Abstract
Acquired antibody immunity to Streptococcus pneumoniae (pneumococcus) has been linked to serotype (ST)-specific opsonic antibodies to the relevant pneumococcal capsular polysaccharide (PPS) that mediate protection by enhancing the bactericidal effect of host phagocytes. Despite the well-recognized role of opsonic IgG in host defense against pneumococcus, PPS-specific monoclonal antibodies (MAbs) that mediate protection against lethal challenge with ST3 pneumococcus in mice but do not promote phagocytic killing in vitro (nonopsonic antibodies) have been described. In this study, we sought to determine the biological activity of one such MAb, A7 (a human PPS3-specific IgM), and the mechanism by which it mediates protection. In vitro studies demonstrated that coincubation of A7 with ST3 in the absence of phagocytes or a complement source resulted in a reduction in CFU on blood agar plates that was largely reversible by sonication. A chromogenic cellular proliferation assay demonstrated that A7 did not affect replication of ST3 in liquid culture. The ability of A7 to induce aggregation of ST3 was confirmed by fluorescence microscopy and flow cytometry: A7 induced aggregation of ST3, and in the presence of a complement source, A7 promoted deposition of complement component 3 (C3) on aggregated bacteria in a dose-dependent fashion. Similarly, administration of preincubated mixtures of A7 and ST3 intraperitoneally to mice protected them from the lethality of ST3 in a dose-dependent fashion. These findings suggest that A7-mediated aggregation enhances resistance to ST3, most likely by enhancing C3 deposition on the ST3 capsule, thereby promoting host antipneumococcal activity in vivo.
Despite the availability of antibiotics and two vaccines for Streptococcus pneumoniae (pneumococcus), mortality attributable to pneumococcal disease remains a significant global problem (1, 23). Concerns about ongoing pneumococcal antibiotic resistance, poor vaccine immunogenicity in immunocompromised patients, and serotype (ST) replacement stemming from use of the conjugate vaccine (23, 25, 29, 38) underscore the critical need for a better understanding of correlates of pneumococcal immunity. The efficacy of pneumococcal capsular polysaccharide (PPS)-based vaccines has been linked to their ability to elicit serotype-specific IgG that promotes phagocytosis and killing of the homologous pneumococcal ST in vitro (13, 27, 43, 46). However, given that ST3 has emerged as a replacement ST that causes severe disease (23, 25), it is concerning that an investigational conjugate vaccine containing an ST3 moiety did not protect against ST3 disease in children (37). The foregoing, together with evidence that ST3 is associated with a higher risk of severe disease and death than other serotypes (3, 23, 35), suggests the need for new strategies to prevent disease with ST3.
The importance of opsonic, ST-specific antibody in protection against pneumococcal disease is incontrovertible (42). Nonetheless, PPS-specific monoclonal antibodies (MAbs) that protect against lethal pneumococcal disease in mice but do not promote phagocyte killing in vitro have been identified (9, 14, 50). For one such MAb, the human IgM A7, protection against lethal systemic challenge requires an intact complement pathway (10) and is associated with a decrease in blood and tissue CFU and a reduction in the inflammatory response (14). Complement is required for natural resistance to experimental pneumococcal disease (7, 28) and in some mouse models of antibody-mediated protection against lethal pneumococcal infection (5, 10, 45, 53), but not others (50). However, complement was required for IgM-mediated protection against experimental pneumococcal infection (10, 53). In this report, we investigated the mechanism of efficacy of A7 against lethal systemic infection by probing its interactions with and its ability to aggregate ST3 and to promote complement deposition on the ST3 capsule.
MATERIALS AND METHODS
Bacteria.
S. pneumoniae ST3 strain 6303 and ST6B (designated DS-2189-94) (American Type Culture Collection, Manassas, VA) were grown in tryptic soy broth (TSB; Difco Laboratories, Sparks, MD) to mid-log phase in 5% CO2 at 37°C, frozen in TSB in 10% glycerol, and stored at −80°C until they were used as described previously (10, 14). ATCC 6303 is a mucoid strain of ST3 that has been used extensively in mouse models of pneumococcal infection (14, 16, 31, 48). Prior to use, pneumococci were rapidly thawed, placed on ice, and diluted in TSB to the desired concentration. To confirm the desired concentration, diluted pneumococci were plated onto a Trypticase agar plate containing 5% sheep's blood (Becton Dickinson, Franklin Lakes, NJ), incubated overnight at 5% CO2 and 37°C, and counted the following day.
Antibodies.
A7 [IgM(κ)] is a human MAb derived from XenoMouse mice that was previously shown to bind to PPS3 and to protect mice from death after intraperitoneal (i.p.) challenge (10, 14). A7 was purified by affinity chromatography using anti-human IgM-coated beads (Sigma-Aldrich, St. Louis, MO). 54B11 and 57E2 (IgM), which are human PPS8-specific IgM MAbs, and G19 (IgM), which is a human IgM MAb to Cryptococcus neoformans glucuronoxylomannan (GXM) (17) derived from XenoMouse mice, were used as controls. A human myeloma IgM (Calbiochem, San Diego, CA) was used as an additional negative control.
Mice.
Male wild-type C57BL/6 mice (6 to 8 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were maintained by the Institute for Animal Studies at the Albert Einstein College of Medicine, Bronx, NY, in accordance with the rules and regulations of animal welfare at the Albert Einstein College of Medicine.
Quellung reaction.
Ten micrograms of A7 was incubated at 37°C with 5% CO2 for 5 min in 50 μl PBS with one colony from an overnight culture grown on Trypticase soy agar with 5% sheep's blood (Becton Dickinson, Franklin Lakes, NJ). After incubation, 3 μl of the mixture was placed on a glass microscope slide with 1.5 μl India ink (Becton Dickinson, Franklin Lakes, NJ) and viewed with an Axio Imager microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
In vitro aggregation assay.
