Abstract
We showed previously that diploid human fibroblasts that express a transfected HRAS oncogene from the human bladder carcinoma cell line T24 exhibit several characteristics of transformed cells but do not acquire an infinite life-span and are not tumorigenic. To extend these studies of the T24 HRAS in human cells, we have utilized an infinite life-span, but otherwise phenotypically normal, human fibroblast cell strain, MSU-1.1, developed in this laboratory after transfection of diploid fibroblasts with a viral v-myc oncogene. Transfection of MSU-1.1 cells with the T24 HRAS flanked by two transcriptional enhancer elements (pHO6T1) yielded foci of morphologically transformed cells. No such transformation occurred if the plasmid containing T24 HRAS had only one enhancer or none at all or if the normal human HRAS gene was transfected in the pHO6 vector (pHO6N1). Cell strains derived from such foci expressed high levels of T24 HRAS product p21, formed colonies in soft agar at high frequency, proliferated rapidly in serum-free medium that does not support growth of the parental cell line, and formed progressively growing, invasive fibrosarcomas. These foci-derived T24 HRAS-transformed cell strains, as well as cells from the tumors derived from them, had the same near-diploid karyotype as that of the parental MSU-1.1 cells. Transfection of pHO6T1 into two other infinite life-span human fibroblast cell lines, cells that had not been transfected with v-myc, also resulted in malignant transformation, suggesting that the infinite life-span phenotype of MSU-1.1 cells, and not necessarily expression of the v-myc oncogene, was the factor that complemented T24 HRAS expression to cause malignant transformation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andeol Y., Nardeux P. C., Daya-Grosjean L., Brison O., Cebrian J., Suarez H. Both N-ras and c-myc are activated in the SHAC human stomach fibrosarcoma cell line. Int J Cancer. 1988 May 15;41(5):732–737. doi: 10.1002/ijc.2910410516. [DOI] [PubMed] [Google Scholar]
- Andres A. C., Schönenberger C. A., Groner B., Hennighausen L., LeMeur M., Gerlinger P. Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1299–1303. doi: 10.1073/pnas.84.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bettger W. J., Boyce S. T., Walthall B. J., Ham R. G. Rapid clonal growth and serial passage of human diploid fibroblasts in a lipid-enriched synthetic medium supplemented with epidermal growth factor, insulin, and dexamethasone. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5588–5592. doi: 10.1073/pnas.78.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R., Marshall C. J., Pennie S. G., Hall A. Mechanism of activation of an N-ras gene in the human fibrosarcoma cell line HT1080. EMBO J. 1984 Jun;3(6):1321–1326. doi: 10.1002/j.1460-2075.1984.tb01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger J., Di Paolo J. A., Popescu N. C. Transformation of Bloom's syndrome fibroblasts by DNA transfection. Science. 1983 Dec 9;222(4628):1144–1146. doi: 10.1126/science.6648529. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Maruyama K., Garrels J. I., Ruley H. E. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell. 1986 Feb 14;44(3):409–418. doi: 10.1016/0092-8674(86)90462-9. [DOI] [PubMed] [Google Scholar]
- Fry D. G., Hurlin P. J., Maher V. M., McCormick J. J. Transformation of diploid human fibroblasts by transfection with the v-sis, PDGF2/c-sis, or T24 H-ras genes. Mutat Res. 1988 Jun;199(2):341–351. doi: 10.1016/0027-5107(88)90213-8. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Hurlin P. J., Fry D. G., Maher V. M., McCormick J. J. Morphological transformation, focus formation, and anchorage independence induced in diploid human fibroblasts by expression of a transfected H-ras oncogene. Cancer Res. 1987 Nov 1;47(21):5752–5757. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- McCormick J. J., Maher V. M. Towards an understanding of the malignant transformation of diploid human fibroblasts. Mutat Res. 1988 Jun;199(2):273–291. doi: 10.1016/0027-5107(88)90209-6. [DOI] [PubMed] [Google Scholar]
