Abstract
To begin to understand the surprising survival of macrophage-specific lipopolysaccharide-induced tumor necrosis factor alpha factor-deficient (macLITAF−/−) animals after a lethal dose of lipopolysaccharide (LPS), as reported earlier, the present follow-up study focuses on the role of LITAF in the regulation of inflammatory cytokines secreted in response to lethal or sublethal doses of LPS administered to wild-type (WT) and macLITAF−/− mice. A time course study of kinase expression in peritoneal macrophages revealed increased phosphorylation of prosurvival kinases Akt, Erk1/2, and ribosomal S6 kinase (RSK) in macLITAF−/− mice compared to that in WT mice (n = 8), confirming their role in LPS-mediated diseases. macLITAF−/− mice (n = 8) survived a lethal dose of LPS plus d-galactosamine (d-GalN), expressing lower serum levels of pro- and anti-inflammatory cytokines than the WT levels. To extend our knowledge on LPS-induced inflammatory events, an effective sublethal dose of LPS was administered to the animals (n = 14). WT animals exhibited an acute inflammatory response that decreased after 4 h. Interestingly, macLITAF−/− mice exhibited an initial delay in the secretion of proinflammatory cytokines that peaked after 8 h and reached WT levels after 18 h. Anti-inflammatory cytokine secretions were initially delayed but increased after 4 h and remained elevated compared to WT levels, even after 18 h. Our results demonstrate that LITAF deficiency in vivo affects cytokines other than TNF-α and influences the balance between the pro- and anti-inflammatory cytokines, which protects the animals from the deleterious effects of an LPS-induced inflammatory response, resulting in a beneficial host regulation of inflammatory cytokines and in enhanced survival. Therapeutic intervention aimed at reducing LITAF via kinase modulators may prove useful in preventing LPS-induced mortality.
Tumor necrosis factor alpha (TNF-α) is a prominent inflammatory cytokine that is vital in activating beneficial inflammatory reactions involved in the surveillance and defense of the innate immune system, including stimulation of autocrine secretion as well as secretion of other cytokines. It also alters the activation and expression of adhesion molecules, regulates survival, proliferation, migration, and differentiation of cells, and protects the host against a few neurological diseases (5, 12). On the other hand, it has been documented as a critical mediator of septic shock (18, 25) and plays a role in chronic inflammatory conditions such as arthritis (15), inflammatory bowel disease (26), psoriasis (9), and cachexia (1).
Lipopolysaccharide-induced TNF-α factor (LITAF) is a transcription factor that binds to a regulatory element, CTCCC, on the TNF-α promoter and induces the transcription of TNF-α (22). Lipopolysaccharide (LPS)-induced LITAF and STAT 6B form a complex in the cytoplasm that translocates into the nucleus and induces the release of TNF-α, interleukin-1α (IL-1α), IL-10, gamma interferon (IFN-γ), macrophage chemoattractant protein 2 (MCP-2), and RANTES (23). To extend our knowledge of the role that LITAF plays in regulating the secretion of various cytokines, Tang and colleagues developed mice deficient in LITAF, specifically in macrophages (macLITAF−/− mice), using the Cre-LoxP system.
Importantly, they reported the surprising finding that the macLITAF−/− animals survived a high dose of LPS that is lethal to control mice (24).
In this follow-up study, we extended our search to identify serine/threonine kinases affected by the deficiency of LITAF. Prosurvival kinases Akt, Erk1/2, and ribosomal S6 kinase (RSK) showed increased phosphorylation in peritoneal macrophages obtained from macLITAF−/− mice compared to that in cells from wild-type (WT) mice when the cells were stimulated with LPS. To begin to understand this resistance to LPS toxicity afforded by LITAF deficiency, we made a time course comparison of the secretion of multiple cytokines in macLITAF−/− animals and WT mice exposed to a lethal or sublethal dose of LPS.
MATERIALS AND METHODS
Mice.
LITAF macrophage conditional knockout mice were generated on a C57BL/6 background by use of the Cre-LoxP system as previously described (24). LITAF and LysMCre deficiencies were confirmed by PCR and Western blot analyses of lysates of the peritoneal macrophages. Mice were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were maintained at the Boston University transgenic facility. Mice used in the experiments were 10 to 14 weeks of age and were kept under strict specific-pathogen-free (SPF) conditions. The Institutional Animal Care and Use Committee at Boston University Medical Center approved all animal procedures.