To test for aggregation in vitro, ST3 was incubated with A7 as described for opsonophagocytosis assays (9, 14, 50), as follows. Ten microliters of ST3 (∼2,000 CFU) was incubated with 40 μl containing 10 μg, 5 μg, or 1 μg of A7 or an isotype control IgM for 15 min at room temperature and then for 45 min at 37°C with shaking. All dilutions and incubations were performed in Veronal-buffered saline (VBS; Fisher Scientific, Pittsburgh, PA). This was followed by sonication in a mini Ultrasonik water bath sonicator (Neytech, Bloomfield, CT) for 2 min. Sonication did not cause death of the bacteria (Fig. 1). Bacterial cells were enumerated by plating on Trypticase agar plates containing 5% sheep's blood (Becton Dickinson, Franklin Lakes, NJ). Colonies were counted on the following day.
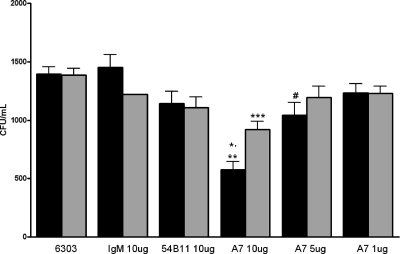
FIG. 1.
A7 induces reversible reduction in CFU of ST3 on solid medium. The black bars represent CFU of ST3 (ATCC 6303) without sonication; gray bars represent CFU after 2 min of sonication. Each bar represents the mean for the designated group; the error bars show the standard errors of the means. *, P < 0.0001, comparing ST3 (no sonication) to 10 μg A7 (no sonication) (t test); **, P < 0.0022, comparing 10 μg A7 (no sonication) to 10 μg A7 (sonication) (t test); ***, P < 0.0001, comparing ST3 (sonication) to 10 μg A7 (sonication) (t test); #, P = 0.0223, comparing ST3 (no sonication) to 5 μg A7 (no sonication) (Mann-Whitney U test). The data shown are combined from four separate experiments performed in duplicate, except for data obtained with 5 μg A7, for which experiments were performed two times.
Aggregation was also determined by microscopy as described previously (15), with the following modifications. A total of ∼108 CFU of ST3 was first labeled with Oregon Green (see “Flow cytometry”), after which the bacteria were centrifuged at 8,000 × g for 20 min and washed with VBS three times. Next, 10 μl of ST3 was incubated with 40 μl of A7, an isotype control MAb, or PBS for 15 min at room temperature, after which the final volume was brought up to 1 ml in VBS. The mixtures were then added to slides previously coated with poly-l-lysine (Sigma-Aldrich, St. Louis, MO) for 1 h, after which the slides were washed three times with VBS. All fluorescent images were taken on an Axio Imager microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Chromogenic killing assay.
To determine if bacteria are directly killed by A7 in vitro, we used a commercially available cell proliferation kit (cell proliferation kit II [XTT]; Roche Diagnostics GmbH, Mannheim, Germany). This kit incorporates XTT {3,3′-[1(30)-3,4-tetrazolium]-bis[4-methoxy-6-nitro] benzene sulfonic acid hydrate}, a yellow tetrazolium salt which is converted to an orange formazan dye only upon metabolism by living cells. The experiment was carried out according to the manufacturer's directions and as modified by Lin et al. (34), as follows. Twenty microliters of ST3 (∼106 CFU) was incubated with 80 μl of diluted antibody for 15 min at room temperature and then for 45 min at 37°C with shaking. All dilutions and incubations were performed in VBS. Samples were then added to a 96-well polystyrene microplate. Fifty microliters of a mixture of XTT and the electron coupling reagent provided in the kit was added to the samples. The absorbance was read at 450 nm.
Flow cytometry.
Flow cytometry was used to determine if A7 induced aggregation of ST3, as follows. A total of ∼108 CFU of ST3 was labeled with Oregon Green 488-carboxylic acid diacetate, succinimidyl ester (carboxy-DFFDA, SE) mixed isomers (Invitrogen, Carlsbad, CA) (600:1) overnight at 4°C. The following day, live labeled ST3 was centrifuged at 8,000 × g for 20 min and washed three times with VBS. Ten microliters of ST3 and 40 μl of A7 (10 μg or 1 μg) or an isotype control IgM were coincubated for 15 min at room temperature, after which the final volume was brought up to 1 ml in VBS. This mixture was then analyzed in a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Bacterial cells positive for Oregon Green labeling were gated and analyzed by forward scattering.
Complement deposition.
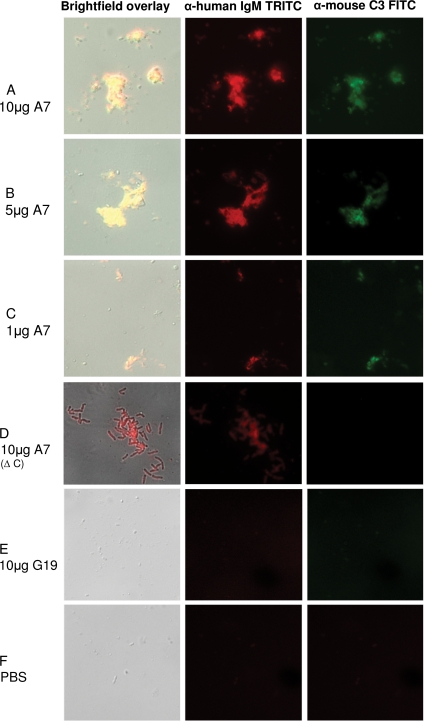
The ability of A7 to mediate complement deposition on the ST3 capsule was determined by fluorescence microscopy, as follows. A total of 10 μg/0.04 ml A7, isotype control MAb 54B11 (ST8-specific IgM MAb) or G19 (GXM-specific IgM MAb), or PBS and 10 μl of fresh mouse serum were incubated with 106 CFU ST3/0.01 ml for 45 min at room temperature in VBS. After incubation, the mixture was diluted to 1 ml in VBS and immunostained with tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-human IgM (Southern Biotech, Birmingham, AL) and 1 μl fluorescein (FITC)-conjugated goat F(ab′)2 fragment to mouse complement C3 (Molecular Bioproducts, San Diego, CA) for 30 min on ice. After centrifugation, the mixture was washed three times, suspended in VBS, transferred to poly-l-lysine-coated slides, washed, and Gram stained (Sigma Diagnostic Accustain Gram stain). Slides were visualized by bright-field Nomarski DIC and fluorescence microscopy with an Axioskop 2 Plus microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Intraperitoneal infection model and mouse protection studies.
