Abstract
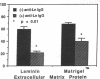
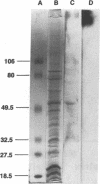
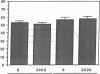
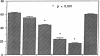
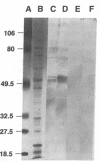
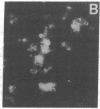
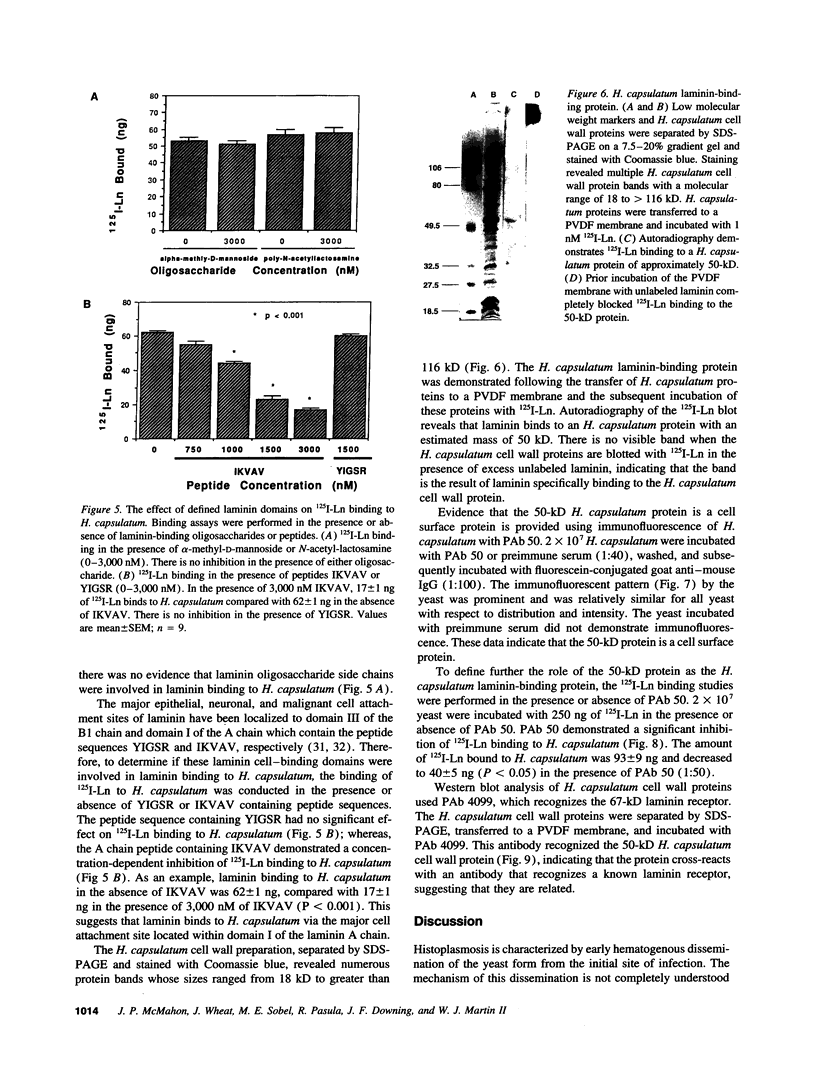
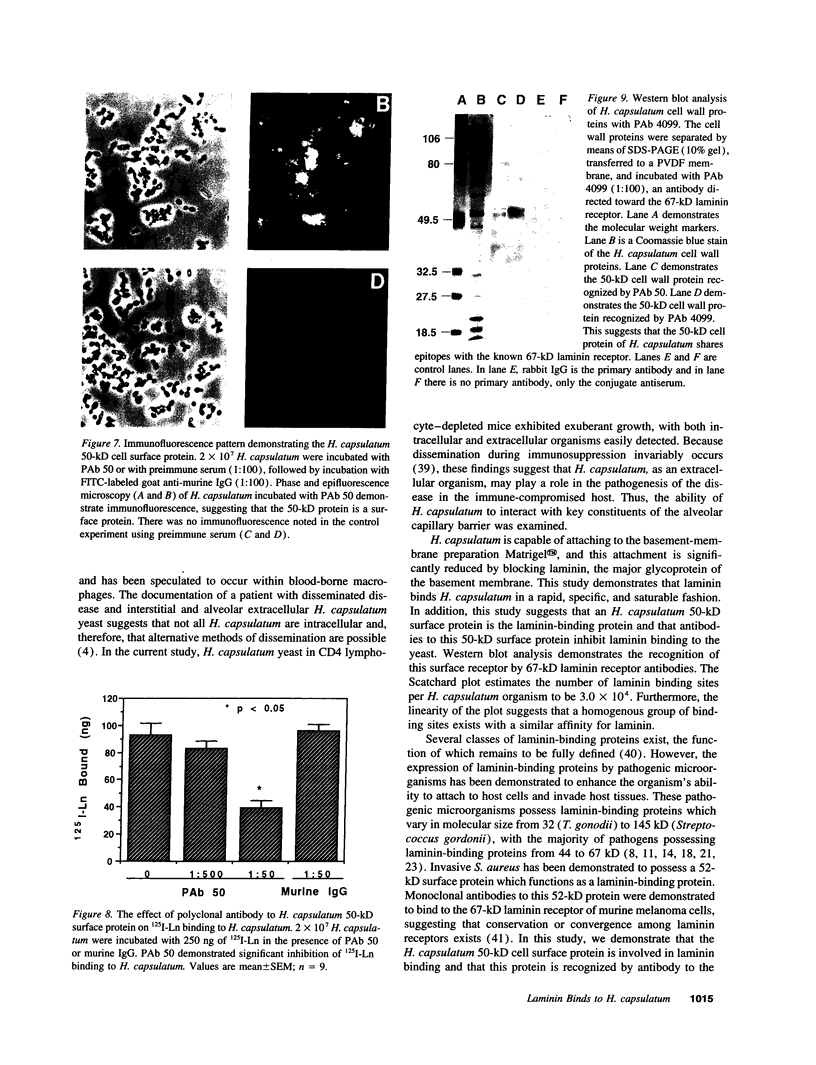
Histoplasmosis, an increasingly important opportunistic infection in immunosuppressed subjects, is characterized by hematogenous dissemination of the yeast from the lung. The mechanism of this dissemination is not fully understood. Laminin, the major glycoprotein of the extracellular matrix, is known to mediate the attachment of various invasive pathogens to host tissues. In the current study, laminin is demonstrated to bind to Histoplasma capsulatum in a rapid, specific, and saturable manner. Scatchard analysis with 125I-labeled laminin revealed an estimated 3.0 x 10(4) binding sites per yeast with an apparent Kd for laminin binding of 1.6 x 10(-9) M. Laminin binding to H. capsulatum was decreased from 62 +/- 1 to 17 +/- 1 ng (P < 0.001) in the presence of 3,000 nM of Ile-Lys-Val-Ala-Val, a pentapeptide within one major cell attachment site of laminin. A 50-kD H. capsulatum laminin-binding protein was demonstrated using an 125I-Ln blot of H. capsulatum cell wall proteins. The 50-kD protein is also recognized by antibodies directed at the 67-kD laminin receptor, suggesting they are related. This study proposes a possible mechanism for H. capsulatum attachment to laminin, an important first step required for the yeast to recognize and traverse the basement membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bao Z. Z., Muschler J., Horwitz A. F. LBL, a novel, developmentally regulated, laminin-binding lectin. J Biol Chem. 1992 Mar 5;267(7):4974–4980. [PubMed] [Google Scholar]
- Beck K., Hunter I., Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990 Feb 1;4(2):148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronovo V., Taraboletti G., Sobel M. E. Functional domains of the 67-kDa laminin receptor precursor. J Biol Chem. 1991 Oct 25;266(30):20440–20446. [PubMed] [Google Scholar]
- Castronovo V., Taraboletti G., Sobel M. E. Laminin receptor complementary DNA-deduced synthetic peptide inhibits cancer cell attachment to endothelium. Cancer Res. 1991 Oct 15;51(20):5672–5678. [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado G. C., Slowik M., Kleinman H. K., Joiner K. A. Laminin enhances binding of Toxoplasma gondii tachyzoites to J774 murine macrophage cells. Infect Immun. 1992 Jun;60(6):2337–2342. doi: 10.1128/iai.60.6.2337-2342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Iwamoto Y., Sasaki M., Martin G. R., Kleinman H. K., Robey F. A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987 Mar 27;48(6):989–996. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Kamel S. M., Wheat L. J., Garten M. L., Bartlett M. S., Tansey M. R., Tewari R. P. Production and characterization of murine monoclonal antibodies to Histoplasma capsulatum yeast cell antigens. Infect Immun. 1989 Mar;57(3):896–901. doi: 10.1128/iai.57.3.896-901.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen M., Raunio T., Virkola R., Lähteenmäki K., Mäkelä P. H., Klemm P., Clegg S., Korhonen T. K. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type I fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol Microbiol. 1993 Jan;7(2):229–237. doi: 10.1111/j.1365-2958.1993.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- López-Ribot J. L., Casanova M., Monteagudo C., Sepúlveda P., Martínez J. P. Evidence for the presence of a high-affinity laminin receptor-like molecule on the surface of Candida albicans yeast cells. Infect Immun. 1994 Feb;62(2):742–746. doi: 10.1128/iai.62.2.742-746.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Mecham R. P. Receptors for laminin on mammalian cells. FASEB J. 1991 Aug;5(11):2538–2546. doi: 10.1096/fasebj.5.11.1651264. [DOI] [PubMed] [Google Scholar]
- Medoff G., Kobayashi G. S., Painter A., Travis S. Morphogenesis and pathogenicity of Histoplasma capsulatum. Infect Immun. 1987 Jun;55(6):1355–1358. doi: 10.1128/iai.55.6.1355-1358.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota G. F., Carneiro C. R., Gomes L., Lopes J. D. Monoclonal antibodies to Staphylococcus aureus laminin-binding proteins cross-react with mammalian cells. Infect Immun. 1988 Jun;56(6):1580–1584. doi: 10.1128/iai.56.6.1580-1584.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Bucher C., Rhodes J., Bullock W. E. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J Clin Invest. 1990 Jan;85(1):223–230. doi: 10.1172/JCI114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCKNOW J. J., PAGE M. I., LOOSLI C. G. Early pathogenesis of experimental histoplasmosis. Arch Pathol. 1960 Apr;69:413–426. [PubMed] [Google Scholar]
- Paulsson M., Ljungh A., Wadström T. Rapid identification of fibronectin, vitronectin, laminin, and collagen cell surface binding proteins on coagulase-negative staphylococci by particle agglutination assays. J Clin Microbiol. 1992 Aug;30(8):2006–2012. doi: 10.1128/jcm.30.8.2006-2012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Rao C. N., Castronovo V., Schmitt M. C., Wewer U. M., Claysmith A. P., Liotta L. A., Sobel M. E. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. 1989 Sep 5;28(18):7476–7486. doi: 10.1021/bi00444a047. [DOI] [PubMed] [Google Scholar]