Reagents and kits.
Escherichia coli LPS serotype O111:B4 was purchased from InvivoGen, San Diego, CA. d-Galactosamine (d-GalN) was purchased from Sigma Aldrich, St. Louis, MO. Mouse monoclonal anti-LITAF antibody was obtained from BD Transduction Laboratories, Boston, MA. Primary antibodies to p-RSK, p-Akt, p-Erk1/2, total RSK, total Akt, and total Erk1/2 were obtained from Cell Signaling Technologies, Beverly, MA. The secondary anti-mouse and anti-rabbit antibodies were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Penicillin-streptomycin, phosphate-buffered saline (PBS), fetal bovine serum (FBS), and RPMI 1640 culture medium were purchased from Invitrogen, Carlsbad, CA. The 23-plex mouse cytokine kits were purchased from Bio-Rad Laboratories, CA. The cytokines measured in the experiments were a selected representative of three groups of cytokines: proinflammatory cytokines, chemokines, and anti-inflammatory cytokines.
In vivo experiments.
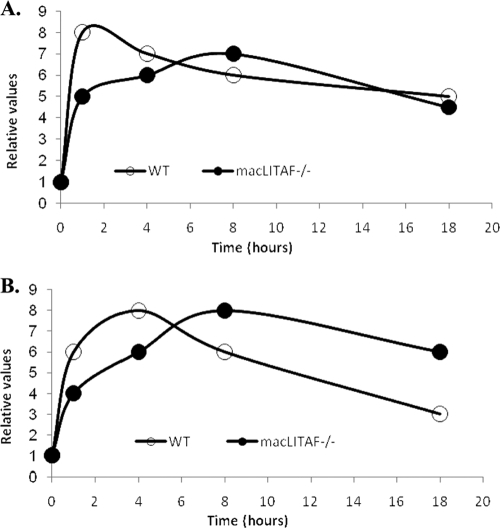
A lethal dose of LPS (12.5 ng LPS per gram body weight plus 1.25 mg/g body weight of d-GalN) was previously defined as the dose that results in death of the animal at 8 h (10). The sublethal dose (100 μg LPS per 30 g body weight) chosen causes an inflammatory response that does not result in the death of the animal. After the administration of a lethal or sublethal dose of LPS, blood samples were collected from mice by tail vein incision and/or mandible bleeds (facial vein) and cardiac puncture. Serum was obtained after 1 hour, by centrifugation at 800 × g for 15 min at 4°C. The supernatants were then decanted, aliquoted, and stored at −80°C until analysis. In experiments in which lethal doses of LPS were injected, blood was collected 2 h and 6 h after an intraperitoneal injection of LPS and the serum samples were assayed using 23-plex mouse cytokine kits. The 2-h time point was chosen based on previous reports that serum TNF-α levels peak 1 to 2 h after LPS challenge (19). Since most of the mice died 8 h after LPS-d-GalN administration (24), we chose 6 h as our second time point in order to assay serum levels of cytokines shortly before the death of the animal. In experiments in which animals were injected via the tail vein with sublethal doses of LPS, blood samples were collected at the times indicated and serum samples were assayed using 23-plex mouse cytokine kits and read using a Bio-Plex 200 system platform (Bio-Rad Laboratories, CA). Samples were assayed in duplicate, and each experiment was performed three times. For Fig. 4, the proinflammatory and anti-inflammatory cytokines were analyzed and grouped according to their response to LPS, and the average relative fold change observed at each time point was plotted against time.
FIG. 4.
