Abstract
There are few options for prophylaxis after exposure to Bacillus anthracis, especially in children and women of childbearing potential. Faropenem is a β-lactam in the penem subclass that is being developed as an oral prodrug, faropenem medoxomil, for the treatment of respiratory tract infections. Faropenem was shown to have in vitro activity against B. anthracis strains that variably express the bla1 β-lactamase (MIC range, ≤0.06 to 1 μg/ml). In this study we evaluated the pharmacokinetic-pharmacodynamic (PK-PD) relationships between the plasma faropenem free-drug (f) concentrations and efficacy against B. anthracis in a murine postexposure prophylaxis inhalation model. The plasma PKs and PKs-PDs of faropenem were evaluated in BALB/c mice following the intraperitoneal (i.p.) administration of doses ranging from 2.5 to 160 mg/kg of body weight. For the evaluation of efficacy, mice received by inhalation aerosol doses of B. anthracis (Ames strain; faropenem MIC, 0.06 μg/ml) at 100 times the 50% lethal dose. The faropenem dosing regimens (10, 20, 40, and 80 mg/kg/day) were administered i.p. at 24 h postchallenge at 4-, 6-, and 12-h intervals for 14 days. The sigmoid maximum-threshold-of-efficacy (Emax) model fit the survival data, in which the free-drug area under the concentration-time curve (fAUC)/MIC ratio, the maximum concentration of free drug in plasma (fCmax)/MIC ratio, and the cumulative percentage of a 24-h period that the free-drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (f %TMIC) were each evaluated. Assessment of f %TMIC demonstrated the strongest correlation with survival (R2 = 0.967) compared to the correlations achieved by assessment of fAUC/MIC or fCmax/MIC, for which minimal correlations were observed. The 50% effective dose (ED50), ED90, and ED99 corresponded to f %TMIC values of 10.6, 13.4, and 16.4%, respectively, and Emax was 89.3%. Overall, faropenem demonstrated a high level of activity against B. anthracis in the murine postexposure prophylaxis inhalation model.
One of the most feared scenarios regarding a terrorist attack is the dispersal of an aerosolized lethal bacterium, such as Bacillus anthracis. Only a limited number of antibiotics are currently approved by the United States Food and Drug Administration (FDA) for treatment after exposure to this bacterial agent. Of these, only penicillin is considered safe for use by children and pregnant women. Bacterial resistance to β-lactams is frequently attributed to the production of β-lactamase enzymes, which cleave the amide bond of the β-lactam ring, rendering the agent inactive (2). Unfortunately, B. anthracis contains two genes coding for β-lactamases, the bla1 gene (group 2a penicillinase) and the bla2 gene (group 3 metalloenzyme) (4, 5). The bla1 gene is variably expressed, while the level of expression of the bla2 gene remains unknown. The expression of such genes puts anyone receiving a β-lactam susceptible to β-lactamase degradation at risk. Faropenem is an oral penem with broad-spectrum activity that is stable to many commonly encountered β-lactamases (3, 9). Faropenem medoxomil has been evaluated in phase III clinical trials and has been shown to have activity for the treatment of respiratory and skin infections, including those caused by β-lactamase-producing organisms (16, 17). One objective of the study described here was to evaluate the in vitro activities of faropenem and other agents against a diverse collection of B. anthracis strains that contain and variably express the bla1 and/or bla2 gene. The second objective of the study was to evaluate the in vivo activity of faropenem against a single strain (B. anthracis Ames) following aerosol challenge in a mouse model.
MATERIALS AND METHODS
Bacterial isolates.
The B. anthracis isolates used in this study were part of a diverse collection of β-lactamase-producing isolates that contain and variably express the bla1 (group 2a penicillinase) and/or bla2 (group 3 metalloenzymes) gene. The isolate collection included strains from all eight genotype families of Keim et al. (13).
Antimicrobial susceptibility testing.
All 29 isolates of B. anthracis were tested for their susceptibilities to faropenem, amoxicillin-clavulanate, penicillin, meropenem, azithromycin, doxycycline, ciprofloxacin, and tetracycline. Antimicrobial susceptibility testing was conducted by broth microdilution with frozen panels that were prepared in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (6) and was conducted in the laboratory of Henry S. Heine (U.S. Army Medical Research Institute of Infectious Disease [USAMRIID], Fort Detrick, MD). Quality control of the antimicrobial agents was established by using Staphylococcus aureus ATCC 29213 and established control ranges (6).
