) recovered one or more obligate anaerobic species from sputum from 41 of 45 (91%) CF patients. A total of 168 species belonging to 16 genera were identified, with the most common species being Staphylococcus saccharolyticus and Peptostreptococcus prevotii. Quantitative cultures again revealed bacterial densities comparable to those found with P. aeruginosa and S. aureus. However, in contrast to the study by Tunney et al., the presence of P. aeruginosa did not increase the likelihood of recovery of anaerobic species. Studies such as these indicate that anaerobic cultures of respiratory tract specimens from the majority of CF patients would be expected to be positive for a variety of species. However, expectorated sputum and even BAL fluid samples are likely contaminated with bacteria residing in the upper airways, oropharynx, and sinuses. Rogers and colleagues (255) used 16S rRNA-based microbial profiling to demonstrate marked differences in the microbial communities in mouthwash samples compared with those in sputum samples from CF patients, suggesting that expectorated sputum is not significantly contaminated by bacteria present in the oral cavity. Nevertheless, in the absence of sterile sampling of the lower airways and/or a more direct detection of anaerobes at these sites and better correlation between the presence of anaerobic species and disease progression, questions about the relevance of these species in CF will persist.
FUNGI
A variety of yeast and filamentous fungi have been recovered from cultures of respiratory specimens from persons with CF. Among these, A. fumigatus has received the most attention, being the most common filamentous fungus species isolated and capable of precipitating a chronic allergic inflammatory response or invasive infection after lung transplantation. Other fungal species, including Exophiala dermatitidis and Scedosporium species, are being increasingly recognized as being capable of causing chronic colonization or infection of the airways of CF patients. The epidemiology and clinical significance of filamentous fungi in CF were recently reviewed (238). Salient features of the epidemiology of yeasts and molds commonly identified in CF cultures will be highlighted in the following sections.
Aspergillus Species
A. fumigatus is the most common filamentous fungus involved in CF lung disease. Reported prevalence rates vary from 6% to nearly 60% (12, 18, 19, 48, 172, 202, 211, 220, 284, 285, 315). This wide range likely results from differences in culture methodology, particularly the type of medium used to recover fungi, the age of the patients studied, culture frequencies, definitions of “Aspergillus positive,” and the periods of observation used. Bakare and colleagues (12) used Sabouraud glucose agar to recover A. fumigatus from 43 of 94 (46%) adult CF patients (median age 28 years) receiving care in a German center during a 6-month period. A similar rate was reported by Valenza and colleagues (315), who recovered A. fumigatus from 47 of 60 (78%) adult and pediatric patients (median age, 18 years) attending another German CF care center during 2006. In the United States, the prevalence of patients reported to the CFF Patient Registry from whom Aspergillus has been recovered is considerably lower but has increased from 11% in 1999 to 14% in 2008 (72).
Most studies in which the prevalence of A. fumigatus is stratified by age indicate that infection is relatively uncommon in young children and increases steadily with age (238). In two multicenter studies from France, Pihet and colleagues (238) showed that the mean ages of patients when A. fumigatus was first isolated were 12.3 and 14.1 years, respectively. Genotyping analyses indicate that patients may be sequentially or simultaneously infected with several different strains of A. fumigatus (52). However, in recurrently or chronically infected patients, a single strain eventually becomes dominant. Recently, Vanhee and colleagues (331) used a high-resolution microsatellite-based method to genotype 256 A. fumigatus isolates recovered from eight chronically infected French and Belgian CF patients. A total of 161 distinct genotypes were found. Only four types were shared between patients, and all patients were found to be coinfected with multiple strains. For seven of the eight patients, one or more strains persisted in sequential cultures obtained at least 5 months apart, indicating that chronic colonization with the same strain for prolonged periods of time is not uncommon.
Although invasive infection with A. fumigatus can occur in persons with CF, particularly after lung transplantation, the most common complication of Aspergillus infection is allergic bronchopulmonary aspergillosis (ABPA) (141). ABPA manifests as a worsening of pulmonary disease with wheezing, shortness of breath, cough, and chest pain. Estimates of the frequency of ABPA vary widely owing to differences in the definitions of ABPA used by various studies. Currently, the diagnosis of APBA depends upon the presence of an acute or subacute clinical deterioration not attributable to another etiology and associated with (i) an elevated total serum IgE titer, (ii) an immediate cutaneous reactivity to A. fumigatus or the presence of specific serum IgE, and (iii) the presence of specific serum IgG or recent signs of infection upon chest radiography that do not respond to physiotherapy or antibacterial treatment (296).
Other Aspergillus species may also be recovered from cultures of CF respiratory secretions. These species include Aspergillus flavus, Aspergillus niger, Aspergillus nidulans, and Aspergillus terreus. Among these species, A. terreus was reported to be most common (238).
Other Fungal Species
Scedosporium species.
The genus Scedosporium (formerly known as the anamorph, or asexual state, of the genus Pseudallescheria) currently contains five species: Scedosporium apiospermum, Scedosporium boydii, Scedosporium prolificans, Scedosporium aurantiacum, and Scedosporium dehoogii (106). These are saprophytic filamentous fungi that are found to be widely distributed in the natural environment, including in soil, sewage, contaminated water, and farm animal manure (81, 300). Among these species, S. aurantiacum and S. dehoogii have been only recently described; consequently, reports of these species causing human infection are lacking (106). In contrast, several reports described S. apiospermum (or its former anamorph, Pseudallescheria boydii, which is now placed in the distinct species S. boydii) and S. prolificans as emerging opportunistic pathogens in immunocompromised hosts (42, 121, 253). These two species have also been described as being recovered from CF patients, although there are few good estimates of infection frequency in this population. As many as 10% of CF patients receiving care in an Australian care center were reported as having had Scedosporium species recovered from sputum cultures during a 17-month period in 1997 and 1998 (351). Around the same time, a 5-year prospective study from France recovered S. apiospermum from sputum samples from 11 of 128 (8.6%) CF patients, making this the most common filamentous fungus identified after A. fumigatus (50). More recently, Scedosporium species were recovered from 6 of 42 (14%) CF patients in a German CF care center (137). In contrast, although Aspergillus and Candida species were recovered from more than half of the 60 CF patients studied from January to December 2006 at another German CF care center, no Scedosporium species were identified (315). This difference in rates of recovery likely results from differences in the culture methodologies used in these studies. The use of a semiselective medium such as yeast extract-peptone-dextrose agar plus cycloheximide or dichloran-rose bengal-chloramphenicol agar plus benomyl significantly facilitates the recovery of Scedosporium (238).
A recent review of 162 cases of S. prolificans infection reported in the English-, Spanish-, and French-language literature found that CF was second only to malignancy as the most common underlying condition associated with infection; 19 of 162 cases (12%) occurred in CF patients (253). The risk factors for the acquisition of Scedosporium species by CF patients are not clear. S. apiospermum has been found in polluted soil and water and, notably, in the soil of 36 of 55 (65%) potted plants sampled in the homes of six CF patients infected with this species (81, 238). There is little evidence of the interpatient spread of S. apiospermum between CF patients. Defontaine and colleagues (79) performed genotyping analysis of 129 isolates recovered from nine CF patients receiving care in three French centers between 1998 and 1999. No shared strains were found, and most patients were chronically infected with a single strain.
Exophiala (Wangiella) dermatitidis.
Exophiala (Wangiella) dermatitidis grows as a black yeast at 37°C and as a filamentous fungus at room temperature. The taxonomy and nomenclature of this species are controversial. While it is most often referred to as E. dermatitidis, it is considered by many taxonomists to be more properly classified as the single species in the genus Wangiella. Nevertheless, this species was first described as causing infection in persons with CF in 1990 (124). Several more case reports describing the recovery of this species from CF patients, primarily in Germany, subsequently appeared (25, 87, 123, 138, 169, 216, 248, 274). Reported prevalence rates among CF patients in Europe range between 1% and 16% (12, 138, 238); however, reports of this species causing infection in CF patients in North America are lacking. This apparent discrepancy in prevalences most likely stems from differences in culture methodologies. Recovery of E. dermatitidis from cultures requires incubation times as long as 4 weeks. Furthermore, a selective medium such as erythritol-chloramphenicol agar (ECA) may be necessary for recovery, especially in the presence of other fungal and bacterial species. Horré and colleagues (138) found ECA to be superior to other media, including Sabouraud glucose agar, for the recovery of E. dermatitidis from 5 of 81 CF patients (6%) whose ages ranged from 9 to 35 years (mean, 22 years). E. dermatitidis can survive for many months in hot and moist conditions and was described as having a particularly high prevalence in sauna facilities (198).
Candida species.
Candida species, especially Candida albicans, are often recovered from sputum cultures from CF patients. Indeed, Bakare and colleagues (12) isolated C. albicans specimens from 71 of 94 (75%) CF patients studied during a 6-month period. In a prospective study employing six different culture media, Valenza and colleagues (315) recovered C. albicans isolates from 47 of 60 (78%) children and adults with CF studied during a 12-month period. However, given the high frequency with which C. albicans inhabits the skin and oropharynx, the relevance of isolating this species in CF sputum cultures is not clear. Other Candida species, including Candida glabrata, Candida parapsilosis, Candida dubliniensis, and Candida tropicalis, are much less frequently recovered (27, 35, 192, 217, 234).
Miscellaneous fungal species.
Other fungal species that were reported as having been recovered from CF respiratory cultures include Penicillium emersonii (49) and Acrophialophora fusispora (51, 113). Both species are inhabitants of soil and have rarely been associated with human infection. A. fusispora was recently reported as being recovered from three children and one adult with CF in France (51). The difficulty in identifying this species may result in its occurrence being underestimated in CF.
VIRUSES
The role of viruses in contributing to the exacerbation of pulmonary symptoms and progression of lung disease in CF has been the subject of numerous studies during the past three decades (1, 8, 69, 125, 132, 136, 228, 236, 240, 246, 286, 340). Most of these studies have relied on viral detection in clinical specimens by using standard tissue culture, immunofluorescence, or serological assays, and, not surprisingly, most found a positive correlation between the presence of respiratory viruses and worsening of symptoms. In general, typical respiratory viruses have been identified in these studies, although their relative frequencies vary widely among studies, most likely due to differences in detection methods, patient ages, and seasons. Respiratory syncytial virus (RSV) has typically been the most common virus identified, followed by influenza A and B viruses (328). Parainfluenza virus and adenovirus have usually been found at lower frequencies, and older studies did not include assays for the detection of coronavirus or human metapneumovirus. Several studies have shown that although the frequency of infection, seasonal occurrence, and distribution of viruses involved do not differ significantly between CF patients and non-CF controls, infections of CF patients are often more severe and have a longer duration (132, 246, 340).
More recently, nucleic acid-based detection methods, including virus-specific PCR and microarray-based assays, have been used to investigate the epidemiology of viral respiratory infection in CF patients. Punch and colleagues (241) used a multiplex reverse transcriptase PCR assay capable of detecting influenza A and B viruses; parainfluenza viruses 1, 2, and 3; RSV; and adenovirus to investigate 52 sputum samples from 38 Australian adults with CF who were hospitalized with exacerbations of respiratory symptoms. Overall, 12 (23%) of the samples were PCR positive: 4 samples were positive for influenza B virus, 3 each were positive for parainfluenza and influenza A viruses, and 2 were positive for RSV. A study by Olesen and colleagues (225) employed PCR assays for the same viruses as well as for human rhinovirus and human metapneumovirus in a prospective study of 75 Danish children (median age, 8 years) whose sputum samples were tested monthly during a 12-month period in 2002 and 2003. A total of 96 specimens from 45 patients were virus PCR positive, with rhinovirus accounting for the great majority of virus isolates (87%). Parainfluenza virus accounted for 6% of the positive specimens, influenza A virus accounted for 3% of the positive specimens, and adenovirus and RSV each accounted for 2% of positive tests; no specimens were PCR positive for influenza B virus or metapneumovirus. In contrast, Garcia and colleagues (104) used serological assays to detect metapneumovirus infection in 20 of 42 (48%) children aged 7 to 18 years with acute respiratory illness studied prospectively at a Texas CF care center during a 7-month period in the winter of 1998 and 1999.
Another recent prospective study by Wat and colleagues (341) assessed the incidence of respiratory viruses in 71 Welsh children (median age, 9 years) with CF during a 17-month period between 2002 and 2004. Nasal swabs were obtained whenever subjects had an exacerbation of respiratory symptoms and/or when subjects were asymptomatic during bimonthly clinic visits. Specimens were examined for the presence of respiratory viruses, including influenza A and B viruses, RSV, parainfluenza virus types 1 to 4, rhinovirus, and coronavirus by using real-time nucleic acid sequence-based amplification. Among the 138 specimens obtained during episodes of respiratory exacerbation, 63 (46%) were positive for one of the viruses included in the test panel. In contrast, 23 of 136 (17%) specimens from asymptomatic patients were positive. Rhinovirus was the most common virus detected for both groups, and significantly more influenza viruses were detected in children with exacerbations than in those without exacerbations.
The observation made by several older studies that respiratory viral infections are no more frequent in individuals with CF than in non-CF controls was again demonstrated recently in a prospective study from the Netherlands (327). Twenty young children with CF (mean age, 3.5 years) and 18 age-matched, healthy, non-CF control subjects were contacted twice weekly during a 6-month winter period. Nasal swabs were collected when children had respiratory symptoms or at least every 2 weeks and examined by using PCR assays to detect rhinovirus, enterovirus, coronavirus, adenovirus, metapneumovirus, RSV, and influenza A and B viruses. No differences between the CF patients and controls in either the frequency of respiratory viral infection or the distribution of the viruses involved were found. Rhinovirus was detected in all of the study subjects; coronavirus and enterovirus were the next most common viruses detected. Metapneumovirus and influenza viruses were found in only seven and three children, respectively.
Although it seems clear that the epidemiology of respiratory virus infection among persons with CF does not differ significantly from that of the general population, the impact that such infections have on the progression of pulmonary disease in CF remains an active area of investigation. Evidence from both in vitro and in vivo models strongly suggests that synergy between viruses and bacteria plays an important role in promoting bacterial colonization of the respiratory epithelium and possibly in decreasing bacterial clearance from lungs (142, 240, 246, 286, 294, 329, 330, 340). Recent studies also suggest that respiratory viruses interact with CF epithelial cells to potentiate the proinflammatory effects of bacterial infection, possibly through facilitating the dispersal of bacteria from biofilms within the airway lumen (267).
CAVEATS TO ASSESSING CHANGES IN MICROBIAL EPIDEMIOLOGY IN CF
Perception is real even when it is not reality.
—Edward de Bono, Maltese physician, educator, and author.
Is There an Expanding Spectrum of Microbial Species Involved in Infection in CF?
Many reports published during the past couple decades described various microbial species as having “emerged” as new respiratory pathogens in persons with CF. The implication is that certain species, such as B. cepacia, S. maltophilia, and A. xylosoxidans, have only recently become pathogens in this patient population. However, the mechanisms underlying this apparent phenomenon have not been clearly articulated. While it is well established that microbial species may acquire an enhanced capacity to cause human infection (i.e., through the acquisition of genes encoding virulence factors), firm evidence that this is primarily responsible for the emergence of “new” pathogens in CF is lacking. More commonly, the emergence of new pathogens in CF is attributed to changes in the CF host population that have been driven by changes in the care and treatment provided to persons with CF (203); that is, while the possibility exists that alterations in microbial species account for their ability to emerge as new pathogens in CF, it is more certain that changes in the management of persons with CF have altered this patient population, potentially creating a greater opportunity for more species to cause infection therein.
Most notable among these changes has been the increase in life expectancy of persons with CF during the last 20 years. The median predicted rate of survival for persons born with CF in the United States has increased from approximately 28 years of age in 1990 to nearly 40 years of age today (Fig. 2). It is anticipated that soon, approximately half of all persons living with CF will be adults aged 18 years or older. This growing population of adults with CF effectively represents a novel ecological niche, which one could argue provides an expanded opportunity for human colonization or infection by typically nonpathogenic microbial species. Furthermore, much of the success in increasing life expectancy in CF has been attributed to the greater availability and more aggressive use of antibiotics (164). New therapies, such as quinolone and inhaled antibiotics, have become a standard part of CF care. There has also been a steady movement away from the episodic treatment of respiratory exacerbations that typified CF care for many years toward chronic suppressive antibiotic therapy as well as early aggressive treatment intended to eradicate initial respiratory tract colonization/infection. These strategies have arguably placed greater selective pressures on the microbes causing infection in CF, possibly facilitating the emergence of species with multidrug resistance phenotypes.
FIG. 2.
