Abstract
Synergy time-kill studies against 40 methicillin-resistant Staphylococcus aureus (MRSA) strains of differing resistance phenotypes were conducted. Subinhibitory concentrations of telavancin were combined with sub-MIC concentrations of other antimicrobial agents that might be used in combination with telavancin to provide Gram-negative coverage. The highest incidence of synergy was found after 24 h with gentamicin (90% of strains), followed by ceftriaxone (88%), rifampin and meropenem (each 65%), cefepime (45%), and ciprofloxacin (38%) for combinations tested at or below the intermediate breakpoint for each agent.
Methicillin-resistant Staphylococcus aureus (MRSA) strains are increasingly encountered and cannot be treated with available β-lactams. Most methicillin-resistant (and also some methicillin-susceptible) strains are resistant to all available quinolones, and vancomycin-heterointermediate (hVISA), vancomycin-intermediate (VISA), and vancomycin-resistant (VRSA) S. aureus strains have appeared (7-9, 16, 31). Most multidrug-resistant S. aureus strains are nosocomially acquired and cause an array of site-specific infections in hospitalized patients, including bloodstream infections, pneumonia, surgical site infections, and urinary tract infections (11). However, in the past few years, there has been an increase in the incidence of community-acquired MRSA strains, which at this time are susceptible to most other agents but are more virulent than hospital strains (1, 15, 17, 18, 22, 24, 30). The mechanism of this increased virulence may lie at least in part with production of toxins such as Panton-Valentine leukocidin (10, 29) and phenol-soluble modulin alpha 3 (20).
Development of S. aureus strains with diminished susceptibility to vancomycin is at least partially caused by the selective pressure of vancomycin use in the community. The increase in the number of infections due to community-acquired MRSA will likely lead to more glycopeptide use in the community setting, therefore increasing the selective pressure for vancomycin resistance. Although alternative therapeutic modalities to vancomycin for treatment of systemic staphylococcal infections already exist, the situation will not remain stable and development of new drugs is therefore needed (3, 14).
VISA strains with thickened cell walls are often not susceptible to daptomycin, dalbavancin, or oritavancin at established or proposed breakpoints (21; P. C. Appelbaum, unpublished information). In contrast, telavancin, an investigational lipoglycopeptide with a dual mechanism of action, is potent, with MICs of ≤1 μg/ml against all MRSA phenotypes (including hVISA and VISA) with the exception of some VRSA strains (12, 13, 19, 23). Telavancin was recently approved in the United States and Canada for treatment of complicated skin and skin structure infections due to Gram-positive pathogens (26-28), including MRSA, and is under investigation as a once-daily treatment for hospital-acquired pneumonia caused by Gram-positive bacteria (25).
This study was performed to examine the synergistic activity of telavancin when combined with rifampin, gentamicin, cefepime, ceftriaxone, oxacillin, meropenem, and ciprofloxacin against 40 MRSA strains with various resistotypes. The 40 MRSA strains studied were recent isolates and comprised 15 community-acquired MRSA strains and 12 nosocomially acquired MRSA strains with known and differing resistance phenotypes (S. aureus ATCC 33591 was included among the nosocomially acquired MRSA strains), 2 hVISA strains (Hershey isolates, screened by the Etest macromethod and confirmed by population analyses), 8 VISA strains (4 Hershey Medical Center isolates, 4 from the Network on Antimicrobial Resistance in Staphylococcus aureus), and 3 VRSA strains (Detroit, Hershey, and NYC isolates).
In vitro methodology for the detection of synergy between two antibacterials has not been standardized. Although checkerboard analyses have been used extensively in the past, we feel that they are neither as sensitive nor as discriminatory as time-kill analysis to accurately detect synergy. Thus, we have conducted our assessment of telavancin synergy employing time-kill methodology (4, 6).