To determine whether aggregation of ST3 affects the lethality of ST3 in mice, 20 μl of ST3 (∼2,000 CFU) was incubated with 80 μl of A7 or an isotype control MAb (myeloma), containing 10 μg, 5 μg, or 1 μg of the MAb, for 15 min at room temperature, after which the mixture was injected immediately into mice i.p. Survival was monitored for 7 days.
Statistical analysis.
The bacterial counts (CFU) enumerated in in vitro aggregation assays were compared for different MAbs with an unpaired t or Mann-Whitney test if normality was not passed. Mouse survival data were analyzed with the Kaplan-Meier log rank survival test. All statistical analyses were performed using Prism (v.4.02 for Windows; GraphPad Software, San Diego, CA). A P value of <0.05 was used for statistical significance.
RESULTS
In vitro aggregation.
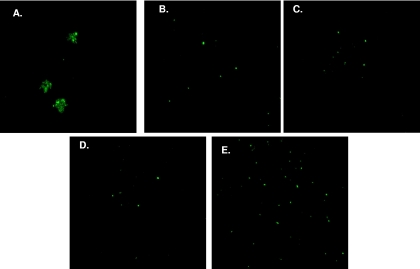
Incubation of 10 μg A7 with ST3 resulted in a significant decrease in CFU compared to that with ST3 alone, 10 μg isotype control 54B11, or 10 μg human myeloma IgM, and incubation of 5 μg A7 with ST3 resulted in a significant decrease in CFU compared to that with ST3 alone (Fig. 1). In comparison to 10 or 5 μg A7, incubation of 1 μg A7 with ST3 did not result in a decrease in CFU. There was a larger number of ST3 CFU for sonicated 10-μg A7-ST3 mixtures than for nonsonicated mixtures. No significant differences in CFU were observed in other groups after sonication. To visualize the interaction of A7 with ST3, Oregon Green-labeled ST3 was incubated with A7 or an isotype control IgM and examined by fluorescence microscopy. Ten micrograms of A7 aggregated ST3 (Fig. 2A) and induced a quellung reaction (not shown). However, PBS, 10 or 1 μg isotype control IgM, or 1 μg A7 did not result in aggregation (Fig. 2B to E) or induce a quellung reaction (not shown).
FIG. 2.
A7 induces aggregation of ST3. Oregon Green-labeled ST3 (ATCC 6303) was imaged by fluorescence microscopy. Total magnification, ×400 (40× objective lens × 10× ocular lens). Microscopic images represent 10 μg A7 (A), ST3 alone (B), 10 μg (human myeloma) IgM (C), 1 μg human IgM (D), and 1 μg A7 (E).
Chromogenic cell proliferation.
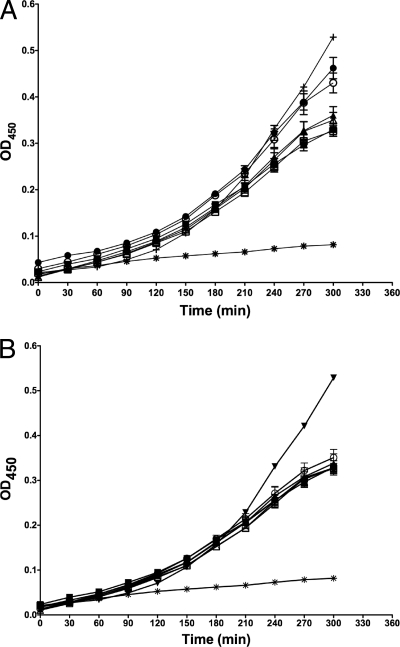
To determine whether ST3 can grow in the presence of A7, we performed a chromogenic assay that measures microbial metabolism as a function of conversion of the tetrazolium salt XTT. The conversion of XTT to a formazan dye is detected in a microplate reader. The optical density increased over time in the presence of 10 μg of A7, indicating that A7 did not affect metabolism (Fig. 3A). There was a similar pattern when 1 μg of A7 was coincubated with ST3 (Fig. 3B). A negative control, heat-killed ST3, did not result in a significant increase in optical density over time. As a positive control, serotype 6B was used (Fig. 3A and B); this serotype was shown previously to validate the XTT assay for ST6. Incubation of XTT with A7 alone did not result in an increase in optical density, suggesting that the observed increases were due to ST3 alone (data not shown).
FIG. 3.
A7 has no effect on growth of ST3 in liquid culture. The figures depict the optical density at 450 nm (OD450) (y axis) versus the time of assay development (x axis). The wells contained an initial inoculum of ∼106 CFU of ST3 (ATCC 6303). Optical densities were determined at the indicated times after addition of XTT, with or without sonication and with or without 10 μg (A) or 1 μg (B) of A7. Closed symbols represent OD450 values obtained without sonication; open symbols represent OD450 values obtained after 2 min of sonication. Symbols: ▪, ST3 alone; □, ST3 alone; •, A7 + ST3; ○, A7 + ST3; ▴, 57E2 (human IgM to PPS8) + ST3; ▵, 57E2 + ST3; *, heat-killed ST3 alone; +, ST6B alone. Data represent OD450 values from four separate experiments performed in duplicate.