Median predicted survival age for U.S. CF patients born in the years indicated. (Based on data derived from reference 72.)
These arguments explaining the possible emergence of new species in CF notwithstanding, there are other factors that have likely contributed to the perception that the spectrum of infectious agents in CF has expanded recently. Perhaps chief among these are the refinements in microbial taxonomy that have occurred during the past 20 years, particularly with respect to bacterial species. This has resulted in numerous revisions in species nomenclature and the description of several novel taxa. This is best illustrated by the reassignment of several Pseudomonas species to the novel genera Burkholderia and Ralstonia in the early 1990s (Fig. 3). Bacteria identified as being B. cepacia strains were subsequently found to be comprised of at least 17 distinct species in what is now referred to as the B. cepacia complex. Bacteria that were identified as R. pickettii or “R. pickettii-like” were subsequently recognized as representing at least three novel species, including R. mannitolilytica, R. insidiosa, and R. respiraculi. Further taxonomic evaluation eventually led to the reclassification of the latter species as Cupriavidus respiraculi. Detailed study of “B. cepacia-like” and “R. pickettii-like” bacteria, recovered primarily from CF respiratory specimens, resulted in the description of the new genus Pandoraea, which currently includes five species. Thus, bacteria that were identified as belonging to one of two species, P. cepacia and P. pickettii, 20 years ago are now represented by at least 25 distinct species, most of which may be perceived as novel species that have only recently “emerged” as new pathogens in CF. The development and use of improved methods for the identification of these species contribute to the perception that these species are being found with increasing frequency in CF sputum cultures.
FIG. 3.
Taxonomic changes in CF-relevant species. Solid arrows indicate changes in genus names. Dashed arrows indicate the identification of novel species based on a taxonomic assessment of “atypical” isolates within the species indicated.
In addition to the reclassification of existing species and the development of methods for their accurate identification, the ability to detect a broader range of microbial species in clinical specimens also likely contributes to the sense that the spectrum of species involved in CF infection is expanding. Culture-independent approaches for characterizing the microbial community of the CF respiratory tract suggest that a much greater array of species may be involved in CF infection than previously appreciated (24, 128, 163, 254, 257). As species that heretofore have not been recognized as typical CF pathogens are detected by such methods, they too may well be perceived as having only recently become involved in CF lung disease. The detection of bacteria belonging to the Streptococcus milleri group in CF sputum samples illustrates this point. These fastidious species frequently do not grow on standard laboratory media but have been identified recently in association with acute pulmonary exacerbations in CF patients by using a combination of culture-independent and culture-based approaches (282). If such findings are corroborated in further studies, these species may be added to the list of emerging pathogens in CF. It will remain unclear, however, whether these species have truly only recently become pathogens in CF or have gone unrecognized in CF due primarily to difficulties in their recovery from culture and accurate identification.
Have the Incidence and Prevalence of Infection by Specific Species Really Changed?
As described in the preceding sections, analysis of the information available from small single-center studies, as well as from larger multicenter studies and national patient registries, suggests that several changes have occurred in the incidence and prevalence of infection due to specific microbial species in CF in recent years. A recent analysis of the U.S. CFF Patient Registry, which currently includes in excess of 23,000 registrants, found that the reported annual prevalence of infection due to P. aeruginosa and members of the B. cepacia complex decreased significantly between 1995 and 2005 (249). The reported prevalences of S. aureus, H. influenzae, S. maltophilia, and A. xylosoxidans infection all increased during this interval. A recent analysis of the Royal Brompton Hospital (United Kingdom) CF database, which included 195 patients in 1985 and 391 patients in 2005, similarly showed significant increases in the prevalences of infection due to S. maltophilia and A. xylosoxidans between 1985 and 2005 (203). As in the United States, the proportion of patients reported to be infected with B. cepacia complex strains decreased between 1990 and 2005. In contrast to the data from the United States, however, no significant changes in the proportion of patients infected with P. aeruginosa or S. aureus were found during this interval.
The reasons for these apparent changes in microbial epidemiology in CF are not entirely clear. Again, it is quite possible that changes in patient care and demographics have played a role in altering infection frequencies. The increasingly aggressive use of antibiotics targeting P. aeruginosa in both chronic suppressive and early eradication strategies may be contributing to declines in the prevalence of infection due to this species (203, 249). The increasing median age of patients with CF may be providing new opportunities for infection by S. maltophilia, A. xylosoxidans, NTM, and other species, as described above. Better adherence to rigorous infection control measures may have resulted in a decrease in the incidences of P. aeruginosa and certain B. cepacia complex species.
However, data gleaned from patient registries and single-center databases must be interpreted with caution. Although such data repositories provide an invaluable resource, there are several pitfalls that may confound interpretations of the data therein (272). Not the least among these is confounding resulting from missing data (i.e., variables that were not initially considered for inclusion in the registry). An illustration is provided by MRSA, the prevalence of which has clearly increased among U.S. CF patients during the past decade. Nevertheless, the calculation of the statistical significance of the changes in MRSA prevalence rates based on the rate of 0.1% reported to the CFF Patient Registry in 1995 is problematic insofar as a data field for MRSA was not included in the registry at that time (110, 114).
The methods employed for collecting and reporting data for inclusion in such registries have an enormous bearing on the quality and interpretation of the data that they provide. Discrepancies between data reported to the CFF Patient Registry and data collected by a separate active surveillance program at the same institution have been described (68). Moreover, registries that are limited to annual data entry (one entry per year) lack the granularity of information in registries that provide for real-time or encounter-based data entry. As registries evolve to enable more opportunities for data entry and better standardization of input data, the limitations of earlier data must be taken into account when longitudinal trends are assessed.
Historical differences in the methods used for specimen processing and species identification also contribute to the relative reliability of data included in patient registries. Recommendations for and adherence to protocols for processing of CF respiratory specimens (e.g., the use of selective media for Burkholderia and, more recently, for staphylococci) have varied considerably during the past decade (281, 361). High rates of misidentification of several CF-relevant species by commercially available microbial identification systems have been well described (29, 66, 158, 201, 265, 280, 291, 292, 334). In some surveys, for example, more than 1 in 10 isolates referred to reference laboratories initially identified as B. cepacia complex or A. xylosoxidans isolates had been misidentified (201, 265). Data from the CFF Patient Registry indicate that in 1996, 1.9% of U.S. CF patients were infected with A. xylosoxidans; however, this species was detected by a reference laboratory in 8.7% of 595 CF patients enrolled in a 6-month antibiotic trial at approximately the same time (35).
The role that species misidentification may have in confounding interpretations of infection rates derived from patient registries is further illustrated by recently reported trends in the rates of B. cepacia complex infection. Data from the CFF Patient Registry indicate that the incidence of B. cepacia complex infection in U.S. CF patients decreased significantly, from 1.3% to 0.8% between 1995 and 2005 (249). Analysis of isolates referred to the BcRLR (University of Michigan) indicates that during the period from 2000 to 2008, an average of 41 patients per year (range, 34 to 47 patients) were initially misidentified as being infected with B. cepacia complex isolates by U.S. CF care centers. Confirmatory testing of isolates effectively decreased the number of B. cepacia complex-infected patients reported to the CFF Patient Registry during these years. Thus, it may be argued that the availability of reference laboratories specializing in CF microbiology has improved the reliability of data provided to patient repositories and may account for some of the trends observed in rates of infection for specific species. As specimen processing is standardized and identification systems improve, particularly for uncommon human pathogens, the rates at which these species are identified in the clinical laboratory would be expected to increase (i.e., unless the actual rate of infection has decreased). Comparing current rates of infection to rates observed before the widespread acceptance of recommended protocols for sample processing and the availability of reliable identification methods is inherently problematic.
The frequency with which surveillance cultures are obtained from patients is yet another factor that is critical in determining rates of infection, particularly for species such as S. maltophilia and A. xylosoxidans that often transiently infect the respiratory tracts of persons with CF (82). Data from the CFF Patient Registry indicate that only 4% of U.S. CF patients were culture positive for S. maltophilia in 1996 (72). During this time, surveillance sputum cultures were generally obtained from U.S. CF patients on an annual or semiannual basis. In a large multicenter antibiotic clinical trial conducted around that time, wherein sputum cultures were obtained several times during the course of 6 months and processed by a single reference laboratory, 30% of subjects had at least one sputum culture positive for S. maltophilia (35, 118). Most patients were only intermittently culture positive; indeed, patients who were culture positive at any time point were more likely than not to be culture negative at the next time point. Although these results could have been biased by selective pressure from the antibiotic being studied, longitudinal studies have shown similar results. Ballestero and colleagues (16), for example, found that 30% of CF patients were sputum culture positive for S. maltophilia at least once during a 5-year study period. A study at the pediatric and adult CF units in Leeds, United Kingdom, indicated that although only 13 of 557 (2.3%) patients were defined as being chronically infected (culture positive on at least three occasions over a 6-month period) with A. xylosoxidans during the years 1992 to 1999, a further 31 (5.5%) patients were intermittently colonized (306). A single-center study from Belgium, in which sputum culture samples were obtained from all patients every 3 months, found that 17.9% of 140 patients had at least a single positive culture, but only 5.3% of the patients were chronically infected with A. xylosoxidans, having had at least three positive cultures during at least 9 months (78). A study from Italy reported that 22 (29.3%) of 75 patients had at least one positive culture during the period between 2003 and 2007 (247). Rates of infection with NTM species are similarly dependent on culture frequency. In fact, Levy and colleagues (177) found that the frequency of sputum culture was the most significant predictor for the isolation of NTM strains among patients in a large multicenter survey.
CONCLUDING REMARKS
Despite the advances made in the care of persons with CF and the impressive increases in life expectancy observed during the last 20 years, infection of the respiratory tract remains the leading cause of morbidity and mortality for this patient population. A better understanding of the microbial epidemiology of CF would be expected to provide further opportunities to improve treatment strategies and refine infection control measures. Further definition of the types of microbial species causing infection in CF and analysis of the rates with which such infections occur are fundamental to these efforts.
Case reports and relatively small-scale, single-center studies provided much of the descriptive data that constituted our understanding of CF microbial epidemiology in the 1980s and early 1990s. Since then, several factors have contributed to the impression that there have been marked changes in this epidemiology in recent years. Most significant among these have been refinements in bacterial taxonomy, CF respiratory sample processing, and species identification methodologies, all of which have led to a marked expansion of the breadth of microbial species that are now recognized as being involved in infection of CF airways. At the same time, however, changes in patient care, particularly the more aggressive use of antibiotic therapy, the implementation of more stringent infection control measures, and the dramatic increase in life expectancy in CF, have provided a rationale to interpret this expansion of “CF-relevant” species as involving more than ascertainment artifact.
The assessment of changes in the incidence and prevalence of infection due to specific microbial species in CF, particularly those that occur in a relatively small proportion of patients, requires the use of large multicenter, national, or international registries. Indeed, such registries are critical, often providing the only opportunity to power statistically sound analyses of changes in infection frequency. As such, they provide the observational data needed to assess the impact of new therapies and infection control measures, to identify risk factors for infection, and to generate hypotheses regarding apparent trends in rates of infection. However, the design and operation of such registries require great care and prescient consideration of factors that may confound future analyses. As important, the pitfalls inherent in interpreting data derived from such registries must be respected by investigators.
Moving forward, it is likely that further changes in patient care (e.g., the introduction of new therapies and changes in the frequency of routine surveillance cultures) and improvements in microbial detection and identification will drive additional changes in the microbial epidemiology of respiratory tract infection in CF. The use of culture-independent microbial detection methods in particular has the potential to provide an unprecedented view of the microbial ecology of the CF respiratory tract. Future efforts to better understand the microbial epidemiology of CF will need to move beyond a one-dimensional perspective to account for the polymicrobial nature of CF respiratory infection. Tracking the relative frequencies with which various combinations of microbes colonize and infect the CF airways will be the next challenge.
Acknowledgments
This work was made possible by support provided by the Cystic Fibrosis Foundation. I am very grateful to Hebe Quinton and Bruce Marshall for providing data and analyses from the CFF Patient Registry. I thank Linda Kalikin, Ted Spilker, and Judith Green for their expert help in preparing the manuscript. I acknowledge the generosity of persons with CF and their families and caregivers for providing the data that are the basis for this review. I apologize to colleagues whose work I was unable to cite but who have made important contributions to our understanding of respiratory tract infection in CF.
J.J.L. receives grant funding support from the Cystic Fibrosis Foundation and the National Institutes of Health.
Biography
 John J. LiPuma received an M.D. degree from St. Louis University School of Medicine. He completed pediatric residency training and then a fellowship in pediatric infectious diseases at the University of Michigan Health System, before joining the faculty of the Medical College of Pennsylvania (currently Drexel University College of Medicine). He is currently Professor of Pediatrics at the University of Michigan Medical School and Professor of Epidemiology at the University of Michigan School of Public Health. He directs the CFF Burkholderia cepacia Research Laboratory and Repository at the University of Michigan. Dr. LiPuma has investigated the epidemiology, natural history, and pathogenesis of respiratory tract infection in persons with cystic fibrosis for 25 years.
John J. LiPuma received an M.D. degree from St. Louis University School of Medicine. He completed pediatric residency training and then a fellowship in pediatric infectious diseases at the University of Michigan Health System, before joining the faculty of the Medical College of Pennsylvania (currently Drexel University College of Medicine). He is currently Professor of Pediatrics at the University of Michigan Medical School and Professor of Epidemiology at the University of Michigan School of Public Health. He directs the CFF Burkholderia cepacia Research Laboratory and Repository at the University of Michigan. Dr. LiPuma has investigated the epidemiology, natural history, and pathogenesis of respiratory tract infection in persons with cystic fibrosis for 25 years.