- McCormick J. J., Yang D. J., Maher V. M., Farber R. A., Neuman W., Peterson W. D., Jr, Pollack M. S. The HuT series of 'carcinogen-transformed' human fibroblast cell lines are derived from the human fibrosarcoma cell line 8387. Carcinogenesis. 1988 Nov;9(11):2073–2079. doi: 10.1093/carcin/9.11.2073. [DOI] [PubMed] [Google Scholar]
- Morgan T. L., Maher V. M., McCormick J. J. Optimal parameters for the polybrene-induced DNA transfection of diploid human fibroblasts. In Vitro Cell Dev Biol. 1986 Jun;22(6):317–319. doi: 10.1007/BF02623404. [DOI] [PubMed] [Google Scholar]
- Namba M., Nishitani K., Fukushima F., Kimoto T., Nose K. Multistep process of neoplastic transformation of normal human fibroblasts by 60Co gamma rays and Harvey sarcoma viruses. Int J Cancer. 1986 Mar 15;37(3):419–423. doi: 10.1002/ijc.2910370314. [DOI] [PubMed] [Google Scholar]
- Namba M., Nishitani K., Fukushima F., Kimoto T., Yuasa Y. Multi-step neoplastic transformation of normal human fibroblasts by Co-60 gamma rays and Ha-ras oncogenes. Mutat Res. 1988 Jun;199(2):415–423. doi: 10.1016/0027-5107(88)90218-7. [DOI] [PubMed] [Google Scholar]
- Namba M., Nishitani K., Hyodoh F., Fukushima F., Kimoto T. Neoplastic transformation of human diploid fibroblasts (KMST-6) by treatment with 60Co gamma rays. Int J Cancer. 1985 Feb 15;35(2):275–280. doi: 10.1002/ijc.2910350221. [DOI] [PubMed] [Google Scholar]
- Namba M., Nishitani K., Kimoto T. Carcinogenesis in tissue culture. 29: Neoplastic transformation of a normal human diploid cell strain, WI-38, with Co-60 gamma rays. Jpn J Exp Med. 1978 Aug;48(4):303–311. [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- O'Brien W., Stenman G., Sager R. Suppression of tumor growth by senescence in virally transformed human fibroblasts. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8659–8663. doi: 10.1073/pnas.83.22.8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Paterson H., Reeves B., Brown R., Hall A., Furth M., Bos J., Jones P., Marshall C. Activated N-ras controls the transformed phenotype of HT1080 human fibrosarcoma cells. Cell. 1987 Dec 4;51(5):803–812. doi: 10.1016/0092-8674(87)90103-6. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Ryan P. A., Maher V. M., McCormick J. J. Modification of MCDB 110 medium to support prolonged growth and consistent high cloning efficiency of diploid human fibroblasts. Exp Cell Res. 1987 Oct;172(2):318–328. doi: 10.1016/0014-4827(87)90390-9. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr Human cell transformation by simian virus 40--a review. In Vitro. 1981 Jan;17(1):1–19. doi: 10.1007/BF02618025. [DOI] [PubMed] [Google Scholar]
- Sager R., Tanaka K., Lau C. C., Ebina Y., Anisowicz A. Resistance of human cells to tumorigenesis induced by cloned transforming genes. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7601–7605. doi: 10.1073/pnas.80.24.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn E., Muller W., Pattengale P., Tepler I., Wallace R., Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987 May 22;49(4):465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
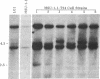
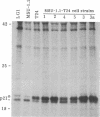
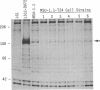
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spandidos D. A. Mechanism of carcinogenesis: the role of oncogenes, transcriptional enhancers and growth factors. Anticancer Res. 1985 Sep-Oct;5(5):485–498. [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Bennett P. V., Freeman A. G., Moore S. P., Strickland P. T. Transformation of human cells by DNAs ineffective in transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2399–2403. doi: 10.1073/pnas.82.8.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo R. A., Rheinwald J. G. Normal human mesothelial cells and fibroblasts transfected with the EJras oncogene become EGF-independent, but are not malignantly transformed. Oncogene Res. 1987 Sep-Oct;1(4):407–421. [PubMed] [Google Scholar]
- Yunis J. J., Chandler M. E. High-resolution chromosome analysis in clinical medicine. Prog Clin Pathol. 1978;7:267–288. [PubMed] [Google Scholar]