- Reynolds R. J., 3rd, Penn R. L., Grafton W. D., George R. B. Tissue morphology of Histoplasma capsulatum in acute histoplasmosis. Am Rev Respir Dis. 1984 Aug;130(2):317–320. doi: 10.1164/arrd.1984.130.2.317. [DOI] [PubMed] [Google Scholar]
- Shellito J., Suzara V. V., Blumenfeld W., Beck J. M., Steger H. J., Ermak T. H. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest. 1990 May;85(5):1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Piotrowski J., Sengupta S., Slomiany A. Inhibition of gastric mucosal laminin receptor by Helicobacter pylori lipopolysaccharide. Biochem Biophys Res Commun. 1991 Mar 29;175(3):963–970. doi: 10.1016/0006-291x(91)91659-z. [DOI] [PubMed] [Google Scholar]
- Sommer P., Gleyzal C., Guerret S., Etienne J., Grimaud J. A. Induction of a putative laminin-binding protein of Streptococcus gordonii in human infective endocarditis. Infect Immun. 1992 Feb;60(2):360–365. doi: 10.1128/iai.60.2.360-365.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Tashiro K., Sephel G. C., Weeks B., Sasaki M., Martin G. R., Kleinman H. K., Yamada Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989 Sep 25;264(27):16174–16182. [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Larcher G., Lissitzky J. C., Chabasse D. Interaction between Aspergillus fumigatus and basement membrane laminin: binding and substrate degradation. Biol Cell. 1993;77(2):201–208. doi: 10.1016/s0248-4900(05)80189-3. [DOI] [PubMed] [Google Scholar]
- Trust T. J., Doig P., Emödy L., Kienle Z., Wadström T., O'Toole P. High-affinity binding of the basement membrane proteins collagen type IV and laminin to the gastric pathogen Helicobacter pylori. Infect Immun. 1991 Dec;59(12):4398–4404. doi: 10.1128/iai.59.12.4398-4404.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkonen K. H., Ringner M., Ljungh A., Wadström T. High-affinity binding of laminin by Helicobacter pylori: evidence for a lectin-like interaction. FEMS Immunol Med Microbiol. 1993 Jun;7(1):29–37. doi: 10.1111/j.1574-695X.1993.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Valkonen K. H., Veijola J., Dagberg B., Uhlin B. E. Binding of basement-membrane laminin by Escherichia coli. Mol Microbiol. 1991 Sep;5(9):2133–2141. doi: 10.1111/j.1365-2958.1991.tb02143.x. [DOI] [PubMed] [Google Scholar]
- Vicentini A. P., Gesztesi J. L., Franco M. F., de Souza W., de Moraes J. Z., Travassos L. R., Lopes J. D. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect Immun. 1994 Apr;62(4):1465–1469. doi: 10.1128/iai.62.4.1465-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat L. J., Connolly-Stringfield P. A., Baker R. L., Curfman M. F., Eads M. E., Israel K. S., Norris S. A., Webb D. H., Zeckel M. L. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990 Nov;69(6):361–374. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]