Abstract
Studies of the genetics of G2/M checkpoints in budding and fission yeasts have produced many of the defining concepts of checkpoint biology. Recent progress in the biochemistry of the checkpoint gene products is adding a mechanistic understanding to our models and identifying the components of the normal cell cycle machinery that are targeted by checkpoints.
Introduction
The paradigm that checkpoints arrest the cell cycle to allow time for completion of a previous cell cycle event or repair of cellular damage has come to be seen as a fundamental part of cell cycle regulation. This is as a result of an appreciation of the widespread occurrence of checkpoints and the realization that distinctions between checkpoints and the mechanisms that normally control the timing of cell cycle transitions are often semantic. In the past few years checkpoint research has blossomed in breadth [1] and depth [2]. Checkpoints have been identified that regulate all of the major mitotic cell cycle transitions, and several of the meiotic cell cycle transitions. Checkpoint proteins also have roles in transcriptional regulation, induction of DNA repair, recovery from cell cycle arrest, adaptation and, in metazoans, apoptosis. Increasingly detailed genetic, molecular and biochemical analyses of the gene products involved in checkpoints are rapidly revealing the mechanisms by which they exert their effects.
This review covers recent developments in this field from work on Saccharomyces cerevisiae and Schizosaccharomyces pombe. We focus on the cell cycle arrest functions of the G2/M DNA damage checkpoint that prevents mitosis in the presence of damaged DNA, and the S/M replication checkpoint that prevents mitosis when DNA replication is blocked, dealing primarily with experiments that show a direct effect on cell cycle progression.
G2/M checkpoints prevent cells from passing through mitosis. In some cases, such as the G2 DNA-damage checkpoint in S. pombe and vertebrates, cells arrest in G2 [3,4], while in others, such as the DNA-damage and spindle checkpoints in S. cerevisiae, cells arrest during M phase at the metaphase to anaphase transition [5,6]. But, as near as we can tell, the general purpose of G2/M checkpoints is to prevent sister chromatid separation which, in the presence of either DNA damage or incomplete replication, is the irreversible step that turns a temporary setback into a lethal event (Figure 1a). Therefore, G2/M checkpoints need to target an event just before sister chromatid separation. In S. pombe and vertebrates, the G2/M transition, regulated by the inhibitory tyrosine phosphorylation of Cdc2sp, serves this purpose [7••,8••,9]. In S. cerevisiae, however, the events regulated by the tyrosine phosphorylation of Cdc28sc, such as spindle pole body separation and the bud formation checkpoint, occur much earlier in the cell cycle, at around the end of S phase [10,11] (Figure b). Thus, the period between S phase and mitosis in S. cerevisiae is, in some ways, more akin to metaphase than to G2. Instead of targeting the transition, the S. cerevisiae checkpoints target the metaphase to anaphase transition [5,12]. To help distinguish between the overlapping nomenclature of the two yeasts, in this review S. cerevisiae and S. pombe gene and protein names are identified by super scripted sc and sp, respectively.
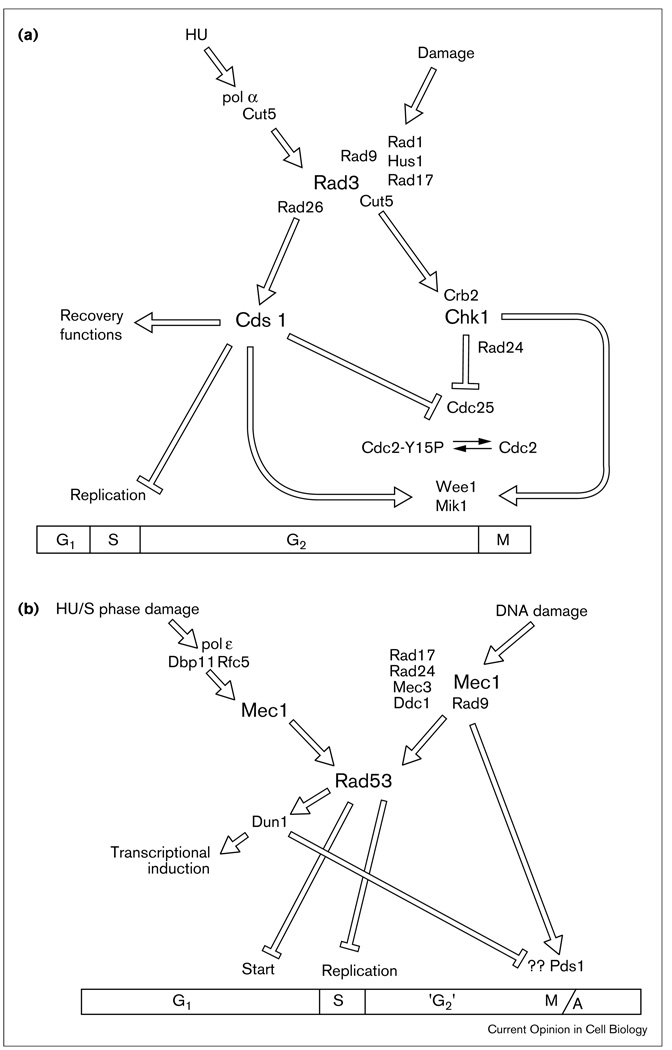
Figure 1.
Models of checkpoint control in (a) S. pombe and (b) S. cerevisiae. These models of the checkpoint regulatory pathways represent the interpretation of a variety of genetic, molecular and biochemical data. The proximity of protein names to each other does not necessarily imply a physical interaction. (a) The position of Cut5sp in two places reflects an uncertainty about its exact role. (b) The question marks represent an unknown target of the Rad53sc/Dun1sc pathway. It is unknown if this target is independent of the role of Dun1sc in transcriptional induction.
The Schizosaccharomyces pombe G2 DNA damage checkpoint
The proteins involved in the G2 DNA damage checkpoint can be divided into three groups: the checkpoint rad proteins that are also involved in the S/M replication checkpoint, the G2 DNA-damage checkpoint-specific proteins, and the target genes that are part of the normal mitotic machinery. The checkpoint rad genes include rad1sp, rad3sp, rad9sp, rad17sp, rad26sp, and hus1sp. These are required for all the known S. pombe DNA checkpoints [3,13,14]. At the very least, the checkpoint rad gene products serve as signal transducers between the primary checkpoint signal and the cell cycle machinery. They may also be directly involved in the recognition of DNA damage, since several are similar to proteins involved in DNA metabolism (Table 1). Rad1sp is similar to the Ustilago Rec1 exonuclease, the putative human Rad1sp homolog (hRad1) displays exonuclease activity [15,16•], and Rad17sp has limited similarity to replication factor C (RFC) [17]. Furthermore, S. cerevisiae homologs of Rad1 and Rad17 have been implicated in the processing of DNA damage [18]. The most interesting similarity is that of Rad3sp to DNA-dependent protein kinase (DNA-PK) [19,20]. DNA-PK is a three-subunit enzyme that is activated by binding to double- and single-strand DNA breaks [21] suggesting that DNA-PK-like kinases may be directly involved in recognition of damaged DNA. The catalytic subunit of DNA-PK is a large and unusual protein kinase that has structural similarities to lipid kinase [19]. Other members of the DNA-PK-like family involved in checkpoint regulation include ATM (ataxia telangiectasia mutated) and ATR (ATM related) in humans, and Mec1 in S. cerevisiae [19,22–24]. Efficient activation of DNA-PK by DNA breaks, however, requires two regulatory subunits, Ku70 and Ku86 [21]. KU homologs have not been found in S. pombe, and the KU subunits in S. cerevisiae are not involved in DNA damage checkpoints [25•].