Antibiotics and bacteria for efficacy testing.
Faropenem sodium was supplied by Replidyne, Inc. (Louisville, CO), and ciprofloxacin was obtained from Bayer Healthcare AG (West Haven, CT). All dosing solutions were prepared in saline for injection at 10 ml/kg of body weight. Spores of the B. anthracis Ames strain were prepared and challenge aerosols were generated as described previously (14). The bacteria were counted in triplicate by serial dilution in sterile water and plating onto sheep blood agar plates (SBAP). The plates were incubated at 35°C for 18 h, and the colonies were counted.
Animal research.
The research conducted at USAMRIID was performed in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals (15). USAMRIID is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Murine PKs.
The pharmacokinetics (PKs) of faropenem in the plasma of female BALB/c mice were evaluated following the intraperitoneal (i.p.) administration of a single dose of faropenem sodium. The faropenem sodium doses evaluated were 2.5, 10, 11.7, 40, and 160 mg/kg of body weight. Each dose group consisted of 27 mice; and blood samples were collected (3 mice per time point) at 0.083, 0.25, 0.5, 1, 1.5, 2, 3, 4, and 6 h postadministration. Blood samples were processed to obtain plasma and were stored at −70°C. Plasma faropenem concentrations were determined within 8 weeks of collection by a validated liquid chromatography-tandem mass spectrometry assay with a lower limit of quantitation of 10 ng/ml and an upper limit of quantitation of 50,000 ng/ml. Noncompartmental pharmacokinetic parameters were determined for each dose group by using the median faropenem plasma concentrations at each time point. The linear-log trapezoidal rule was used for determination of the area under the concentration-time curve (AUC).
Pharmacokinetic modeling.
A noncompartmental model was developed to assess the plasma faropenem concentrations by using the plasma concentration data for all dose groups (2.5 to 160 mg/kg). This model was based on the assumption of dose linear pharmacokinetics and the dose-normalized median plasma concentrations calculated at each time point. All of the plasma concentration data were normalized to a dose of 1 mg/kg, and the median values were assessed if greater than 50% of the values were above the limit of quantitation at each time point. For calculation of the medians, values that were below the limit of quantitation were assigned a value of zero. For assessment of the plasma faropenem concentrations at times beyond the last time point with a measurable median value, the median elimination half-life (t1/2) value across the dose groups was used. This model was then used to assess the plasma faropenem concentration-time profiles associated with different faropenem doses and dosing schedules.
Serum protein binding.
The level of faropenem protein binding in mouse serum was assessed by ultrafiltration with 14C-radiolabeled faropenem. Fifty microliters of faropenem in phosphate-buffered saline, pH 7.4, was added to 540 μl of CD-1 mouse serum to attain final faropenem concentrations of 5, 20, 50, 100, 250, 500, and 1,000 μg/ml. After incubation at 37°C for 15 min, ultrafiltration was performed by centrifugation at 1,000 × g for 15 min at 25°C by using a micropartition system (MPS-3; Amicon). Counts of the radioactivity, before and after ultrafiltration, for total and free faropenem were determined by liquid scintillation counting (Tricarb 2200CA; Packard Instrument Co., Ltd.), and the percentage of faropenem bound to serum was calculated as follows: [1 − (free faropenem concentration/total faropenem concentration)] × 100. To determine the relationship between the total faropenem concentration and the percentage of faropenem bound to serum, the data were fit to a four-parameter logistic equation (GraphPad Prism).
Murine postexposure prophylaxis inhalation model.
For evaluation of the efficacy of faropenem, mice (10 per group) received aerosol inhalation of B. anthracis (Ames strain; faropenem MIC, 0.06 μg/ml) at 100 times the challenge dose that kills 50% of the animals (i.e., the 50% lethal dose [LD50], which was 3.4 × 106 CFU) nebulized for 20 min (11). The faropenem doses (10, 20, 40, and 80 mg/kg/day) were administered i.p. at 24 h postchallenge at 4-, 6-, and 12-h intervals for 14 days. For the negative control, animals received the vehicle i.p. every 12 h; and for the positive control, 30 mg/kg of ciprofloxacin was administered i.p. every 12 h for 14 days. Survival was assessed daily for 27 days. On day 27, the surviving mice were killed by exposure to CO2 and the bacterial burdens in lung tissue were determined. The lungs were aseptically removed, weighed, homogenized in 1 ml of sterile water, and then serially diluted 1:10 in sterile water; 100-μl aliquots were then plated on agar plates (SBAPs). The presence of B. anthracis spores was determined by heat shocking aliquots of the lung homogenates for 30 min at 65°C, and the homogenates were serially diluted and plated as described above. The SBAPs were incubated at 37°C for 18 h, the colonies were counted, and the counts were standardized to the tissue weight (11).