Deficiency of LITAF delays inflammatory response. The deficiency in the inflammatory response in LITAF-deficient mice was clarified after averaging fold changes observed at each time point for proinflammatory and anti-inflammatory cytokines, which were grouped according to their response to LPS. Time courses comparing the expression levels of proinflammatory cytokines (cytokines and chemokines) (A) and anti-inflammatory cytokines (B) between the WT and LITAF-deficient animals in response to a sublethal dose of LPS were plotted accordingly. The following are the mediators included in this plotting: proinflammatory cytokines that showed a decrease in macLITAF−/− mice compared to WT mice at 1 h, TNF-α, IL-1β, IL-2, IL-3, IL-6, IL-12 (p40), IFN-γ, MIP-1α, MIP-1β, RANTES, MCP-1, and KC; at 4 h, TNF-α, IL-1α, IL-1β, IL-5, IL-6, GM-CSF, MIP-1α, MIP-1β, RANTES, and KC; at 8 h, IL-5, IL-6, IL-12 (p40), MIP-1β, RANTES, MCP-1, KC, and eotaxin; at 18 h, IL-6, G-CSF, MIP-1β, RANTES, MCP-1, KC, and eotaxin; proinflammatory cytokines that showed no difference in concentration between the WT and macLITAF−/− animals at 1 h, IL-5, IL-9, IL-12 (p70), IL-17, GM-CSF, and eotaxin; at 4 h, IL-12 (p40), IL-12 (p70), IFN-γ, IL-17, G-CSF, eotaxin, and MCP-1; at 8 h, IL-1α, IL-1β, IL-9, G-CSF, and MIP-1α; at 18 h, TNF-α, IL-1α, IL-1β, IL-2, IL-3, IL-9, IL-12 (p40), IL-12 (p70), IFN-γ, IL-17, GM-CSF, and MIP-1α; anti-inflammatory cytokines that showed a decrease in the macLITAF−/− mice compared to the WT mice at 1 h, IL-10 and IL-4; anti-inflammatory cytokines that showed no difference in concentration between the WT and macLITAF−/− mice at 1 h, IL-13; at 4 h, IL-4 and at 8 h, IL-4 and IL-13.
In vitro experiments.
WT and macLITAF−/− mice were stimulated with 2 ml of 3% sterile thioglycolate medium (Fisher Scientific, Pittsburgh, PA). Cells were harvested by peritoneal lavage with PBS 3 days after injection. Cells were centrifuged at 800 × g for 5 min, and RBC lysing buffer (Sigma-Aldrich, MO) was used according to the manufacturer's instructions to remove any contamination by red blood cells. Macrophages were seeded in 6-well plates at 2 × 106 cells per well, cultured overnight in RPMI 1640 medium containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, and maintained at 37°C in a humidified atmosphere containing 5% CO2. Confluent cells (80 to 90%) were then serum starved for 12 to 15 h (RPMI + 1% FBS). The medium was replaced with fresh RPMI 1640 medium with or without LPS. The WT and macLITAF−/− peritoneal macrophages were stimulated with 0.1 μg/ml E. coli LPS for 0, 5, 10, 20, 30, 60, 120, and 180 min. At each time point, cells were washed with ice-cold PBS and resuspended in cell lysis buffer (1% Triton X-100; 10 mM Tris-HCl, pH 7.4; 5 mM EDTA; 50 mM NaCl; 50 mM NaF) containing sodium vanadate and proteinase inhibitor cocktail. Lysates were sonicated, and the protein concentration for each sample was determined using the Bradford assay (2).
Phosphoprotein and total protein analyses by Western blotting.
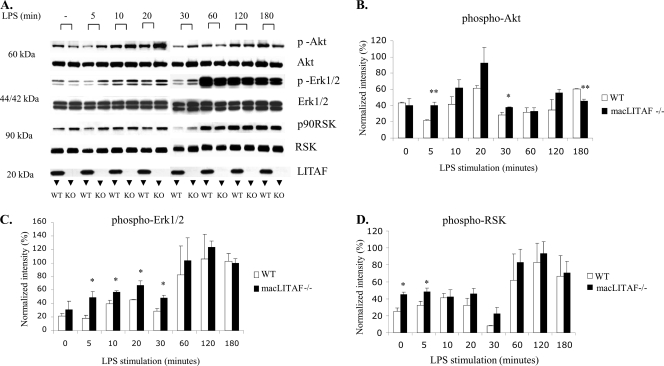
Protein samples (25 μg) were separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane by use of Towbin's transfer buffer with 15% methanol at 0.23 A for 120 min. The PVDF membrane was blocked using Tween-Tris-buffered saline (TBST) containing 10% bovine serum albumin (BSA) and washed using TBST. The membrane was then incubated for 1 h with a 1:1,000 dilution of primary antibody against p90 RSK, p-Akt, or p-Erk1/2, followed by a wash with TBST, incubated for 30 min with either anti-mouse or anti-rabbit antibody at a 1:2,000 dilution, and washed again. Antigen was detected using Western blotting Luminol reagent (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were stripped and reprobed for the corresponding total proteins. Immunoblots were quantified using VersaDoc scanning densitometry (Bio-Rad, CA). The experiment was repeated three times, using 4 animals from each group per experiment. Figure 1A required two separate gels, and the comparisons were made only within the same gel and not between the early and late time points.