Table 1.
Yeast mitotic DNA checkpoint genes.
| Checkpoint homologs* | Function/similarity | References | ||
|---|---|---|---|---|
| Other yeast | Human | |||
| S. pombe | ||||
| Damage and replication | ||||
| Rad1sp | Rad17sc | hRad1‡ | Exonuclease | [3,15,16•] |
| Rad9sp | Ddc1sc | hRad9‡ | [3,81] | |
| Rad17sp | Rad24sc | hRad17‡ | Limited similarity to RFC | [3,17,82•] |
| Rad26sp | [14,72•,74••] | |||
| Hus1sp | hHus1‡ | [13,26••] | ||
| Cut5sp† | Dpb11sc | XRCC1 like repeats | [27,28,29•] | |
| Damage | ||||
| Chk1sp | hChk1 | Serine/threonine protein kinase | [30,83••] | |
| Crb2sp | Rad9sc | BRCT motif | [33••,34] | |
| Rad24sp | 14–3–3 | 14–3–3 | [43] | |
| Replication | ||||
| Pol1sp | DNA pol α | [67] | ||
| Cds1sp | Rad53sc | Serine/threonine protein kinase with FHA | [53,73••,74••,75] | |
| Targets | ||||
| Wee1sp | hWee1‡ | Cdc2-Y15 kinase | [36] | |
| Cdc25sp | Cdc25C | Cdc2-Y15 phosphatase | [36] | |
| S. cerevisiae | ||||
| Damage and replication | ||||
| Mec1sc | Rad3sp | ATM | DNA-PK like protein kinase | [2,52] |
| Rad53sc | Cds1sp | Serine/threonine protein kinase with 2 FHA | [48,52,53,57••] | |
| Damage | ||||
| Rad9sc | Crb2sp | BRCT motif | [18,34,44,51•,57••,86] | |
| Rad17sc | Rad1sp | hRad1‡ | Exonuclease | [12,87] |
| Rad24sc | Rad17sp | hRad17‡ | Limited similarity to RFC | [12,87] |
| Ddc1sc | Rad9sp | hRad9‡ | ||
| Mec3sc | [12,87] | |||
| Replication | ||||
| Pol2sc | DNA pol ε | [78] | ||
| Dpb11sc | Cut5sp | XRCC1 like repeats | [79] | |
| Rfc5sc | RFC small subunit | [80] | ||
| Targets | ||||
| Pds1sc | Anaphase inhibitor | [5,58••] | ||
Homologs that are known not to have a checkpoint function are not listed.
May not be required for all damage checkpoints.
Not shown to have checkpoint function.
Are the other checkpoint Rad proteins regulatory partners of Rad3sp? It is too early to say, but there is accumulating evidence that at least some of the checkpoint Rad proteins associate in vivo. Rad1sp and Hus1sp are bound together in vivo, and this interaction depends on Rad9sp, suggesting that they may form a heterotrimer [26••].
Furthermore, Hus1sp is phosphorylated in response to DNA damage and this phosphorylation depends on Rad1sp and Rad9sp, as well as Rad3sp [26••]. Deletions of the other checkpoint rad genes do not effect the Rad1sp-–Hus1sp complex. Therefore, Rad1sp, Hus1sp and maybe Rad9sp form a stable complex independent of the other checkpoint rad genes.
A seventh gene, cut5sp, may also belong to the checkpoint rad class but its analysis is complicated by the fact that it is essential for replication [27]. Thus, cut5spΔ cells are inviable and most analysis has been done with temperature sensitive alleles that may retain some residual function. Cut5sp is required for both the S/M and the γ-radiation induced G2 DNA damage checkpoint [28,29•] but, surprisingly, not for the UV-radiation induced G2 DNA damage checkpoint [28,29•]. Whether these results indicate a real difference between the γ and UV damage checkpoints, or a complication of using non-null ts cut5sp mutations, awaits to be determined.
One role of the checkpoint Rad proteins in the G2 DNA damage checkpoint is to regulate the serine/threonine kinase Chk1sp. Deletion of Chk1sp completely inactivates the G2 DNA damage checkpoint [14]. Cells with the chk1spΔ mutation are not as sensitive to DNA damage as cells with a deletion of any of the checkpoint rad genes, suggesting that the checkpoint Rad proteins also have a Chk1sp independent role, perhaps in activating DNA repair [14,30]. Furthermore, deletion of Chk1sp does not affect the S/M checkpoint, indicating that the checkpoint rad genes have other targets in the S/M checkpoint, as discussed below [14,30]. Chk1sp is phosphorylated in response to DNA damage and this phosphorylation depends on the checkpoint Rad proteins [31].
The nature of the interaction between Chk1sp and the checkpoint Rad proteins has remained obscure, but analysis of the newly identified Crb2sp protein promises to shed light on the problem. Crb2sp, like Chk1sp, is specific to the G2 DNA damage checkpoint and acts downstream of the checkpoint Rad proteins [32•,33••]. Like Rad9sc it contains a BRCA1 carboxy-terminal (BRCT) motif [32•,33••] also found in the p53 binding protein 1 (53BP1) and the breast cancer susceptibility gene BRCA1 [34,35]. Although it is intriguing that the BRCT motif is found in a number of otherwise unrelated proteins involved in checkpoints and cancer, its role is as yet unclear. Crb2sp is phosphorylated in response to DNA damage in a checkpoint Rad protein dependent but Chk1sp independent manner [33••]. This phosphorylation may release Crb2sp from a large complex, since Crb2sp found in lower molecular weight fractions after DNA damage. Conversely, Chk1sp phosphorylation is Crb2sp dependent [33••]. These data suggest that Crb2sp acts downstream of the checkpoint Rad proteins and upstream of Chk1sp. Thus, Crb2sp may act a mediator between the checkpoint Rad proteins and Chk1sp. Consistent with this idea, Chk1sp and Crb2sp interact in a two-hybrid assay, although this interaction has not been seen in vivo [33••]. Crb2sp also interacts with Cut5sp in vitro, providing a potential link between Crb2sp/Chk1sp and the checkpoint Rad proteins [33••].
The ultimate target of the G2 DNA damage checkpoint is the tyrosine phosphorylation of the cyclin-dependent kinase Cdc2sp [7••]. The timing of mitosis in a normal cell cycle is controlled by the phosphorylation of Cdc2sp on Tyr15 which maintains Cdc2sp–cyclin B complexes at an interphase level of activity, high enough to initiate replication and prevent rereplication, but too low to trigger mitosis [36]. Phosphorylation of Tyr15 is catalyzed by the Wee1sp and Mik1sp kinases and is removed by the Cdc25sp phosphatase, and to a lesser extent the Pyp3sp phosphatase [36]. At the G2→M transition, the balance of kinase and phosphatase activity shifts in favor of dephosphorylation, Cdc2sp is activated, and mitosis ensues. During DNA-damage-induced checkpoint-arrest, Cdc2sp is maintained in its tyrosine phosphorylated form [7••,37•]. Mutation of Tyr15 to Phe (which cannot be phosphorylated) abrogates the checkpoint, showing that Tyr15 phosphorylation of Cdc2sp is required for the checkpoint arrest [7••]. The fact that there is no residual checkpoint in cdc2-Y15F (in the single letter code for amino acids) cells also shows that S. pombe does not have a significant metaphase to anaphase DNA damage checkpoint.