PK-PD modeling.
For each dosage regimen, plasma faropenem concentration-time profiles were determined by noncompartmental modeling. To correct for murine serum protein binding, the total faropenem concentrations were used to estimate plasma free-drug (f) concentrations on the basis of the four-parameter logistic equation derived for serum protein binding. By using the free-drug plasma concentration-time profiles derived from noncompartmental modeling and the MIC of the infecting organism, the ratio of the area under the concentration-time curve from 0 to 24 h for the free drug (fAUC0-24) to the MIC (fAUC0-24/MIC), the ratio of the free-drug maximum plasma concentration (fCmax) to the MIC (fCmax/MIC), and the cumulative percentage of a 24-h period that the free-drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (f%TMIC) were determined for each dosing regimen. For the noncompartmental model, the linear-log trapezoidal rule was applied for determination of fAUC0-24 and f%TMIC values.
To evaluate potential PK-PD relationships for efficacy, the sigmoid maximum-threshold-of-efficacy (Emax) model fit the survival data, in which the fAUC/MIC and fCmax/MIC ratios and f%TMIC were each evaluated separately as independent variables.
RESULTS
In vitro susceptibility.
The results in Table 1 show the activities of faropenem and the comparator agents against 29 isolates of B. anthracis. The activity of faropenem was evaluated against all isolates. The MIC values ranged from ≤0.06 to >64 μg/liter, and the MIC90 value was 0.5 μg/ml. The MIC90 value for faropenem was lower than that observed for other β-lactams, such as meropenem, amoxicillin-clavulanate, and penicillin, for which the MIC90 values were 4, 4, and 8 μg/ml, respectively. The MIC values for faropenem against strains known to variably express bla1 ranged from ≤0.06 to 1 μg/ml, whereas the MIC ranges were 1 to 4 μg/ml for penicillin, ≤0.06 to 8 μg/ml for meropenem, and 0.25 to 4 μg/ml for amoxicillin-clavulanate. As with the other β-lactams, faropenem was not active against the single strain of B. anthracis (strain SK57) that is known to express bla2, which encodes a metallo-β-lactamase (MIC, 64 μg/ml). The MIC90 values of the non-β-lactam agents were ≤0.06 μg/ml for doxycycline and tetracycline, 0.25 μg/ml for ciprofloxacin, and 4 μg/ml for azithromycin. As expected, the activities of the non-β-lactam agents were not compromised by the production of β-lactamases. The B. anthracis Ames strain has been established for evaluation in the murine postexposure prophylaxis inhalation model (1, 11) and was susceptible to faropenem (MIC value ≤ 0.06 μg/ml). Subsequent confirmation susceptibility testing showed that the MIC value of faropenem for the B. anthracis Ames strain was 0.06 μg/ml.
TABLE 1.
