Abstract
Visual perceptual learning (VPL) is defined as a long-term improvement in performance on a visual task. In recent years, the idea that conscious effort is necessary for VPL to occur has been challenged by research suggesting the involvement of more implicit processing mechanisms, such as reinforcement-driven processing and consolidation. In addition, we have learnt much about the neural substrates of VPL and it has become evident that changes in visual areas and regions beyond the visual cortex can take place during VPL.
The adult neural system can achieve long-term enhanced performance on a visual task as a result of visual experience1. This process is known as visual perceptual learning (VPL). An expert in X-ray analysis, for example, can identify a tumour from the pattern of gray and black spots on an X-ray scan without much difficulty, whereas it is impossible for an untrained person to perform the task.
Investigating the neural changes that are associated with VPL will lead to an increased understanding of plasticity in the adult visual system. When carrying out such studies, it is crucial to distinguish between the processes that lead to VPL and the changes that occur in association with the completion of VPL (that is, the areas involved in the process of training on a task may not necessarily be altered with VPL). As such, we discuss these issues separately.
A dominant view on the processes that lead to VPL has been that they require conscious effort on the part of the learner2,3. This view has been challenged by recent lines of research suggesting that implicit processing without conscious effort during and after training has a significantly more fundamental role in VPL. There is also wide acceptance that brain changes associated with VPL occur in the primary visual cortex (V1) and higher-level areas of the visual cortex. In addition, the results of some more recent studies suggest that changes in association with VPL occur beyond the visual areas — that is, in the connections between the visual and ‘decision-making’ areas of the brain or in the decision-making areas themselves4,5.
Here we review the current understanding of implicit and conscious processing during and after VPL training and the changes associated with completion of VPL. We focus on VPL of primitive visual features, such as orientation, motion, luminance contrast and Vernier acuity, and aim to complement excellent recent reviews of auditory learning6, multisensory learning7, learning of higher cognitive features and aspects8, and visual statistical learning9,10.
Processing during VPL training
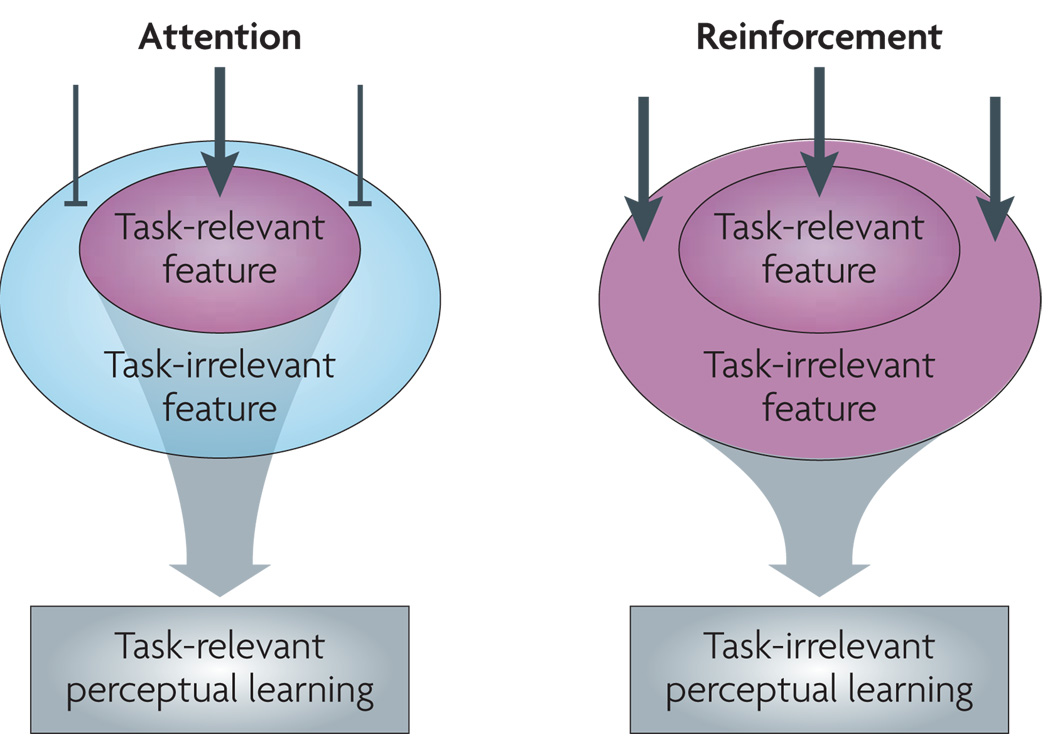
Our eyes are constantly bombarded with visual information. In order to continue to process visual signals, the visual system must maintain a certain degree of stability and thus cannot be changed by every bit of information it receives. At the same time, the visual system needs to be able to adapt to important novel environmental input. This creates what has been called the plasticity–stability dilemma11,12. To deal with these conflicting demands, plasticity must be gated so that only the features in the new environment that are important to the observer are learnt. Various studies have indicated that focused attention functions as a gate to ensure that VPL occurs only in response to features to which attention is directed (task-relevant features). However, it has recently been reported that focused attention on a feature is not necessary for VPL to occur and that rewards function as a gate to regulate VPL of both task-relevant features and features to which attention is not directed (task-irrelevant features) (FIG. 1).