MICs were determined by broth macrodilution after 24 h of incubation. The kill kinetics of each drug were tested alone by incubating an initial inoculum of 5 × 105 to 5 × 106 CFU/ml with drug concentrations at the MIC, two dilutions above the MIC (2 and 4× MIC), and three dilutions below the MIC (one-half [1/2], 1/4, and 1/8× MIC) (4, 6). Testing of oxacillin was performed in medium supplemented with 2% NaCl. Viability counts were performed after 0, 3, 6, 12, and 24 h of incubation at 35°C in a shaking water bath by plating undiluted and 10-fold serially diluted samples onto Trypticase soy-5% sheep blood agar plates (Becton Dickinson, Inc., Sparks, MD). Plates were incubated at 35°C for 24 to 48 h, and colony counts were determined. Quality control strains, as recommended by the Clinical and Laboratory Standards Institute (CLSI), were included (5).
Synergy testing was performed by time-kill methodology by combining telavancin with each of the seven antimicrobial agents listed above as follows: combinations were tested for each strain by pairing telavancin at 1/2× MIC for that strain with the comparator at 1/2× MIC in one tube, along with a second tube containing telavancin at 1/4× MIC with the comparator at 1/4× MIC, a third tube containing 1/2× telavancin MIC and 1/4× comparator MIC, and a fourth tube containing 1/4× telavancin MIC and 1/2× comparator MIC. All four of these combinations were tested against each strain, and synergy was noted when observed in each case. Due to the high MICs of some of the comparator drugs, we made the following exceptions: drugs with MICs of >128 μg/ml were tested in combinations using 64 μg/ml and 32 μg/ml instead of 1/2× and 1/4× their MICs. Synergy was defined as a ≥2 log10 decrease in CFU/ml between the combination and its most active constituent after 3, 6, 12, or 24 h (all time periods were evaluated), with the number of surviving organisms in the presence of the combination ≥2 log10 CFU/ml below the starting inoculum. Synergy time-kills are usually read after 24 h of incubation, but we feel that assessment of viability at earlier time periods may also have clinical significance. This entire field has not been standardized, and we followed procedures accepted and published in previous reports (4, 6).
Results of antimicrobial susceptibility by strain phenotype are presented in Table 1. Thirty-eight of the isolates (including 1 VRSA strain) had telavancin MICs of 0.125 to 1 μg/ml; two VRSA strains had telavancin MICs of 4 μg/ml. Telavancin was tested alone in time-kill studies at 1×, 2×, and 4× MIC against all 40 isolates. A summary of these data is shown in Table 2. Telavancin was bactericidal at 24 h against 36 of the 40 strains when tested at 2× MIC and against 38 of the 40 strains when tested at 4× MIC. The two strains against which telavancin was not bactericidal at 24 h at 4× MIC were one VISA strain (−1.7 Δlog10 CFU/ml) and one vancomycin-susceptible S. aureus (VSSA) strain (−2.8 Δlog10 CFU/ml).
TABLE 1.
In vitro antimicrobial activities of telavancin and other agents used in combination assay
| Isolate | No. tested | MIC range (μg/ml)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Telavancin | Gentamicin | Ceftriaxone | Rifampin | Meropenem | Cefepime | Ciprofloxacin | Oxacillin | ||
| All | 40 | 0.125-4 | 0.5->512 | 16->512 | 0.002->512 | 0.25-128 | 4-512 | 0.5-512 | 4-512 |
| VSSA | 27 | 0.125-1 | 0.5-4 | 64->512 | 0.004-0.03 | 2-128 | 16-512 | 0.5-512 | 32-512 |
| hVISA | 2 | 0.5-1 | 1 | >512 | 0.004-0.016 | 32-64 | 512 | 128-256 | 512 |
| VISA | 8 | 0.5-1 | 1->512 | 16->512 | 0.004->512 | 0.25-64 | 4-512 | 0.5-512 | 4-512 |
| VRSA | 3 | 1-4 | 2-128 | 512->512 | 0.002->512 | 4-64 | 128-512 | 64-256 | 128-256 |
Determined by broth macrodilution.
TABLE 2.