In order to maintain Cdc2sp in its tyrosine phosphorylated interphase form, the checkpoint must target either Cdc25sp, Wee1sp, Mik1sp or some combination of the three. Consistent with this, Chk1sp can phosphorylate both Wee1sp and Cdc25sp in vitro, although the effect of this in vivo has yet to be determined [37•,38••]. The role of Cdc25sp as a target of the G2 DNA damage checkpoint has been demonstrated by genetic analysis [38••]; furthermore, dephosphorylation of Cdc2sp by Cdc25sp is inhibited by the checkpoint [7••]. Whether such inhibition is caused by inactivation of Cdc25sp, or by a mechanism that prevents access to Cdc2sp of Cdc25sp, was not addressed. While these data show that Cdc25sp is a target of the DNA damage checkpoint, they certainly do not exclude the possibility that Wee1sp or Mik1sp could also be targeted.
The regulation of Cdc25sp by the G2 DNA damage checkpoint may involve the 14-3-3 protein Rad24sp. The name 14-3-3 describes a large family of proteins that bind to a phosphorylated consensus site in a variety of proteins, including Cdc25 [39,40,41•,42••]. Deletion of rad24sp advances mitosis, which is consistent with Rad24 acting as a negative regulator of Cdc25 during normal growth, but only partially impairs the G2 DNA damage checkpoint [43]. The remaining checkpoint function in rad24spΔ cells may be due to Rad25sp, another 14-3-3 protein and a high copy suppressor of rad24spΔ [43]; however, rad25spΔ has no checkpoint defect and rad24spΔ rad25spΔ cells are inviable, suggesting these genes have important functions that are unrelated to checkpoints [43].
The Saccharomyces cerevisiae metaphase DNA damage checkpoint
Five genes in S. cerevisiae, RAD9sc, RAD17sc, RAD24sc, MEC3sc, and DDC1sc, are required for the metaphase DNA damage checkpoint, but not the S/M replication checkpoint [12,44,45••]. These genes can be divided into two groups, with RAD9sc in one and the rest in another, referred to as the RAD24sc epistasis group. This distinction is made on the basis of the additive phenotypes of rad9scΔ in combination with deletions of members of the RAD24sc epistasis group [18,46•,47••]. The other two genes required for the metaphase DNA damage checkpoint, MEC1sc and RAD53sc, are also required for the S/M checkpoint [12,48]. They also differ in that they are essential for viability but this role is independent of their mitotic checkpoint functions (SJ Elledge, personal communication).
Mec1sc, like Rad3sp, is a member of the DNA-PK family of protein kinases [2]. Another member this family is Tel1sc, a protein involved in telomere maintenance [49]. While, Tel1sc is not required for checkpoints in S. cerevisiae, it can substitute for Mec1sc in a number of situations [50,51•,52]. Rad53sc is also a protein kinase and it contains two forkhead associated (FHA) domains, one on either end of the protein [48,53], which may be involved in binding regulatory partners. Dun1sc is a Rad53sc homolog that is also involved in checkpoints [54]. While the major role of Dun1sc is thought to be downstream of Rad53sc in transcriptional induction of repair genes, dun1scΔ cells do have a partial checkpoint arrest defect which may or may not be related to the role of Dun1sc in transcription [54,55]. Rad53sc is phosphorylated in response to DNA damage and this phosphorylation is dependent on Mec1sc, Rad9sc, and the proteins of the Rad24sc epistasis group [52,56]. Whether Rad53sc is a direct substrate of Mec1sc remains to be determined.
Both Rad9sc and the proteins of the Rad24sc group were thought to function upstream of Mec1sc [2]. Several proteins of the Rad24sc group are structurally similar to proteins involved in DNA metabolism (Table 1), and are required for the processing of damaged DNA [18]. Both Rad9sc and Ddc1sc, however, are phosphorylated after DNA damage in a Mec1sc dependent manner, indicating that they lie downstream of Mec1sc [45••,51•,57••]. A simple interpretation of these results is that Rad9sc and Ddc1sc associate with Mec1sc in such a way that they are required for Mec1sc activation and serve as Mec1sc substrates. This is a common situation for the regulatory subunits of kinases; however, the differences between the roles of Rad9sc and Ddc1sc suggest that they may be in different Mec1sc complexes [47••]. There is much work to be done to substantiate such a model. Initial work has led to the characterization of Rad9sc and Ddc1sc. Ddc1sc interacts in vivo with Mec3sc, and this association requires Rad17sc [47••]. This is reminiscent of the observation that S. pombe homologs of Rad17sc and Ddc1sc, Rad1sp and Rad9sp, may also interact [26••]. Phosphorylation of Ddc1sc is dependent on the other members of the Rad24sc group, but not Rad9sc, consistent with the additive roles of Rad9sc and the Rad24sc group [47••]. The phosphorylation of Rad9sc leads to its association with Rad53sc, through the second of the two Rad53sc FHA domains [51•,57••]. It is plausible that Rad9sc acts as an adapter, such that, when phosphorylated by Mec1sc, it recruits Rad53sc to be phosphorylated in turn by Mec1sc. This cannot be the only role of Rad9sc, however, because phosphorylation of Pds1sc is dependent on Rad9sc but not on Rad53sc [58••].
In order to arrest cells, the S. cerevisiae metaphase DNA damage checkpoint targets the stability of Pds1sc. Pds1sc is a rate-limiting negative regulator of anaphase that is degraded at the metaphase to anaphase transition. Expression of a nondegradable Pds1sc prevents anaphase, while deletion of PDS1sc allows premature sister chromosome separation [59,60] and also compromises the metaphase DNA damage checkpoint [5]. Pds1sc is phosphorylated in response to DNA damage in a Rad9sc and Mec1sc dependent manner [58••]. Although the effect of Pds1sc phosphorylation is unknown, it is tempting to speculate that it stabilizes the protein, leading to metaphase arrest. The phosphorylation of Pds1sc is not dependent on Rad53sc or Ddc1sc [47••,58••]. Consistent with this observation, mutations in neither RAD53sc or PDS1sc alone completely eliminate the metaphase DNA damage checkpoint, although both mutations together do (R Gardner, C Putnam, T Weinert, personal communication). Hence, Rad53sc is likely to have a Pds1sc independent target. This Rad53sc dependent arrest is also dependent on Dun1sc. In this context, it is interesting to note that maintenance of Cdc28sc activity seems to be required for metaphase arrest in S. cerevisiae. Mutations that decrease Cdc28 activity compromise metaphase arrest [61,62]. These results have led to a model in which both Cdc28sc activity and Pds1sc stability are required to prevent anaphase [62]. If this is true, the metaphase arrest checkpoint may act both to stabilize Pds1sc via a Rad53sc independent manner and to maintain the activity of Cdc28sc via a Rad53sc dependent mechanism.