FIG. 1.
Increased activation of serine/threonine kinases in the absence of LITAF. (A) Western blot depicting the patterns of expression of Akt, Erk1/2, and RSK in elicited peritoneal macrophages obtained from WT and macLITAF−/− mice (n = 8). The cells (2 × 106 cells/well) were exposed to E. coli LPS (0.1 μg/ml) for the times indicated. The bracket for each time point compares LITAF-deficient with wild-type protein extracts. Note the complete absence of LITAF protein in the samples obtained from the macLITAF−/− mice. Densitometric quantitative analyses of the phosphorylated proteins, normalized against their respective total proteins, were performed for Akt (B), Erk1/2 (C), and RSK (D), using Bio-Rad QuantityOne software. Note the increased phosphorylation of all three kinases studied in the LITAF-deficient animals compared to the WT. The data are means ± SEM for three independent experiments. *, statistical significance (P < 0.05); **, statistical significance (P < 0.01).
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM) for the results obtained from three independent experiments. Student's two-tailed t test was used for statistical analysis, and a P value of <0.05 was considered significant.
RESULTS
Identification of kinases affected in the absence of LITAF.
Our search for kinases affected by LITAF deficiency included a primary screen using commercially available Bio-Plex phosphoprotein and total target assays specific for Akt, Erk1/2, IκBα, p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), and RSK (Bio-Rad Laboratories, CA). On the basis of the primary screen, only three serine/threonine kinases, Akt, Erk, and RSK, demonstrated significance (data not shown) and hence were pursued and validated using Western blotting. Western blot analyses were performed to determine the phosphorylation levels of Akt, Erk1/2, and RSK (Fig. 1A). Akt, a 60-kDa serine/threonine kinase, showed a trend depicting a significantly increased level of phosphorylation at 5 and 30 min in the LPS-stimulated macLITAF−/− samples compared to the WT samples; however, the level was significantly decreased 180 min after LPS stimulation (Fig. 1B). The phosphorylation levels of Erk1/2 increased significantly in the macLITAF−/− samples at early time points (5, 10, 20, and 30 min) (Fig. 1C). RSK, a kinase downstream of Erk1/2, was subsequently increased at baseline as well as 5 min after LPS stimulation in the LITAF-deficient samples compared to the WT samples (Fig. 1D).
Comparison of serum levels of inflammatory cytokines in wild-type versus macLITAF−/− mice after a lethal dose of LPS.