Flow cytometry.
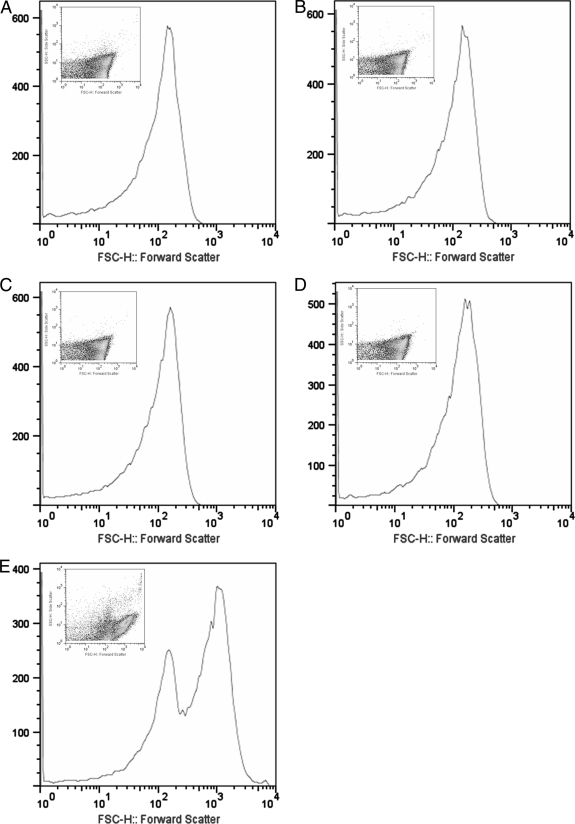
To determine if A7 formed complexes with ST3, we examined mixtures of Oregon Green-labeled ST3 after coincubation with A7 or an isotype control IgM (57E2) by flow cytometry. Forward scattering analysis revealed one distinct peak when ST3 was incubated with PBS, an isotype control IgM, or 1 μg of A7 (Fig. 4A, B, C, and D). However, when ST3 was incubated with 10 μg A7, a second, larger peak appeared that was related to the presence of ST3 aggregates, and a decrease in the size of the first peak was observed with the appearance of the second, larger peak (Fig. 4E).
FIG. 4.
A7 mediates aggregation of ST3. Flow cytometric analysis was performed on mixtures of MAbs and Oregon Green-labeled ST3 (ATCC 6303). Cell size and forward scatter were determined for ST3 alone (A), 10 μg 57E2 (human MAb to PPS8) (B), 1 μg 57E2 (C), 1 μg A7 (D), and 10 μg A7 (E). Insets depict scatter plot analyses of cellular size.
Complement deposition on ST3.
Previous work demonstrated that A7 did not promote opsonophagocytosis in the presence of complement (14). In the study reported herein, we determined the ability of A7 to promote C3 deposition on ST3 organisms. The sera were tested for antibody reactivity with PPS3 prior to use and were found to have undetectable reactivity. Incubation of ST3 with 10, 5, or 1 μg A7 and mouse sera resulted in ST3 aggregation and C3 deposition on ST3, exhibiting a dose dependence of aggregate size and the degree of C3 deposition (Fig. 5A to C). There was aggregation and A7 deposition on ST3, but no C3 deposition, when the complement source was heat inactivated (Fig. 5D), and there was no aggregation or C3 deposition on ST3 when the bacteria were incubated with a control MAb or PBS (Fig. 5E and F). Similar results were obtained with a human complement source (data not shown).
FIG. 5.
A7 mediates aggregation and complement deposition on ST3. Binding of IgM (red, middle) and C3 (green, right) and merged DIC bright-field images (overlay, left) are shown for the designated mixtures of ST3 (ATCC 6303), A7, and mouse serum (used as a complement source). Δ C, heat-inactivated complement source; G19, human IgM MAb to GXM. Magnification, ×100 (all images). The appearance of the A7-ST3 aggregate in panel D differs from that in panels A to C due to focusing on the aggregate, rather than the bacteria, in the bright-field image.
Mouse protection.
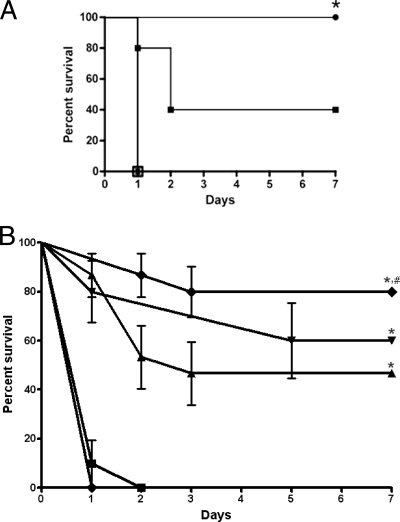
To determine the influence of aggregates of A7 and ST3 on the lethality of ST3 in vivo, mice were injected i.p. with ST3 that had been preincubated with either PBS, an isotype control IgM, or different amounts of A7. Mice injected with mixtures of ST3 and 10 or 1 μg A7 survived significantly longer than did mice injected with PBS or mixtures with an isotype control MAb (P = 0.001 for 10 μg A7 and 0.14 for 1 μg A7) (Fig. 6A). In a dose-response experiment, mice injected with mixtures of ST3 and 10, 5, or 1 μg A7 survived significantly longer than did mice injected with mixtures of ST3 and either PBS or 0.1 μg A7 (P = 0.0001) (Fig. 6B). The survival of mice that received ST3 with 10 μg A7 was significantly longer than that of mice that received ST3 with 1 μg A7 (P = 0.048).