Activities of faropenem and comparator agents against Bla1 and Bla2 β-lactamase-producing B. anthracis strains
| B. anthracis strain | MIC (μg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Faropenem | Ciprofloxacin | Penicillin | Doxycycline | Meropenem | Azithromycin | Amoxicillin-clavulanate | Tetracycline | |
| Ames | ≤0.06 | ≤0.06 | 4 | ≤0.06 | 0.06 | 2 | 1 | ≤0.06 |
| Vollum 1B | 0.25 | 0.12 | 4 | ≤0.06 | 0.12 | 2 | 1 | ≤0.06 |
| Sterne | ≤0.06 | ≤0.06 | 4 | ≤0.06 | ≤0.06 | 2 | 0.25 | ≤0.06 |
| SK57 | >64 | ≤0.06 | 32 | ≤0.06 | 32 | 4 | 32 | ≤0.06 |
| Texas | ≤0.06 | 0.25 | 1 | ≤0.06 | ≤0.06 | 1 | 0.12 | ≤0.06 |
| Ohio | 0.06 | ≤0.06 | 1 | ≤0.06 | <0.06 | 2 | 0.5 | ≤0.06 |
| NH | 0.25 | ≤0.06 | 2 | ≤0.06 | 0.5 | 2 | 0.5 | ≤0.06 |
| Africa 33 | 0.12 | ≤0.06 | 8 | ≤0.06 | 4 | 2 | 1 | ≤0.06 |
| Colorado | ≤0.06 | ≤0.06 | 8 | ≤0.06 | 0.12 | 2 | 2 | ≤0.06 |
| K7978 | 0.25 | 0.25 | 2 | 0.25 | 0.25 | 4 | 1 | ≤0.06 |
| K5926 | 0.12 | 0.12 | 1 | ≤0.06 | 0.12 | 4 | 0.5 | ≤0.06 |
| 02AS-1 | 0.12 | 0.06 | 2 | ≤0.06 | 0.12 | 4 | 0.25 | ≤0.06 |
| K9724 | 0.12 | 0.12 | 2 | ≤0.06 | 0.12 | 4 | 0.25 | ≤0.06 |
| K4539 | 0.12 | 0.12 | 1 | ≤0.06 | 0.12 | 4 | 1 | ≤0.06 |
| BA1007 | 0.12 | ≤0.06 | 2 | ≤0.06 | 0.12 | 4 | 0.5 | ≤0.06 |
| 17T5 | 0.12 | ≤0.06 | 2 | ≤0.06 | 0.12 | 4 | 2 | ≤0.06 |
| V770 | ≤0.06 | 0.12 | 1 | ≤0.06 | 0.12 | 4 | 0.5 | ≤0.06 |
| V770-NP1-R | 0.12 | 0.12 | 1 | ≤0.06 | 0.12 | 4 | 0.25 | ≤0.06 |
| 003BS | 0.25 | 0.12 | 2 | ≤0.06 | 0.25 | 4 | 0.5 | ≤0.06 |
| BA1086 | 1 | ≤0.06 | 2 | ≤0.06 | 8 | 4 | 2 | ≤0.06 |
| K1938 | 0.12 | ≤0.06 | 2 | ≤0.06 | 0.12 | 4 | 1 | ≤0.06 |
| K7038 | 0.12 | 0.12 | 1 | ≤0.06 | 0.25 | 4 | 0.25 | ≤0.06 |
| SPS 0053 | 0.12 | ≤0.06 | 8 | ≤0.06 | 0.25 | 4 | 4 | ≤0.06 |
| BA1024 | 0.5 | 0.25 | 4 | ≤0.06 | 0.25 | 4 | 4 | ≤0.06 |
| BA0018 | ≤0.06 | 1 | 2 | ≤0.06 | 0.25 | 4 | 1 | ≤0.06 |
| Texas 2 | ≤0.06 | ≤0.06 | 4 | ≤0.06 | 0.12 | 4 | 2 | ≤0.06 |
| K8091 | ≤0.06 | ≤0.06 | 4 | ≤0.06 | 0.12 | 2 | 0.5 | ≤0.06 |
| B126 | 0.12 | 0.12 | 4 | ≤0.06 | 0.25 | 2 | 1 | ≤0.06 |
| B133 | ≤0.06 | ≤0.06 | 2 | ≤0.06 | 0.12 | 4 | 1 | ≤0.06 |
| MIC range | ≤0.06->64 | ≤0.06-1 | 1-32 | ≤0.06-0.25 | ≤0.06-32 | 1-4 | 0.12-32 | ≤0.06 |
| MIC50 | 0.12 | ≤0.06 | 2 | ≤0.06 | 0.12 | 4 | 1 | ≤0.06 |
| MIC90 | 0.5 | 0.25 | 8 | ≤0.06 | 4 | 4 | 4 | ≤0.06 |
The mode MIC for faropenem against all isolates was 0.12 μg/ml, whereas the mode MICs were 1 μg/ml for amoxicillin-clavulanate and 2 μg/ml for penicillin. B. anthracis SK57 (bla2; metallo-β-lactamase producer) accounted for the outlier MIC values of >64 μg/ml for faropenem and 32 μg/ml for amoxicillin-clavulanate and penicillin. For the strains known to only variably express bla1, no isolates with faropenem MICs of >1 μg/ml were observed.
Pharmacokinetics.