Figure 1. Processing during VPL training.
According to the model presented, visual perceptual learning (VPL) of the presented visual feature occurs when a bottom-up signal from the feature is boosted by attention (left) or by reinforcement signals (right). Attention enhances task-relevant signals and inhibits task-irrelevant signals, leading to task-relevant VPL. By contrast, reinforcement signals are diffusive and enhance signals from any stimulus feature presented in the visual field, irrespective of whether the feature is task-relevant or task-irrelevant.
Attention as a gate to task-relevant VPL
Early studies of VPL found that a conscious effort to direct focused attention plays a fundamental part in gating visual plasticity. For example, when two features were presented simultaneously in a task that required the subject to pay attention to one feature of the presented stimulus there was no VPL of the feature to which the subject did not pay attention. This suggested that only the feature to which the subject actively paid attention was learnt3,13,14. In addition, some studies indicated that VPL is task-dependent. VPL of a particular feature did not transfer from the task on which the subject was originally trained to another task involving the same or similar stimuli but using a different procedure15,16. These findings led some vision scientists to conclude that conscious effort when performing a task, such as focused attention to the task-relevant feature during training (or to the task procedure), is necessary for the feature (or the task) to be learnt.
An important role of conscious effort was also demonstrated by research examining the effects of giving subjects feedback on the correctness of their responses on VPL. VPL of a Vernier acuity task was facilitated by trial-by-trial feedback (response feedback) and also by feedback regarding the percentage of correct responses in a block consisting of around a hundred trials (block feedback)17. In other studies, the criteria that subjects used to make decisions during training on a Vernier acuity task seemed to be shifted by incorrect feedback. Subjects were trained to carry out a three-dot Vernier discrimination task in which they had to judge whether a central dot was shifted towards the right or left as compared with the dots that were vertically aligned18,19 (FIG. 2a). When the central dot was slightly shifted to one side, the subject was given incorrect response feedback, whereas when the central dot was shifted to the other side, they were given correct response feedback. As a result of this training, the subjects tended to respond according to the feedback information, irrespective of whether the feedback was actually correct or incorrect. However, the subjects that had set their criteria according to incorrect feedback gave correct responses as soon as they were provided with correct feedback. These feedback studies suggest an important role of top-down processing, probably attention, in VPL18. In addition, the rapid modification of the response or decision criteria suggests that the modification is temporary, rather than a long-term change associated with VPL. A recent study found that VPL was facilitated by positive fake feedback indicating that the subject’s performance was improving from one block of trials to another regardless of the actual performance change. By contrast, negative fake feedback did not influence performance improvement. This finding suggests that feedback works as a positive reward20. Although feedback facilitates VPL, in many cases VPL can also occur in the absence of feed-back3,21,22, indicating that feedback is not necessary for VPL. However, the process of training without feedback should not be regarded as implicit as it still requires the subject to make efforts to perform the task.
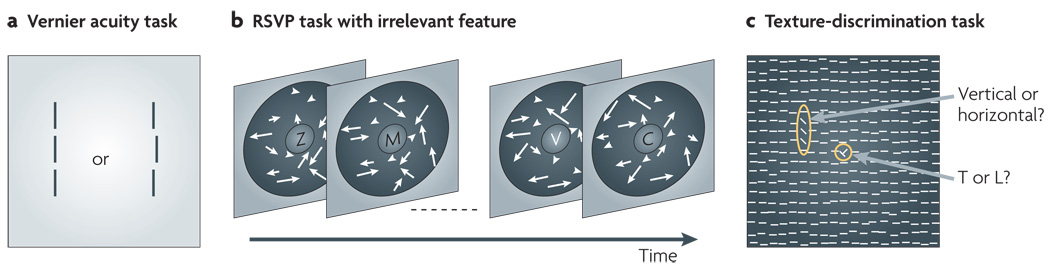
Figure 2. Typical tasks used in VPL studies.