Time-kill results for 40 MRSA strains exposed to telavancin
| Phenotype and telavancin concn | No. of strains with Δlog10 CFU/ml at: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 h |
6 h |
12 h |
24 h |
|||||||||
| −1a | −2a | −3a | −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | |
| All (n = 40) | ||||||||||||
| 4× MIC | 27 | 4 | 0 | 39 | 17 | 0 | 40 | 33 | 10 | 40 | 39 | 38 |
| 2× MIC | 23 | 3 | 0 | 37 | 4 | 0 | 40 | 32 | 8 | 40 | 39 | 36 |
| 1× MIC | 19 | 0 | 0 | 35 | 4 | 0 | 36 | 24 | 5 | 34 | 31 | 24 |
| VSSA (n = 27) | ||||||||||||
| 4× MIC | 16 | 0 | 0 | 26 | 13 | 0 | 27 | 26 | 9 | 27 | 27 | 26 |
| 2× MIC | 13 | 1 | 0 | 25 | 0 | 0 | 27 | 25 | 8 | 27 | 27 | 25 |
| 1× MIC | 10 | 0 | 0 | 24 | 3 | 0 | 27 | 20 | 5 | 23 | 21 | 16 |
| hVISA+VISA (n = 10) | ||||||||||||
| 4× MIC | 8 | 3 | 0 | 10 | 3 | 0 | 10 | 4 | 0 | 10 | 9 | 9 |
| 2× MIC | 7 | 2 | 0 | 9 | 3 | 0 | 10 | 4 | 0 | 10 | 9 | 8 |
| 1× MIC | 7 | 0 | 0 | 8 | 1 | 0 | 7 | 3 | 0 | 9 | 8 | 6 |
| VRSA (n = 3) | ||||||||||||
| 4× MIC | 3 | 1 | 0 | 3 | 1 | 0 | 3 | 3 | 1 | 3 | 3 | 3 |
| 2× MIC | 3 | 0 | 0 | 3 | 1 | 0 | 3 | 3 | 0 | 3 | 3 | 3 |
| 1× MIC | 2 | 0 | 0 | 3 | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 2 |
−1, 90% killing; −2, 99% killing; −3, 99.9% killing.
Synergy time-kill data obtained with each of the 40 isolates at the different time periods earlier than 24 h are listed in Table S1 in the supplemental material. The concentrations listed were the lowest at which synergy was observed. Table 3 shows the number of strains out of 40 tested isolates demonstrating synergy with the combination of telavancin and comparators, each at sub-MIC concentrations, at the 24-h time point. Results were also assessed for the number of strains showing synergy at concentrations of the comparator that were at or below the intermediate breakpoint for each comparator. All synergy with comparators at concentrations less than or equal to intermediate breakpoints was observed at concentrations of telavancin that were ≤1 μg/ml (the susceptibility breakpoint for S. aureus).
TABLE 3.
Results of in vitro antimicrobial combinations with telavancin studied by time-kill assay at sub-MIC concentrations of both agents
| Phenotype | No of strainsa at 24 h showing synergy for telavancin tested in combination with: |
||||||
|---|---|---|---|---|---|---|---|
| Gentamicin | Ceftriaxone | Rifampin | Meropenem | Cefepime | Ciprofloxacin | Oxacillin | |
| All (n = 40) | 38 | 39 | 28 | 39 | 37 | 33 | 24 |
| VSSA (n = 27) | 27 | 27 | 20 | 27 | 26 | 24 | 12 |
| hVISA (n = 2) | 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| VISA (n = 8) | 7 | 7 | 5 | 7 | 7 | 6 | 8 |
| VRSA (n = 3) | 2 | 3 | 1 | 3 | 2 | 2 | 2 |
No. of strains showing synergy at concentrations of comparator that were at or below the intermediate breakpoint are presented in the text.
