Abstract
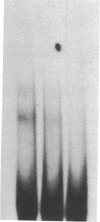
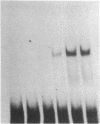
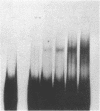
A DNA-binding protein has been identified that recognizes runs of deoxyadenines and/or deoxythymines (dA/dT sequences) and purified from a chromatographic fraction containing the multiprotein DNA polymerase alpha-primase complex of HeLa cells by successive steps of chromatography on oligo(dT)-cellulose and Q-Sepharose. Polyacrylamide gel electrophoresis of the purified dA/dT sequence-binding protein in the presence of NaDodSO4 showed a single protein band of 62 kDa. Nitrocellulose filter binding assays using homopolydeoxynucleotides indicated that the purified protein preferentially binds to dA/dT sequences in single-stranded or duplex DNAs. Gel mobility shift assays with a variety of DNAs showed that the purified protein specifically binds to a fragment of simian virus 40 DNA containing the minimal (core) origin for replication. The binding occurred in a protein-dependent manner and in the presence of a vast excess of competing DNAs lacking the simian virus replication origin. The origin binding was reduced, however, when DNA fragments from simian virus 40 deletion mutants containing deletions within the 17-base-pair A + T-rich tract in the core DNA replication origin were used in the assays. These results indicate that the dA/dT sequence-binding protein preferentially binds to the 17-base-pair A + T-rich tract and suggest a possible role for the protein in the initiation of DNA replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baur C. P., Knippers R. Protein-induced bending of the simian virus 40 origin of replication. J Mol Biol. 1988 Oct 20;203(4):1009–1019. doi: 10.1016/0022-2836(88)90125-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chiu R. W., Baril E. F. Nuclear DNA polymerases and the HeLa cell cycle. J Biol Chem. 1975 Oct 10;250(19):7951–7957. [PubMed] [Google Scholar]
- Deb S., DeLucia A. L., Koff A., Tsui S., Tegtmeyer P. The adenine-thymine domain of the simian virus 40 core origin directs DNA bending and coordinately regulates DNA replication. Mol Cell Biol. 1986 Dec;6(12):4578–4584. doi: 10.1128/mcb.6.12.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. Purification of a yeast protein that binds to origins of DNA replication and a transcriptional silencer. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2120–2124. doi: 10.1073/pnas.85.7.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P., Lehman I. R. Interaction of origin binding protein with an origin of replication of herpes simplex virus 1. Proc Natl Acad Sci U S A. 1988 May;85(9):2959–2963. doi: 10.1073/pnas.85.9.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M., Lapidot J., Weisman-Shomer P. A DNA template recognition protein: partial purification from mouse liver and stimulation of DNA polymerase alpha. Biochemistry. 1985 Dec 17;24(26):7549–7556. doi: 10.1021/bi00347a007. [DOI] [PubMed] [Google Scholar]
- Fry M., Perrino F. W., Levy A., Loeb L. A. Factor D is a selective single-stranded oligodeoxythymidine binding protein. Nucleic Acids Res. 1988 Jan 11;16(1):199–211. doi: 10.1093/nar/16.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Furth M. E., McLeester C., Dove W. F. Specificity determinants for bacteriophage lambda DNA replication. I. A chain of interactions that controls the initiation of replication. J Mol Biol. 1978 Dec 5;126(2):195–225. doi: 10.1016/0022-2836(78)90359-5. [DOI] [PubMed] [Google Scholar]
- Gerard R., Gluzman Y. Functional analysis of the role of the A + T-rich region and upstream flanking sequences in simian virus 40 DNA replication. Mol Cell Biol. 1986 Dec;6(12):4570–4577. doi: 10.1128/mcb.6.12.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz G. Z., Young M. R., Mertz J. E. The A+T-rich sequence of the simian virus 40 origin is essential for replication and is involved in bending of the viral DNA. J Virol. 1987 Jul;61(7):2322–2325. doi: 10.1128/jvi.61.7.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Li J. J., Peden K. W., Dixon R. A., Kelly T. Functional organization of the simian virus 40 origin of DNA replication. Mol Cell Biol. 1986 Apr;6(4):1117–1128. doi: 10.1128/mcb.6.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes W., Bonin P., Baril E. Exonuclease activity associated with a multiprotein form of HeLa cell DNA polymerase alpha. Purification and properties of the exonuclease. J Biol Chem. 1986 May 15;261(14):6629–6636. [PubMed] [Google Scholar]
- Stillman B., Gerard R. D., Guggenheimer R. A., Gluzman Y. T antigen and template requirements for SV40 DNA replication in vitro. EMBO J. 1985 Nov;4(11):2933–2939. doi: 10.1002/j.1460-2075.1985.tb04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Traut W., Fanning E. Sequence-specific interactions between a cellular DNA-binding protein and the simian virus 40 origin of DNA replication. Mol Cell Biol. 1988 Feb;8(2):903–911. doi: 10.1128/mcb.8.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Purification of bacteriophage lambda O protein that specifically binds to the origin of replication. Mol Gen Genet. 1981;181(3):325–331. doi: 10.1007/BF00425606. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. Electrophoretic assay for DNA-binding proteins. Methods Enzymol. 1987;151:551–565. doi: 10.1016/s0076-6879(87)51044-8. [DOI] [PubMed] [Google Scholar]
- Vishwanatha J. K., Coughlin S. A., Wesolowski-Owen M., Baril E. F. A multiprotein form of DNA polymerase alpha from HeLa cells. Resolution of its associated catalytic activities. J Biol Chem. 1986 May 15;261(14):6619–6628. [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Interaction of Escherichia coli dnaB and dnaC(D) gene products in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):921–925. doi: 10.1073/pnas.72.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., DePamphilis M. L. DNA binding site for a factor(s) required to initiate simian virus 40 DNA replication. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1646–1650. doi: 10.1073/pnas.83.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Binding and bending of the lambda replication origin by the phage O protein. EMBO J. 1985 Dec 16;4(13A):3605–3616. doi: 10.1002/j.1460-2075.1985.tb04124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Rapaport E., Baril E. F. Priming of DNA synthesis by diadenosine 5',5"'-P1,P4-tetraphosphate with a double-stranded octadecamer as a template and DNA polymerase alpha. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1791–1794. doi: 10.1073/pnas.79.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]