a | In the Vernier acuity task, a configuration of two or three vertical lines (or dots) is presented. The subject is asked to indicate whether the lines (or the dots) are aligned. b | The RSVP (rapid serial visual presentation) task with moving dots during training is used to examine visual perceptual learning (VPL) of task-irrelevant coherent motion. The subject is asked to identify two target items (such as white letters) within a sequence of non-target items (such as black letters) at the centre of the display. The background display consists of coherent motion (dots moving in the same direction at the same speed) and random motion (dots moving in random directions with random speed). The arrows represent the velocity of the coherent motion. In test stages before and after training, only coherent motion is displayed (not shown here) to determine how performance in motion-discrimination or -detection tasks is changed by training. c | A texture-discrimination task is the most frequently used task in VPL studies. The subject is first asked to respond according to whether a ‘T’ (as shown in the figure) or an ‘L’ is presented in the centre of the display to ensure fixation at the centre, and then to indicate whether the orientation of the target (the three elements with orientation that differs from that of the rest of the elements) is vertical (as shown in the figure) or horizontal. VPL of the target orientation is examined. Part b is modified, with permission, from REF. 29 © (2001) Macmillan Publishers Ltd. All rights reserved. Part c is modified, with permission, from REF. 71 © (1991) National Academy of Sciences.
Task-irrelevant VPL
The research described above indicates that conscious effort, including attention, has a significant role in VPL. However, several studies have indicated that, although important, such effort is not necessary for VPL to occur23–31. In one study29, subjects were asked to identify a letter in the centre of a display while a motion display, consisting of moving dots, was presented in a peripheral field (FIG. 2b). In the motion display, 5% of the dots moved coherently and the remaining dots moved randomly32. The percentage of dots moving coherently was so small that subjects were able to discriminate or detect the coherent-motion direction with only chance-level performance before (pre-test) and after (post-test) the exposure period. Nevertheless, when subjects were subsequently tested with a supra-threshold (10%) coherent-motion display, their ability to discriminate or detect coherent motion was enhanced for motion in the direction to which they were previously exposed. These results indicate that conscious effort such as paying attention to a feature is not necessary for VPL of the feature to occur. This type of VPL is known as task-irrelevant VPL and has been reported by a number of research groups23–26.
Interestingly, it has also been shown that although VPL of a task-irrelevant feature occurred in the experiments described above, performance is enhanced to a greater extent when attention is directed to the feature25,26. This finding serves as evidence that attention plays an important part in gating VPL26,33,34. However, the task-irrelevant VPL findings suggest that there may be a factor other than attention that gates VPL to control the balance between the plasticity and stability of the visual system. One possible factor is reinforcement processing, as we discuss below.
Reward and reinforcement signals
Recent studies indicate that reinforcement signals gate task-irrelevant VPL. VPL of task-irrelevant coherent-motion direction occurred only when the motion was paired with the presentation of a target item (such as white letters) in a sequence of otherwise distractors30 (such as black letters as in FIG. 2b). Furthermore, task-irrelevant VPL of coherent motion occurred only when the target was successfully perceived or recognized35. These findings suggest that successful task performance led to a sense of accomplishment that functioned as an internal reward (as opposed to an externally provided physical reward). Unlike focused attention, which relatively enhances signals directed to a specific spatial location in the brain, reward is known to be a trigger for reinforcement signals that are spatially distributed in the brain. Such spatially diffusive reinforcement signals boost incoming sensory signals related to the presented feature, irrespective of whether the feature is task-relevant or task-irrelevant, and result in the learning of the feature36.