REFERENCES
- 1.Abman, S. H., J. W. Ogle, N. Butler-Simon, C. M. Rumack, and F. J. Accurso. 1988. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J. Pediatr. 113:826-830. [DOI] [PubMed] [Google Scholar]
- 2.Agger, W. A., T. H. Cogbill, H. Busch, Jr., J. Landercasper, and S. M. Callister. 1986. Wounds caused by corn-harvesting machines: an unusual source of infection due to Gram-negative bacilli. Rev. Infect. Dis. 8:927-931. [DOI] [PubMed] [Google Scholar]
- 3.Agodi, A., E. Mahenthiralingam, M. Barchitta, V. Giannino, A. Sciacca, and S. Stefani. 2001. Burkholderia cepacia complex infection in Italian patients with cystic fibrosis: prevalence, epidemiology, and genomovar status. J. Clin. Microbiol. 39:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aitken, M. L., W. Burke, G. McDonald, C. Wallis, B. Ramsey, and C. Nolan. 1993. Nontuberculous mycobacterial disease in adult cystic fibrosis patients. Chest 103:1096-1099. [DOI] [PubMed] [Google Scholar]
- 5.Al-Aloul, M., J. Crawley, C. Winstanley, C. A. Hart, M. J. Ledson, and M. J. Walshaw. 2004. Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax 59:334-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfieri, N., K. Ramotar, P. Armstrong, M. E. Spornitz, G. Ross, J. Winnick, and D. R. Cook. 1999. Two consecutive outbreaks of Stenotrophomonas maltophilia (Xanthomonas maltophilia) in an intensive-care unit defined by restriction fragment-length polymorphism typing. Infect. Control Hosp. Epidemiol. 20:553-556. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong, D., S. Bell, M. Robinson, P. Bye, B. Rose, C. Harbour, C. Lee, H. Service, M. Nissen, M. Syrmis, and C. Wainwright. 2003. Evidence for spread of a clonal strain of Pseudomonas aeruginosa among cystic fibrosis clinics. J. Clin. Microbiol. 41:2266-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong, D., K. Grimwood, J. B. Carlin, R. Carzino, J. Hull, A. Olinsky, and P. D. Phelan. 1998. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr. Pulmonol. 26:371-379. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong, D. S., K. Grimwood, J. B. Carlin, R. Carzino, J. P. Gutierrez, J. Hull, A. Olinsky, E. M. Phelan, C. F. Robertson, and P. D. Phelan. 1997. Lower airway inflammation in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 156:1197-1204. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong, D. S., G. M. Nixon, R. Carzino, A. Bigham, J. B. Carlin, R. M. Robins-Browne, and K. Grimwood. 2002. Detection of a widespread clone of Pseudomonas aeruginosa in a pediatric cystic fibrosis clinic. Am. J. Respir. Crit. Care Med. 166:983-987. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson, R. M., J. J. LiPuma, D. B. Rosenbluth, and W. M. Dunne, Jr. 2006. Chronic colonization with Pandoraea apista in cystic fibrosis patients determined by repetitive-element-sequence PCR. J. Clin. Microbiol. 44:833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakare, N., V. Rickerts, J. Bargon, and G. Just-Nubling. 2003. Prevalence of Aspergillus fumigatus and other fungal species in the sputum of adult patients with cystic fibrosis. Mycoses 46:19-23. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin, A., E. Mahenthiralingam, P. Drevinek, P. Vandamme, J. R. Govan, D. J. Waine, J. J. LiPuma, L. Chiarini, C. Dalmastri, D. A. Henry, D. P. Speert, D. Honeybourne, M. C. Maiden, and C. G. Dowson. 2007. Environmental Burkholderia cepacia complex isolates in human infections. Emerg. Infect. Dis. 13:458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldwin, A., E. Mahenthiralingam, K. M. Thickett, D. Honeybourne, M. C. Maiden, J. R. Govan, D. P. Speert, J. J. LiPuma, P. Vandamme, and C. G. Dowson. 2005. Multilocus sequence typing scheme that provides both species and strain differentiation for the Burkholderia cepacia complex. J. Clin. Microbiol. 43:4665-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkhy, H. H., G. Cunningham, C. Francis, M. A. Almuneef, G. Stevens, N. Akkad, A. Elgammal, A. Alassiri, E. Furukawa, F. K. Chew, M. Sobh, D. Daniel, G. Poff, and Z. A. Memish. 2005. A National Guard outbreak of Burkholderia cepacia infection and colonization secondary to intrinsic contamination of albuterol nebulization solution. Am. J. Infect. Control 33:182-188. [DOI] [PubMed] [Google Scholar]
- 16.Ballestero, S., I. Virseda, H. Escobar, L. Suarez, and F. Baquero. 1995. Stenotrophomonas maltophilia in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 14:728-729. [DOI] [PubMed] [Google Scholar]
- 17.Bange, F. C., B. A. Brown, C. Smaczny, R. J. Wallace, Jr., and E. C. Bottger. 2001. Lack of transmission of Mycobacterium abscessus among patients with cystic fibrosis attending a single clinic. Clin. Infect. Dis. 32:1648-1650. [DOI] [PubMed] [Google Scholar]
- 18.Bauernfeind, A., R. M. Bertele, K. Harms, G. Horl, R. Jungwirth, C. Petermuller, B. Przyklenk, and C. Weisslein-Pfister. 1987. Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection 15:270-277. [DOI] [PubMed] [Google Scholar]
- 19.Becker, J. W., W. Burke, G. McDonald, P. A. Greenberger, W. R. Henderson, and M. L. Aitken. 1996. Prevalence of allergic bronchopulmonary aspergillosis and atopy in adult patients with cystic fibrosis. Chest 109:1536-1540. [DOI] [PubMed] [Google Scholar]
- 20.Bernhardt, S. A., T. Spilker, T. Coffey, and J. J. LiPuma. 2003. Burkholderia cepacia complex in cystic fibrosis: frequency of strain replacement during chronic infection. Clin. Infect. Dis. 37:780-785. [DOI] [PubMed] [Google Scholar]
- 21.Berthelot, P., F. Grattard, P. Mahul, R. Jospe, B. Pozzetto, A. Ros, O. G. Gaudin, and C. Auboyer. 1993. Ventilator temperature sensors: an unusual source of Pseudomonas cepacia in nosocomial infection. J. Hosp. Infect. 25:33-43. [DOI] [PubMed] [Google Scholar]
- 22.Biddick, R., T. Spilker, A. Martin, and J. J. LiPuma. 2003. Evidence of transmission of Burkholderia cepacia, Burkholderia multivorans and Burkholderia dolosa among persons with cystic fibrosis. FEMS Microbiol. Lett. 228:57-62. [DOI] [PubMed] [Google Scholar]
- 23.Bittar, F., A. Leydier, E. Bosdure, A. Toro, M. Reynaud-Gaubert, S. Boniface, N. Stremler, J. C. Dubus, J. Sarles, D. Raoult, and J. M. Rolain. 2008. Inquilinus limosus and cystic fibrosis. Emerg. Infect. Dis. 14:993-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bittar, F., H. Richet, J. C. Dubus, M. Reynaud-Gaubert, N. Stremler, J. Sarles, D. Raoult, and J. M. Rolain. 2008. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One 3:e2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaschke-Hellmessen, R., I. Lauterbach, K. D. Paul, K. Tintelnot, and G. Weissbach. 1994. Detection of Exophiala dermatitidis (Kano) De Hoog 1977 in septicemia of a child with acute lymphatic leukemia and in patients with cystic fibrosis. Mycoses 37(Suppl. 1):89-96. [PubMed] [Google Scholar]
- 26.Blessing, J., J. Walker, and B. Maybury. 1979. Pseudomonas cepacia and maltophilia in the cystic fibrosis patient. Am. Rev. Respir. Dis. Suppl. 119:262. [Google Scholar]
- 27.Bouchara, J. P., H. Y. Hsieh, S. Croquefer, R. Barton, V. Marchais, M. Pihet, and T. C. Chang. 2009. Development of an oligonucleotide array for direct detection of fungi in sputum samples from patients with cystic fibrosis. J. Clin. Microbiol. 47:142-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutros, N., N. Gonullu, A. Casetta, M. Guibert, D. Ingrand, and L. Lebrun. 2002. Ralstonia pickettii traced in blood culture bottles. J. Clin. Microbiol. 40:2666-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brisse, S., S. Stefani, J. Verhoef, A. Van Belkum, P. Vandamme, and W. Goessens. 2002. Comparative evaluation of the BD Phoenix and VITEK 2 automated instruments for identification of isolates of the Burkholderia cepacia complex. J. Clin. Microbiol. 40:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brook, I., and R. Fink. 1983. Transtracheal aspiration in pulmonary infection in children with cystic fibrosis. Eur. J. Respir. Dis. 64:51-57. [PubMed] [Google Scholar]
- 31.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15:716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdge, D. R., E. M. Nakielna, and M. A. Noble. 1993. Case-control and vector studies of nosocomial acquisition of Pseudomonas cepacia in adult patients with cystic fibrosis. Infect. Control Hosp. Epidemiol. 14:127-130. [DOI] [PubMed] [Google Scholar]
- 33.Burdge, D. R., M. A. Noble, M. E. Campbell, V. L. Krell, and D. P. Speert. 1995. Xanthomonas maltophilia misidentified as Pseudomonas cepacia in cultures of sputum from patients with cystic fibrosis: a diagnostic pitfall with major clinical implications. Clin. Infect. Dis. 20:445-448. [DOI] [PubMed] [Google Scholar]
- 34.Burkholder, W. H. 1950. Sour skin, a bacterial rot of onion bulbs. Phytopathology 40:115-118. [Google Scholar]
- 35.Burns, J. L., J. Emerson, J. R. Stapp, D. L. Yim, J. Krzewinski, L. Louden, B. W. Ramsey, and C. R. Clausen. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27:158-163. [DOI] [PubMed] [Google Scholar]
- 36.Butler, W. R., M. M. Floyd, J. M. Brown, S. R. Toney, M. I. Daneshvar, R. C. Cooksey, J. Carr, A. G. Steigerwalt, and N. Charles. 2005. Novel mycolic acid-containing bacteria in the family Segniliparaceae fam. nov., including the genus Segniliparus gen. nov., with descriptions of Segniliparus rotundus sp. nov. and Segniliparus rugosus sp. nov. Int. J. Syst. Evol. Microbiol. 55:1615-1624. [DOI] [PubMed] [Google Scholar]
- 37.Butler, W. R., C. A. Sheils, B. A. Brown-Elliott, N. Charles, A. A. Colin, M. J. Gant, J. Goodill, D. Hindman, S. R. Toney, R. J. Wallace, Jr., and M. A. Yakrus. 2007. First isolations of Segniliparus rugosus from patients with cystic fibrosis. J. Clin. Microbiol. 45:3449-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cade, A., M. Denton, K. G. Brownlee, N. Todd, and S. P. Conway. 1999. Acute bronchopulmonary infection due to Streptococcus milleri in a child with cystic fibrosis. Arch. Dis. Child. 80:278-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campana, S., G. Taccetti, N. Ravenni, F. Favari, L. Cariani, A. Sciacca, D. Savoia, A. Collura, E. Fiscarelli, G. De Intinis, M. Busetti, A. Cipolloni, A. d'Aprile, E. Provenzano, I. Collebrusco, P. Frontini, G. Stassi, M. Trancassini, D. Tovagliari, A. Lavitola, C. J. Doherty, T. Coenye, J. R. Govan, and P. Vandamme. 2005. Transmission of Burkholderia cepacia complex: evidence for new epidemic clones infecting cystic fibrosis patients in Italy. J. Clin. Microbiol. 43:5136-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canton, R., M. I. Morosini, S. Ballestero, M. E. Alvarez, H. Escobar, L. Maiz, and F. Baquero. 1997. Lung colonization with Enterobacteriaceae producing extended-spectrum beta-lactamases in cystic fibrosis patients. Pediatr. Pulmonol. 24:213-217. [DOI] [PubMed] [Google Scholar]
- 41.Carmeli, Y., and M. H. Samore. 1997. Comparison of treatment with imipenem vs. ceftazidime as a predisposing factor for nosocomial acquisition of Stenotrophomonas maltophilia: a historical cohort study. Clin. Infect. Dis. 24:1131-1134. [DOI] [PubMed] [Google Scholar]
- 42.Castiglioni, B., D. A. Sutton, M. G. Rinaldi, J. Fung, and S. Kusne. 2002. Pseudallescheria boydii (anamorph Scedosporium apiospermum). Infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine (Baltimore) 81:333-348. [DOI] [PubMed] [Google Scholar]
- 43.Chen, J. S., K. A. Witzmann, T. Spilker, R. J. Fink, and J. J. LiPuma. 2001. Endemicity and inter-city spread of Burkholderia cepacia genomovar III in cystic fibrosis. J. Pediatr. 139:643-649. [DOI] [PubMed] [Google Scholar]
- 44.Chen, W. M., S. Laevens, T. M. Lee, T. Coenye, P. De Vos, M. Mergeay, and P. Vandamme. 2001. Ralstonia taiwanensis sp. nov., isolated from root nodules of Mimosa species and sputum of a cystic fibrosis patient. Int. J. Syst. Evol. Microbiol. 51:1729-1735. [DOI] [PubMed] [Google Scholar]
- 45.Cheng, K., R. L. Smyth, J. R. Govan, C. Doherty, C. Winstanley, N. Denning, D. P. Heaf, H. van Saene, and C. A. Hart. 1996. Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 348:639-642. [DOI] [PubMed] [Google Scholar]
- 46.Chiron, R., H. Marchandin, F. Counil, E. Jumas-Bilak, A. M. Freydiere, G. Bellon, M. O. Husson, D. Turck, F. Bremont, G. Chabanon, and C. Segonds. 2005. Clinical and microbiological features of Inquilinus sp. isolates from five patients with cystic fibrosis. J. Clin. Microbiol. 43:3938-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowdhury, P. R., and J. A. Heinemann. 2006. The general secretory pathway of Burkholderia gladioli pv. agaricicola BG164R is necessary for cavity disease in white button mushrooms. Appl. Environ. Microbiol. 72:3558-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cimon, B., J. Carrère, J. P. Chazalette, J. L. Ginies, P. Six, J. F. Vinatier, D. Chabasse, and J. P. Bouchara. 1995. Fungal colonization and immune response to fungi in cystic fibrosis. J. Mycol. Med. 5:211-216. [Google Scholar]
- 49.Cimon, B., J. Carrère, J. P. Chazalette, J. F. Vinatier, D. Chabasse, and J. P. Bouchara. 1999. Chronic airway colonization by Penicillium emersonii in a patient with cystic fibrosis. Med. Mycol. 37:291-293. [PubMed] [Google Scholar]
- 50.Cimon, B., J. Carrère, J. F. Vinatier, J. P. Chazalette, D. Chabasse, and J. P. Bouchara. 2000. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 19:53-56. [DOI] [PubMed] [Google Scholar]
- 51.Cimon, B., S. Challier, H. Beguin, J. Carrère, D. Chabasse, and J. P. Bouchara. 2005. Airway colonization by Acrophialophora fusispora in patients with cystic fibrosis. J. Clin. Microbiol. 43:1484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cimon, B., F. Symoens, R. Zouhair, D. Chabasse, N. Nolard, A. Defontaine, and J. P. Bouchara. 2001. Molecular epidemiology of airway colonisation by Aspergillus fumigatus in cystic fibrosis patients. J. Med. Microbiol. 50:367-374. [DOI] [PubMed] [Google Scholar]
- 53.Reference deleted.
- 54.Coenye, T., E. Falsen, B. Hoste, M. Ohlen, J. Goris, J. R. Govan, M. Gillis, and P. Vandamme. 2000. Description of Pandoraea gen. nov. with Pandoraea apista sp. nov., Pandoraea pulmonicola sp. nov., Pandoraea pnomenusa sp. nov., Pandoraea sputorum sp. nov. and Pandoraea norimbergensis comb. nov. Int. J. Syst. Evol. Microbiol. 50(Pt. 2):887-899. [DOI] [PubMed] [Google Scholar]
- 55.Coenye, T., E. Falsen, M. Vancanneyt, B. Hoste, J. R. Govan, K. Kersters, and P. Vandamme. 1999. Classification of Alcaligenes faecalis-like isolates from the environment and human clinical samples as Ralstonia gilardii sp. nov. Int. J. Syst. Bacteriol. 49:405-413. [DOI] [PubMed] [Google Scholar]
- 56.Coenye, T., J. Goris, P. De Vos, P. Vandamme, and J. J. LiPuma. 2003. Classification of Ralstonia pickettii-like isolates from the environment and clinical samples as Ralstonia insidiosa sp. nov. Int. J. Syst. Evol. Microbiol. 53:1075-1080. [DOI] [PubMed] [Google Scholar]
- 57.Coenye, T., J. Goris, T. Spilker, P. Vandamme, and J. J. LiPuma. 2002. Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinus limosus gen. nov., sp. nov. J. Clin. Microbiol. 40:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coenye, T., S. Laevens, A. Willems, M. Ohlen, W. Hannant, J. R. Govan, M. Gillis, E. Falsen, and P. Vandamme. 2001. Burkholderia fungorum sp. nov. and Burkholderia caledonica sp. nov., two new species isolated from the environment, animals and human clinical samples. Int. J. Syst. Evol. Microbiol. 51:1099-1107. [DOI] [PubMed] [Google Scholar]
- 59.Coenye, T., and J. J. LiPuma. 2002. Multilocus restriction typing: a novel tool for studying global epidemiology of Burkholderia cepacia complex infection in cystic fibrosis. J. Infect. Dis. 185:1454-1462. [DOI] [PubMed] [Google Scholar]
- 60.Coenye, T., and J. J. LiPuma. 2002. Population structure analysis of Burkholderia cepacia genomovar III: varying degrees of genetic recombination characterize major clonal complexes. Microbiology 149:77-88. [DOI] [PubMed] [Google Scholar]
- 61.Coenye, T., and J. J. LiPuma. 2002. Use of the gyrB gene for the identification of Pandoraea species. FEMS Microbiol. Lett. 208:15-19. [DOI] [PubMed] [Google Scholar]
- 62.Coenye, T., L. Liu, P. Vandamme, and J. J. LiPuma. 2001. Identification of Pandoraea species by 16S ribosomal DNA-based PCR assays. J. Clin. Microbiol. 39:4452-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coenye, T., E. Mahenthiralingam, D. Henry, J. J. LiPuma, S. Laevens, M. Gillis, D. P. Speert, and P. Vandamme. 2001. Burkholderia ambifaria sp. nov., a novel member of the Burkholderia cepacia complex including biocontrol and cystic fibrosis-related isolates. Int. J. Syst. Evol. Microbiol. 51:1481-1490. [DOI] [PubMed] [Google Scholar]
- 64.Coenye, T., T. Spilker, R. Reik, P. Vandamme, and J. J. LiPuma. 2005. Use of PCR analyses to define the distribution of Ralstonia species recovered from patients with cystic fibrosis. J. Clin. Microbiol. 43:3463-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coenye, T., T. Spilker, A. Van Schoor, J. J. LiPuma, and P. Vandamme. 2004. Recovery of Burkholderia cenocepacia strain PHDC from cystic fibrosis patients in Europe. Thorax 59:952-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coenye, T., P. Vandamme, and J. J. LiPuma. 2002. Infection by Ralstonia species in cystic fibrosis patients: identification of R. pickettii and R. mannitolilytica by polymerase chain reaction. Emerg. Infect. Dis. 8:692-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coenye, T., P. Vandamme, and J. J. LiPuma. 2003. Ralstonia respiraculi sp. nov., isolated from the respiratory tract of cystic fibrosis patients. Int. J. Syst. Evol. Microbiol. 53:1339-1342. [DOI] [PubMed] [Google Scholar]
- 68.Coffin, S., S. Rettig, L. Bell, T. Scanlin, and K. St. John. 2004. Comparison of prevalence calculations using infection control surveillance methods and Cystic Fibrosis Foundation Patient Registry guidelines. Am. J. Infect. Control 32:E85. [Google Scholar]
- 69.Collinson, J., K. G. Nicholson, E. Cancio, J. Ashman, D. C. Ireland, V. Hammersley, J. Kent, and C. O'Callaghan. 1996. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax 51:1115-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corral, D. M., A. L. Coates, Y. C. Yau, R. Tellier, M. Glass, S. M. Jones, and V. J. Waters. 2008. Burkholderia pseudomallei infection in a cystic fibrosis patient from the Caribbean: a case report. Can. Respir. J. 15:237-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Creech, C. B., II, D. S. Kernodle, A. Alsentzer, C. Wilson, and K. M. Edwards. 2005. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr. Infect. Dis. J. 24:617-621. [DOI] [PubMed] [Google Scholar]
- 72.Cystic Fibrosis Foundation. 2008. Patient registry 2008. Annual data report to the center directors. Cystic Fibrosis Foundation, Bethesda, MD.