When telavancin was combined with gentamicin, 38 of the strains (95%) showed synergy; 36 isolates (90%) showed synergy at concentrations less than or equal to intermediate breakpoint concentrations for gentamicin (≤8 μg/ml; synergistic concentrations, 0.25 to 4 μg/ml). One of the strains originally resistant to gentamicin with an MIC of 16 μg/ml showed synergy at a susceptible gentamicin concentration of 4 μg/ml when combined with 0.25 μg/ml of telavancin. The combination of telavancin with ceftriaxone led to synergy in 39 isolates (98%). Thirty-five strains (88%) showed synergy at ≤32 μg/ml of ceftriaxone (intermediate breakpoint; synergistic concentrations, 4 to 32 μg/ml). Thirty-four strains had resistant ceftriaxone MICs of 64 to >512 μg/ml but showed synergy at concentrations of 16 to 32 μg/ml when combined with telavancin, while one strain with an intermediate ceftriaxone MIC of 16 μg/ml showed synergy at 4 μg/ml. When telavancin and rifampin were combined, synergy was observed in 28 isolates (70%), with 26 strains (65%) showing synergy at concentrations less than or equal to the intermediate rifampin breakpoint (≤2 μg/ml; synergistic concentrations, 0.001 to 0.016 μg/ml). Combinations of telavancin and meropenem showed synergy in 39 of the strain tested (98%), while 26 strains (65%) showed synergy when tested at or below the meropenem intermediate breakpoint (≤8 μg/ml; synergistic concentrations, 0.06 to 8 μg/ml). One strain with a resistant meropenem MIC of 16 μg/ml showed synergy at 4 μg/ml, three strains with resistant MICs of 32 μg/ml showed synergy at meropenem intermediate concentrations of 8 μg/ml, and ten strains with intermediate MICs of 8 μg/ml had synergy at susceptible concentrations of 2 to 4 μg/ml. Synergy between telavancin and cefepime occurred in 37 isolates (93%), of which 18 strains (45%) showed synergy at cefepime concentrations less than or equal to the intermediate breakpoint (16 μg/ml: synergistic concentrations 2 to 16 μg/ml). Fifteen strains with resistant cefepime MICs of 64 μg/ml showed synergy at the intermediate breakpoint of 16 μg/ml, and two strains with intermediate MICs of 16 μg/ml showed synergy at 4 μg/ml. Telavancin combined with ciprofloxacin showed synergy in 33 strains (83%). Fifteen strains (38%) showed synergy at or below the intermediate ciprofloxacin concentration (≤2 μg/ml; synergistic concentrations, 0.125 to 0.25 μg/ml). When telavancin was combined with oxacillin, 24 strains (60%) showed synergy, but only 1 strain (3%) showed synergy at a concentration of oxacillin less than or equal to the susceptible breakpoint of 2 μg/ml. This strain was resistant to oxacillin (MIC of 4 μg/ml) but showed synergy at 1 μg/ml.
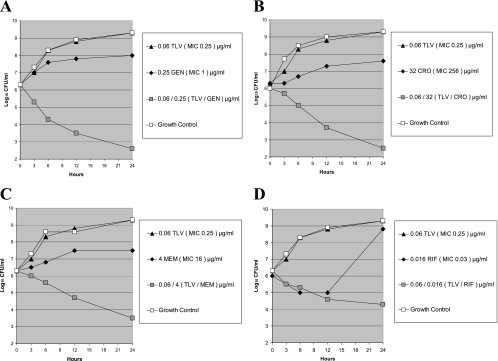
Figure 1 shows synergy time-kill results observed in one nosocomially isolated MRSA strain when tested with four different drug combinations. As can be seen, synergy was observed with telavancin in combination with gentamicin, ceftriaxone, meropenem, and rifampin at sub-MIC concentrations of all compounds.
FIG. 1.
Time-kill curves for telavancin (TLV) and four comparator agents tested alone and in combination against a nosocomially acquired MRSA isolate (strain SA 1324). (A) TLV plus gentamicin (GEN). (B) TLV plus ceftriaxone (CRO). (C) TLV plus meropenem (MEM). (D) TLV plus rifampin (RIF).
Since there is no universally accepted definition of antagonism or indifference using synergy time-kill methodology (see Antimicrobial Agents and Chemotherapy Instructions to Authors [2]), we have described our findings as synergistic or nonsynergistic. None of the combinations tested yielded colony counts of >2 log10 CFU/ml higher than those seen with the more active single drug tested by itself; thus, no indication of what could be interpreted as antagonism was observed with any combination studied. The highest synergy rates were found at 24 h when subinhibitory concentrations of telavancin were combined with clinically relevant, subinhibitory concentrations of gentamicin (90%), ceftriaxone (88%), meropenem (65%), and rifampin (65%). The clinical significance of these high rates of synergy observed in vitro remains to be ascertained.