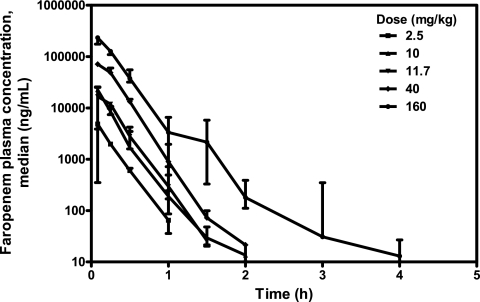
Across the dose groups, the plasma faropenem concentration profiles showed similar behaviors in mice following i.p. administration, with the time of the maximum plasma concentration in plasma (Tmax) being observed at 5 min, followed by a decline in the concentration that was predominantly monoexponential, with the t1/2 being approximate 0.19 h (Fig. 1 and Table 2). At the highest dose, a second elimination phase for which t1/2 was 0.5 h was observed. At doses of 2.5, 10, 11.7, 40, and 160 mg/kg, the faropenem Cmaxs were 4.87, 22.6, 16.7, 71.1, and 234 μg/ml, respectively; and the corresponding values of the AUC from time zero to infinity (AUC0-∞) were 1.16, 4.80, 5.24, 22.1, and 66.2 μg·h/ml. Linear pharmacokinetics were observed for both AUC0-∞ and Cmax across the dose range evaluated.
FIG. 1.
Median faropenem plasma concentration-versus-time curves after i.p. administration to female BALB/c mice. The medians (closed symbols) and ranges (bars) are shown (n = 3 mice per time point).
TABLE 2.
Noncompartmental plasma pharmacokinetic parameters of faropenem following i.p. administration to female BALB/c mice
| Pharmacokinetic parametera | Value for the following dose (mg/kg)b: |
||||
|---|---|---|---|---|---|
| 2.5 | 10 | 11.7 | 40 | 160 | |
| Cmax (μg/ml) | 4.87 | 22.6 | 16.7 | 71.1 | 234 |
| Tmax (h) | 0.0833 | 0.0833 | 0.0833 | 0.0833 | 0.0833 |
| AUC0-∞ (μg·h/ml) | 1.16 | 4.80 | 5.24 | 22.1 | 66.2 |
| AUC%EXP | 1.23 | 0.106 | 0.106 | 0.026 | 0.0149 |
| CL/F (ml/h/kg) | 1750 | 1690 | 1810 | 1460 | 1960 |
| t1/2 (h) | 0.153 | 0.262 | 0.148 | 0.186 | 0.528 |
AUC%EXP, percentage of AUC extrapolated between AUC0-∞ and the last AUC measured; CL/F, clearance.
Dose based on the use of faropenem sodium.
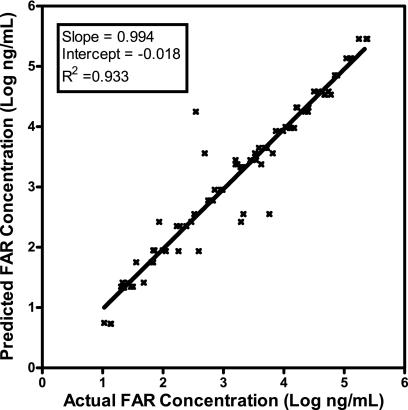
Since dose linear pharmacokinetics were observed in mice, a noncompartmental model was calculated on the basis of the dose-normalized median values at each time point (for the 1-mg/kg dose, the values were 2,190 ng/ml at 0.0833 h, 1,050 ng/ml at 0.25 h, 294 ng/ml at 0.5 h, 27.8 ng/ml at 1 h, 2.74 ng/ml at 1.5 h, and 0.664 ng/ml at 2 h); the median t1/2 was 0.186 h. Comparison of the actual plasma faropenem concentrations versus the predicted values from the noncompartmental model are shown in Fig. 2. The R2 value of 0.933, the slope of 0.994, and a y intercept of −0.018 indicate that this is an acceptable model for prediction of the plasma faropenem concentrations over the dose range of 2.5 to 160 mg/kg.
FIG. 2.
Actual plasma faropenem (FAR) concentrations versus predicted plasma faropenem concentrations determined by use of the noncompartmental model (linear fit to log values; n = 80). Plasma faropenem concentrations were measurable at between 0.0833 and 2 h for the 2.5- to 160-mg/kg dose groups.
Serum protein binding.