- 73.Dance, D. A., M. D. Smith, H. M. Aucken, and T. L. Pitt. 1999. Imported melioidosis in England and Wales. Lancet 353:208. [DOI] [PubMed] [Google Scholar]
- 74.Daneshvar, M. I., D. G. Hollis, A. G. Steigerwalt, A. M. Whitney, L. Spangler, M. P. Douglas, J. G. Jordan, J. P. MacGregor, B. C. Hill, F. C. Tenover, D. J. Brenner, and R. S. Weyant. 2001. Assignment of CDC weak oxidizer group 2 (WO-2) to the genus Pandoraea and characterization of three new Pandoraea genomospecies. J. Clin. Microbiol. 39:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dasen, S. E., J. J. LiPuma, J. R. Kostman, and T. L. Stull. 1994. Characterization of PCR-ribotyping for Burkholderia (Pseudomonas) cepacia. J. Clin. Microbiol. 32:2422-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davies, J. C., and B. K. Rubin. 2007. Emerging and unusual Gram-negative infections in cystic fibrosis. Semin. Respir. Crit. Care Med. 28:312-321. [DOI] [PubMed] [Google Scholar]
- 77.De Baere, T., S. Steyaert, G. Wauters, P. Des Vos, J. Goris, T. Coenye, T. Suyama, G. Verschraegen, and M. Vaneechoutte. 2001. Classification of Ralstonia pickettii biovar 3/′thomasii' strains (Pickett 1994) and of new isolates related to nosocomial recurrent meningitis as Ralstonia mannitolytica sp. nov. Int. J. Syst. Evol. Microbiol. 51:547-558. [DOI] [PubMed] [Google Scholar]
- 78.De Baets, F., P. Schelstraete, S. Van Daele, F. Haerynck, and M. Vaneechoutte. 2007. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J. Cyst. Fibros. 6:75-78. [DOI] [PubMed] [Google Scholar]
- 79.Defontaine, A., R. Zouhair, B. Cimon, J. Carrere, E. Bailly, F. Symoens, M. Diouri, J. N. Hallet, and J. P. Bouchara. 2002. Genotyping study of Scedosporium apiospermum isolates from patients with cystic fibrosis. J. Clin. Microbiol. 40:2108-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Degand, N., E. Carbonnelle, B. Dauphin, J. L. Beretti, M. Le Bourgeois, I. Sermet-Gaudelus, C. Segonds, P. Berche, X. Nassif, and A. Ferroni. 2008. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of nonfermenting Gram-negative bacilli isolated from cystic fibrosis patients. J. Clin. Microbiol. 46:3361-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Hoog, G. S., F. D. Marvin-Sikkema, G. A. Lahpoor, J. C. Gottschall, R. A. Prins, and E. Gueho. 1994. Ecology and physiology of the emerging opportunistic fungi Pseudallescheria boydii and Scedosporium prolificans. Mycoses 37:71-78. [DOI] [PubMed] [Google Scholar]
- 82.Demko, C. A., R. C. Stern, and C. F. Doershuk. 1998. Stenotrophomonas maltophilia in cystic fibrosis: incidence and prevalence. Pediatr. Pulmonol. 25:304-308. [DOI] [PubMed] [Google Scholar]
- 83.Denton, M., and K. G. Kerr. 1998. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 11:57-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Denton, M., A. Rajgopal, L. Mooney, A. Qureshi, K. G. Kerr, V. Keer, K. Pollard, D. G. Peckham, and S. P. Conway. 2003. Stenotrophomonas maltophilia contamination of nebulizers used to deliver aerosolized therapy to inpatients with cystic fibrosis. J. Hosp. Infect. 55:180-183. [DOI] [PubMed] [Google Scholar]
- 85.Denton, M., N. J. Todd, K. G. Kerr, P. M. Hawkey, and J. M. Littlewood. 1998. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J. Clin. Microbiol. 36:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Denton, M., N. J. Todd, and J. M. Littlewood. 1996. Role of anti-pseudomonal antibiotics in the emergence of Stenotrophomonas maltophilia in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 15:402-405. [DOI] [PubMed] [Google Scholar]
- 87.Diemert, D., D. Kunimoto, C. Sand, and R. Rennie. 2001. Sputum isolation of Wangiella dermatitidis in patients with cystic fibrosis. Scand. J. Infect. Dis. 33:777-779. [DOI] [PubMed] [Google Scholar]
- 88.Drevinek, P., O. Cinek, J. Melter, L. Langsadl, Y. Navesnakova, and V. Vavrova. 2003. Genomovar distribution of the Burkholderia cepacia complex differs significantly between Czech and Slovak patients with cystic fibrosis. J. Med. Microbiol. 52:603-604. [DOI] [PubMed] [Google Scholar]
- 89.Drevinek, P., S. Vosahlikova, O. Cinek, V. Vavrova, J. Bartosova, P. Pohunek, and E. Mahenthiralingam. 2005. Widespread clone of Burkholderia cenocepacia in cystic fibrosis patients in the Czech Republic. J. Med. Microbiol. 54:655-659. [DOI] [PubMed] [Google Scholar]
- 90.Duggan, J. M., S. J. Goldstein, C. E. Chenoweth, C. A. Kauffman, and S. F. Bradley. 1996. Achromobacter xylosoxidans bacteremia: report of four cases and review of the literature. Clin. Infect. Dis. 23:569-576. [DOI] [PubMed] [Google Scholar]
- 91.Dunne, W. M., Jr., and S. Maisch. 1995. Epidemiological investigation of infections due to Alcaligenes species in children and patients with cystic fibrosis: use of repetitive-element-sequence polymerase chain reaction. Clin. Infect. Dis. 20:836-841. [DOI] [PubMed] [Google Scholar]
- 92.Elizur, A., R. C. Orscheln, T. W. Ferkol, J. J. Atkinson, W. M. Dunne, Jr., R. S. Buller, J. R. Armstrong, E. R. Mardis, G. A. Storch, and C. L. Cannon. 2007. Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus lung infection in patients with cystic fibrosis. Chest 131:1718-1725. [DOI] [PubMed] [Google Scholar]
- 93.Elizur, A., R. C. Orscheln, T. W. Ferkol, W. M. Dunne, Jr., G. A. Storch, and C. L. Cannon. 2007. Transmission of Panton-Valentine leukocidin-positive Staphylococcus aureus between patients with cystic fibrosis. J. Pediatr. 151:90-92. [DOI] [PubMed] [Google Scholar]
- 94.Elting, L. S., N. Khardori, G. P. Bodey, and V. Fainstein. 1990. Nosocomial infection caused by Xanthomonas maltophilia: a case-control study of predisposing factors. Infect. Control Hosp. Epidemiol. 11:134-138. [DOI] [PubMed] [Google Scholar]
- 95.Esther, C. R., Jr., M. M. Henry, P. L. Molina, and M. W. Leigh. 2005. Nontuberculous mycobacterial infection in young children with cystic fibrosis. Pediatr. Pulmonol. 40:39-44. [DOI] [PubMed] [Google Scholar]
- 96.Estivariz, C. F., L. I. Bhatti, R. Pati, B. Jensen, M. J. Arduino, D. Jernigan, J. J. LiPuma, and A. Srinivasan. 2006. An outbreak of Burkholderia cepacia associated with contamination of albuterol and nasal spray. Chest 130:1346-1353. [DOI] [PubMed] [Google Scholar]
- 97.Falkinham, J. O., III. 1996. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 9:177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fauroux, B., B. Delaisi, A. Clement, C. Saizou, D. Moissenet, C. Truffot-Pernot, G. Tournier, and H. V. Thien. 1997. Mycobacterial lung disease in cystic fibrosis: a prospective study. Pediatr. Infect. Dis. J. 16:354-358. [DOI] [PubMed] [Google Scholar]
- 99.Fiore, A., S. Laevens, A. Bevivino, C. Dalmastri, S. Tabacchioni, P. Vandamme, and L. Chiarini. 2001. Burkholderia cepacia complex: distribution of genomovars among isolates from the maize rhizosphere in Italy. Environ. Microbiol. 3:137-143. [DOI] [PubMed] [Google Scholar]
- 100.Fisher, M. C., J. J. LiPuma, S. E. Dasen, G. C. Caputo, J. E. Mortensen, K. L. McGowan, and T. L. Stull. 1993. Source of Pseudomonas cepacia: ribotyping of isolates from patients and from the environment. J. Pediatr. 123:745-747. [DOI] [PubMed] [Google Scholar]
- 101.Forslow, U., A. Geborek, L. Hjelte, B. Petrini, and N. Heurlin. 2003. Early chemotherapy for non-tuberculous mycobacterial infections in patients with cystic fibrosis. Acta Paediatr. 92:910-915. [DOI] [PubMed] [Google Scholar]
- 102.Francis, J. S., M. C. Doherty, U. Lopatin, C. P. Johnston, G. Sinha, T. Ross, M. Cai, N. N. Hansel, T. Perl, J. R. Ticehurst, K. Carroll, D. L. Thomas, E. Nuermberger, and J. G. Bartlett. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin. Infect. Dis. 40:100-107. [DOI] [PubMed] [Google Scholar]
- 103.Funke, G., T. Hess, A. von Graevenitz, and P. Vandamme. 1996. Characteristics of Bordetella hinzii strains isolated from a cystic fibrosis patient over a 3-year period. J. Clin. Microbiol. 34:966-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garcia, D. F., P. W. Hiatt, A. Jewell, S. L. Schoonover, S. G. Cron, M. Riggs, S. Grace, C. M. Oermann, and P. A. Piedra. 2007. Human metapneumovirus and respiratory syncytial virus infections in older children with cystic fibrosis. Pediatr. Pulmonol. 42:66-74. [DOI] [PubMed] [Google Scholar]
- 105.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 106.Gilgado, F., J. Cano, J. Gene, D. A. Sutton, and J. Guarro. 2008. Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. J. Clin. Microbiol. 46:766-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gillis, M., T. V. Van, R. Bardin, et al. 1995. Pholyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus and proposition of Burkholderia vietnamiensis sp. nov. for N2-fixing isolates from rice in Vietnam. Int. J. Syst. Bacteriol. 45:274. [Google Scholar]
- 108.Girón, R. M., L. Máiz, I. Barrio, M. T. Martínez, A. Salcedo, and C. Prados. 2008. Nontuberculous mycobacterial infection in patients with cystic fibrosis: a multicenter prevalence study. Arch. Bronconeumol. 44:679-684. (In Spanish.) [PubMed] [Google Scholar]
- 109.Givney, R., A. Vickery, A. Holliday, M. Pegler, and R. Benn. 1997. Methicillin-resistant Staphylococcus aureus in a cystic fibrosis unit. J. Hosp. Infect. 35:27-36. [DOI] [PubMed] [Google Scholar]
- 110.Glikman, D., J. D. Siegel, M. Z. David, N. M. Okoro, S. Boyle-Vavra, M. L. Dowell, and R. S. Daum. 2008. Complex molecular epidemiology of methicillin-resistant Staphylococcus aureus isolates from children with cystic fibrosis in the era of epidemic community-associated methicillin-resistant S. aureus. Chest 133:1381-1387. [DOI] [PubMed] [Google Scholar]
- 111.Goerke, C., K. Kraning, M. Stern, G. Doring, K. Botzenhart, and C. Wolz. 2000. Molecular epidemiology of community-acquired Staphylococcus aureus in families with and without cystic fibrosis patients. J. Infect. Dis. 181:984-989. [DOI] [PubMed] [Google Scholar]
- 112.Gold, R., E. Jin, H. Levison, A. Isles, and P. C. Fleming. 1983. Ceftazidime alone and in combination in patients with cystic fibrosis: lack of efficacy in treatment of severe respiratory infections caused by Pseudomonas cepacia. J. Antimicrob. Chemother. 12(Suppl. A):331-336. [DOI] [PubMed] [Google Scholar]
- 113.Gonzalez-Escalada, A., A. Del Palacio, M. T. Calvo, J. Gene, and J. Guarro. 2000. Two cases of scalp wound colonization and respiratory tract by mycelial fungi. Rev. Iberoam. Micol. 17:149-151. (In Spanish.) [PubMed] [Google Scholar]
- 114.Goodrich, J. S., T. N. Sutton-Shields, A. Kerr, J. P. Wedd, M. B. Miller, and P. H. Gilligan. 2009. Prevalence of community-associated methicillin-resistant Staphylococcus aureus in patients with cystic fibrosis. J. Clin. Microbiol. 47:1231-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Govan, J. R., A. R. Brown, and A. M. Jones. 2007. Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol. 2:153-164. [DOI] [PubMed] [Google Scholar]
- 116.Govan, J. R., P. H. Brown, J. Maddison, C. J. Doherty, J. W. Nelson, M. Dodd, A. P. Greening, and A. K. Webb. 1993. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet 342:15-19. [DOI] [PubMed] [Google Scholar]
- 117.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Graff, G. R., and J. L. Burns. 2002. Factors affecting the incidence of Stenotrophomonas maltophilia isolation in cystic fibrosis. Chest 121:1754-1760. [DOI] [PubMed] [Google Scholar]
- 119.Graham, P. L., III, A. S. Morel, J. Zhou, F. Wu, P. Della-Latta, D. Rubenstein, and L. Saiman. 2002. Epidemiology of methicillin-susceptible Staphylococcus aureus in the neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 23:677-682. [DOI] [PubMed] [Google Scholar]
- 120.Grothues, D., U. Koopmann, H. von der Hardt, and B. Tummler. 1988. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J. Clin. Microbiol. 26:1973-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guarro, J., A. S. Kantarcioglu, R. Horré, J. L. Rodriguez-Tudela, M. Cuenca Estrella, J. Berenguer, and G. S. de Hoog. 2006. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 44:295-327. [DOI] [PubMed] [Google Scholar]
- 122.Haas, J. P., A. M. Evans, K. E. Preston, and E. L. Larson. 2005. Risk factors for surgical site infection after cardiac surgery: the role of endogenous flora. Heart Lung 34:108-114. [DOI] [PubMed] [Google Scholar]
- 123.Haase, G., H. Skopnik, T. Groten, G. Kusenbach, and H. G. Posselt. 1991. Long-term fungal culture of sputum from patients with cystic fibrosis. Mycoses 34(Suppl. 1):49-52. [PubMed] [Google Scholar]
- 124.Haase, G., H. Skopnik, and G. Kusenbach. 1990. Exophiala dermatitidis infection in cystic fibrosis. Lancet 336:188-189. [DOI] [PubMed] [Google Scholar]
- 125.Hament, J. M., J. L. Kimpen, A. Fleer, and T. F. Wolfs. 1999. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol. Med. Microbiol. 26:189-195. [DOI] [PubMed] [Google Scholar]
- 126.Hamill, R. J., E. D. Houston, P. R. Georghiou, C. E. Wright, M. A. Koza, R. M. Cadle, P. A. Goepfert, D. A. Lewis, G. J. Zenon, and J. E. Clarridge. 1995. An outbreak of Burkholderia (formerly Pseudomonas) cepacia respiratory tract colonization and infection associated with nebulized albuterol therapy. Ann. Intern. Med. 122:762-766. [DOI] [PubMed] [Google Scholar]
- 127.Hanes, S. D., K. Demirkan, E. Tolley, B. A. Boucher, M. A. Croce, G. C. Wood, and T. C. Fabian. 2002. Risk factors for late-onset nosocomial pneumonia caused by Stenotrophomonas maltophilia in critically ill trauma patients. Clin. Infect. Dis. 35:228-235. [DOI] [PubMed] [Google Scholar]
- 128.Harris, J. K., M. A. De Groote, S. D. Sagel, E. T. Zemanick, R. Kapsner, C. Penvari, H. Kaess, R. R. Deterding, F. J. Accurso, and N. R. Pace. 2007. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc. Natl. Acad. Sci. U. S. A. 104:20529-20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hauben, L., L. Vauterin, E. R. Moore, B. Hoste, and J. Swings. 1999. Genomic diversity of the genus Stenotrophomonas. Int. J. Syst. Bacteriol. 49(Pt. 4):1749-1760. [DOI] [PubMed] [Google Scholar]
- 130.Hayes, D., Jr., B. S. Murphy, R. J. Kuhn, M. I. Anstead, and D. J. Feola. 2009. Mucoid Inquilinus limosus in a young adult with cystic fibrosis. Pediatr. Pulmonol. 44:619-621. [DOI] [PubMed] [Google Scholar]
- 131.Henry, R. L., C. M. Mellis, and L. Petrovic. 1992. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr. Pulmonol. 12:158-161. [DOI] [PubMed] [Google Scholar]