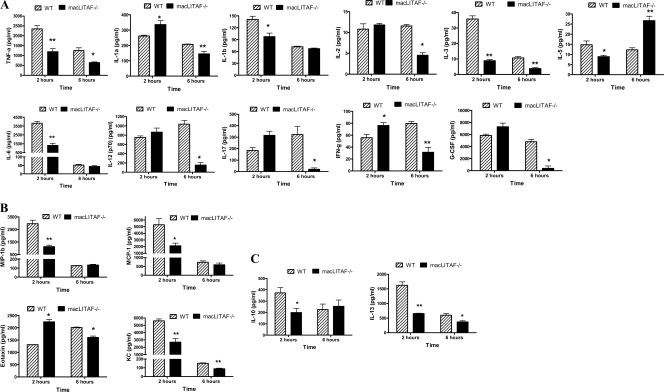
WT and macLITAF−/− animals were subjected to endotoxic shock by being pretreated with d-GalN, a known factor that sensitizes mice to the lethal effects of LPS and causes death due to TNF-α toxicity (13), followed by an LPS injection. Two hours after the injection of LPS plus d-GalN, serum samples from macLITAF−/− mice showed significant reductions in the proinflammatory cytokines TNF-α, IL-1β, IL-3, IL-5, and IL-6 (Fig. 2A), the chemokines macrophage inflammatory protein-1β (MIP-1β), KC, and MCP-1 (Fig. 2B), and the anti-inflammatory cytokines IL-10 and IL-13 (Fig. 2C). In contrast, levels of the proinflammatory cytokines and chemokines IL-1α, IL-17, IFN-γ, and eotaxin were significantly increased compared to those in WT mice. At 6 h, serum samples from macLITAF−/− mice exhibited significant reductions in the proinflammatory cytokines TNF-α, IL-1α, IL-2, IL-3, IL-12 (p70), IL-17, granulocyte colony-stimulating factor (G-CSF), and IFN-γ as well as in the chemokines eotaxin and KC and the anti-inflammatory cytokine IL-13. It is noteworthy that IL-5 was significantly increased in the macLITAF−/− animals compared to that in the WT animals (Fig. 2A, B, and C).
FIG. 2.
Comparison of levels of inflammatory cytokines in WT and macLITAF−/− mice (n = 8) in response to a lethal dose of LPS plus d-GalN for serum samples collected at 2 h and 6 h, using 23-plex mouse cytokine kits. The data are means ± SEM for three independent experiments. *, statistical significance (P < 0.05); **, statistical significance (P < 0.01). (A) Proinflammatory cytokines. (B) Chemokines. (C) Anti-inflammatory cytokines.
Comparison of time courses of serum levels of inflammatory cytokines in wild-type versus macLITAF−/− mice after a sublethal dose of LPS.
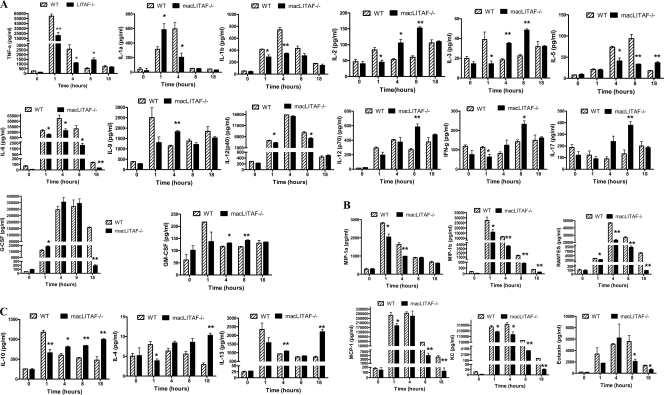
One hour after the administration of an effective but sublethal dose of LPS, there was a significant diminution in the secretion of both proinflammatory and anti-inflammatory cytokines in macLITAF−/− animals (Fig. 3A, B, and C).
FIG. 3.
Comparison of levels of inflammatory cytokines in WT and macLITAF−/− animals (n = 14) in response to a sublethal dose of E. coli LPS for serum samples collected at 0, 1, 4, 8, and 18 h, using 23-plex mouse cytokine kits. The data are means ± SEM for three independent experiments. *, statistical significance (P < 0.05); **, statistical significance (P < 0.01). (A) Proinflammatory cytokines. (B) Chemokines. (C) Anti-inflammatory cytokines.
After 4 h, there were detectable increases in the secretion of both the proinflammatory and anti-inflammatory cytokines, although they were found to be comparatively lower than those in the WT animals. The proinflammatory cytokines and chemokines TNF-α, IL-1β, IL-5, IL-6, KC, RANTES, MIP-1α, and MIP-1β were significantly reduced in the macLITAF−/− mice compared to those in the WT mice. The proinflammatory cytokines IL-1α, IL-2, IL-3, IL-9, and granulocyte-macrophage colony-stimulating factor (GM-CSF) showed an increase, whereas there was no significant difference with regard to IL-12, G-CSF, IL-17, IFN-γ, eotaxin, and MCP-1 in comparison to the WT levels. The anti-inflammatory cytokines IL-10 and IL-13 were significantly decreased in the LITAF-deficient mice compared to those in the WT mice.