According to this hypothesis, signal strength gates plasticity. If this is true, when reinforcement signals are internally released while the stimulus is presented, learning should occur automatically and implicitly, irrespective of whether the feature is task-relevant or task-irrelevant37. This hypothesis is supported by the results of a recent study that examined whether an invisible feature paired with reward was learnt31. In the exposure stage of the experiment, Gabor patches that contained oriented grating structures were repeatedly exposed to one eye of water-deprived subjects. During the presentations of Gabor patches, different images of randomly placed coloured patches were flashed successively at approximately 10 Hz into the other eye. The presentation of the dynamic coloured patches eliminated the perception of the Gabor patches38. Gabor patches containing gratings with two different orientations were alternately presented, only one of which was paired with water as a reward. Performance on a task in which the subjects had to indicate the orientation of the grating after exposure was higher only when they had to indicate the orientation previously paired with the water. This result supports the hypothesis that VPL occurs as a result of a bottom-up stimulus signal being boosted by a reinforcement signal. Furthermore, performance enhancement was observed when the exposed eye (to which the orientation was presented) was used in test stages, but not when the unexposed eye was used. This suggests that such learning involves monocular processing that may mainly occur in V1 (REF. 39). This view is in accordance with the finding that reward can affect cells in V1 of rats40 and humans41.
Comparing task-relevant and task-irrelevant VPL
Do task-relevant and task-irrelevant VPL occur through the same mechanism? Furthermore, why does task-irrelevant VPL occur only under certain conditions3,13,14,29? Task-irrelevant VPL in detecting coherent-motion direction did not occur when the percentage of coherent-motion signals was 50%, making the coherent motion conspicuous. However, task-irrelevant VPL of coherent-motion did occur when the percentage was just below the perception threshold42. This finding is in accordance with the results of a brain imaging experiment suggesting that signals from a task-irrelevant display containing a high percentage of coherent motion (50%) are effectively suppressed by regions that control attention42, such as the lateral prefrontal cortex (LPFC)43. Signals from a motion display with a low percentage of coherence (5%) are not detected and therefore not suppressed by the LPFC44. These findings suggest that in some conditions task-irrelevant learning will occur only if the irrelevant feature is inconspicuous enough to avoid detection by the attention system.
It was generally thought that the failure to learn conspicuous irrelevant features was a result of the need for focused attention in order for VPL to occur3,13,14. However, the results of the above studies42,44 suggest that the failure was due merely to attentional inhibition of a conspicuous task-irrelevant feature. That is, a conspicuous task-irrelevant feature is detected and inhibited by the attention system and therefore learning of the feature does not occur. This is in accordance with the finding that training on a task-relevant feature reduced sensitivity to a task-irrelevant feature22,25,45 and the blood oxygen level-dependent (BOLD) signal it generated in response46.
The view that focused attention to a feature is necessary for VPL of the feature to occur has also been refuted by the results of several studies demonstrating that task-irrelevant VPL can occur for a conspicuous irrelevant feature23–26. In one such study, performance in a Vernier acuity task in a test stage was higher after a mere exposure to a multitude of stimuli with no task performance24. As no task was performed during the exposure, there was no reason for attention to inhibit signals from the stimuli. Therefore, task-irrelevant learning may have occurred. In a second study, VPL of not only a moving grating to which the subject attended but also of a non-attended grating (moving in the same direction as the attended grating but presented in a different location) occurred. However, a grating moving in a different direction and presented in a different location was not learnt25. This suggests that there may be a factor, such as feature-based attention, that enhances signals from stimuli with the same feature presented in different locations. This contrasts with spatial attention, which enhances signals from a stimuli in the location to which attention is directed47,48. These studies indicate that task-irrelevant learning can occur under a number of different conditions, which should be systematically examined in order to fully understand this phenomenon.
In summary, VPL is gated by both attention that is driven by task demands and reinforcement signals that are triggered by rewards, but in different ways. Attention enhances task-relevant signals and inhibits task-irrelevant signals, leading to task-relevant VPL (FIG. 1a). Conversely, reinforcement signals enhance bottom-up visual signals irrespective of whether the visual signals come from a task-relevant or task-irrelevant feature, leading to both task-relevant and task-irrelevant VPL (FIG. 1b). Although attention and reinforcement signals usually work cooperatively to enhance task-relevant VPL49, in cases in which attention is defective or ineffective the task-irrelevant signal may not be inhibited and task-irrelevant VPL may take place.
Processing during VPL consolidation
Most research on the processing that leads to VPL has concentrated on examining the processing that occurs during training. However, the consolidation of VPL after training has recently attracted attention. This is partly due to the great progress in research on sleep and memory consolidation in the past few years, as well as the increased interest in implicit processing during and after training. Research on some types of learning has demonstrated that memory and/or learning is so fragile immediately after training that it is necessary to allow processing time — a period known as consolidation — to stabilize it (that is, to make it permanent). Importantly, consolidation occurs without the subject’s knowledge or effort and is therefore implicit. There is evidence that consolidation occurs both during wakefulness immediately after training and during subsequent sleep35,50–68. Recently there has been increasing interest in the mechanisms of consolidation in VPL.