- 132.Hiatt, P. W., S. C. Grace, C. A. Kozinetz, S. H. Raboudi, D. G. Treece, L. H. Taber, and P. A. Piedra. 1999. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics 103:619-626. [DOI] [PubMed] [Google Scholar]
- 133.Hjelte, L., B. Petrini, G. Kallenius, and B. Strandvik. 1990. Prospective study of mycobacterial infections in patients with cystic fibrosis. Thorax 45:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Høiby, N. 1974. Epidemiological investigations of the respiratory tract bacteriology in patients with cystic fibrosis. Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 82:541-550. [PubMed] [Google Scholar]
- 135.Holmes, A., M. Ganner, S. McGuane, T. L. Pitt, B. D. Cookson, and A. M. Kearns. 2005. Staphylococcus aureus isolates carrying Panton-Valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J. Clin. Microbiol. 43:2384-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hordvik, N. L., P. Konig, B. Hamory, M. Cooperstock, C. Kreutz, D. Gayer, and G. Barbero. 1989. Effects of acute viral respiratory tract infections in patients with cystic fibrosis. Pediatr. Pulmonol. 7:217-222. [DOI] [PubMed] [Google Scholar]
- 137.Horré, R., G. Marklein, R. Siekmeier, S. Nidermajer, and S. M. Reiffert. 2009. Selective isolation of Pseudallescheria and Scedosporium species from respiratory tract specimens of cystic fibrosis patients. Respiration 77:320-324. [DOI] [PubMed] [Google Scholar]
- 138.Horré, R., K. P. Schaal, R. Siekmeier, B. Sterzik, G. S. de Hoog, and N. Schnitzler. 2004. Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. A prospective study. Respiration 71:360-366. [DOI] [PubMed] [Google Scholar]
- 139.Hutchinson, J., W. Runge, M. Mulvey, G. Norris, M. Yetman, N. Valkova, R. Villemur, and F. Lepine. 2004. Burkholderia cepacia infections associated with intrinsically contaminated ultrasound gel: the role of microbial degradation of parabens. Infect. Control Hosp. Epidemiol. 25:291-296. [DOI] [PubMed] [Google Scholar]
- 140.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 141.Iversen, M., C. M. Burton, S. Vand, L. Skovfoged, J. Carlsen, N. Milman, C. B. Andersen, M. Rasmussen, and M. Tvede. 2007. Aspergillus infection in lung transplant patients: incidence and prognosis. Eur. J. Clin. Microbiol. Infect. Dis. 26:879-886. [DOI] [PubMed] [Google Scholar]
- 142.Jennings, L. C., T. P. Anderson, A. M. Werno, K. A. Beynon, and D. R. Murdoch. 2004. Viral etiology of acute respiratory tract infections in children presenting to hospital: role of polymerase chain reaction and demonstration of multiple infections. Pediatr. Infect. Dis. J. 23:1003-1007. [DOI] [PubMed] [Google Scholar]
- 143.Jewes, L. A., and R. C. Spencer. 1990. The incidence of anaerobes in the sputum of patients with cystic fibrosis. J. Med. Microbiol. 31:271-274. [DOI] [PubMed] [Google Scholar]
- 144.Johnson, L. N., J. Y. Han, S. M. Moskowitz, J. L. Burns, X. Qin, and J. A. Englund. 2004. Pandoraea bacteremia in a cystic fibrosis patient with associated systemic illness. Pediatr. Infect. Dis. J. 23:881-882. [DOI] [PubMed] [Google Scholar]
- 145.Johnson, W. M., S. D. Tyler, and K. R. Rozee. 1994. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J. Clin. Microbiol. 32:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jones, A. M., M. E. Dodd, C. J. Doherty, J. R. Govan, and A. K. Webb. 2002. Increased treatment requirements of patients with cystic fibrosis who harbour a highly transmissible strain of Pseudomonas aeruginosa. Thorax 57:924-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jones, A. M., J. R. Govan, C. J. Doherty, M. E. Dodd, B. J. Isalska, T. N. Stanbridge, and A. K. Webb. 2001. Spread of a multiresistant strain of Pseudomonas aeruginosa in an adult cystic fibrosis clinic. Lancet 358:557-558. [DOI] [PubMed] [Google Scholar]
- 148.Jönsson, B. E., M. Gilljam, A. Lindblad, M. Ridell, A. E. Wold, and C. Welinder-Olsson. 2007. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 45:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jordan, P. W., T. Stanley, F. M. Donnelly, J. S. Elborn, R. B. McClurg, B. C. Millar, C. E. Goldsmith, and J. E. Moore. 2007. Atypical mycobacterial infection in patients with cystic fibrosis: update on clinical microbiology methods. Lett. Appl. Microbiol. 44:459-466. [DOI] [PubMed] [Google Scholar]
- 150.Jorgensen, I. M., H. K. Johansen, B. Frederiksen, T. Pressler, A. Hansen, P. Vandamme, N. Hoiby, and C. Koch. 2003. Epidemic spread of Pandoraea apista, a new pathogen causing severe lung disease in cystic fibrosis patients. Pediatr. Pulmonol. 36:439-446. [DOI] [PubMed] [Google Scholar]
- 151.Kalish, L. A., D. A. Waltz, M. Dovey, G. Potter-Bynoe, A. J. McAdam, J. J. Lipuma, C. Gerard, and D. Goldmann. 2006. Impact of Burkholderia dolosa on lung function and survival in cystic fibrosis. Am. J. Respir. Crit. Care Med. 173:421-425. [DOI] [PubMed] [Google Scholar]
- 152.Kanellopoulou, M., S. Pournaras, H. Iglezos, N. Skarmoutsou, E. Papafrangas, and A. N. Maniatis. 2004. Persistent colonization of nine cystic fibrosis patients with an Achromobacter (Alcaligenes) xylosoxidans clone. Eur. J. Clin. Microbiol. Infect. Dis. 23:336-339. [DOI] [PubMed] [Google Scholar]
- 153.Kasperbauer, S. H., and C. L. Daley. 2008. Diagnosis and treatment of infections due to Mycobacterium avium complex. Semin. Respir. Crit. Care Med. 29:569-576. [DOI] [PubMed] [Google Scholar]
- 154.Kay, S. E., R. A. Clark, K. L. White, and M. M. Peel. 2001. Recurrent Achromobacter piechaudii bacteremia in a patient with hematological malignancy. J. Clin. Microbiol. 39:808-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kerem, E., M. Corey, R. Gold, and H. Levison. 1990. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J. Pediatr. 116:714-719. [DOI] [PubMed] [Google Scholar]
- 156.Khardori, N., L. Elting, E. Wong, B. Schable, and G. P. Bodey. 1990. Nosocomial infections due to Xanthomonas maltophilia (Pseudomonas maltophilia) in patients with cancer. Rev. Infect. Dis. 12:997-1003. [DOI] [PubMed] [Google Scholar]
- 157.Kiratisin, P., P. Koomanachai, P. Kowwigkai, S. Pattanachaiwit, N. Aswapokee, and A. Leelaporn. 2006. Early-onset prosthetic valve endocarditis caused by Inquilinus sp. Diagn. Microbiol. Infect. Dis. 56:317-320. [DOI] [PubMed] [Google Scholar]
- 158.Kiska, D. L., A. Kerr, M. C. Jones, J. A. Caracciolo, B. Eskridge, M. Jordan, S. Miller, D. Hughes, N. King, and P. H. Gilligan. 1996. Accuracy of four commercial systems for identification of Burkholderia cepacia and other Gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Klausner, J. D., C. Zukerman, A. P. Limaye, and L. Corey. 1999. Outbreak of Stenotrophomonas maltophilia bacteremia among patients undergoing bone marrow transplantation: association with faulty replacement of handwashing soap. Infect. Control Hosp. Epidemiol. 20:756-758. [DOI] [PubMed] [Google Scholar]
- 160.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 161.Klinger, J. D., and M. J. Thomassen. 1985. Occurrence and antimicrobial susceptibility of Gram-negative nonfermentative bacilli in cystic fibrosis patients. Diagn. Microbiol. Infect. Dis. 3:149-158. [DOI] [PubMed] [Google Scholar]
- 162.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kolak, M., F. Karpati, H. J. Monstein, and J. Jonasson. 2003. Molecular typing of the bacterial flora in sputum of cystic fibrosis patients. Int. J. Med. Microbiol. 293:309-317. [DOI] [PubMed] [Google Scholar]
- 164.Konstan, M. W., L. Rasouliyan, D. J. Pasta, W. J. Morgan, J. R. Jacobs, and J. R. Wagener. 2008. Trends in the use of routine therapies in cystic fibrosis: 1995-2005, abstr. 487, p. 376. Abstr. 22nd Ann. N. Am. Cyst. Fibros. Conf., Orlando, FL.
- 165.Kosorok, M. R., L. Zeng, S. E. West, M. J. Rock, M. L. Splaingard, A. Laxova, C. G. Green, J. Collins, and P. M. Farrell. 2001. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr. Pulmonol. 32:277-287. [DOI] [PubMed] [Google Scholar]
- 166.Krzewinski, J. W., C. D. Nguyen, J. M. Foster, and J. L. Burns. 2001. Use of random amplified polymorphic DNA PCR to examine epidemiology of Stenotrophomonas maltophilia and Achromobacter (Alcaligenes) xylosoxidans from patients with cystic fibrosis. J. Clin. Microbiol. 39:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kuehnert, M. J., D. Kruszon-Moran, H. A. Hill, G. McQuillan, S. K. McAllister, G. Fosheim, L. K. McDougal, J. Chaitram, B. Jensen, S. K. Fridkin, G. Killgore, and F. C. Tenover. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J. Infect. Dis. 193:172-179. [DOI] [PubMed] [Google Scholar]
- 168.Kumar, A., S. Dietrich, W. Schneider, R. Jacobson, F. P. Downes, B. E. Robinson-Dunn, R. Honicky, J. Smith, and R. Martin. 1997. Genetic relatedness of Burkholderia (Pseudomonas) cepacia isolates from five cystic fibrosis centers in Michigan. Respir. Med. 91:485-492. [DOI] [PubMed] [Google Scholar]
- 169.Kusenbach, G., H. Skopnik, G. Haase, F. Friedrichs, and H. Dohmen. 1992. Exophiala dermatitidis pneumonia in cystic fibrosis. Eur. J. Pediatr. 151:344-346. [DOI] [PubMed] [Google Scholar]
- 170.Kutty, P. K., B. Moody, J. S. Gullion, M. Zervos, M. Ajluni, R. Washburn, R. Sanderson, M. A. Kainer, T. A. Powell, C. F. Clarke, R. J. Powell, N. Pascoe, A. Shams, J. J. LiPuma, B. Jensen, J. Noble-Wang, M. J. Arduino, and L. C. McDonald. 2007. Multistate outbreak of Burkholderia cenocepacia colonization and infection associated with the use of intrinsically contaminated alcohol-free mouthwash. Chest 132:1825-1831. [DOI] [PubMed] [Google Scholar]
- 171.Laraya-Cuasay, L. R., M. Lipstein, and N. N. Huang. 1977. Pseudomonas cepacia in the respiratory flora of patients with cystic fibrosis. Pediatr. Pulmonol. 11:502. [Google Scholar]
- 172.Laufer, P., J. N. Fink, W. T. Bruns, G. F. Unger, J. H. Kalbfleisch, P. A. Greenberger, and R. Patterson. 1984. Allergic bronchopulmonary aspergillosis in cystic fibrosis. J. Allergy Clin. Immunol. 73:44-48. [DOI] [PubMed] [Google Scholar]
- 173.Ledson, M. J., M. J. Gallagher, J. E. Corkill, C. A. Hart, and M. J. Walshaw. 1998. Cross infection between cystic fibrosis patients colonized with Burkholderia cepacia. Thorax 53:432-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee, T. W. 2009. Eradication of early Pseudomonas infection in cystic fibrosis. Chron. Respir. Dis. 6:99-107. [DOI] [PubMed] [Google Scholar]
- 175.Lee, T. W., K. G. Brownlee, S. P. Conway, M. Denton, and J. M. Littlewood. 2003. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J. Cyst. Fibros. 2:29-34. [DOI] [PubMed] [Google Scholar]
- 176.Leitritz, L., M. Griese, A. Roggenkamp, A. M. Geiger, V. Fingerle, and J. Heesemann. 2004. Prospective study on nontuberculous mycobacteria in patients with and without cystic fibrosis. Med. Microbiol. Immunol. (Berl.) 193:209-217. [DOI] [PubMed] [Google Scholar]
- 177.Levy, I., G. Grisaru-Soen, L. Lerner-Geva, E. Kerem, H. Blau, L. Bentur, M. Aviram, J. Rivlin, E. Picard, A. Lavy, Y. Yahav, and G. Rahav. 2008. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg. Infect. Dis. 14:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.LiPuma, J. J. 2003. Burkholderia and emerging pathogens in cystic fibrosis. Semin. Respir. Crit. Care Med. 24:681-692. [DOI] [PubMed] [Google Scholar]
- 179.LiPuma, J. J. 2002. Preventing Burkholderia cepacia complex infection in cystic fibrosis: is there a middle ground? J. Pediatr. 141:467-469. [DOI] [PubMed] [Google Scholar]
- 180.LiPuma, J. J. 2005. Update on the Burkholderia cepacia complex. Curr. Opin. Pulm. Med. 11:528-533. [DOI] [PubMed] [Google Scholar]
- 181.LiPuma, J. J., S. E. Dasen, D. W. Nielson, R. C. Stern, and T. L. Stull. 1990. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet 336:1094-1096. [DOI] [PubMed] [Google Scholar]
- 182.LiPuma, J. J., J. E. Mortensen, S. E. Dasen, T. D. Edlind, D. V. Schidlow, J. L. Burns, and T. L. Stull. 1988. Ribotype analysis of Pseudomonas cepacia from cystic fibrosis treatment centers. J. Pediatr. 113:859-862. [DOI] [PubMed] [Google Scholar]
- 183.LiPuma, J. J., T. Spilker, T. Coenye, and C. F. Gonzalez. 2002. An epidemic Burkholderia cepacia complex strain identified in soil. Lancet 359:2002-2003. [DOI] [PubMed] [Google Scholar]
- 184.LiPuma, J. J., T. Spilker, L. H. Gill, P. W. Campbell III, L. Liu, and E. Mahenthiralingam. 2001. Disproportionate distribution of Burkholderia cepacia complex species and transmissibility markers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 164:92-96. [DOI] [PubMed] [Google Scholar]
- 185.Liu, L., T. Coenye, J. L. Burns, P. W. Whitby, T. L. Stull, and J. J. LiPuma. 2002. Ribosomal DNA-directed PCR for identification of Achromobacter (Alcaligenes) xylosoxidans recovered from sputum samples from cystic fibrosis patients. J. Clin. Microbiol. 40:1210-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Loukil, C., C. Saizou, C. Doit, P. Bidet, P. Mariani-Kurkdjian, Y. Aujard, F. Beaufils, and E. Bingen. 2003. Epidemiologic investigation of Burkholderia cepacia acquisition in two pediatric intensive care units. Infect. Control Hosp. Epidemiol. 24:707-710. [DOI] [PubMed] [Google Scholar]
- 187.Lynch, J. P., III. 2009. Burkholderia cepacia complex: impact on the cystic fibrosis lung lesion. Semin. Respir. Crit. Care Med. 30:596-610. [DOI] [PubMed] [Google Scholar]
- 188.Mahenthiralingam, E., M. E. Campbell, J. Foster, J. S. Lam, and D. P. Speert. 1996. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mahenthiralingam, E., M. E. Campbell, D. A. Henry, and D. P. Speert. 1996. Epidemiology of Burkholderia cepacia infection in patients with cystic fibrosis: analysis by randomly amplified polymorphic DNA fingerprinting. J. Clin. Microbiol. 34:2914-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144-156. [DOI] [PubMed] [Google Scholar]
- 191.Mahenthiralingam, E., P. Vandamme, M. E. Campbell, D. A. Henry, A. M. Gravelle, L. T. Wong, A. G. Davidson, P. G. Wilcox, B. Nakielna, and D. P. Speert. 2001. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clin. Infect. Dis. 33:1469-1475. [DOI] [PubMed] [Google Scholar]
- 192.Máiz, L., M. Cuevas, S. Quirce, J. F. Cañón, A. Pacheco, A. Sousa, and H. Escobar. 2002. Serologic IgE immune responses against Aspergillus fumigatus and Candida albicans in patients with cystic fibrosis. Chest 121:782-788. [DOI] [PubMed] [Google Scholar]