FIG. 6.
A7-ST3 mixtures enhance mouse survival after i.p. infection with ST3. The percent survival was determined for mice that received mixtures of ST3 (ATCC 6303) preincubated with PBS, control IgM MAbs, 10 μg A7, or 1 μg A7 (A) or with different amounts of A7 (B) prior to i.p. administration. (A) *, P = 0.003 for comparison of 10 μg A7 to controls, P = 0.14 for comparison of 1 μg A7 to PBS, and P = 0.051 for comparison of 10 to 1 μg A7. Symbols: □, PBS; ▴, 10 μg IgM (myeloma); ▾, 1 μg IgM (myeloma); •, 10 μg A7; ▪, 1 μg A7. Note that PBS, 10 μg IgM, and 1 μg IgM produced overlapping curves. (B) *, P < 0.0001 for comparison of 10, 5, and 1 μg A7 to PBS and 0.1 μg A7; #, P = 0.048 for comparison of 10 to 1 μg A7. Symbols: •, PBS; ⧫, 10 μg A7; ▾, 5 μg IgM; ▴, 1 μg A7; •, 10 μg A7; ▪, 0.1 μg A7. Survival was analyzed by the Kaplan-Meier log rank test (n = 5 [A] and 10 to 15 [B] mice per group).
DISCUSSION
The experiments reported herein were undertaken to determine how A7, a PPS3-specific human IgM MAb that does not promote phagocyte-mediated killing (opsonophagocytosis) in vitro (14), affects the growth and state of ST3. When this study began, it had already been established that A7 does not require CD4 or CD8 T cells, B cells, or naturally occurring IgM to mediate protection against lethal i.p. challenge with ST3 in mice (14). The dispensability of cellular subsets of acquired immunity, which are required for antibody immunity to another encapsulated pathogen (41, 52), and natural IgM, which is required for protection of naïve mice against pneumococcus and other pathogens (2, 7), led us to consider other mechanisms by which A7 could mediate protection.
The starting point of this study was that A7 does not promote phagocyte-mediated opsonophagocytosis (14), as described for PPS-specific IgG (44). Nonetheless, in working with this MAb, we have consistently noted that compared to PBS and isotype control MAbs, the addition of ≥10 μg A7 to ST3 resulted in a smaller number of ST3 CFU on solid medium, resembling the reduction in CFU that is observed with bacterial killing (14). Hence, we set out to establish whether or not this phenomenon reflected direct antibody-mediated bacterial killing. We considered it unlikely that this was the case, because it is generally held that the Gram-positive bacterial cell wall is not damaged by antibody. However, we note that inhibition of in vitro growth of Gram-positive bacteria, including pneumococcus, has been described for an anti-idiotypic MAb (11, 12), with the caveat that the MAb functioned like an antimicrobial peptide. Nonetheless, given that a fungal polysaccharide-specific MAb was shown to inhibit fungal growth (39, 51) and that fungi are also thought to be resistant to direct antibody action, we decided to delve deeper into the effect of A7 on pneumococcal growth.
We used different methods to determine the effect of A7 on bacterial growth and metabolism. First, we determined the effect of sonication on the number of CFU obtained from mixtures of A7 and ST3. After sonication, the number of CFU obtained for A7 (10 μg)-ST3 mixtures was significantly larger than that obtained with nonsonicated mixtures. However, the number of CFU obtained for A7 (10 μg)-ST3 mixtures after sonication was smaller than that observed for controls. This might have reflected reaggregation, which we noted at 30 min postsonication. However, this seems unlikely, because the sonicated mixtures were plated immediately postsonication. For mixtures containing 5 μg A7, the number of CFU prior to sonication was smaller than that of ST3 alone, but postsonication CFUs were similar to those under control conditions. For mixtures containing 1 μg A7, there was no reduction in CFU compared to controls. Hence, the reduction in CFU observed for A7-ST3 mixtures is a dose-dependent manifestation of A7-induced aggregation, with the caveat that an effect on bacterial growth cannot be ruled out for mixtures containing 10 μg A7. In this regard, we note reports of the phenomenon of pneumococcal fratricide (19, 24) and wonder whether there are parallels between bacterial clumping and MAb-mediated aggregation. To establish that A7 does not affect the growth of ST3 in liquid culture, we performed a chromogenic cell proliferation assay. This method was previously validated as comparable to CFU-based assays for assessment of pneumococcal growth (34). A7 did not inhibit ST3 growth in this assay. Hence, our data demonstrate that A7 does not inhibit growth of ST3 in liquid culture or on solid medium.