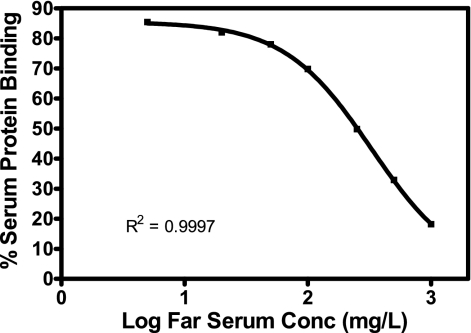
Assessment of faropenem mouse serum protein binding by ultrafiltration displayed concentration-dependent binding, with approximately 85% being bound at 5 μg/ml; with increasing faropenem concentrations, the level of protein binding decreased to approximately 18% bound at a faropenem concentration of 1,000 μg/ml (Fig. 3). Similar results were determined for human serum, with 90% binding occurring with a faropenem concentration of 5 μg/ml and 21.3% binding occurring with one of 1,000 μg/ml.
FIG. 3.
Percentage of faropenem (Far) bound to mouse serum proteins for various faropenem concentrations. The values are means (n = 2 to 3). The fitted line represents the four-parameter logistic fit.
Pharmacodynamics (PDs).
The survival of mice infected with B. anthracis was assessed according to the different faropenem doses and dosing regimens used. Figure 4 shows a representative Kaplan-Meier survival plot for animals that received faropenem at 20 mg/kg/day by the use of various dose schedules. Mice treated with vehicle alone died by day 4. Animals treated with faropenem at 20 mg/kg/day administered every 4 h and animals treated with ciprofloxacin at 30 mg/kg/day administered every 12 h had comparable survival rates. The day 27 survival data for the controls and the groups treated with faropenem at different doses and on different schedules are shown in Table 3.
FIG. 4.
Assessment of survival in postexposure prophylaxis B. anthracis treatment model in BALB/c mice.
TABLE 3.
Anthrax postexposure prophylaxis murine model survival dataa
| Test article | Dose (mg/kg/day) | Schedule | % survival on day 27 |
|---|---|---|---|
| Control | q12h | 0 | |
| Faro | 10 | q4h | 77.8 |
| Faro | 20 | q4h | 80 |
| Faro | 40 | q4h | 80 |
| Faro | 80 | q4h | 100 |
| Faro | 10 | q6h | 30 |
| Faro | 20 | q6h | 60 |
| Faro | 40 | q6h | 90 |
| Faro | 80 | q6h | 90 |
| Faro | 10 | q12h | 0 |
| Faro | 20 | q12h | 10 |
| Faro | 40 | q12h | 20 |
| Faro | 80 | q12h | 22.2 |
| Cipro | 60 | q12h | 90 |
Abbreviations: Faro, faropenem; Cipro, ciprofloxacin; q4h, every 4 h; q6h, every 6 h; q12h, every 12 h.
In addition to assessment of the rates of survival, the B. anthracis spore loads in the lungs of the surviving animals from all experimental groups were evaluated on day 27. The surviving faropenem-treated animals had spore levels that were within the range of those for the surviving ciprofloxacin-treated animals and below the established threshold for the reestablishment of disease (11), indicating that postexposure protection had been achieved.
PK-PD modeling.
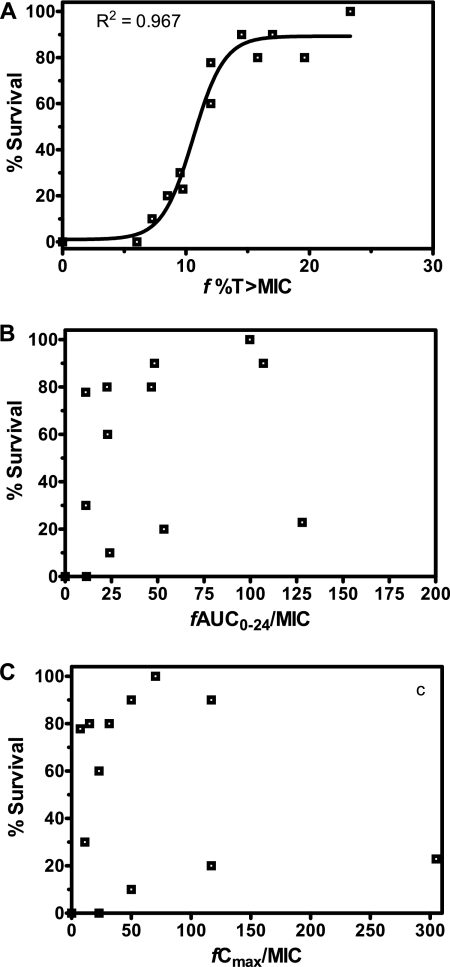
The relationships between the percentage of mice surviving on day 27 following postexposure prophylaxis with faropenem against B. anthracis and each of the PK-PD measures, f%TMIC, the fAUC/MIC ratio, and the fCmax/MIC ratio, are shown in Fig. 5A to C, respectively. Of these, only the faropenem f%TMIC demonstrated a significant correlation with percent survival (R2 = 0.967). On the basis of this relationship, the 50% effective dose (ED50), ED90, and ED99 corresponded to f%TMIC values of 10.6, 13.4, and 16.4%, respectively, and Emax was 89.3%. The Emax value of 89.3% was similar to the overall rate of survival observed for the ciprofloxacin-positive control group (90%).