After 8 h in macLITAF−/− animals, a relative shift in the secretion of both proinflammatory and anti- inflammatory cytokines was observed to surpass that in the WT animals. The proinflammatory cytokines TNF-α, IL-2, IL-3, IL-12 (p70), IL-17, GM-CSF, and IFN-γ were significantly increased, whereas the proinflammatory cytokines and chemokines IL-5, IL-6, IL-12 (p40), eotaxin, KC, RANTES, MCP-1, and MIP-1β were significantly decreased in macLITAF−/− mice in comparison to those in the WT mice, whereas IL-1α, IL-1β, IL-9, G-CSF, and MIP-1α showed no significant difference between the two groups. The anti-inflammatory cytokine IL-10 was significantly increased in the macLITAF−/− mice compared to that in the WT mice.
After 18 h, although the secretion of most of the proinflammatory cytokines in the macLITAF−/− mice was similar to that seen in the WT mice, a few others were significantly reduced. Interestingly, the secretion of anti-inflammatory cytokines showed a sustained and significant increase compared to that in the WT mice. There was no significant difference between the WT and macLITAF−/− mice with regard to TNF-α, IL-1α, IL-1β, IL-2, IL-3, IL-9, IL-12, IL-17, GM-CSF, IFN-γ, and MIP-1α. However, IL-6, G-CSF, eotaxin, KC, RANTES, MCP-1, and MIP-1β were significantly reduced, and IL-5 was the only proinflammatory cytokine that was increased significantly in the LITAF-deficient mice. The anti-inflammatory cytokines IL-10, IL-13, and IL-4 were significantly increased in macLITAF−/− mice compared to those in WT mice.
DISCUSSION
The present paper is a follow-up study that extends our current knowledge on the role of LITAF in the regulation of multiple cytokines. This was accomplished by comparing the cytokine response following a lethal dose of LPS with that of a sublethal dose. The LITAF-deficient mice have provided insight into the alterations in cytokine secretion that occur in the absence of LITAF. The inflammatory response to LPS (Fig. 4) can be summarized as follows: the initial surge in inflammatory cytokine secretion seen in WT animals is replaced by a slow but gradual increase over a prolonged period in the LITAF-deficient animals, allowing them to better manage the early deleterious effects of LPS-induced inflammation.
Although LITAF has been characterized functionally, it is not yet established whether LITAF undergoes posttranslational modifications. To address this question, LITAF protein sequences for both humans (GenBank accession number NP_004853) and mice (GenBank accession number NP_064364) were analyzed for potential phosphorylation sites. By analyzing the sequence homology and using bioinformatic tools, two potential serine/threonine phosphorylation sites were predicted in the LITAF protein sequence. The bioinformatic software available at http://scansite.mit.edu/ recognized Thr18 and Thr22 in the mouse LITAF sequence and Ser18 and Ser22 in the human LITAF sequence as inherent phosphorylation sites. It is interesting that Akt was identified bioinformatically as a potential candidate to phosphorylate Ser18 and Thr18 on human and mouse LITAF, respectively. This led us to investigate the involvement of various kinases affected by the absence of LITAF.
On the basis of our preliminary results as well as data initially explored (24) using a kinase array that identified Erk as a potential candidate to be involved, we focused on a few of the prominent serine/threonine kinases, including Akt, Erk1/2, and RSK.
In light of current evidence, the prosurvival role of Akt signaling has been shown to affect cytokine secretion favoring cell survival (3, 4, 6, 10, 17). Western blot analyses of peritoneal macrophages obtained from LITAF−/− mice showed an increase in Akt activation. Erk, similar to Akt, exerts an antiapoptotic effect and promotes cell survival (4, 7, 8, 20). Our Western blot analyses showed an increase in Erk1/2 activation, suggesting that Erk1/2, along with Akt, contributes to the survival of LITAF-deficient animals after a challenge with an otherwise lethal dose of LPS. Concurrently, activation of RSK, a molecule downstream of Erk, is also increased (11). The survival of the animals can be attributed to multiple other ramifications that have yet to be identified as sequelae to LITAF deficiency and need to be delineated.