Given that the A7-induced reduction in ST3 CFU on solid medium was largely reversible and that A7 did not inhibit ST3 growth in liquid culture, we hypothesized that A7-induced reductions in CFU on solid medium were due to bacterial aggregation. By flow cytometry, we demonstrated that the addition of 10 μg A7 to ST3 resulted in the appearance of a second peak that was not observed with ST3 alone, an isotype control IgM MAb, or 1 μg of A7. As demonstrated by another group for an IgA to ST2 (15), this peak most likely represented A7-ST3 aggregates. Fluorescence microscopy provided additional evidence that A7 induces the formation of ST3 aggregates and that this phenomenon is a function of antibody specificity and amount. The degree of aggregation was dose dependent, with 10 μg A7 inducing more and larger aggregates than 5 μg A7 and 5 μg A7 inducing more and larger aggregates than 1 μg A7. Previous work demonstrated that 10 μg of A7 was more protective in mice than 1 μg, but both doses were protective (10). The data presented herein demonstrate that A7-ST3 aggregates abrogate the lethality of ST3 in mice in a dose-dependent fashion. Although 1 μg A7 did not induce a CFU reduction upon plating, 1 μg A7 did form aggregates and promoted C3 deposition in vitro, providing a plausible explanation for its efficacy in vivo. At present, we do not know whether or how A7-induced ST3 aggregation is recapitulated in vivo or the precise mechanism by which A7 mediates protection in vivo. Nonetheless, in light of our data showing that A7 binding induces capsular swelling (quellung reaction), capsular rearrangement could affect virulence factor activity and/or ST3 interaction with host receptors, as described for MAbs to another encapsulated pathogen (36, 49). Although we did not examine bacteremia in this study, there are ample data showing that mice die of bacteremia/sepsis in the i.p. model that was used in this study (8, 14). A previous study established that A7 prevented ST3 bacteremia and sepsis and was associated with lower levels of inflammatory cytokines (14). The findings reported herein demonstrate an association between A7-mediated aggregation in vitro and protection in vivo and suggest that in vitro aggregation could be a surrogate for antibody efficacy in vivo for certain nonopsonic MAbs.
PPS-specific antibody-induced aggregation has been examined previously; however, its relationship to protection in vivo has not been investigated. Reed et al. demonstrated that PPS3-specific rabbit IgG induced ST3 agglutination, but in contrast to A7, PPS3-specific rabbit IgG promoted opsonophagocytosis of ST3 in vitro, and antibody efficacy was not examined in vivo (40). PPS antibody-induced aggregation was also explored by Fasching et al. for PPS-specific IgA (15). Like multivalent IgM (A7), divalent IgA induced aggregation of ST2, but unlike A7, IgA was opsonic and induced ST2 killing in vitro; however, its efficacy was not determined in vivo. An agglutinating β-1,2-mannotriose-specific mouse IgM MAb was shown to protect mice against intravenous challenge with Candida, but other agglutinating IgMs did not (21, 22), underscoring the importance of specificity in antibody-mediated protection in vivo. In contrast to A7, a protective human IgM MAb to Cryptococcus neoformans GXM, 2E9, was opsonic and promoted fungal killing in vitro (17, 54). Given that there is limited evidence for a phagocytic IgM Fc receptor in mice (18, 30, 47), 2E9-induced phagocytosis was thought to stem from MAb-dependent C3 deposition on GXM, enabling binding to host phagocyte complement receptors (54).
Our data show that A7 is required for (mouse or human) serum-mediated C3 deposition on the ST3 capsule in vitro. A previous study established that A7 mediated C3 deposition on solid-phase PPS3 and that C3 was required for A7 efficacy in vivo (10). The data presented herein demonstrate that only A7 mediated C3 deposition on ST3 and that nonspecific IgM and mouse and human sera did not. This is consistent with classic papers in the pneumococcal field, which established that complement is required for clearance of pneumococci from the bloodstream (4, 26) and that only anticapsular antibody can mediate C3 deposition on the pneumococcal capsule (6). In light of these tenets and previous work which demonstrated that A7 is not opsonic in vitro (14) and requires C3 to mediate protection in mice (10), our data suggest that the mechanism by which A7 mediates protection in vivo is by promoting C3 deposition on the ST3 capsule. Clearance of C3-opsonized bacteria is enhanced in vivo (4, 5), and immune adherence of C3-opsonized bacteria to red blood cells via complement receptors is thought to enhance intravascular clearance to the reticuloendothelial system (20, 32, 33). The phenomenon of immune adherence has been reported for PPS-specific IgG, but to our knowledge, it has not been demonstrated for IgM. It is possible that A7-mediated C3 deposition promotes ST3-macrophage binding by immune adherence (33), though we note that the significance of MAb-mediated immune adherence has not been established in vivo. Studies to address these questions are under way in our laboratory.
The results reported herein show that a protective, nonopsonic, PPS3-specific human IgM MAb induces ST3 aggregation in vitro and that aggregation correlates with protection against ST3 lethality in vivo. Although we recognize that correlation is not causation, we hypothesize that A7-mediated aggregation and C3 deposition could be in vitro surrogates for protection in vivo, whereby the mechanism of protection involves intravascular clearance of bacteria rather than opsonophagocytosis. More work is needed to validate this hypothesis and the question of whether A7 binding to the ST3 capsule alters the expression and/or function of other pneumococcal virulence factors. Experiments to address these hypotheses were beyond the scope of the current study; however, they hold promise for unraveling mechanisms of nonopsonic antibody-mediated immunity to pneumococcal disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01AI045459 and R01AI044374) to L.P. J.D.N. is supported in part by NIH grant AI52733, NIH grant AI056070-01A2, and a Hirschl/Weill-Caulier Trust Research Award. A.J.G. is supported in part by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (NIH D43-TW007129).
Footnotes
Published ahead of print on 3 March 2010.
