Abstract
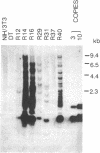
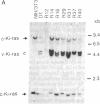
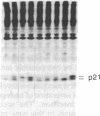
Seven morphologically nontransformed (flat) revertants with reduced tumorigenicity in vivo have been isolated from populations of Kirsten sarcoma virus-transformed NIH 3T3 cells transfected with a cDNA expression library of normal human fibroblasts. Each revertant harbors 1-10 recombinant plasmids per cell and retains a rescuable transforming virus as well as high level expression of v-Ki-ras-specific RNA and the viral oncogene product, p21v-Ki-ras. Transformed phenotypes are suppressed in cell hybrids generated by fusing each revertant to v-Ki-ras-transformed NIH 3T3 cells. From two of the revertant lines, plasmids capable of giving rise to flat secondary transfectants have been recovered. Thus, in some, if not all, of the revertants, transfected cDNAs seem to be responsible for the suppression of specific transformed phenotypes.
Full text
PDF
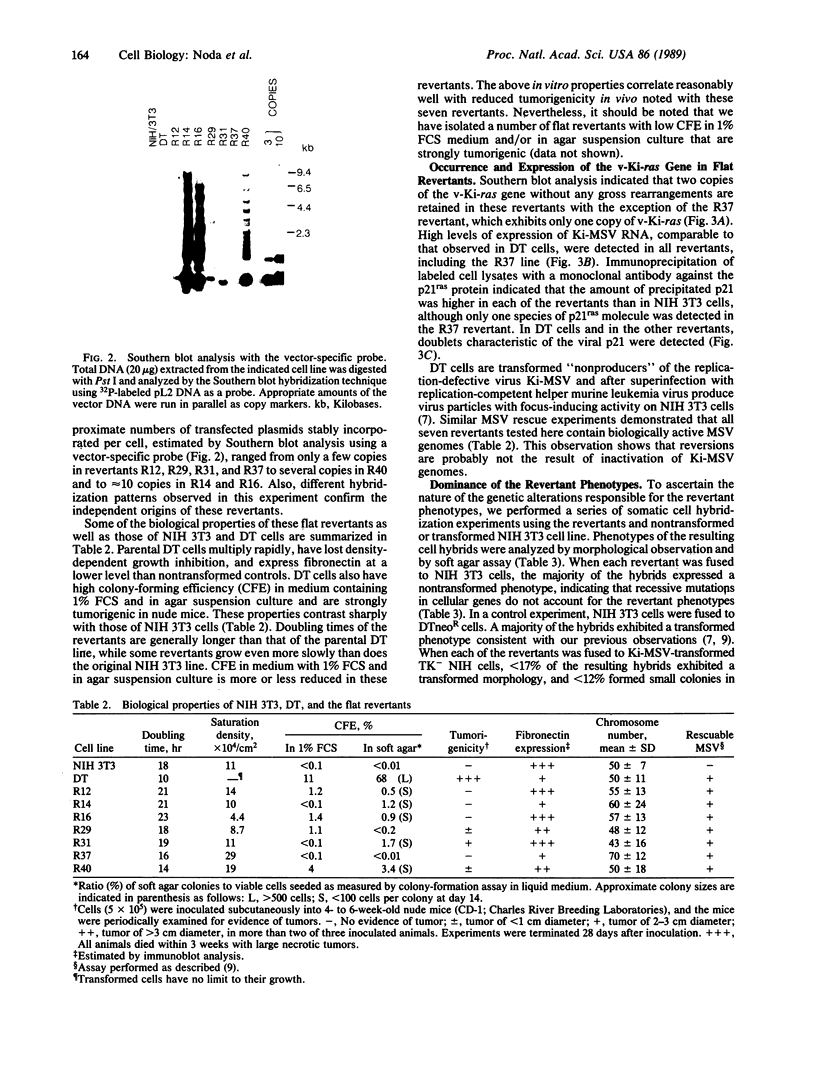


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from simian virus 40-transformed cells. 3. Concanavalin A-selected revertant cells. J Virol. 1972 Apr;9(4):611–620. doi: 10.1128/jvi.9.4.611-620.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Thomas K., Capecchi M. R. Nonreciprocal exchanges of information between DNA duplexes coinjected into mammalian cell nuclei. Mol Cell Biol. 1985 Jan;5(1):59–69. doi: 10.1128/mcb.5.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Kamata T., Sullivan N. F., Wooten M. W. Reduced protein kinase C activity in a ras-resistant cell line derived from Ki-MSV transformed cells. Oncogene. 1987 Mar;1(1):37–46. [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Faras A. J., Spector D. H. Post-transcriptional control of avian oncornavirus transforming gene sequences in mammalian cells. Nature. 1977 Sep 8;269(5624):175–179. doi: 10.1038/269175a0. [DOI] [PubMed] [Google Scholar]
- Morris A., Clegg C., Jones J., Rodgers B., Avery R. J. The isolation and characterization of a clonally related series of murine retrovirus-infected mouse cells. J Gen Virol. 1980 Jul;49(1):105–113. doi: 10.1099/0022-1317-49-1-105. [DOI] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimura M., Gilmer T. M., Barrett J. C. Nonrandom loss of chromosome 15 in Syrian hamster tumours induced by v-Ha-ras plus v-myc oncogenes. Nature. 1985 Aug 15;316(6029):636–639. doi: 10.1038/316636a0. [DOI] [PubMed] [Google Scholar]
- Ozer H. L., Jha K. K. Malignancy and transformation: expression in somatic cell hybrids and variants. Adv Cancer Res. 1977;25:53–93. doi: 10.1016/s0065-230x(08)60632-6. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D. S., Zwiebel J. A., Noda M., Bassin R. H. Flat revertants derived from Kirsten murine sarcoma virus-transformed cells produce transforming growth factors. J Cell Physiol. 1984 Oct;121(1):22–30. doi: 10.1002/jcp.1041210105. [DOI] [PubMed] [Google Scholar]
- Schaefer R., Iyer J., Iten E., Nirkko A. C. Partial reversion of the transformed phenotype in HRAS-transfected tumorigenic cells by transfer of a human gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1590–1594. doi: 10.1073/pnas.85.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer E. S., Kelly R. B. Selective packaging of human growth hormone into synaptic vesicles in a rat neuronal (PC12) cell line. J Cell Biol. 1985 Aug;101(2):667–676. doi: 10.1083/jcb.101.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Aaronson S. A. Characterization of morphologic revertants of murine and avian sarcoma virus-transformed cells. J Virol. 1973 Feb;11(2):218–222. doi: 10.1128/jvi.11.2.218-222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Wyke J. Revertants of an ASV-transformed rat cell line have lost the complete provius or sustained mutations in src. Virology. 1981 Jan 15;108(1):28–46. doi: 10.1016/0042-6822(81)90525-0. [DOI] [PubMed] [Google Scholar]
- Vogel A., Risser R., Pollack R. Isolation and characterization of revertant cell lines. 3. Isolation of density-revertants of SV40-transformed 3T3 cells using colchicine. J Cell Physiol. 1973 Oct;82(2):181–188. doi: 10.1002/jcp.1040820206. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Latreille J., Jolicoeur P. Revertants of v-fos-transformed fibroblasts have mutations in cellular genes essential for transformation by other oncogenes. Cell. 1987 Nov 6;51(3):357–369. doi: 10.1016/0092-8674(87)90632-5. [DOI] [PubMed] [Google Scholar]