The potential role of the kinases in cytokine secretion during an inflammatory process in the absence of LITAF was subsequently explored. To focus on the potential effect of LITAF on TNF-α, animals were subjected to endotoxic shock by being pretreated with d-GalN, a known factor that sensitizes mice to the lethal effects of LPS and causes death due to TNF-α toxicity (13), followed by an LPS injection. Sepsis is characterized by an early phase, called the systemic inflammatory response syndrome (SIRS), portrayed by uncontrolled release of proinflammatory mediators (hyperinflammatory state), followed by a compensatory anti-inflammatory response syndrome (CARS) phase, characterized by the release of anti-inflammatory mediators (hypoinflammatory state) that limit the SIRS. These overlapping phenomena are called mixed anti-inflammatory response syndrome (MARS) (16). Our results are consistent with the literature describing the time courses of pro- and anti-inflammatory mediators in WT animals exposed to LPS (16). The fact that multiple proinflammatory as well as anti-inflammatory mediators are affected by the deficiency of LITAF extends the sphere of influence of LITAF to multiple other inflammatory mediators. Indeed, our laboratory has identified the presence of the LITAF binding sequence CTCCC not only in the promoter of TNF-α (21) but also in the promoters of IL-10 and MCP-1 (unpublished data), whose levels were found to be altered similarly to that of TNF-α.
In contrast to the reductions in the expression levels of most of the cytokines, there were a few that were increased, which can be ascribed to a compensatory mechanism functioning in the absence of LITAF. We speculate that alternative signaling pathways may be triggered when there is a dearth of LITAF, suggesting the presence of multiple signaling mechanisms contributing to the survival of macLITAF−/− animals following an otherwise lethal challenge with LPS-d-GalN.
We reasoned that a sublethal dose of LPS would trigger a different pattern of cytokine secretion, not just a TNF-α-mediated inflammatory response. The initial phase of the inflammatory response observed in the LITAF-deficient animals was similar to that seen in response to the lethal challenge, i.e., there were declines in the production of both proinflammatory and anti-inflammatory mediators. The rest of the time course showed a delay in response for both the proinflammatory and anti-inflammatory cytokines in the macLITAF−/− mice that apparently contributed to their enhanced survival.
To better elucidate the role of LITAF in LPS-induced events, we compared the time courses of proinflammatory and anti-inflammatory cytokines (Fig. 4). The data demonstrate that the secretion of both pro- and anti-inflammatory cytokines was substantially reduced during the early phase of LPS-induced events after a sublethal dose in the LITAF-deficient mice compared to that in the WT mice. As the inflammatory response progressed in macLITAF−/− animals, a delayed but gradual increase in both pro- and anti-inflammatory cytokines was seen, demonstrating a transient shift in the inflammatory response that gradually returned to the levels attained by the WT mice 18 h after LPS injection with regard to the proinflammatory mediators, while the anti-inflammatory mediators remained elevated. Interestingly, we observed that in the absence of LITAF, the levels of Akt and Erk were elevated. Since Akt and Erk have been reported to stimulate the secretion of the anti-inflammatory cytokine IL-10 (10, 14, 17), this observation supports the notion that the altered levels of kinases in LITAF-deficient animals contribute to the levels of anti-inflammatory cytokines remaining elevated for a significant period. Furthermore, given that the same trend was observed for these kinases in WT cells, but at lower levels, this finding further supports our observation pointing at a delay in the response to LPS challenge for the macLITAF−/− animals compared to the WT animals.
The significant reduction in anti-inflammatory cytokines in response to both lethal and, at 1 h, sublethal doses of LPS can be attributed to the multiple complex signaling pathways involved in the absence of LITAF and to the fact that a reduction in the proinflammatory mediators does not stimulate the anti-inflammatory mediators.
This observation may play an important role in the physiology associated with increased survival of LITAF-deficient mice in response to a lethal dose of LPS. Our data support a novel therapeutic intervention by which inhibition of LITAF function would allow improved regulation of the inflammatory response, possibly by the use of kinase modulators.
Acknowledgments
We thank Chelcie F. Heaney and John O'Hara for their technical assistance.
This work was supported by NHI/NIDCR grant R01 DE014079 (S.A.).