- 193.Makkar, N. S., and L. E. Casida. 1987. Cupriavidus necator gen. nov., sp. nov.: a nonobligate bacterial predator of bacteria in soil. Int. J. Syst. Bacteriol. 37:323-326. [Google Scholar]
- 194.Manno, G., C. Dalmastri, S. Tabacchioni, P. Vandamme, R. Lorini, L. Minicucci, L. Romano, A. Giannattasio, L. Chiarini, and A. Bevivino. 2004. Epidemiology and clinical course of Burkholderia cepacia complex infections, particularly those caused by different Burkholderia cenocepacia strains, among patients attending an Italian cystic fibrosis center. J. Clin. Microbiol. 42:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marchac, V., A. Equi, C. Le Bihan-Benjamin, M. Hodson, and A. Bush. 2004. Case-control study of Stenotrophomonas maltophilia acquisition in cystic fibrosis patients. Eur. Respir. J. 23:98-102. [DOI] [PubMed] [Google Scholar]
- 196.Maree, C. L., R. S. Daum, S. Boyle-Vavra, K. Matayoshi, and L. G. Miller. 2007. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg. Infect. Dis. 13:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Marzuillo, C., M. De Giusti, D. Tufi, A. Giordano, A. Del Cimmuto, S. Quattrucci, C. Mancini, and P. Villari. 2009. Molecular characterization of Stenotrophomonas maltophilia isolates from cystic fibrosis patients and the hospital environment. Infect. Control Hosp. Epidemiol. 30:753-758. [DOI] [PubMed] [Google Scholar]
- 198.Matos, T., G. S. de Hoog, A. G. de Boer, I. de Crom, and G. Haase. 2002. High prevalence of the neurotrope Exophiala dermatitidis and related oligotrophic black yeasts in sauna facilities. Mycoses 45:373-377. [DOI] [PubMed] [Google Scholar]
- 199.McCallum, S. J., J. Corkill, M. Gallagher, M. J. Ledson, C. A. Hart, and M. J. Walshaw. 2001. Superinfection with a transmissible strain of Pseudomonas aeruginosa in adults with cystic fibrosis chronically colonised by P. aeruginosa. Lancet 358:558-560. [DOI] [PubMed] [Google Scholar]
- 200.McCallum, S. J., M. J. Gallagher, J. E. Corkill, C. A. Hart, M. J. Ledson, and M. J. Walshaw. 2002. Spread of an epidemic Pseudomonas aeruginosa strain from a patient with cystic fibrosis (CF) to non-CF relatives. Thorax 57:559-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.McMenamin, J. D., T. M. Zaccone, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Misidentification of Burkholderia cepacia in US cystic fibrosis treatment centers: an analysis of 1,051 recent sputum isolates. Chest 117:1661-1665. [DOI] [PubMed] [Google Scholar]
- 202.Milla, C. E., C. L. Wielinski, and W. E. Regelmann. 1996. Clinical significance of the recovery of Aspergillus species from the respiratory secretions of cystic fibrosis patients. Pediatr. Pulmonol. 21:6-10. [DOI] [PubMed] [Google Scholar]
- 203.Millar, F. A., N. J. Simmonds, and M. E. Hodson. 8 September 2009, posting date. Trends in pathogens colonising the respiratory tract of adult patients with cystic fibrosis, 1985-2005, J. Cyst. Fibros. doi: 10.1016/j.jcf.2009.08.003. [DOI] [PubMed]
- 204.Miller, S. C., J. J. LiPuma, and J. L. Parke. 2002. Culture-based and non-growth-dependent detection of the Burkholderia cepacia complex in soil environments. Appl. Environ. Microbiol. 68:3750-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moissenet, D., A. Baculard, M. Valcin, V. Marchand, G. Tournier, A. Garbarg-Chenon, and H. Vu-Thien. 1997. Colonization by Alcaligenes xylosoxidans in children with cystic fibrosis: a retrospective clinical study conducted by means of molecular epidemiological investigation. Clin. Infect. Dis. 24:274-275. [DOI] [PubMed] [Google Scholar]
- 206.Molina-Cabrillana, J., M. Bolanos-Rivero, E. E. Alvarez-Leon, A. M. Martin Sanchez, M. Sanchez-Palacios, D. Alvarez, and J. A. Saez-Nieto. 2006. Intrinsically contaminated alcohol-free mouthwash implicated in a nosocomial outbreak of Burkholderia cepacia colonization and infection. Infect. Control Hosp. Epidemiol. 27:1281-1282. [DOI] [PubMed] [Google Scholar]
- 207.Moore, J. E., A. Reid, B. C. Millar, X. Jiru, J. McCaughan, C. E. Goldsmith, J. Collins, P. G. Murphy, and J. S. Elborn. 2002. Pandoraea apista isolated from a patient with cystic fibrosis: problems associated with laboratory identification. Br. J. Biomed. Sci. 59:164-166. [DOI] [PubMed] [Google Scholar]
- 208.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, and D. A. Talan. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666-674. [DOI] [PubMed] [Google Scholar]
- 209.Moroney, S. M., L. C. Heller, J. Arbuckle, M. Talavera, and R. H. Widen. 2007. Staphylococcal cassette chromosome mec and Panton-Valentine leukocidin characterization of methicillin-resistant Staphylococcus aureus clones. J. Clin. Microbiol. 45:1019-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mortensen, J. E., M. C. Fisher, and J. J. LiPuma. 1995. Recovery of Pseudomonas cepacia and other Pseudomonas species from the environment. Infect. Control Hosp. Epidemiol. 16:30-32. [DOI] [PubMed] [Google Scholar]
- 211.Mroueh, S., and A. Spock. 1994. Allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Chest 105:32-36. [DOI] [PubMed] [Google Scholar]
- 212.Muder, R. R., A. P. Harris, S. Muller, M. Edmond, J. W. Chow, K. Papadakis, M. W. Wagener, G. P. Bodey, and J. M. Steckelberg. 1996. Bacteremia due to Stenotrophomonas (Xanthomonas) maltophilia: a prospective, multicenter study of 91 episodes. Clin. Infect. Dis. 22:508-512. [DOI] [PubMed] [Google Scholar]
- 213.Muhdi, K., F. P. Edenborough, L. Gumery, S. O'Hickey, E. G. Smith, D. L. Smith, and D. E. Stableforth. 1996. Outcome for patients colonised with Burkholderia cepacia in a Birmingham adult cystic fibrosis clinic and the end of an epidemic. Thorax 51:374-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mussaffi, H., J. Rivlin, I. Shalit, M. Ephros, and H. Blau. 2005. Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur. Respir. J. 25:324-328. [DOI] [PubMed] [Google Scholar]
- 215.Nadesalingam, K., S. P. Conway, and M. Denton. 2005. Risk factors for acquisition of methicillin-resistant Staphylococcus aureus (MRSA) by patients with cystic fibrosis. J. Cyst. Fibros. 4:49-52. [DOI] [PubMed] [Google Scholar]
- 216.Nagano, Y., J. S. Elborn, B. C. Millar, C. E. Goldsmith, J. Rendall, and J. E. Moore. 2008. Development of a novel PCR assay for the identification of the black yeast, Exophiala (Wangiella) dermatitidis from adult patients with cystic fibrosis (CF). J. Cyst. Fibros. 7:576-580. [DOI] [PubMed] [Google Scholar]
- 217.Nagano, Y., B. C. Millar, E. Johnson, C. E. Goldsmith, J. S. Elborn, J. Rendall, and J. E. Moore. 2007. Fungal infections in patients with cystic fibrosis. Rev. Med. Microbiol. 18:11-16. [Google Scholar]
- 218.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976-2984. [DOI] [PubMed] [Google Scholar]
- 219.Nasser, R. M., A. C. Rahi, M. F. Haddad, Z. Daoud, N. Irani-Hakime, and W. Y. Almawi. 2004. Outbreak of Burkholderia cepacia bacteremia traced to contaminated hospital water used for dilution of an alcohol skin antiseptic. Infect. Control Hosp. Epidemiol. 25:231-239. [DOI] [PubMed] [Google Scholar]
- 220.Nelson, L. A., M. L. Callerame, and R. H. Schwartz. 1979. Aspergillosis and atopy in cystic fibrosis. Am. Rev. Respir. Dis. 120:863-873. [DOI] [PubMed] [Google Scholar]
- 221.Nixon, G. M., D. S. Armstrong, R. Carzino, J. B. Carlin, A. Olinsky, C. F. Robertson, and K. Grimwood. 2001. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J. Pediatr. 138:699-704. [DOI] [PubMed] [Google Scholar]
- 222.Nolan, G., P. Moivor, H. Levison, P. C. Fleming, M. Corey, and R. Gold. 1982. Antibiotic prophylaxis in cystic fibrosis: inhaled cephaloridine as an adjunct to oral cloxacillin. J. Pediatr. 101:626-630. [DOI] [PubMed] [Google Scholar]
- 223.O'Carroll, M. R., T. J. Kidd, C. Coulter, H. V. Smith, B. R. Rose, C. Harbour, and S. C. Bell. 2003. Burkholderia pseudomallei: another emerging pathogen in cystic fibrosis. Thorax 58:1087-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.O'Carroll, M. R., M. W. Syrmis, C. E. Wainwright, R. M. Greer, P. Mitchell, C. Coulter, T. P. Sloots, M. D. Nissen, and S. C. Bell. 2004. Clonal strains of Pseudomonas aeruginosa in paediatric and adult cystic fibrosis units. Eur. Respir. J. 24:101-106. [DOI] [PubMed] [Google Scholar]
- 225.Olesen, H. V., L. P. Nielsen, and P. O. Schiotz. 2006. Viral and atypical bacterial infections in the outpatient pediatric cystic fibrosis clinic. Pediatr. Pulmonol. 41:1197-1204. [DOI] [PubMed] [Google Scholar]
- 226.Olivier, K. N., D. J. Weber, R. J. Wallace, Jr., A. R. Faiz, J. H. Lee, Y. Zhang, B. A. Brown-Elliot, A. Handler, R. W. Wilson, M. S. Schechter, L. J. Edwards, S. Chakraborti, and M. R. Knowles. 2003. Nontuberculous mycobacteria I: multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 167:828-834. [DOI] [PubMed] [Google Scholar]
- 227.Olivier, K. N., J. R. Yankaskas, and M. R. Knowles. 1996. Nontuberculous mycobacterial pulmonary disease in cystic fibrosis. Semin. Respir. Infect. 11:272-284. [PubMed] [Google Scholar]
- 228.Ong, E. L., M. E. Ellis, A. K. Webb, K. R. Neal, M. Dodd, E. O. Caul, and S. Burgess. 1989. Infective respiratory exacerbations in young adults with cystic fibrosis: role of viruses and atypical microorganisms. Thorax 44:739-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Palleroni, N. J., and J. F. Bradbury. 1993. Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) Swings et al., 1983. Int. J. Syst. Bacteriol. 43:606-609. [DOI] [PubMed] [Google Scholar]
- 230.Pamukcu, A., A. Bush, and R. Buchdahl. 1995. Effects of Pseudomonas aeruginosa colonization on lung function and anthropometric variables in children with cystic fibrosis. Pediatr. Pulmonol. 19:10-15. [DOI] [PubMed] [Google Scholar]
- 231.Panlilio, A. L., C. M. Beck-Sague, J. D. Siegel, R. L. Anderson, S. Y. Yetts, N. C. Clark, P. N. Duer, K. A. Thomassen, R. W. Vess, and B. C. Hill. 1992. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin. Infect. Dis. 14:1078-1083. [DOI] [PubMed] [Google Scholar]
- 232.Parkins, M. D., C. D. Sibley, M. G. Surette, and H. R. Rabin. 2008. The Streptococcus milleri group—an unrecognized cause of disease in cystic fibrosis: a case series and literature review. Pediatr. Pulmonol. 43:490-497. [DOI] [PubMed] [Google Scholar]
- 233.Pegues, C. F., D. A. Pegues, D. S. Ford, P. L. Hibberd, L. A. Carson, C. M. Raine, and D. C. Hooper. 1996. Burkholderia cepacia respiratory tract acquisition: epidemiology and molecular characterization of a large nosocomial outbreak. Epidemiol. Infect. 116:309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Peltroche-Llacsahuanga, H., H. Dohmen, and G. Haase. 2002. Recovery of Candida dubliniensis from sputum of cystic fibrosis patients. Mycoses 45:15-18. [PubMed] [Google Scholar]
- 235.Peltroche-Llacsahuanga, H., G. Haase, and H. Kentrup. 1998. Persistent airway colonization with Alcaligenes xylosoxidans in two brothers with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 17:132-134. [DOI] [PubMed] [Google Scholar]
- 236.Petersen, N. T., N. Hoiby, C. H. Mordhorst, K. Lind, E. W. Flensborg, and B. Bruun. 1981. Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma—possible synergism with Pseudomonas aeruginosa. Acta Paediatr. Scand. 70:623-628. [DOI] [PubMed] [Google Scholar]
- 237.Pierre-Audigier, C., A. Ferroni, I. Sermet-Gaudelus, M. Le Bourgeois, C. Offredo, H. Vu-Thien, B. Fauroux, P. Mariani, A. Munck, E. Bingen, D. Guillemot, G. Quesne, V. Vincent, P. Berche, and J. L. Gaillard. 2005. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J. Clin. Microbiol. 43:3467-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pihet, M., J. Carrere, B. Cimon, D. Chabasse, L. Delhaes, F. Symoens, and J. P. Bouchara. 2009. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—a review. Med. Mycol. 47:387-397. [DOI] [PubMed] [Google Scholar]
- 239.Pitt, T. L., M. E. Kaufmann, P. S. Patel, L. C. Benge, S. Gaskin, and D. M. Livermore. 1996. Type characterization and antibiotic susceptibility of Burkholderia (Pseudomonas) cepacia isolates from patients with cystic fibrosis in the United Kingdom and the Republic of Ireland. J. Med. Microbiol. 44:203-210. [DOI] [PubMed] [Google Scholar]
- 240.Pribble, C. G., P. G. Black, J. A. Bosso, and R. B. Turner. 1990. Clinical manifestations of exacerbations of cystic fibrosis associated with nonbacterial infections. J. Pediatr. 117:200-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Punch, G., M. W. Syrmis, B. R. Rose, C. Harbour, P. T. Bye, M. D. Nissen, M. R. Elkins, and T. P. Sloots. 2005. Method for detection of respiratory viruses in the sputa of patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 24:54-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Purcell, K., and J. Fergie. 2005. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at Driscoll Children's Hospital. Arch. Pediatr. Adolesc. Med. 159:980-985. [DOI] [PubMed] [Google Scholar]
- 243.Rahal, J. J., and C. Urban. 2000. Acinetobacter. Semin. Respir. Crit. Care Med. 21:341-348. [DOI] [PubMed] [Google Scholar]
- 244.Ramette, A., J. J. LiPuma, and J. M. Tiedje. 2005. Species abundance and diversity of Burkholderia cepacia complex in the environment. Appl. Environ. Microbiol. 71:1193-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ramsey, A. H., P. Skonieczny, D. T. Coolidge, T. A. Kurzynski, M. E. Proctor, and J. P. Davis. 2001. Burkholderia cepacia lower respiratory tract infection associated with exposure to a respiratory therapist. Infect. Control Hosp. Epidemiol. 22:423-426. [DOI] [PubMed] [Google Scholar]
- 246.Ramsey, B. W., E. J. Gore, A. L. Smith, M. K. Cooney, G. J. Redding, and H. Foy. 1989. The effect of respiratory viral infections on patients with cystic fibrosis. Am. J. Dis. Child. 143:662-668. [DOI] [PubMed] [Google Scholar]
- 247.Raso, T., O. Bianco, B. Grosso, M. Zucca, and D. Savoia. 2008. Achromobacter xylosoxidans respiratory tract infections in cystic fibrosis patients. APMIS 116:837-841. [DOI] [PubMed] [Google Scholar]
- 248.Rath, P. M., K. D. Muller, H. Dermoumi, and R. Ansorg. 1997. A comparison of methods of phenotypic and genotypic fingerprinting of Exophiala dermatitidis isolated from sputum samples of patients with cystic fibrosis. J. Med. Microbiol. 46:757-762. [DOI] [PubMed] [Google Scholar]