In addition to the well studied mitotic DNA damage checkpoint, a second, mid-anaphase, checkpoint has been identified [63••]. This Rad9sc dependent checkpoint is triggered by the stretching of dicentric chromosomes as anaphase begins. This suggests that there is independent regulation of anaphase A and B. The mid-anaphase checkpoint may provide a means of dissecting that regulation.
The S. pombe S/M replication checkpoint
The S/M replication checkpoint in S. pombe is defined as the checkpoint that prevents mitosis if replication is blocked by hydroxyurea (HU) [64]. HU is a competitive inhibitor of ribonucleotide reductase, which blocks replication after initiation by preventing nucleotide synthesis. Unreplicated DNA per se is insufficent to activate the S/M checkpoint, as mutants that cannot initiate replication nevertheless undero mitosis [65]. Thus, to show a replication protein has a direct role in the S/M checkpoint, it is important to show that it is required for the checkpoint under conditions in which it is not required for replication. In S. pombe, temperature sensitive mutations in replication proteins such as polymerases and ligase often lead to a checkpoint dependent arrest with close to fully replicated DNA content. This arrest, unlike an HU induced arrest, is dependent on Chk1 and Crb2, and is genetically the same as the G2 DNA damage checkpoint [33••,66]. While this type of checkpoint arrest is sometimes referred to as a replication checkpoint, it is distinct from the HU induced S/M replication checkpoint. A simple explanation for this type of checkpoint arrest is that the mutant cells complete bulk DNA replication and therefore do not activate the S/M checkpoint, but leave the DNA in a imperfectly replicated state that then triggers the G2 DNA damage checkpoint.
The primary signal in the S/M checkpoint probably emanates from the replication forks themselves. In S. pombe, the DNA polymerase responsible for replication initiation, DNA polymerase α (encoded by pol1sp), seems to be one of the proteins involved in sending that signal [67]. Cells with polspΔ mutations (derived from germinated spores of pol1sp/pol1spΔ diploids) partially replicate their DNA, probably using residual maternal pol1sp protein, but lack an S/M checkpoint. Thus, DNA polymerase a is required for the S/M checkpoint even after replication is initiated. The role of Cut5sp in the S/M checkpoint is also independent of its requirement for replication [28,29•]. Therefore, Cut5sp may also play a role in generating the S/M checkpoint signal, although this will probably remain unclear until the essential role of Cut5sp in replication is understood. Unlike S. cerevisiae, DNA polymerase ε is not required for the S. pombe S/M checkpoint [68•].
As in the G2 DNA damage checkpoint, the S/M checkpoint prevents mitosis by preventing the tyrosine dephosphorylation of Cdc2sp [69•]. This mechanism does not reduce the activity of Cdc2, but rather maintains it at its interphase level [70]. Genetic studies have implicated Cdc25sp as a target of the S/M checkpoint [64]; moreover, as is the case for DNA damage during G2, HU treatment decreases the rate of Cdc2sp tyrosine dephosphorylation by Cdc25sp [69•]. Since, as described below, Wee1 is also phosphorylated by an S/M checkpoint kinase in vitro, the two G2/M checkpoints have overlapping, and possibly identical, cell cycle targets.
In addition to having the same ultimate target, the S/M checkpoint shares the upstream checkpoint Rad proteins with the G2 DNA damage checkpoint [13,14]. It is possible that all the checkpoint rad genes are required for both G2/M checkpoints because they sense the same primary signal in both cases, perhaps single stranded DNA. Two observations argue against this idea. First, DNA polymerase asp seems to be specifically involved in generating the S/M signal. Second, the S. cerevisiae homologs of Rad1sp, Rad9sp, and Rad17sp are not required for the S. cerevisiae S/M checkpoint. Alternatively, it has been suggested that the checkpoint Rad proteins form a complex that has separate roles in the two checkpoints, and that although different subunits function in only one or other checkpoint, they all are required for the integrity of the complex [14]. The hypothesis is supported by the fact that there are alleles of rad1sp and rad26sp that disrupt only the S/M checkpoint [14,71,72•], suggesting that they have separate roles in the two checkpoints.
Although the checkpoint Rad proteins are required for both S/M and G2 DNA damage checkpoints, they have different downstream effectors for the different checkpoint signals. Specifically, Chk1sp is not phosphorylated in response to HU [31]. While this does not necessarily mean that Chk1sp is not activated by HU, Chk1sp is not normally required for an HU induced arrest [14,30]. Instead, the protein kinase Cds1sp plays the analogous role in the S/M checkpoint [73••,74••,75]. Cds1sp is the putative homolog of Rad53sc, although Cds1sp lacks the second FHA domain that is required for the metaphase DNA checkpoint function of Rad53sc [75]. This observation may explain the fact that while Rad53sc acts in both checkpoints, Cds1sp is specific to the S/M checkpoint. If FHA domains are involved in binding regulatory partners in a phosphorylation dependent manner, one such partner of Cds1sp could be Rad26sp. Rad26sp interacts with Cds1 in vivo, although not in vitro [74••]. The lack of in vitro interaction may indicate an indirect interaction, or the need for Rad26sp phosphorylation. Cds1sp is phosphorylated and activated by HU treatment in a checkpoint Rad dependent manner [73••,74••]. Furthermore, HU treatment causes Cds1sp to bind Wee1sp and the bound Cds1sp can phosphorylate Wee1sp in vitro [73••]. The fact that the S/M checkpoint also appears to effect Cdc25sp suggests that Cdc25sp might also be a target of Cds1sp [69•]. Deletion of cds1sp does not cause an apparent checkpoint defect because in the absence of Cds1sp, HU treatment leads to Chk1sp phosphorylation and causes cells to arrest in a Chk1 dependent manner [73••,74••]. Thus, the role of Cds1sp in preventing mitosis in response to replication arrest is masked by the ability of Chk1sp to substitute in its absence. In the absence of Chk1sp, however, Cds1sp is required for cells to arrest in response to HU, revealing its true role as a checkpoint kinase.
There are plausible reasons why the checkpoint signals should be split into S phase and G2 branches, even if they then reconverge on the same checkpoint targets. Cds1sp has roles in HU resistance that are independent of its checkpoint function. In the absence of Cds1sp, or any of the checkpoint Rad proteins, cells rapidly lose viability, independent of the failure to prevent mitosis [13,75]. It is thought that this loss of viability reflects a role for Cds1sp, termed its recovery function, in stabilizing stalled replication forks. In addition, Cds1sp is required to slow replication when DNA is damaged during S phase [74••,76•]. Clearly, these HU recovery and S phase DNA damage functions of Cds1sp would have no purpose during G2. Conversely, Chk1sp may have a role in the induction of DNA repair and such induction in every S phase may be detrimental to efficient replication.
Chk1sp is required for a prolonged cell cycle arrest in HU at 37°C, close to the maximum temperature at which S. pombe can grow [77]. Given the dependence of the HU arrest on Chk1sp in the absence of Cds1sp, this observation may indicate that the Cds1sp dependent S/M arrest function fails at 37°C.