Supplementary Material
Acknowledgments
This study was sponsored by Theravance, Inc., South San Francisco, CA.
Footnotes
Published ahead of print on 16 February 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Adern, P. V., C. P. Montgomery, A. N. Husain, T. K. Koogler, V. Arangelovich, M. Humilier, S. Boyle-Vavra, and R. S. Daum. 2005. Staphylococcus aureus sepsis and the Waterhouse-Freiderichsen syndrome in children. N. Engl. J. Med. 353:1245-1251. [DOI] [PubMed] [Google Scholar]
- 2.American Society for Microbiology. 2010. Instructions to authors. Antimicrob. Agents Chemother. 54:1-23. [Google Scholar]
- 3.Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Clark, C. L., M. R. Jacobs, and P. C. Appelbaum. 1999. Activities of clinafloxacin, alone and in combination with other compounds, against 45 Gram-positive and -negative organisms for which clinafloxacin MICs are high. Antimicrob. Agents Chemother. 43:2295-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing. Approved standard M100-S19. Nineteenth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Credito, K., G. Lin, and P. C. Appelbaum. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 51:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui, L., A. Iwamoto, J. Q. Lian, H. M. Neoh, T. Murayama, Y. Horikawa, and K. Hiramatsu. 2006. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui, L., E. Tominaga, H. M. Neoh, and K. Hiramatsu. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lassence, A., N. Hidri, J. F. Timsit, M.-L. Joly-Guillou, G. Thiery, A. Boyer, P. Lable, A. Blivet, H. Kalinowski, Y. Martin, J. P. Lajonchere, and D. Dreyfuss. 2006. Control and outcome of a large outbreak of colonization and infection with glycopeptide-intermediate Staphylococcus aureus in an intensive care unit. Clin. Infect. Dis. 42:170-178. [DOI] [PubMed] [Google Scholar]
- 10.Denis, O., A. Deplano, H. de Beenhouwer, M. Hallin, G. Huysmans, M. G. Garrino, Y. Glupczynski, X. Malaviolle, A. Vergison, and M. J. Streulens. 2005. Polyclonal emergence and importation of community-acquired methicillin-resistant Staphylococcus aureus strains harbouring Panton-Valentine leukocidin genes in Belgium. J. Antimicrob. Chemother. 56:1103-1106. [DOI] [PubMed] [Google Scholar]
- 11.Deresinski, S. 2005. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin. Infect. Dis. 40:562-573. [DOI] [PubMed] [Google Scholar]
- 12.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. Comparative surveillance study of telavancin activity against recently collected gram-positive clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:2383-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draghi, D. C., B. M. Benton, K. M. Krause, C. Thornsberry, C. Pillar, and D. F. Sahm. 2008. In vitro activity of telavancin against recent Gram-positive clinical isolates: results of the 2004-05 Prospective European Surveillance Initiative. J. Antimicrob. Chemother. 62:116-121. [DOI] [PubMed] [Google Scholar]
- 14.Fischbach, M. A., and C. T. Walsh. 2009. Antibiotics for emerging pathogens. Science 325:1089-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis, J. S., M. C. Doherty, U. Lopatin, C. P. Johnston, G. Sinha, T. Ross, M. Cai, N. N. Hansel, T. Perl, J. R. Ticehurst, K. Carroll, D. L. Thomas, E. Nuermberger, and J. G. Bartlett. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin. Infect. Dis. 40:100-107. [DOI] [PubMed] [Google Scholar]
- 16.Garnier, F., D. Chainier, T. Walsh, A. Karlsson, A. Bolmström, C. Grelaud, M. Mounier, F. Denis, and M. C. Ploy. 2006. A 1 year surveillance study of glycopeptide-intermediate Staphylococcus aureus strains in a French hospital. J. Antimicrob. Chemother. 57:146-149. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez, B. E., K. G. Hulten, M. K. Dishop, L. B. Lamberth, W. A. Hammerman, E. O. Mason, Jr., and S. L. Kaplan. 2005. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus. Clin. Infect. Dis. 41:583-590. [DOI] [PubMed] [Google Scholar]