- 249.Razvi, S., L. Quittell, A. Sewall, H. Quinton, B. Marshall, and L. Saiman. 2009. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995-2005. Chest 136:1554-1560. [DOI] [PubMed] [Google Scholar]
- 250.Reboli, A. C., R. Koshinski, K. Arias, K. Marks-Austin, D. Stieritz, and T. L. Stull. 1996. An outbreak of Burkholderia cepacia lower respiratory tract infection associated with contaminated albuterol nebulization solution. Infect. Control Hosp. Epidemiol. 17:741-743. [DOI] [PubMed] [Google Scholar]
- 251.Reik, R., T. Spilker, and J. J. LiPuma. 2005. Distribution of Burkholderia cepacia complex species among isolates recovered from persons with or without cystic fibrosis. J. Clin. Microbiol. 43:2926-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ritz, N., R. A. Ammann, C. C. Aebischer, F. Franziska Schoeni-Affolter, and M. H. Schoeni. 2005. Risk factors for allergic bronchopulmonary aspergillosis and sensitisation to Aspergillus fumigatus in patients with cystic fibrosis. Eur. J. Pediatr. 164:577-582. [DOI] [PubMed] [Google Scholar]
- 253.Rodriguez-Tudela, J. L., J. Berenguer, J. Guarro, A. S. Kantarcioglu, R. Horre, G. S. de Hoog, and M. Cuenca-Estrella. 2009. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med. Mycol. 47:359-370. [DOI] [PubMed] [Google Scholar]
- 254.Rogers, G. B., M. P. Carroll, D. J. Serisier, P. M. Hockey, G. Jones, and K. D. Bruce. 2004. Characterization of bacterial community diversity in cystic fibrosis lung infections by use of 16S ribosomal DNA terminal restriction fragment length polymorphism profiling. J. Clin. Microbiol. 42:5176-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rogers, G. B., M. P. Carroll, D. J. Serisier, P. M. Hockey, G. Jones, V. Kehagia, G. J. Connett, and K. D. Bruce. 2006. Use of 16S rRNA gene profiling by terminal restriction fragment length polymorphism analysis to compare bacterial communities in sputum and mouthwash samples from patients with cystic fibrosis. J. Clin. Microbiol. 44:2601-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rogers, G. B., M. P. Carroll, D. J. Serisier, P. M. Hockey, V. Kehagia, G. R. Jones, and K. D. Bruce. 2005. Bacterial activity in cystic fibrosis lung infections. Respir. Res. 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rogers, G. B., C. A. Hart, J. R. Mason, M. Hughes, M. J. Walshaw, and K. D. Bruce. 2003. Bacterial diversity in cases of lung infection in cystic fibrosis patients: 16S ribosomal DNA (rDNA) length heterogeneity PCR and 16S rDNA terminal restriction fragment length polymorphism profiling. J. Clin. Microbiol. 41:3548-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Román, F., R. Cantón, M. Pérez-Vázquez, F. Baquero, and J. Campos. 2004. Dynamics of long-term colonization of respiratory tract by Haemophilus influenzae in cystic fibrosis patients shows a marked increase in hypermutable strains. J. Clin. Microbiol. 42:1450-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Romero-Gomez, M. P., M. I. Quiles-Melero, P. Pena Garcia, A. Gutierrez Altes, M. A. Garcia de Miguel, C. Jimenez, S. Valdezate, and J. A. Saez Nieto. 2008. Outbreak of Burkholderia cepacia bacteremia caused by contaminated chlorhexidine in a hemodialysis unit. Infect. Control Hosp. Epidemiol. 29:377-378. [DOI] [PubMed] [Google Scholar]
- 260.Romling, U., B. Fiedler, J. Bosshammer, D. Grothues, J. Greipel, H. von der Hardt, and B. Tummler. 1994. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J. Infect. Dis. 170:1616-1621. [DOI] [PubMed] [Google Scholar]
- 261.Romling, U., J. Wingender, H. Muller, and B. Tummler. 1994. A major Pseudomonas aeruginosa clone common to patients and aquatic habitats. Appl. Environ. Microbiol. 60:1734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rønne Hansen, C., T. Pressler, N. Høiby, and M. Gormsen. 2006. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J. Cyst. Fibros. 5:245-251. [DOI] [PubMed] [Google Scholar]
- 263.Rosenstein, B. J., and D. E. Hall. 1980. Pneumonia and septicemia due to Pseudomonas cepacia in a patient with cystic fibrosis. Johns Hopkins Med. J. 147:188-189. [PubMed] [Google Scholar]
- 264.Sagel, S. D., R. L. Gibson, J. Emerson, S. McNamara, J. L. Burns, J. S. Wagener, and B. W. Ramsey. 2009. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J. Pediatr. 154:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Saiman, L., Y. Chen, S. Tabibi, P. San Gabriel, J. Zhou, Z. Liu, L. Lai, and S. Whittier. 2001. Identification and antimicrobial susceptibility of Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. J. Clin. Microbiol. 39:3942-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Saiman, L., and J. Siegel. 2003. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect. Control Hosp. Epidemiol. 24:S6-S52. [DOI] [PubMed] [Google Scholar]
- 267.Sajjan, U. S., Y. Zhao, A. Goldsmith, and M. B. Hershenson. 2008. Mucoid P. aeruginosa and rhinovirus coinfection in CF mice, abstr. 391, p. 340. Abstr. 22nd Ann. N. Am. Cyst. Fibros. Conf., Orlando, FL.
- 268.Samra, Z., L. Kaufman, S. Pitlik, I. Shalit, and J. Bishara. 2005. Emergence of Mycobacterium simiae in respiratory specimens. Scand. J. Infect. Dis. 37:838-841. [DOI] [PubMed] [Google Scholar]
- 269.Sanyal, S. C., and E. M. Mokaddas. 1999. The increase in carbapenem use and emergence of Stenotrophomonas maltophilia as an important nosocomial pathogen. J. Chemother. 11:28-33. [DOI] [PubMed] [Google Scholar]
- 270.Sattler, C. A., E. O. Mason, Jr., and S. L. Kaplan. 2000. Nonrespiratory Stenotrophomonas maltophilia infection at a children's hospital. Clin. Infect. Dis. 31:1321-1330. [DOI] [PubMed] [Google Scholar]
- 271.Schaumann, R., K. Stein, C. Eckhardt, G. Ackermann, and A. C. Rodloff. 2001. Infections caused by Stenotrophomonas maltophilia—a prospective study. Infection 29:205-208. [DOI] [PubMed] [Google Scholar]
- 272.Schechter, M. S. 2008. Patient registry analyses: seize the data, but caveat lector. J. Pediatr. 153:733-735. [DOI] [PubMed] [Google Scholar]
- 273.Schlichting, C., C. Branger, J. M. Fournier, W. Witte, A. Boutonnier, C. Wolz, P. Goullet, and G. Doring. 1993. Typing of Staphylococcus aureus by pulsed-field gel electrophoresis, zymotyping, capsular typing, and phage typing: resolution of clonal relationships. J. Clin. Microbiol. 31:227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schmalreck, A. F., P. Trankle, E. Vanca, and R. Blaschke-Hellmessen. 1998. Differentiation and characterization of yeasts pathogenic for humans (Candida albicans, Exophiala dermatitidis) and algae pathogenic for animals (Prototheca spp.) using Fourier transform infrared spectroscopy (FTIR) in comparison with conventional methods. Mycoses 41(Suppl. 1):71-77. [DOI] [PubMed] [Google Scholar]
- 275.Schmoldt, S., P. Latzin, J. Heesemann, M. Griese, A. Imhof, and M. Hogardt. 2006. Clonal analysis of Inquilinus limosus isolates from six cystic fibrosis patients and specific serum antibody response. J. Med. Microbiol. 55:1425-1433. [DOI] [PubMed] [Google Scholar]
- 276.Schülin, T., and I. Steinmetz. 2001. Chronic melioidosis in a patient with cystic fibrosis. J. Clin. Microbiol. 39:1676-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Scott, F. W., and T. L. Pitt. 2004. Identification and characterization of transmissible Pseudomonas aeruginosa strains in cystic fibrosis patients in England and Wales. J. Med. Microbiol. 53:609-615. [DOI] [PubMed] [Google Scholar]
- 278.Segonds, C., T. Heulin, N. Marty, and G. Chabanon. 1999. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J. Clin. Microbiol. 37:2201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sermet-Gaudelus, I., M. Le Bourgeois, C. Pierre-Audigier, C. Offredo, D. Guillemot, S. Halley, C. Akoua-Koffi, V. Vincent, V. Sivadon-Tardy, A. Ferroni, P. Berche, P. Scheinmann, G. Lenoir, and J. L. Gaillard. 2003. Mycobacterium abscessus and children with cystic fibrosis. Emerg. Infect. Dis. 9:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shelly, D. B., T. Spilker, E. J. Gracely, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Utility of commercial systems for identification of Burkholderia cepacia complex from cystic fibrosis sputum culture. J. Clin. Microbiol. 38:3112-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shreve, M. R., S. Butler, H. J. Kaplowitz, H. R. Rabin, D. Stokes, M. Light, and W. E. Regelmann. 1999. Impact of microbiology practice on cumulative prevalence of respiratory tract bacteria in patients with cystic fibrosis. J. Clin. Microbiol. 37:753-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sibley, C. D., M. D. Parkins, H. R. Rabin, K. Duan, J. C. Norgaard, and M. G. Surette. 2008. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 105:15070-15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Simmonds, N. J., P. Cullinan, and M. E. Hodson. 2009. Growing old with cystic fibrosis—the characteristics of long-term survivors of cystic fibrosis. Respir. Med. 103:629-635. [DOI] [PubMed] [Google Scholar]
- 284.Skov, M., C. Koch, C. M. Reimert, and L. K. Poulsen. 2000. Diagnosis of allergic bronchopulmonary aspergillosis (ABPA) in cystic fibrosis. Allergy 55:50-58. [DOI] [PubMed] [Google Scholar]
- 285.Skov, M., K. McKay, C. Koch, and P. J. Cooper. 2005. Prevalence of allergic bronchopulmonary aspergillosis in cystic fibrosis in an area with a high frequency of atopy. Respir. Med. 99:887-893. [DOI] [PubMed] [Google Scholar]
- 286.Smyth, A. R., R. L. Smyth, C. Y. Tong, C. A. Hart, and D. P. Heaf. 1995. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch. Dis. Child. 73:117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Speert, D. P., M. E. Campbell, D. A. Henry, R. Milner, F. Taha, A. Gravelle, A. G. Davidson, L. T. Wong, and E. Mahenthiralingam. 2002. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis in British Columbia, Canada. Am. J. Respir. Crit. Care Med. 166:988-993. [DOI] [PubMed] [Google Scholar]
- 288.Speert, D. P., D. Henry, P. Vandamme, M. Corey, and E. Mahenthiralingam. 2002. Epidemiology of Burkholderia cepacia complex in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 8:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Spencer, R. C. 1995. The emergence of epidemic, multiple-antibiotic-resistant Stenotrophomonas (Xanthomonas) maltophilia and Burkholderia (Pseudomonas) cepacia. J. Hosp. Infect. 30(Suppl.):453-464. [DOI] [PubMed] [Google Scholar]
- 290.Spilker, T., A. Baldwin, A. Bumford, C. G. Dowson, E. Mahenthiralingam, and J. J. LiPuma. 2009. Expanded multilocus sequence typing for Burkholderia species. J. Clin. Microbiol. 47:2607-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Spilker, T., A. A. Liwienski, and J. J. LiPuma. 2008. Identification of Bordetella spp. in respiratory specimens from individuals with cystic fibrosis. Clin. Microbiol. Infect. 14:504-506. [DOI] [PubMed] [Google Scholar]
- 292.Spilker, T., A. Z. Uluer, F. M. Marty, W. W. Yeh, J. H. Levison, P. Vandamme, and J. J. LiPuma. 2008. Recovery of Herbaspirillum species from persons with cystic fibrosis. J. Clin. Microbiol. 46:2774-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Springman, A. C., J. L. Jacobs, V. S. Somvanshi, G. W. Sundin, M. H. Mulks, T. S. Whittam, P. Viswanathan, R. L. Gray, J. J. LiPuma, and T. A. Ciche. 2009. Genetic diversity and multihost pathogenicity of clinical and environmental strains of Burkholderia cenocepacia. Appl. Environ. Microbiol. 75:5250-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Stark, J. M., M. A. Stark, G. N. Colasurdo, and A. M. LeVine. 2006. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J. Med. Virol. 78:829-838. [DOI] [PubMed] [Google Scholar]
- 295.Steinkamp, G., B. Wiedemann, E. Rietschel, A. Krahl, J. Gielen, H. Barmeier, and F. Ratjen. 2005. Prospective evaluation of emerging bacteria in cystic fibrosis. J. Cyst. Fibros. 4:41-48. [DOI] [PubMed] [Google Scholar]
- 296.Stevens, D. A., R. B. Moss, V. P. Kurup, A. P. Knutsen, P. Greenberger, M. A. Judson, D. W. Denning, R. Crameri, A. S. Brody, M. Light, M. Skov, W. Maish, and G. Mastella. 2003. Allergic bronchopulmonary aspergillosis in cystic fibrosis—state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 37(Suppl. 3):S225-S264. [DOI] [PubMed] [Google Scholar]
- 297.Stone, A., and L. Saiman. 2007. Update on the epidemiology and management of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 13:515-521. [DOI] [PubMed] [Google Scholar]
- 298.Stranden, A. M., R. Frei, H. Adler, U. Fluckiger, and A. F. Widmer. 2009. Emergence of SCCmec type IV as the most common type of methicillin-resistant Staphylococcus aureus in a university hospital. Infection 37:44-48. [DOI] [PubMed] [Google Scholar]
- 299.Stryjewski, M. E., J. J. LiPuma, R. H. Messier, Jr., L. B. Reller, and B. D. Alexander. 2003. Sepsis, multiple organ failure, and death due to Pandoraea pnomenusa infection after lung transplantation. J. Clin. Microbiol. 41:2255-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Summerbell, R. C., S. Krajden, and J. Kane. 1989. Potted plants in hospitals as reservoirs of pathogenic fungi. Mycopathologia 106:13-22. [DOI] [PubMed] [Google Scholar]
- 301.Swings, J., P. De Vos, M. Van den Mooter, and J. De Ley. 1983. Transfer of Pseudomonas maltophilia Hugh 1981 to the genus Xanthomonas as Xanthomonas maltophilia (Hugh 1981) comb. nov. Int. J. Syst. Bacteriol. 33:409-413. [Google Scholar]
- 302.Tablan, O. C., T. L. Chorba, D. V. Schidlow, J. W. White, K. A. Hardy, P. H. Gilligan, W. M. Morgan, L. A. Carson, W. J. Martone, and J. M. Jason. 1985. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J. Pediatr. 107:382-387. [DOI] [PubMed] [Google Scholar]
- 303.Tablan, O. C., W. J. Martone, C. F. Doershuk, R. C. Stern, M. J. Thomassen, J. D. Klinger, J. W. White, L. A. Carson, and W. R. Jarvis. 1987. Colonization of the respiratory tract with Pseudomonas cepacia in cystic fibrosis. Risk factors and outcomes. Chest 91:527-532. [DOI] [PubMed] [Google Scholar]
- 304.Takigawa, K., J. Fujita, K. Negayama, Y. Yamagishi, Y. Yamaji, K. Ouchi, K. Yamada, M. Abe, T. Nakazawa, and K. Kawanishi. 1993. Nosocomial outbreak of Pseudomonas cepacia respiratory infection in immunocompromised patients associated with contaminated nebulizer devices. Kansenshogaku Zasshi 67:1115-1125. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 305.Talmaciu, I., L. Varlotta, J. Mortensen, and D. V. Schidlow. 2000. Risk factors for emergence of Stenotrophomonas maltophilia in cystic fibrosis. Pediatr. Pulmonol. 30:10-15. [DOI] [PubMed] [Google Scholar]