The S. cerevisiae S/M replication checkpoint
The S/M checkpoint signal in S. cerevisiae seems to be sent by DNA polymerase ε. The role of DNA polymerase ε was revealed by mutations that effect the carboxyl terminus of the protein [78]. This region contains a zinc-finger motif dispensable for catalytic activity that may play a role in subunit interactions. In addition, two other proteins, RFC5sc (the small subunit of RFC) and Dpb11sc (the homolog of Cut5sp) are also required for the checkpoint [79,80]. All three of these proteins have essential functions in replication but, in each case, it has been shown that their role in the S/M checkpoint is independent of their role in bulk replication.
As in the metaphase DNA damage checkpoint, Mec1sc and Rad53sc are both required to transduce the S/M signal [12]. Kinetic analysis of the cell cycle response of MEC1sc or RAD53sc mutant cells has yet to be done, so it is not yet know if these cells retain any checkpoint response. How the S/M checkpoint regulates mitosis is unclear. Pds1sc is not required, but cells arrest with short spindles, indicating that they are blocked at the metaphase to anaphase transition [5,12]. Therefore, there must be a Pds1sc independent mechanism for preventing anaphase. Since destruction of Pds1sc seems to be sufficient for anaphase in the absence of the S/M checkpoint, there may be an active anaphase inhibition induced by the checkpoint that does not play a role in the normal control if mitosis.
Comparison to mammals
As model systems for study of the cell cycle, S. cerevisiae and S. pombe make a well-matched pair. The combination of S. cerevisiae, with its cell cycle controls at Start and metaphase, and S. pombe with its cell cycle control at G2/M, cover all the major cell cycle controls seen in mammalian cells. The same is true of checkpoint control. All of the known mammalian checkpoints are present in one or both of the yeasts and the mechanisms of these checkpoints seem to be conserved. As in the yeasts, a central player in the mammalian checkpoints is a DNA-PK like protein kinase, ATM. ATM was originally cloned as the gene mutated in ataxia telangiectasia, an inherited disease that includes predisposition to cancer [22]. ATM has since been shown to be involved in many mammalian DNA damage checkpoints [24]. In addition to ATM, putative human homologs of various upstream proteins have been identified, including hRad1, hRad9, hRad17, and hHus1 [16•,26••,81,82•]. While the role of these proteins in checkpoint control has yet to be established, hRad1 and hRad17 interact in a two-hybrid assay, hinting that the human checkpoint Rad homologs may function in concert [82•].
The G2/M cell cycle targets also seem to be conserved between the yeasts and mammals. A good example of this is in the control of the G2→M transition. As in S. pombe, mammalian cells regulate the tyrosine phosphorylation of Cdc2to prevent entry into mitosis in the presence of damaged or unreplicated DNA [8••,9]. The human homolog of Chk1sp is phosphorylated in response to DNA damage and can bind to and phosphorylate human Cdc25C in vitro [42••,83••]. This phosphorylation has been mapped to Ser216, the major site of Cdc25C interphase phosphorylation in vivo, and creates a 14-3-3 binding site [41•,42••]. These results inspired a model in which DNA damage leads to the phosphorylation of Cdc25C by hChk1 on Ser216. The inactivation or sequestration of Cdc25C by 14-3-3 binding would then arrest cells in G2 by preventing tyrosine dephosphorylation of Cdc2. This model is supported by the fact that, when expressed in tissue culture, Cdc25C S216A (in the single letter code for amino acids) causes a defect in both the S/M and G2 DNA damage checkpoint [42••]. These effects are modest, however, and it seems likely that there are other levels of checkpoint regulation of Cdc25, or other mammalian G2 checkpoint targets. In any case, much of the S. pombe G2 DNA damage checkpoint seems to be conserved in mammalian cells. A metaphase DNA damage checkpoint has not been identified in mammalian cells, but they do have a metaphase spindle checkpoint that appears to be homologous to the S. cerevisiae spindle checkpoint [61,84,85]. Furthermore, a metaphase DNA damage checkpoint in mammalian cells could explain why overriding the G2/M checkpoint leads to some metaphase events but not an authentic mitosis [8••,9].
Conclusion
The wealth of information on the G2/M checkpoints in S. cerevisiae and S. pombe offers a perfect opportunity to compare and contrast. A comparison of the two yeasts and of vertebrates reveals conserved mechanisms for initiating and sending checkpoint signals. With some plausible speculation, it is possible to build model of how this mechanism may work. At the center of all checkpoints seems to lie a DNA-PK like kinase [2,24]. These kinases probably associate with different regulatory subunits to recognize the different signals generated by DNA damage, replication arrest and perhaps other, as yet unidentified, signals. In S. pombe, we may see a situation where the DNA damage and replication subunits have merged into an interdependent complex. The main function of the DNA-PK like kinase appears to be the activation of one or more downstream kinases that again may associate with regulatory partners. These downstream kinases are then good candidates to have the major signaling function in the checkpoint. At least in the case the Rad53sc and Cds1sp, they seem to have several independent targets. This much of the checkpoint machinery seems to well conserved.
By contrasting the two yeasts, and different checkpoints within the two yeasts, it is clear that conserved checkpoint signals can have diverse targets. The difference between the targets of the G2/M DNA damage checkpoints is an extreme, but useful example. It is not important how sister chromatid separation is delayed; whatever mechanism is used during a normal cell cycle will make a good checkpoint target. A similar situation is seen for the target of the G1 DNA damage checkpoint in S. cerevisiae and mammalian cells [2,4], and we may expect to find flexibility of targets at other cell cycle transitions. To what extent the differences between the checkpoint targets in the two yeast reflects a general plasticity of checkpoint targets remains to be seen. This example is just one of the many interesting questions to be answered by work in other species, inspired by the groundwork laid in yeast.
Acknowledgements
We would like to thank the members of the Scripps Cell Cycle groups, particularly D Clarke and C McGowan, for many interesting discussions on these topics. We are also grateful to T Weinert for allowing us to include unpublished data from his laboratory.
Abbreviations
- ATM
ataxia telangiectasia mutated
- ATR
ATM related
- BRCT
BRCA1 carboxy-terminal motif
- DNA-PK
DNA-dependent protein kinase
- HU
hydroxyurea
- RFC
replication factor C
Footnotes
Note added in proof
The data cited as SJ Elledge, personal communication has now been published [88].
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 2.Weinert T. DNA damage checkpoints update: getting molecular. Curr Opin Genet Dev. 1998;8:185–193. doi: 10.1016/s0959-437x(98)80140-8. [DOI] [PubMed] [Google Scholar]
- 3.al-Khodairy F, Carr AM. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor PM. Mammalian G1 and G2 phase checkpoints. Cancer Surv. 1997;29:151–182. [PubMed] [Google Scholar]
- 5.Yamamoto A, Guacci V, Koshland D. Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s) J Cell Biol. 1996;133:99–110. doi: 10.1083/jcb.133.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 7. Rhind N, Furnari B, Russell P. Cdc2tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504.The nontyrosine phosphorylatable Cdc2sp mutant Tyr15→Phe is not subject to checkpoint control. Elimination of Wee1sp and Mik1sp, the Cdc2sp kinases, in a wee1sp-ts mik1spΔ strain, creates a situation in which the level of Cdc2sp tyrosine phosphorylation is dependent only on its rate of dephosphorylation, a process catalyzed primarily by Cdc25sp. In such a situation, DNA damage reduces the rate of Cdc2sp dephosphorylation, implicating the dephosphorylation of Cdc2sp by Cdc25sp as a checkpoint target.