- 18.Healy, C. M., K. G. Hulten, D. L. Palazzi, J. R. Campbell, and C. J. Baker. 2004. Emergence of new strains of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Clin. Infect. Dis. 39:1460-1466. [DOI] [PubMed] [Google Scholar]
- 19.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hongo, I., T. Baba, K. Oishi, Y. Morimoto, T. Ito, and K. Hiramatsu. 2009. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J. Infect. Dis. 200:715-723. [DOI] [PubMed] [Google Scholar]
- 21.Julian, K., K. Kosowska-Shick, C. Whitener, M. Roos, H. Labischinski, A. Rubio, L. Parent, L. Ednie, L. Koeth, T. Bogdanovich, and P. C. Appelbaum. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazakova, S. V., J. C. Hageman, M. Matava, A. Srinivasan, L. Phelan, B. Garfinkel, T. Boo, S. McAllister, J. Anderson, B. Jensen, D. Dodson, D. Lonsway, L. K. McDougal, M. Arduino, V. J. Fraser, G. Killgore, F. C. Tenover, S. Cody, and D. B. Jernigan. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 23.Leuthner, K. D., C. M. Cheung, and M. J. Rybak. 2006. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:338-343. [DOI] [PubMed] [Google Scholar]
- 24.Miller, L. G., F. Perdreau-Remington, G. Rieg, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein, E., G. R. Corey, M. E. Stryjewski, H. W. Boucher, R. N. Daly, F. C. Gentner, S. L. Barriere, M. M. Kitt, and H. D. Friedland. 2008. Telavancin for hospital-acquired pneumonia, including ventilator-associated pneumonia: the ATTAIN studies, abstr. O75. Abstr. 18th Eur. Congr. Clin. Microbiol. Infect. Dis. Wiley-Blackwell, Oxford, United Kingdom.
- 26.Stryjewski, M. E., D. R. Graham, S. E. Wilson, W. O'Riordan, D. Young, A. Lentnek, D. P. Ross, V. G. Fowler, A. Hopkins, H. D. Friedland, S. L. Barriere, M. M. Kitt, and G. R. Corey on behalf of the Assessment of Telavancin in Complicated Skin and Skin-Structure Infections Study. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683-1693. [DOI] [PubMed] [Google Scholar]
- 27.Stryjewski, M. E., V. H. Chu, W. D. O'Riordan, B. L. Warren, L. M. Dunbar, D. M. Young, M. Vallée, V. G. Fowler, Jr., J. Morganroth, S. L. Barriere, M. M. Kitt, G. R. Corey, and the FAST investigator group. 2006. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by Gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 50:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stryjewski, M. E., W. D. O'Riordan, W. K. Lau, F. D. Pien, L. M. Dunbar, M. Vallee, V. G. Fowler, Jr., V. C. Chu, E. Spencer, S. L. Barriere, M. M. Kitt, C. H. Cabell, G. R. Corey, and the FAST investigator group. 2005. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to Gram-positive bacteria. Clin. Infect. Dis. 40:1601. [DOI] [PubMed] [Google Scholar]
- 29.Takizawa, Y., I. Taneike, S. Nakagawa, T. Oishi, Y. Nitahara, N. Iwakura, K. Ozaki, M. Takano, T. Nakayama, and T. Yamamoto. 2005. A Panton-Valentine leukocidin (PVL)-positive community-acquired methicillin-resistant Staphylococcus aureus (MRSA) strain, another such strain carrying a multiple-drug resistance plasmid, and other more-typical PVL-negative MRSA strains found in Japan. Antimicrob. Agents Chemother. 43:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. Antimicrob. Agents Chemother. 44:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tenover, F. C., L. M. Weigel, P. C. Appelbaum, L. K. McDougal, J. Chaitram, S. McAllister, N. Clark, G. Killgore, C. M. O'Hara, L. Jevitt, J. B. Patel, and B. Bozdogan. 2004. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob. Agents Chemother. 48:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.