FIG. 5.
Relationships between percent survival and the three faropenem PK-PD measures, f%TMIC (A), fAUC/MIC ratio (B), and fCmax/MIC ratio (C).
DISCUSSION
The importance of treating anthrax in humans is underscored by the bioterrorism events of October 2001. While ciprofloxacin has been widely used for prophylaxis following potential exposure to anthrax spores, penicillin has traditionally been the drug of choice because of its well-established safety profile. The Centers for Disease Control and Prevention recommends the use of penicillin, ciprofloxacin, and doxycycline for the treatment of human cases of anthrax. Although penicillin has been widely used, there have been reports from some studies, although rare, of the identification of penicillin-resistant B. anthracis, including β-lactamase-producing strains (4, 5). The isolates of B. anthracis that were used to challenge the activity of faropenem in this study included strains previously shown to contain and variably express the two β-lactamase genes identified in this organism (4). Faropenem exhibited MIC values lower than those of penicillin and amoxicillin-clavulanate against all the bla1 strains of B. anthracis, thus highlighting its potential as a candidate for further evaluation in the murine postexposure prophylaxis model.
The assessment of PK-PD relationships for efficacy and the identification of PK-PD targets associated with a given threshold of effect based on animal studies provide critical information that may be used to guide the selection of dosing regimens for clinical prophylaxis and treatment. In the case of potential agents of bioterrorism, such as B. anthracis, nonclinical animal studies are the only option, as clinical studies with humans are not possible. Only recently have animal PK-PD studies been conducted to assess the appropriate exposure response targets and the associated values for antibiotic prophylaxis and treatment of B. anthracis infections (1).
In the murine B. anthracis inhalation postexposure prophylaxis model described herein, a clear survival response was observed from the faropenem f%TMIC values. The other PK-PD measures evaluated, the fAUC0-24/MIC and fCmax/MIC ratios, showed minimal correlations with survival. This result is consistent with those previously obtained for β-lactams as a class, for which f%TMIC has been shown to be the primary PK-PD parameter describing efficacy against bacterial infections (7). For faropenem, the f%TMIC value required for maximum survival (ED99) against B. anthracis infections was 16.4%. Overall, this low value for f%TMIC required for survival indicates that faropenem has a high level of in vivo potency.
The short f%TMIC value required for the activity of faropenem against B. anthracis on the basis of the data presented herein is consistent with values determined against S. pneumoniae by Craig and Andes in the neutropenic murine thigh infection model (8). Against 13 strains of Streptococcus pneumoniae (MIC values, 0.008 to 2 μg/ml), the mean faropenem f%TMIC for stasis was 13.9% (8). Overall, these two studies are consistent with faropenem demonstrating a high level of potency in the eradication of bacterial infections.
The current treatment options for humans who are exposed to B. anthracis by inhalation require long durations (60 days) and are limited to a few antibiotics. Due to safety concerns, most of these treatments are not considered appropriate for children or women of childbearing potential, and for this population, faropenem medoxomil could provide an appropriate alternative. The pharmacokinetics of faropenem in healthy adults following the administration of 600 mg of faropenem medoxomil showed mean (standard deviation) values for AUC0-∞ of 51.7 (11.0) μg·h/ml, Cmax of 22.4 (7.95) μg/ml, and t1/2 of 1.10 (0.18) h and median values for Tmax of 1.0 h (range, 0.5 to 6.0 h; n = 56) (10). By using a value of 90% protein binding (12), average adult subjects receiving 600 mg of faropenem medoxomil twice daily are estimated to have an f%TMIC of 62% for the B. anthracis Ames strain, which is well in excess of the 16.4% needed on the basis of the findings obtained with the murine model. For MIC values of 0.12, 0.25, and 0.5 μg/ml, the estimated mean f%TMIC values are 51, 44, and 32%, respectively. Clearly, analyses that account for both PK and PD variability are needed to forecast the probability of attaining effective f%TMIC targets in humans. However, given the susceptibility data presented here, faropenem would be expected to be active against many B. anthracis strains, with the exception of those that carry the metallo-β-lactamase. Ultimately, to evaluate the potential of faropenem medoxomil for the treatment of B. anthracis inhalation exposure, additional studies with nonhuman primates will be required to demonstrate efficacy and additional studies with humans will be required to demonstrate adequate safety.