VPL consolidation during wakefulness
Learning of a Vernier acuity task (FIG. 2a) has been shown to be disrupted by training on a different Vernier task within 1 hour of the end of training on the first task69. However, when training on the second task occurred more than 1 hour after training on the first task, no disruption was observed. More recent research70 has demonstrated similar results during VPL of a texture-discrimination task (TDT)71 in which subjects must indicate whether the orientation of a ‘triplet’ consisting of three line segments differs from that of the other line segments (FIG. 2c). These results indicate that the initial consolidation process starts and is completed within 1 hour of training on a perceptual task69.
VPL consolidation during sleep
Another type of consolidation that has attracted a great deal of attention occurs during sleep after training. In typical nocturnal sleep of healthy young adults, rapid eye movement (REM) sleep and non-REM (NREM) sleep alternate four to five times within a 6–8-hour period. According to the conventional sleep scoring method72, NREM sleep is categorized into stages 1, 2, 3 and 4. Stages 3 and 4 are collectively called slow-wave sleep (SWS) in humans. During the early part of a night’s sleep, SWS occurs frequently and the duration of REM sleep tends to be short. In the later part of a night’s sleep, SWS seldom occurs and the duration of REM sleep is longer (see Supplementary information S1 (figure)).
Initial research on the effects of sleep on VPL indicated that depriving an individual of REM sleep after training nullified VPL in a TDT, suggesting that REM sleep plays a crucial part in the consolidation of VPL65. Subsequent research found that depriving subjects of the SWS-rich first half of a night’s sleep after training also nullifies VPL on the same TDT73. Moreover, the amount of SWS during the early part of the sleep cycle and the amount of REM sleep during the later part correlated with improvement in performance on a TDT74. Therefore both SWS and REM sleep seem to have a significant role in VPL consolidation. The timing of sleep is also important for consolidation of learning74–76. Performance on a TDT improved when subjects were tested on the day after initial training having been allowed normal sleep; however, no significant improvement was found when subjects were deprived of sleep for 30 hours after training and then tested after two full nights of recovery sleep74. These results suggest that there is a critical time window for sleep to effectively consolidate VPL. For humans, the window for consolidation seems to be shorter than 30 hours74.
Mechanisms of VPL consolidation
Where in the brain does consolidation of VPL during wakefulness take place? Learning of a Vernier acuity task was disrupted by training on a different Vernier task within 1 hour only if the stimuli used in these tasks had the same orientation or were presented in the same spatial location69. This specificity suggests that consolidation involves early visual processing, although this possibility needs to be physiologically tested.
Sleep-induced consolidation of VPL seems to occur in V1, at least under some conditions. In functional MRI studies, the BOLD signal in the retinotopic region of human V1 corresponding to the visual field location of the trained stimulus (the trained region of V1) was enhanced during wakefulness after sleep subsequent to training compared with during wakefulness before sleep77,78. Furthermore, it was recently shown that the BOLD signal in the trained region of V1 (but not in other regions of V1) was enhanced during the initial NREM sleep after training79. The intensity of the BOLD signal was highly correlated with the increase in performance after sleep. This finding suggests that consolidation during sleep involves a highly localized low-level processing region, changes in which directly correlate with VPL.
Whether other brain areas are involved in sleep consolidation remains an open question. It has been suggested that consolidation of the memory of spatio-temporal patterns activates both the hippocampus and the prefrontal cortex in rats80. Although a neuroimaging study showed a tendency towards prefrontal involvement in VPL of a TDT, it has yet to be clarified whether the prefrontal cortex is also involved in the consolidation of VPL during sleep79.
Two neural models of processing during sleep have been proposed to account for performance enhancement after sleep subsequent to training for learning in general: synaptic homeostasis81,82 and reactivation56,83,84 (BOX 1). However, at present there is no conclusive evidence indicating which model applies to consolidation for VPL.
Box1 | Two models for consolidation during sleep
There are two dominant general theories about the mechanism for consolidation of memory and learning during sleep: the synaptic homeostasis model and the reactivation model.