- 8. Blasina A, Paegle ES, McGowan CH. The role of inhibitory phosphorylation of CDC2following DNA replication block and radiation-induced damage in human cells. Mol Biol Cell. 1997;8:1013–1023. doi: 10.1091/mbc.8.6.1013.Using a nontyrosine phosphorylatable Cdc2 mutant, this work, together with [9], shows that tyrosine phosphorylation of Cdc2 is required for the prevention of Cdc2–cyclin B activation in response to DNA damage or replication arrest in HeLa cells. Lack of Cdc2 tyrosine phosphorylation, however, is not sufficient to induce a morphologically normal mitosis, suggesting that there are other levels of mitotic control in mammalian cells.
- 9.Jin P, Gu Y, Morgan DO. Role of inhibitory CDC2phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lew DJ, Reed SI. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol. 1995;129:739–749. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim HH, Goh PY, Surana U. Spindle pole body separation in Saccharomyces cerevisiae requires dephosphorylation of the tyrosine 19 residue of Cdc28. Mol Cell Biol. 1996;16:6385–6397. doi: 10.1128/mcb.16.11.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- 13.Enoch T, Carr AM, Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 1992;6:2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- 14.al-Khodairy F, Fotou E, Sheldrick KS, Griffiths DJ, Lehmann AR, Carr AM. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long KE, Sunnerhagen P, Subramani S. The Schizosaccharomyces pombe rad1 gene consists of three exons and the cDNA sequence is partially homologous to the Ustilago maydis REC1 cDNA. Gene. 1994;148:155–159. doi: 10.1016/0378-1119(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 16. Parker AE, Van de Weyer I, Laus MC, Oostveen I, Yon J, Verhasselt P, Luyten WHML. A human homologue of the Schizosaccharomyces pombe rad1+ checkpoint gene encodes an exonuclease. J Biol Chem. 1998;273:18332–18339. doi: 10.1074/jbc.273.29.18332.Human Rad1 encodes a bona fide exonuclease with enzymatic characteristics similar to the homologous Ustilago REC1 exonuclease. It maps to a region of the human genome that may contain a tumor suppressor.
- 17.Griffiths DJ, Barbet NC, McCready S, Lehmann AR, Carr AM. Fission yeast rad17: a homologue of budding yeast RAD24 that shares regions of sequence similarity with DNA polymerase accessory proteins. EMBO J. 1995;14:5812–5823. doi: 10.1002/j.1460-2075.1995.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lydall D, Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995;270:1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- 19.Hartley KO, Gell D, Smith GC, Zhang H, Divecha N, Connelly MA, Admon A, Lees-Miller SP, Anderson CW, Jackson SP. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 20.Bentley NJ, Holtzman DA, Flaggs G, Keegan KS, DeMaggio A, Ford JC, Hoekstra M, Carr AM. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 22.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 23.Wright JA, Keegan KS, Herendeen DR, Bentley NJ, Carr AM, Hoekstra MF, Concannon P. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc Natl Acad Sci USA. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoekstra MF. Responses to DNA damage and regulation of cell cycle checkpoints by the ATM protein kinase family. Curr Opin Genet Dev. 1997;7:170–175. doi: 10.1016/s0959-437x(97)80125-6. [DOI] [PubMed] [Google Scholar]
- 25. Laroche T, Martin SG, Gotta M, Gorham HC, Pryde FE, Louis EJ, Gasser SM. Mutation of yeast Ku genes disrupts the subnuclear organization of telomeres. Curr Biol. 1998;8:653–656. doi: 10.1016/s0960-9822(98)70252-0.The Saccharomyces cerevisiae Ku homologs are not required for checkpoint control.
- 26. Kostrub CF, Knudsen K, Subramani S, Enoch T. Hus1p, a conserved fission yeast checkpoint protein, interacts with Rad1p and is phosphorylated in response to DNA damage. EMBO J. 1998;17:2055–2066. doi: 10.1093/emboj/17.7.2055.In fission yeast Hus1sp binds to Rad1sp and maybe Rad9sp. Hus1sp is phosphorylated in a Rad3sp dependent manner. A model is presented in which Hus1sp, Rad1sp and Rad9sp form regulator complex that recruits Rad3sp to DNA damage sites. Putative mouse and human homologs of Hus1sp are reported.
- 27.Saka Y, Yanagida M. Fission yeast cut5+, required for S phase onset and M phase restraint, is identical to the radiation-damage repair gene rad4+ Cell. 1993;74:383–393. doi: 10.1016/0092-8674(93)90428-s. [DOI] [PubMed] [Google Scholar]
- 28.Saka Y, Fantes P, Sutani T, McInerny C, Creanor J, Yanagida M. Fission yeast cut5 links nuclear chromatin and M phase regulator in the replication checkpoint control. EMBO J. 1994;13:5319–5329. doi: 10.1002/j.1460-2075.1994.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McFarlane RJ, Carr AM, Price C. Characterisation of the Schizosaccharomyces pombe rad4/cut5 mutant phenotypes: dissection of DNA replication and G2 checkpoint control function. Mol Gen Genet. 1997;255:332–340. doi: 10.1007/s004380050504.At a semipermissive temperature, fission yeast cells with a cut5sp-ts mutation can replicate but do not arrest in response to hydroxyurea or gamma-irradiation. Thus, the checkpoint role of Cut5sp is independent of its requirement for replication. Under the same condition, the cells do arrest in response to UV-irradiation.
- 30.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 31.Walworth NC, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- 32. Willson J, Wilson S, Warr N, Watts FZ. Isolation and characterization of the Schizosaccharomyces pombe rhp9 gene: a gene required for the DNA damage checkpoint but not the replication checkpoint. Nucleic Acids Res. 1997;25:2138–2146. doi: 10.1093/nar/25.11.2138.See annotation to [33••].
- 33. Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M. Damage and replication checkpoint control in fission yeast is ensured by interactions of Crb2, a protein with BRCT motif, with Cut5 and Chk1. Genes Dev. 1997;11:3387–3400. doi: 10.1101/gad.11.24.3387.This paper, along with [32•], describes the cloning of crb2sp, also known as rhp9sp. Like chk1sp, crb2sp is required for the G2 DNA damage checkpoint, but not the S/M checkpoint. Crb2sp interacts with both Cut5sp and Chk1sp in two-hybrid assays, although interactions were not seen in vivo.
- 34.Bork P, Hofmann K, Bucher P, Neuwald AF, Altschul SF, Koonin EV. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997;11:68–76. [PubMed] [Google Scholar]
- 35.Callebaut I, Mornon JP. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. doi: 10.1016/s0014-5793(96)01312-9. [DOI] [PubMed] [Google Scholar]
- 36.Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 37. O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545.Cdc2sp tyrosine phosphorylation is maintained during DNA damage induced arrest. Wee1sp, a Cdc2sp-Tyr15 kinase, is phosphorylated in vitro by Chk1sp.