Acknowledgments
We thank Yasushi Kanai and Maomi Morozumi (Bio-Pharma Tech Center, Suntory Limited) for their contributions associated with the faropenem serum binding studies.
Footnotes
Published ahead of print on 9 February 2010.
REFERENCES
- 1.Ambrose, P. G., A. Forrest, W. A. Craig, C. M. Rubino, S. M. Bhavnani, G. L. Drusano, and H. S. Heine. 2007. Pharmacokinetics-pharmacodynamics of gatifloxacin in a lethal murine Bacillus anthracis inhalation infection model. Antimicrob. Agents Chemother. 51:4351-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beharry, Z., H. Chen, V. R. Gadhachanda, J. D. Buynak, and T. Palzkill. 2004. Evaluation of penicillin-based inhibitors of the class A and B beta-lactamases from Bacillus anthracis. Biochem. Biophys. Res. Commun. 313:541-545. [DOI] [PubMed] [Google Scholar]
- 3.Black, J. A., E. Smith Moland, T. J. Lockhart, P. D. Lister, and K. S. Thomson. 2001. Faropenem: activity against ESBL, AmpC, and other β-lactamase-producing Enterobacteriaceae, abstr. E-791. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 4.Chen, Y., J. Succi, F. C. Tenover, and T. M. Koehler. 2003. Beta-lactamase genes of the penicillin-susceptible Bacillus anthracis Sterne strain. J. Bacteriol. 185:823-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y., F. C. Tenover, and T. M. Koehler. 2004. Beta-lactamase gene expression in a penicillin-resistant Bacillus anthracis strain. Antimicrob. Agents Chemother. 48:4873-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing, 17th informational supplement. M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. A., and D. R. Andes. 2001. In vivo pharmacodynamic activity of faropenem against Streptococcus pneumoniae, abstr. A-2094. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 9.Dalhoff, A., N. Janjic, and R. Echols. 2006. Redefining penems. Biochem. Pharmacol. 71:1085-1095. [DOI] [PubMed] [Google Scholar]
- 10.Gill, S., and R. Tosiello. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1492.
- 11.Heine, H. S., J. Bassett, L. Miller, J. M. Hartings, B. E. Ivins, M. L. Pitt, D. Fritz, S. L. Norris, and W. R. Byrne. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob. Agents Chemother. 51:1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis, T. C., K. Clawson Stone, I. A. Critchley, S. C. Gill, and N. Janjic. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-6.
- 13.Keim, P., L. B. Price, A. M. Klevytska, K. L. Smith, J. M. Schupp, R. Okinaka, P. J. Jackson, and M. E. Hugh-Jones. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 182:2928-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammed, M. J., C. K. Marston, T. Popovic, R. S. Weyant, and F. C. Tenover. 2002. Antimicrobial susceptibility testing of Bacillus anthracis: comparison of results obtained by using the National Committee for Clinical Laboratory Standards broth microdilution reference and Etest agar gradient diffusion methods. J. Clin. Microbiol. 40:1902-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 16.Siegert, R., O. Berg, P. Gehanno, A. Leiberman, J. L. Martinkenas, P. Nikolaidis, P. Arvis, M. Alefelder, and P. Reimnitz. 2003. Comparison of the efficacy and safety of faropenem daloxate and cefuroxime axetil for the treatment of acute bacterial maxillary sinusitis in adults. Eur. Arch. Otorhinolaryngol. 260:186-194. [DOI] [PubMed] [Google Scholar]
- 17.Upchurch, J., M. Rosemore, R. Tosiello, S. Kowalsky, and R. Echols. 2006. Randomized double-blind study comparing 7- and 10-day regimens of faropenem medoxomil with a 10-day cefuroxime axetil regimen for treatment of acute bacterial sinusitis. Otolaryngol. Head Neck Surg. 135:511-517. [DOI] [PubMed] [Google Scholar]