REFERENCES
- 1.Anonymous. 2008. Progress in introduction of pneumococcal conjugate vaccine—worldwide, 2000-2008. MMWR Morb. Mortal. Wkly. Rep. 57:1148-1151. [PubMed] [Google Scholar]
- 2.Baumgarth, N., J. W. Tung, and L. A. Herzenberg. 2005. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26:347-362. [DOI] [PubMed] [Google Scholar]
- 3.Bender, J. M., K. Ampofo, K. Korgenski, J. Daly, A. T. Pavia, E. O. Mason, and C. L. Byington. 2008. Pneumococcal necrotizing pneumonia in Utah: does serotype matter? Clin. Infect. Dis. 46:1346-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5(Suppl. 4):S797-S805. [DOI] [PubMed] [Google Scholar]
- 5.Brown, E. J., S. W. Hosea, C. H. Hammer, G. Burch, and M. M. Frank. 1982. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J. Clin. Invest. 69:85-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, E. J., K. A. Joiner, R. M. Cole, and M. Berger. 1983. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect. Immun. 39:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. U. S. A. 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchwald, U. K., A. Lees, M. Steinitz, and L. Pirofski. 2005. A peptide mimotope of type 8 pneumococcal capsular polysaccharide induces a protective immune response in mice. Infect. Immun. 73:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns, T., Z. Zhong, M. Steinitz, and L. A. Pirofski. 2003. Modulation of polymorphonuclear cell interleukin-8 secretion by human monoclonal antibodies to type 8 pneumococcal capsular polysaccharide. Infect. Immun. 71:6775-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Q., Z. Zhong, A. Lees, M. Pekna, and L. Pirofski. 2002. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin loci. Infect. Immun. 70:4977-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti, S., W. Magliani, S. Arseni, E. Dieci, R. Frazzi, A. Salati, P. E. Varaldo, and L. Polonelli. 2000. In vitro activity of monoclonal and recombinant yeast killer toxin-like antibodies against antibiotic-resistant gram-positive cocci. Mol. Med. 6:613-619. [PMC free article] [PubMed] [Google Scholar]
- 12.Conti, S., W. Magliani, S. Arseni, R. Frazzi, A. Salati, L. Ravanetti, and L. Polonelli. 2002. Inhibition by yeast killer toxin-like antibodies of oral streptococci adhesion to tooth surfaces in an ex vivo model. Mol. Med. 8:313-317. [PMC free article] [PubMed] [Google Scholar]
- 13.Ekstrom, N., M. Vakevainen, J. Verho, T. Kilpi, and H. Kayhty. 2007. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 75:1794-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabrizio, K., A. Groner, M. Boes, and L. A. Pirofski. 2007. A human monoclonal immunoglobulin M reduces bacteremia and inflammation in a mouse model of systemic pneumococcal infection. Clin. Vaccine Immunol. 14:382-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasching, C. E., T. Grossman, B. Corthesy, A. G. Plaut, J. N. Weiser, and E. N. Janoff. 2007. Impact of the molecular form of immunoglobulin A on functional activity in defense against Streptococcus pneumoniae. Infect. Immun. 75:1801-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira, D. M., M. Darrieux, D. A. Silva, L. C. Leite, J. M. Ferreira, Jr., P. L. Ho, E. N. Miyaji, and M. L. Oliveira. 2009. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin. Vaccine Immunol. 16:636-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleuridor, R., Z. Zhong, and L. Pirofski. 1998. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J. Infect. Dis. 178:1213-1216. [DOI] [PubMed] [Google Scholar]
- 18.Ghumra, A., J. Shi, R. S. Mcintosh, I. B. Rasmussen, R. Braathen, F. E. Johansen, I. Sandlie, P. K. Mongini, T. Areschoug, G. Lindahl, M. J. Lewis, J. M. Woof, and R. J. Pleass. 2009. Structural requirements for the interaction of human IgM and IgA with the human Fcalpha/mu receptor. Eur. J. Immunol. 39:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore, M. S., and W. Haas. 2005. The selective advantage of microbial fratricide. Proc. Natl. Acad. Sci. U. S. A. 102:8401-8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hament, J. M., H. van Dijk, A. Fleer, P. C. Aerts, M. Schoenmakers, M. W. de Snoo, B. H. Dekker, J. L. Kimpen, and T. F. Wolfs. 2003. Pneumococcal immune adherence to human erythrocytes. Eur. J. Clin. Invest. 33:169-175. [DOI] [PubMed] [Google Scholar]
- 21.Han, Y., and J. E. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han, Y., T. R. Kozel, M. X. Zhang, R. S. MacGill, M. C. Carroll, and J. E. Cutler. 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J. Immunol. 167:1550-1557. [DOI] [PubMed] [Google Scholar]
- 23.Harboe, Z. B., R. W. Thomsen, A. Riis, P. Valentiner-Branth, J. J. Christensen, L. Lambertsen, K. A. Krogfelt, H. B. Konradsen, and T. L. Benfield. 2009. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 6:e1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havarstein, L. S., B. Martin, O. Johnsborg, C. Granadel, and J. P. Claverys. 2006. New insights into the pneumococcal fratricide: relationship to clumping and identification of a novel immunity factor. Mol. Microbiol. 59:1297-1307. [DOI] [PubMed] [Google Scholar]
- 25.Hicks, L. A., L. H. Harrison, B. Flannery, J. L. Hadler, W. Schaffner, A. S. Craig, D. Jackson, A. Thomas, B. Beall, R. Lynfield, A. Reingold, M. M. Farley, and C. G. Whitney. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 196:1346-1354. [DOI] [PubMed] [Google Scholar]
- 26.Hosea, S. W., E. J. Brown, and M. M. Frank. 1980. The critical role of complement in experimental pneumococcal sepsis. J. Infect. Dis. 142:903-909. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, S. E., L. Rubin, S. Romero-Steiner, J. K. Dykes, L. B. Pais, A. Rizvi, E. Ades, and G. M. Carlone. 1999. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J. Infect. Dis. 180:133-140. [DOI] [PubMed] [Google Scholar]
- 28.Kadioglu, A., and P. W. Andrew. 2004. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25:143-149. [DOI] [PubMed] [Google Scholar]