- 306.Tan, K., S. P. Conway, K. G. Brownlee, C. Etherington, and D. G. Peckham. 2002. Alcaligenes infection in cystic fibrosis. Pediatr. Pulmonol. 34:101-104. [DOI] [PubMed] [Google Scholar]
- 307.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Thomassen, M. J., C. A. Demko, J. D. Klinger, and R. C. Stern. 1985. Pseudomonas cepacia colonization among patients with cystic fibrosis. A new opportunist. Am. Rev. Respir. Dis. 131:791-796. [DOI] [PubMed] [Google Scholar]
- 309.Thomassen, M. J., J. D. Klinger, S. J. Badger, D. W. van Heeckeren, and R. C. Stern. 1984. Cultures of thoracotomy specimens confirm usefulness of sputum cultures in cystic fibrosis. J. Pediatr. 104:352-356. [DOI] [PubMed] [Google Scholar]
- 310.Torrens, J. K., P. Dawkins, S. P. Conway, and E. Moya. 1998. Non-tuberculous mycobacteria in cystic fibrosis. Thorax 53:182-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tummler, B., U. Koopmann, D. Grothues, H. Weissbrodt, G. Steinkamp, and H. von der Hardt. 1991. Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients. J. Clin. Microbiol. 29:1265-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tunney, M. M., T. R. Field, T. F. Moriarty, S. Patrick, G. Doering, M. S. Muhlebach, M. C. Wolfgang, R. Boucher, D. F. Gilpin, A. McDowell, and J. S. Elborn. 2008. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 177:995-1001. [DOI] [PubMed] [Google Scholar]
- 313.Valdezate, S., A. Vindel, L. Maiz, F. Baquero, H. Escobar, and R. Canton. 2001. Persistence and variability of Stenotrophomonas maltophilia in cystic fibrosis patients, Madrid, 1991-1998. Emerg. Infect. Dis. 7:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Valdezate, S., A. Vindel, P. Martin-Davila, B. S. Del Saz, F. Baquero, and R. Canton. 2004. High genetic diversity among Stenotrophomonas maltophilia strains despite their originating at a single hospital. J. Clin. Microbiol. 42:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Valenza, G., D. Tappe, D. Turnwald, M. Frosch, C. Konig, H. Hebestreit, and M. Abele-Horn. 2008. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 7:123-127. [DOI] [PubMed] [Google Scholar]
- 316.Van Daele, S., R. Verhelst, G. Claeys, G. Verschraegen, H. Franckx, L. Van Simaey, C. de Ganck, F. De Baets, and M. Vaneechoutte. 2005. Shared genotypes of Achromobacter xylosoxidans strains isolated from patients at a cystic fibrosis rehabilitation center. J. Clin. Microbiol. 43:2998-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Vandamme, P., and T. Coenye. 2004. Taxonomy of the genus Cupriavidus: a tale of lost and found. Int. J. Syst. Evol. Microbiol. 54:2285-2289. [DOI] [PubMed] [Google Scholar]
- 318.Vandamme, P., J. Goris, T. Coenye, B. Hoste, D. Janssens, K. Kersters, P. De Vos, and E. Falsen. 1999. Assignment of Centers for Disease Control group IVc-2 to the genus Ralstonia as Ralstonia paucula sp. nov. Int. J. Syst. Bacteriol. 49:663-669. [DOI] [PubMed] [Google Scholar]
- 319.Vandamme, P., D. Henry, T. Coenye, S. Nzula, M. Vancanneyt, J. J. LiPuma, D. P. Speert, J. R. Govan, and E. Mahenthiralingam. 2002. Burkholderia anthina sp. nov. and Burkholderia pyrrocinia, two additional Burkholderia cepacia complex bacteria, may confound results of new molecular diagnostic tools. FEMS Immunol. Med. Microbiol. 33:143-149. [DOI] [PubMed] [Google Scholar]
- 320.Vandamme, P., M. Heyndrickx, M. Vancanneyt, B. Hoste, P. DeVos, E. Falsen, K. Kersters, and K. H. Hinz. 1996. Bordetella trematum sp. nov., isolated from wounds and ear infections in humans, and reassessment of Alcaligenes denitrificans Ruger and Tan 1983. Int. J. Syst. Bacteriol. 46:849-858. [DOI] [PubMed] [Google Scholar]
- 321.Vandamme, P., B. Holmes, T. Coenye, J. Goris, E. Mahenthiralingam, J. J. LiPuma, and J. R. Govan. 2003. Burkholderia cenocepacia sp. nov.—a new twist to an old story. Res. Microbiol. 154:91-96. [DOI] [PubMed] [Google Scholar]
- 322.Vandamme, P., B. Holmes, M. Vancanneyt, T. Coenye, B. Hoste, R. Coopman, H. Revets, S. Lauwers, M. Gillis, K. Kersters, and J. R. Govan. 1997. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int. J. Syst. Bacteriol. 47:1188-1200. [DOI] [PubMed] [Google Scholar]
- 323.Vandamme, P., E. Mahenthiralingam, B. Holmes, T. Coenye, B. Hoste, P. DeVos, D. Henry, and D. P. Speert. 2000. Identification and population structure of Burkholderia stabilis sp. nov. (formerly Burkholderia cepacia genomovar IV). J. Clin. Microbiol. 38:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vaneechoutte, M., T. De Baere, G. Wauters, S. Steyaert, G. Claeys, D. Vogelaers, and G. Verschraegen. 2001. One case each of recurrent meningitis and hemoperitoneum infection with Ralstonia mannitolilytica. J. Clin. Microbiol. 39:4588-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Vaneechoutte, M., P. Kampfer, T. De Baere, E. Falsen, and G. Verschraegen. 2004. Wautersia gen. nov., a novel genus accommodating the phylogenetic lineage including Ralstonia eutropha and related species, and proposal of Ralstonia [Pseudomonas] syzygii (Roberts et al. 1990) comb. nov. Int. J. Syst. Evol. Microbiol. 54:317-327. [DOI] [PubMed] [Google Scholar]
- 327.van Ewijk, B. E., M. M. van der Zalm, T. F. W. Wolfs, A. Fleer, J. L. L. Kimpen, B. Wilbrink, and C. K. van der Ent. 2008. Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics 122:1171-1176. [DOI] [PubMed] [Google Scholar]
- 328.van Ewijk, B. E., M. M. van der Zalm, T. F. W. Wolfs, and C. K. van der Ent. 2005. Viral respiratory infections in cystic fibrosis. J. Cyst. Fibros. 4:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.van Ewijk, B. E., T. F. Wolfs, A. Fleer, J. L. Kimpen, and C. K. van der Ent. 2006. High Pseudomonas aeruginosa acquisition rate in CF. Thorax 61:641-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.van Ewijk, B. E., T. F. W. Wolfs, P. C. Aerts, K. P. M. Van Kessel, A. Fleer, J. L. L. Kimpen, and C. K. Van Der Ent. 2007. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr. Res. 61:398. [DOI] [PubMed] [Google Scholar]
- 331.Vanhee, L. M., F. Symoens, J. P. Bouchara, H. J. Nelis, and T. Coenye. 2008. High-resolution genotyping of Aspergillus fumigatus isolates recovered from chronically colonised patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 27:1005-1007. [DOI] [PubMed] [Google Scholar]
- 332.Vanlaere, E., A. Baldwin, D. Gevers, D. Henry, E. De Brandt, J. J. LiPuma, E. Mahenthiralingam, D. P. Speert, C. Dowson, and P. Vandamme. 2009. Taxon K, a complex within the Burkholderia cepacia complex, comprises at least two novel species, Burkholderia contaminans sp. nov. and Burkholderia lata sp. nov. Int. J. Syst. Evol. Microbiol. 59:102-111. [DOI] [PubMed] [Google Scholar]
- 333.Vanlaere, E., J. J. LiPuma, A. Baldwin, D. Henry, E. De Brandt, E. Mahenthiralingam, D. Speert, C. Dowson, and P. Vandamme. 2008. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int. J. Syst. Evol. Microbiol. 58:1580-1590. [DOI] [PubMed] [Google Scholar]
- 334.van Pelt, C., C. M. Verduin, W. H. Goessens, M. C. Vos, B. Tummler, C. Segonds, F. Reubsaet, H. Verbrugh, and A. van Belkum. 1999. Identification of Burkholderia spp. in the clinical microbiology laboratory: comparison of conventional and molecular methods. J. Clin. Microbiol. 37:2158-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Vermis, K., M. Brachkova, P. Vandamme, and H. Nelis. 2003. Isolation of Burkholderia cepacia complex genomovars from waters. Syst. Appl. Microbiol. 26:595-600. [DOI] [PubMed] [Google Scholar]
- 336.Villarino, M. E., L. E. Stevens, B. Schable, G. Mayers, J. M. Miller, J. P. Burke, and W. R. Jarvis. 1992. Risk factors for epidemic Xanthomonas maltophilia infection/colonization in intensive care unit patients. Infect. Control Hosp. Epidemiol. 13:201-206. [DOI] [PubMed] [Google Scholar]
- 337.Visca, P., G. Cazzola, A. Petrucca, and C. Braggion. 2001. Travel-associated Burkholderia pseudomallei infection (melioidosis) in a patient with cystic fibrosis: a case report. Clin. Infect. Dis. 32:E15-E16. [DOI] [PubMed] [Google Scholar]
- 338.Vu-Thien, H., J. C. Darbord, D. Moissenet, C. Dulot, J. B. Dufourcq, P. Marsol, and A. Garbarg-Chenon. 1998. Investigation of an outbreak of wound infections due to Alcaligenes xylosoxidans transmitted by chlorhexidine in a burns unit. Eur. J. Clin. Microbiol. Infect. Dis. 17:724-726. [DOI] [PubMed] [Google Scholar]
- 339.Vu-Thien, H., D. Moissenet, M. Valcin, C. Dulot, G. Tournier, and A. Garbarg-Chenon. 1996. Molecular epidemiology of Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans in a cystic fibrosis center. Eur. J. Clin. Microbiol. Infect. Dis. 15:876-879. [DOI] [PubMed] [Google Scholar]
- 340.Wang, E., C. Prober, B. Manson, M. Corey, and H. Levison. 1984. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N. Engl. J. Med. 311:1653-1658. [DOI] [PubMed] [Google Scholar]
- 341.Wat, D., C. Gelder, S. Hibbitts, F. Cafferty, I. Bowler, M. Pierrepoint, R. Evans, and I. Doull. 2008. The role of respiratory viruses in cystic fibrosis. J. Cyst. Fibros. 7:320-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wauters, G., G. Claeys, G. Verschraegen, T. De Baere, E. Vandecruys, L. Van Simaey, C. De Ganck, and M. Vaneechoutte. 2001. Case of catheter sepsis with Ralstonia gilardii in a child with acute lymphoblastic leukemia. J. Clin. Microbiol. 39:4583-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Weber, D. J., W. A. Rutala, C. N. Blanchet, M. Jordan, and M. F. Gergen. 1999. Faucet aerators: a source of patient colonization with Stenotrophomonas maltophilia. Am. J. Infect. Control 27:59-63. [DOI] [PubMed] [Google Scholar]
- 344.Weber, D. J., M. B. Wilson, W. A. Rutala, and C. A. Thomann. 1990. Manual ventilation bags as a source for bacterial colonization of intubated patients. Am. Rev. Respir. Dis. 142:892-894. [DOI] [PubMed] [Google Scholar]
- 345.Weems, J. J., Jr. 1993. Nosocomial outbreak of Pseudomonas cepacia associated with contamination of reusable electronic ventilator temperature probes. Infect. Control Hosp. Epidemiol. 14:583-586. [DOI] [PubMed] [Google Scholar]
- 346.Weitkamp, J. H., Y. W. Tang, D. W. Haas, N. K. Midha, and J. E. Crowe, Jr. 2000. Recurrent Achromobacter xylosoxidans bacteremia associated with persistent lymph node infection in a patient with hyper-immunoglobulin M syndrome. Clin. Infect. Dis. 31:1183-1187. [DOI] [PubMed] [Google Scholar]
- 347.Wellinghausen, N., A. Essig, and O. Sommerburg. 2005. Inquilinus limosus in patients with cystic fibrosis, Germany. Emerg. Infect. Dis. 11:457-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Whitby, P. W., K. B. Carter, J. L. Burns, J. A. Royall, J. J. LiPuma, and T. L. Stull. 2000. Identification and detection of Stenotrophomonas maltophilia by rRNA-directed PCR. J. Clin. Microbiol. 38:4305-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Whitby, P. W., L. C. Pope, K. B. Carter, J. J. LiPuma, and T. L. Stull. 2000. Species specific PCR as a tool for the identification of Burkholderia gladioli. J. Clin. Microbiol. 38:282-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Whiteford, M. L., J. D. Wilkinson, J. H. McColl, F. M. Conlon, J. R. Michie, T. J. Evans, and J. Y. Paton. 1995. Outcome of Burkholderia (Pseudomonas) cepacia colonization in children with cystic fibrosis following a hospital outbreak. Thorax 50:1194-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Williamson, E. C., D. Speers, I. H. Arthur, G. Harnett, G. Ryan, and T. J. Inglis. 2001. Molecular epidemiology of Scedosporium apiospermum infection determined by PCR amplification of ribosomal intergenic spacer sequences in patients with chronic lung disease. J. Clin. Microbiol. 39:47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wolz, C., G. Kiosz, J. W. Ogle, M. L. Vasil, U. Schaad, K. Botzenhart, and G. Doring. 1989. Pseudomonas aeruginosa cross-colonization and persistence in patients with cystic fibrosis. Use of a DNA probe. Epidemiol. Infect. 102:205-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Worlitzsch, D., C. Rintelen, K. Bohm, B. Wollschlager, N. Merkel, M. Borneff-Lipp, and G. Doring. 2009. Antibiotic-resistant obligate anaerobes during exacerbations of cystic fibrosis patients. Clin. Microbiol. Infect. 15:454-460. [DOI] [PubMed] [Google Scholar]
- 354.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Yabuuchi, E., Y. Kawamura, T. Ezaki, M. Ikedo, S. Dejsirilert, N. Fujiwara, T. Naka, and K. Kobayashi. 2000. Burkholderia uboniae sp. nov., L-arabinose-assimilating but different from Burkholderia thailandensis and Burkholderia vietnamiensis. Microbiol. Immunol. 44:307-317. [DOI] [PubMed] [Google Scholar]
- 356.Yabuuchi, E., Y. Kawamura, Y. Kosako, and T. Ezaki. 1998. Emendation of genus Achromobacter and Achromobacter xylosoxidans (Yabuuchi and Yano) and proposal of Achromobacter ruhlandii (Packer and Vishniac) comb. nov., Achromobacter piechaudii (Kiredjian et al.) comb. nov., and Achromobacter xylosoxidans subsp. denitrificans (Ruger and Tan) comb. nov. Microbiol. Immunol. 42:429-438. [DOI] [PubMed] [Google Scholar]
- 357.Yabuuchi, E., Y. Kosako, H. Oyaizu, I. Yano, H. Hotta, Y. Hashimoto, T. Ezaki, and M. Arakawa. 1992. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 36:1251-1275. [DOI] [PubMed] [Google Scholar]
- 358.Yabuuchi, E., Y. Kosako, I. Yano, H. Hotta, and Y. Nishiuchi. 1995. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol. Immunol. 39:897-904. [DOI] [PubMed] [Google Scholar]
- 359.Yang, J. H., T. Spilker, and J. J. LiPuma. 2006. Simultaneous coinfection by multiple strains during Burkholderia cepacia complex infection in cystic fibrosis. Diagn. Microbiol. Infect. Dis. 54:95-98. [DOI] [PubMed] [Google Scholar]
- 360.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593-603. [DOI] [PubMed] [Google Scholar]
- 361.Zhou, J., E. Garber, M. Desai, and L. Saiman. 2006. Compliance of clinical microbiology laboratories in the United States with current recommendations for processing respiratory tract specimens from patients with cystic fibrosis. J. Clin. Microbiol. 44:1547-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]