- 38. Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495.Genetic analysis shows that Cdc25sp is a target of the G2 DNA damage checkpoint. Chk1sp binds to Cdc25spin vivo and phosphorylates Cdc25spin vitro.
- 39.Aitken A. 14-3-3 and its possible role in co-ordinating multiple signaling pathways. Trends Cell Biol. 1996;6:341–347. doi: 10.1016/0962-8924(96)10029-5. [DOI] [PubMed] [Google Scholar]
- 40.Conklin DS, Galaktionov K, Beach D. 14-3-3 proteins associate with cdc25 phosphatases. Proc Natl Acad Sci USA. 1995;92:7892–7896. doi: 10.1073/pnas.92.17.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumagai A, Yakowec PS, Dunphy WG. 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell. 1998;9:345–354. doi: 10.1091/mbc.9.2.345.14-3-3 proteins bind Cdc25 in a cell cycle regulated manner. This binding is dependent on phosphorylation of Cdc25 Ser287. Mutation of this site activates Cdc25 in Xenopus embyo extracts, suggesting that 14-3-3 binding negatively regulates Cdc25.
- 42. Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501.Human Cdc25C is phosphorylated on Ser216 in vivo creating a 14-3-3 binding site. This site is phosphorylated by fission yeast protein Chk1spin vitro. Mutation of Ser216 causes mild defects in G2/M checkpoints. See also [83••].
- 43.Ford JC, al-Khodairy F, Fotou E, Sheldrick KS, Griffiths DJ, Carr AM. 14-3-3 protein homologs required for the DNA damage checkpoint in fission yeast. Science. 1994;265:533–535. doi: 10.1126/science.8036497. [DOI] [PubMed] [Google Scholar]
- 44.Weinert TA, Hartwell LH. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988;241:317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- 45. Longhese MP, Paciotti V, Fraschini R, Zaccarini R, Plevani P, Lucchini G. The novel DNA damage checkpoint protein Ddc1p is phosphorylated periodically during the cell cycle and in response to DNA damage in budding yeast. EMBO J. 1997;16:5216–5226. doi: 10.1093/emboj/16.17.5216.DDC1 is a new member of the RAD24 epistasis group, required for DNA damage checkpoints. It is phosphorylated in a Mec3 dependent manner in response to DNA damage.
- 46. de la Torre-Ruiz MA, Green CM, Lowndes NF. RAD9 and RAD24 define two additive, interacting branches of the DNA damage checkpoint pathway in budding yeast normally required for rad53 modification and activation. EMBO J. 1998;17:2687–2698. doi: 10.1093/emboj/17.9.2687.The checkpoint phenotypes of various mutants can be suppressed by over-expression of other checkpoint genes. This work presents a large array of mutant/overexpression combinations and emphasizes the additive nature of RAD9 and RAD24 epistasis groups.
- 47. Paciotti V, Lucchini G, Plevani P, Longhese MP. Mec1p is essential for phosphorylation of the yeast DNA damage checkpoint protein Ddc1p, which physically interacts with Mec3p. EMBO J. 1998;17:4199–4209. doi: 10.1093/emboj/17.14.4199.In budding yeast Ddc1sc binds to Mec3sc and maybe also to Rad17sc. Ddc1sc is phosphorylated in a Mec1sc and Rad24sc group dependent, but Rad9sc independent, manner. Conversely, the Rad9sc dependent phosphorylation of Pds1sc is independent of Ddc1sc. Thus, Ddc1sc (and by extension the other members of the Rad24sc group) and Rad9sc seem to play separate roles in a least some Mec1sc dependent function.
- 48.Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 1994;8:2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- 49.Greenwell PW, Kronmal SL, Porter SE, Gassenhuber J, Obermaier B, Petes TD. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell. 1995;82:823–829. doi: 10.1016/0092-8674(95)90479-4. [DOI] [PubMed] [Google Scholar]
- 50.Morrow DM, Tagle DA, Shiloh Y, Collins FS, Hieter P. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell. 1995;82:831–840. doi: 10.1016/0092-8674(95)90480-8. [DOI] [PubMed] [Google Scholar]
- 51. Emili A. MEC1-dependent phosphorylation of Rad9p in response to DNA damage. Mol Cell. 1998;2:183–189. doi: 10.1016/s1097-2765(00)80128-8.This paper, along with [57••], shows that Rad9 is phosphorylated in a Mec1 dependent manner, and that this phosphorylation leads to Rad53 binding in vivo.
- 52.Sanchez Y, Desany BA, Jones WJ, Liu Q, Wang B, Elledge SJ. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann K, Bucher P. The FHA domain: a putative nuclear signaling domain found in protein kinases and transcription factors. Trends Biochem Sci. 1995;20:347–349. doi: 10.1016/s0968-0004(00)89072-6. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Z, Elledge SJ. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75:1119–1127. doi: 10.1016/0092-8674(93)90321-g. [DOI] [PubMed] [Google Scholar]
- 55.Pati D, Keller C, Groudine M, Plon SE. Reconstitution of a MEC1-independent checkpoint in yeast by expression of a novel human fork head cDNA. Mol Cell Biol. 1997;17:3037–3046. doi: 10.1128/mcb.17.6.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Z, Fay DS, Marini F, Foiani M, Stern DF. Spk1/Rad53 is regulated by Mec1-dependent protein phosphorylation in DNA replication and damage checkpoint pathways. Genes Dev. 1996;10:395–406. doi: 10.1101/gad.10.4.395. [DOI] [PubMed] [Google Scholar]
- 57. Sun Z, Hsiao J, Fay DS, Stern DF. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science. 1998;281:272–274. doi: 10.1126/science.281.5374.272.See annotation to [51•].
- 58. Cohen-Fix O, Koshland D. The anaphase inhibitor of Saccharomyces cerevisiae Pds1p is a target of the DNA damage checkpoint pathway. Proc Natl Acad Sci USA. 1997;94:14361–14366. doi: 10.1073/pnas.94.26.14361.Pds1sc is phosphorylated after DNA damage in a Rad9sc dependent, but Rad53sc independent manner. This suggests that Pds1sc itself, and not part of the machinery that regulates Pds1sc stability, is targeted by the metaphase DNA damage checkpoint.
- 59.Yamamoto A, Guacci V, Koshland D. Pds1p is required for faithful execution of anaphase in the yeast, Saccharomyces cerevisiae. J Cell Biol. 1996;133:85–97. doi: 10.1083/jcb.133.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Cai M. Inactivation of the cyclin-dependent kinase Cdc28 abrogates cell cycle arrest induced by DNA damage and disassembly of mitotic spindles in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:2723–2734. doi: 10.1128/mcb.17.5.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minshull J, Straight A, Rudner AD, Dernburg AF, Belmont A, Murray AW. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr Biol. 1996;6:1609–1620. doi: 10.1016/s0960-9822(02)70784-7. [DOI] [PubMed] [Google Scholar]
- 63. Yang SS, Yeh E, Salmon ED, Bloom K. Identification of a mid-anaphase checkpoint in budding yeast. J Cell Biol. 1997;136:345–354. doi: 10.1083/jcb.136.2.345.Using a conditional centromere to create dicentric chromosomes, the authors identify a Rad9sc dependent mid-anaphase checkpoint that is activated by the stretching or breaking of the dicentric chromosome. The need for time-lapse fluorescence microscopy to assay the checkpoint may hinder future work. But to the extent that it does not, this checkpoint promises to reveal new mechanisms of anaphase regulation.