- 29.Karchmer, A. W. 2004. Increased antibiotic resistance in respiratory tract pathogens: PROTEKT US—an update. Clin. Infect. Dis. 39(Suppl. 3):S142-S150. [DOI] [PubMed] [Google Scholar]
- 30.Kikuno, K., D. W. Kang, K. Tahara, I. Torii, H. M. Kubagawa, K. J. Ho, L. Baudino, N. Nishizaki, A. Shibuya, and H. Kubagawa. 2007. Unusual biochemical features and follicular dendritic cell expression of human Fcalpha/mu receptor. Eur. J. Immunol. 37:3540-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapp, S., J. C. Leemans, S. Florquin, J. Branger, N. A. Maris, J. Pater, N. van Rooijen, and T. van der Poll. 2003. Alveolar macrophages have a protective anti-inflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171-179. [DOI] [PubMed] [Google Scholar]
- 32.Li, J., D. T. Glover, A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 75:5877-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, J., A. J. Szalai, S. K. Hollingshead, M. H. Nahm, and D. E. Briles. 2009. Antibody to the type 3 capsule facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages. Infect. Immun. 77:464-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, J. S., M. K. Park, and M. H. Nahm. 2001. Chromogenic assay measuring opsonophagocytic killing capacities of antipneumococcal antisera. Clin. Diagn. Lab. Immunol. 8:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martens, P., S. W. Worm, B. Lundgren, H. B. Konradsen, and T. Benfield. 2004. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infect. Dis. 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netski, D., and T. R. Kozel. 2002. Fc-dependent and Fc-independent opsonization of Cryptococcus neoformans by anticapsular monoclonal antibodies: importance of epitope specificity. Infect. Immun. 70:2812-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poolman, J., P. Kriz, C. Feron, E. Di-Paolo, I. Henckaerts, A. Miseur, D. Wauters, R. Prymula, and L. Schuerman. 2009. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 27:3213-3222. [DOI] [PubMed] [Google Scholar]
- 38.Porat, N., A. Arguedas, B. G. Spratt, R. Trefler, E. Brilla, C. Loaiza, D. Godoy, N. Bilek, and R. Dagan. 2004. Emergence of penicillin-nonsusceptible Streptococcus pneumoniae clones expressing serotypes not present in the antipneumococcal conjugate vaccine. J. Infect. Dis. 190:2154-2161. [DOI] [PubMed] [Google Scholar]
- 39.Rachini, A., D. Pietrella, P. Lupo, A. Torosantucci, P. Chiani, C. Bromuro, C. Proietti, F. Bistoni, A. Cassone, and A. Vecchiarelli. 2007. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect. Immun. 75:5085-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed, W. P., D. L. Stromquist, and R. C. Williams, Jr. 1983. Agglutination and phagocytosis of pneumococci by immunoglobulin G antibodies of restricted heterogeneity. J. Lab. Clin. Med. 101:847-856. [PubMed] [Google Scholar]
- 41.Rivera, J., O. Zaragoza, and A. Casadevall. 2005. Antibody-mediated protection against Cryptococcus neoformans is dependent on B cells. Infect. Immun. 73:1141-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins, J. B., R. Schneerson, and S. C. Szu. 1995. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 171:1387-1398. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Steiner, S., C. Frasch, N. Concepcion, D. Goldblatt, H. Kayhty, M. Vakevainen, C. Laferriere, D. Wauters, M. H. Nahm, M. F. Schinsky, B. D. Plikaytis, and G. M. Carlone. 2003. Multilaboratory evaluation of a viability assay for measurement of opsonophagocytic antibodies specific to the capsular polysaccharides of Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 10:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero-Steiner, S., C. E. Frasch, G. Carlone, R. A. Fleck, D. Goldblatt, and M. H. Nahm. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saeland, E., G. Vidarsson, J. H. Leusen, E. Van Garderen, M. H. Nahm, H. Vile-Weekhout, V. Walraven, A. M. Stemerding, J. S. Verbeek, G. T. Rijkers, W. Kuis, E. A. Sanders, and J. G. Van de Winkel. 2003. Central role of complement in passive protection by human IgG1 and IgG2 anti-pneumococcal antibodies in mice. J. Immunol. 170:6158-6164. [DOI] [PubMed] [Google Scholar]
- 46.Schuerman, L., R. Prymula, I. Henckaerts, and J. Poolman. 2007. ELISA IgG concentrations and opsonophagocytic activity following pneumococcal protein D conjugate vaccination and relationship to efficacy against acute otitis media. Vaccine 25:1962-1968. [DOI] [PubMed] [Google Scholar]
- 47.Shibuya, A., N. Sakamoto, Y. Shimizu, K. Shibuya, M. Osawa, T. Hiroyama, H. J. Eyre, G. R. Sutherland, Y. Endo, T. Fujita, T. Miyabayashi, S. Sakano, T. Tsuji, E. Nakayama, J. H. Phillips, L. L. Lanier, and H. Nakauchi. 2000. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 1:441-446. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan, M. C., B. W. Cooper, C. H. Nightingale, R. Quintiliani, and M. T. Lawlor. 1993. Evaluation of the efficacy of ciprofloxacin against Streptococcus pneumoniae by using a mouse protection model. Antimicrob. Agents Chemother. 37:234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taborda, C. P., and A. Casadevall. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791-802. [DOI] [PubMed] [Google Scholar]
- 50.Tian, H., S. Weber, P. Thorkildson, T. R. Kozel, and L. A. Pirofski. 2009. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect. Immun. 77:1502-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torosantucci, A., P. Chiani, C. Bromuro, B. F. De, A. S. Palma, Y. Liu, G. Mignogna, B. Maras, M. Colone, A. Stringaro, S. Zamboni, T. Feizi, and A. Cassone. 2009. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS One 4:e5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan, R., A. Casadevall, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify the course of Cryptococcus neoformans in mice. Proc. Natl. Acad. Sci. U. S. A. 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong, Z., and L. Pirofski. 1998. Antifungal activity of a human antiglucuronoxylomannan antibody. Clin. Diagn. Lab. Immunol. 5:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]