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Beutler, B., and A. Cerami. 1989. The biology of cachectin/TNF—a primary mediator of the host response. Annu. Rev. Immunol. 7:625-655. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 4.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 5.Clauss, M., C. Sunderkotter, B. Sveinbjornsson, S. Hippenstiel, A. Willuweit, M. Marino, E. Haas, R. Seljelid, P. Scheurich, N. Suttorp, et al. 2001. A permissive role for tumor necrosis factor in vascular endothelial growth factor-induced vascular permeability. Blood 97:1321-1329. [DOI] [PubMed] [Google Scholar]
- 6.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 7.Domina, A. M., J. A. Vrana, M. A. Gregory, S. R. Hann, and R. W. Craig. 2004. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or Taxol. Oncogene 23:5301-5315. [DOI] [PubMed] [Google Scholar]
- 8.Erhardt, P., E. J. Schremser, and G. M. Cooper. 1999. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol. Cell. Biol. 19:5308-5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ettehadi, P., M. W. Greaves, D. Wallach, D. Aderka, and R. D. Camp. 1994. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin. Exp. Immunol. 96:146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 11.Frodin, M., and S. Gammeltoft. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 151:65-77. [DOI] [PubMed] [Google Scholar]
- 12.Haider, S., and M. Knofler. 2009. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta 30:111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann, V., M. A. Freudenberg, and C. Galanos. 1987. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J. Exp. Med. 165:657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas, M., X. Zhang, V. Prasanna, and D. M. Mosser. 2005. ERK activation following macrophage Fc(gamma)R ligation leads to chromatin modifications at the IL-10 locus. J. Immunol. 175:469-477. [DOI] [PubMed] [Google Scholar]
- 15.Neale, M. L., B. D. Williams, and N. Matthews. 1989. Tumour necrosis factor activity in joint fluids from rheumatoid arthritis patients. Br. J. Rheumatol. 28:104-108. [DOI] [PubMed] [Google Scholar]
- 16.Osuchowski, M. F., K. Welch, J. Siddiqui, and D. G. Remick. 2006. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 177:1967-1974. [DOI] [PubMed] [Google Scholar]
- 17.Pengal, R. A., L. P. Ganesan, G. Wei, H. Fang, M. C. Ostrowski, and S. Tridandapani. 2006. Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol. Immunol. 43:1557-1564. [DOI] [PubMed] [Google Scholar]
- 18.Remick, D. G., R. G. Kunkel, J. W. Larrick, and S. L. Kunkel. 1987. Acute in vivo effects of human recombinant tumor necrosis factor. Lab. Invest. 56:583-590. [PubMed] [Google Scholar]
- 19.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 20.Scheid, M. P., and V. Duronio. 1998. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/Akt: involvement of MEK upstream of Bad phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 95:7439-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiomi, N., F. Myokai, K. Naruishi, K. Oyaizu, K. Senoo, T. Yamaguchi, S. Amar, and S. Takashiba. 2006. Cloning and characterization of lipopolysaccharide-induced tumor necrosis factor α factor promoter. FEMS Immunol. Med. Microbiol. 47:360-368. [DOI] [PubMed] [Google Scholar]
- 22.Tang, X., M. J. Fenton, and S. Amar. 2003. Identification and functional characterization of a novel binding site on TNF-alpha promoter. Proc. Natl. Acad. Sci. U. S. A. 100:4096-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang, X., D. L. Marciano, S. E. Leeman, and S. Amar. 2005. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6 (B) with effects on multiple cytokines. Proc. Natl. Acad. Sci. U. S. A. 102:5132-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang, X., D. Metzger, S. Leeman, and S. Amar. 2006. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: evidence for LITAF-dependent LPS signaling pathways. Proc. Natl. Acad. Sci. U. S. A. 103:13777-13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tracey, K. J., B. Beutler, S. F. Lowry, J. Merryweather, S. Wolpe, I. W. Milsark, R. J. Hariri, T. J. Fahey III, A. Zentella, J. D. Albert, et al. 1986. Shock and tissue injury induced by recombinant human cachectin. Science 234:470-474. [DOI] [PubMed] [Google Scholar]
- 26.Van Deventer, S. J. 1997. Tumour necrosis factor and Crohn's disease. Gut 40:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]