- 64.Enoch T, Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990;60:665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- 65.Humphrey T, Enoch T. Keeping mitosis in check. Curr Biol. 1995;5:376–379. doi: 10.1016/s0960-9822(95)00077-7. [DOI] [PubMed] [Google Scholar]
- 66.Carr AM, Moudjou M, Bentley NJ, Hagan IM. The chk1 pathway is required to prevent mitosis following cell-cycle arrest at ‘start’. Curr Biol. 1995;5:1179–1190. doi: 10.1016/s0960-9822(95)00234-x. [DOI] [PubMed] [Google Scholar]
- 67.D’Urso G, Grallert B, Nurse P. DNA polymerase alpha, a component of the replication initiation complex, is essential for the checkpoint coupling S phase to mitosis in fission yeast. J Cell Sci. 1995;108:3109–3118. doi: 10.1242/jcs.108.9.3109. [DOI] [PubMed] [Google Scholar]
- 68. D’Urso G, Nurse P. Schizosaccharomyces pombe cdc20+ encodes DNA polymerase epsilon and is required for chromosomal replication but not for the S phase checkpoint. Proc Natl Acad Sci USA. 1997;94:12491–12496. doi: 10.1073/pnas.94.23.12491.Cells with mutations in cdc20sp, the gene encoding DNA polymerase ε, arrest in mid-replication, but do not enter mitosis. Furthermore, they arrest properly after exposure to hydroxyurea.
- 69. Rhind N, Russell P. Tyrosine phosphorylation of cdc2 is required for the replication checkpoint in Schizosaccharomyces pombe. Mol Cell Biol. 1998;18:3782–3787. doi: 10.1128/mcb.18.7.3782.Using similar experiments to those in [9] the authors show that tyrosine phosphorylation of Cdc2sp is required for the S/M checkpoint. See [70] for another interpretation of these events.
- 70.Knudsen KE, Knudsen ES, Wang JY, Subramani S. p34cdc2 kinase activity is maintained upon activation of the replication checkpoint in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1996;93:8278–8283. doi: 10.1073/pnas.93.16.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanter-Smoler G, Knudsen KE, Jimenez G, Sunnerhagen P, Subramani S. Separation of phenotypes in mutant alleles of the Schizosaccharomyces pombe cell-cycle checkpoint gene rad1+ Mol Biol Cell. 1995;6:1793–1805. doi: 10.1091/mbc.6.12.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Uchiyama M, Galli I, Griffiths DJ, Wang TS. A novel mutant allele of Schizosaccharomyces pombe rad26 defective in monitoring S-phase progression to prevent premature mitosis. Mol Cell Biol. 1997;17:3103–3115. doi: 10.1128/mcb.17.6.3103.The authors identify an allele of Rad26sp that disrupts the S-M, but not the G2 DNA damage, checkpoint. The phenotype of this allele is suppressed by overexpression of Cdc1sp. A similar allele of Rad26sp is described in [13].
- 73. Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909.This paper describes the identification of Cds1sp as a hydroxyurea stimulated kinase that associates with Wee1sp in vivo. Fission yeasts that are cds1spΔ chk1sp3 double mutants do not arrest in hydroxyurea, demonstrating a checkpoint role for Cds1sp.
- 74. Lindsay HD, Griffiths DJ, Edwards RJ, Christensen PU, Murray JM, Osman F, Walworth N, Carr AM. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382.Cds1sp is activated by hydroxyurea in a checkpoint Rad dependent manner. It is a high-copy suppressor of a rad26sp allele and binds to Rad26sp in vitro. Deletion of cds1sp causes Chk1sp to be phosphorylated in response to hydroxyurea and makes arrest in hydroxyurea Chk1sp dependent. The authors present a different interpretation of these results than is found in this review.
- 75.Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- 76. Rhind N, Russell P. The Schizosaccharomyces pombe S-phase checkpoint differentiates between different types of DNA damage. Genetics. 1998;149 doi: 10.1093/genetics/149.4.1729. 1729-1237.This paper, along with [74••], identifies a role for Cds1sp in slowing the rate of replication in response to DNA damage.
- 77.Francesconi S, Grenon M, Bouvier D, Baldacci G. p56(chk1) protein kinase is required for the DNA replication checkpoint at 37 degrees C in fission yeast. EMBO J. 1997;16:1332–1341. doi: 10.1093/emboj/16.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navas TA, Zhou Z, Elledge SJ. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 79.Araki H, Leem SH, Phongdara A, Sugino A. Dpb11, which interacts with DNA polymerase II(epsilon) in Saccharomyces cerevisiae, has a dual role in S-phase progression and at a cell cycle checkpoint. Proc Natl Acad Sci USA. 1995;92:11791–11795. doi: 10.1073/pnas.92.25.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sugimoto K, Shimomura T, Hashimoto K, Araki H, Sugino A, Matsumoto K. Rfc5, a small subunit of replication factor C complex, couples DNA replication and mitosis in budding yeast. Proc Natl Acad Sci USA. 1996;93:7048–7052. doi: 10.1073/pnas.93.14.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lieberman HB, Hopkins KM, Nass M, Demetrick D, Davey S. A human homolog of the Schizosaccharomyces pombe rad9+ checkpoint control gene. Proc Natl Acad Sci USA. 1996;93:13890–13895. doi: 10.1073/pnas.93.24.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Parker AE, Van de Weyer I, Laus MC, Verhasselt P, Luyten W. Identification of a human homologue of the Schizosaccharomyces pombe rad17(+) checkpoint gene. J Biol Chem. 1998;273:18340–18346. doi: 10.1074/jbc.273.29.18340.Human Rad17 interacts with hRad1 in a two-hybrid assay.
- 83. Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497.Human Chk1 is phosphorylated in response to DNA damage. In vitro Chk1 binds to hCdc25 and phosphorylates it on Ser216. See also [42••].
- 84.Li Y, Gorbea C, Mahaffey D, Rechsteiner M, Benezra R. MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc Natl Acad Sci USA. 1997;94:12431–12436. doi: 10.1073/pnas.94.23.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 86.Schiestl RH, Reynolds P, Prakash S, Prakash L. Cloning and sequence analysis of the Saccharomyces cerevisiae RAD9 gene and further evidence that its product is required for cell cycle arrest induced by DNA damage. Mol Cell Biol. 1989;9:1882–1896. doi: 10.1128/mcb.9.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lydall D, Weinert T. G2/M checkpoint genes of Saccharomyces cerevisiae: further evidence for roles in DNA replication and/or repair. Mol Gen Genet. 1997;256:638–651. doi: 10.1007/s004380050612. [DOI] [PubMed] [Google Scholar]
- 88.Densany BA, Alcasabas AA, Bachant JB, Elledge SJ. Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 1998;12:2956–2970. doi: 10.1101/gad.12.18.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]