According to the synaptic homeostasis model81, slow-wave activity (1–4 Hz spontaneous oscillatory activity that is dominant during slow-wave sleep), which is prominent during early non-rapid eye movement sleep, plays a part in scaling down synapses that are excessively increased in number or strengthened by the learning acquisition process during wakefulness. As a result, only the strongest synapses remain, and performance is higher after sleep. According to this hypothesis, slow-wave activity may therefore act like long-term depression. This model is supported by the presence of increased slow-wave activity near the motor and parietal areas in the right hemisphere during sleep after implicit motor learning115 and also by the finding that the reduction in performance on a texture-discrimination task that can result from excessive training is reduced after sleep82,116–120. In flies, the number of synapses increases during wakefulness in conditions including those in which learning is expected to occur. However, the elevated number of synapses then decreases during sleep121,122.
The reactivation model suggests that neurons that are involved in learning acquisition are reactivated during sleep to strengthen neuronal connections56,83,84. Thus, the reactivation model assumes a process similar to long-term potentiation. In support of this model, firing-rate patterns during episodic memory training in cortical areas including the hippocampus and the medial prefrontal cortex in rats80,123 and humans124 were preserved during sleep after training. It is not clear whether only one of these theories is broadly valid for any type of learning and memory or whether one theory is valid for some types of learning and memory and the other theory for other types.
Changes observed after VPL completion
As described above, we have learnt much about the processes of VPL training and consolidation. But what happens subsequently? That is, what neural changes can be observed after VPL training and consolidation? Although the changes associated with task-irrelevant learning are largely unclear, it is generally widely accepted that task-relevant learning results in changes in the visual cortex. However, recent neurophysiological findings have indicated that some types of VPL are associated with changes in regions of the cortex involved in decision making or in the connectivity between the visual cortex and these areas (FIG. 3).
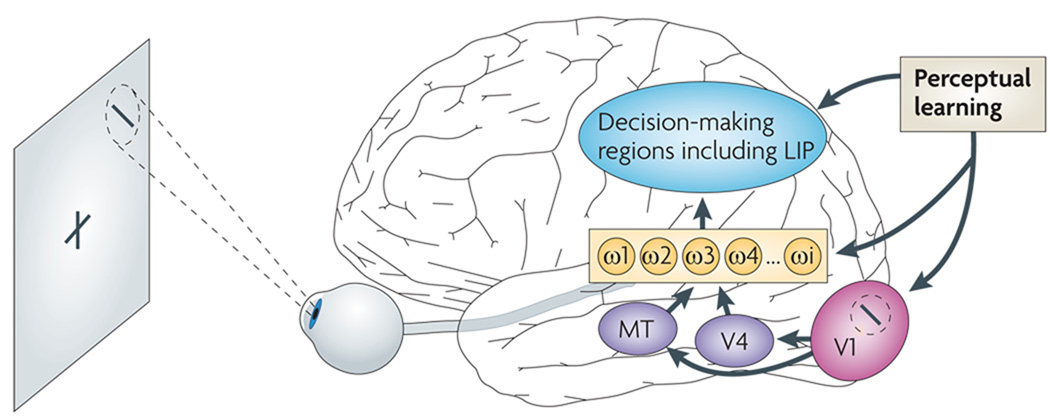
Figure 3. Neural correlates of VPL.
The regions of the brain thought to be altered by visual perceptual learning (VPL). Some experiments have indicated that training on a visual task changes visual representations in the early stages of visual signal processing, such as the tuning properties and activity of the primary visual cortex (V1) region that retinotopically corresponds to the location of the trained stimulus in the visual field. Others have instead suggested that training alters the weight of connections (ω1, ω2 … ωi) between the visual cortex and regions of the brain involved in decision making, or within the decision-making regions themselves. In VPL of motion or a feature carrying spatial information, the weight changes may predominantly occur between areas in the higher visual cortex, such as the middle temporal area (MT) and the lateral intraparietal area (LIP), which is thought to be involved in visual decision-making processes4. MT is usually responsible for coarse binocular disparity (depth) processing; however, when MT is inactivated, decision-making regions may learn to give more weight to signals from areas involved in ventral processing, including V4, when discriminating coarse binocular disparity5.
Changes in the visual cortex
In early studies, several types of VPL were found to be highly specific for the location of the stimulus in the visual field3,21,71,85–89 and for visual features of the stimulus, including orientation14,21,87, motion direction28,90–92 and the eye to which it was presented1,71,93. For example, learning of a stimulus presented at a particular location in the visual field was abolished when the stimulus was spatially shifted by even a few degrees of visual angle3,21,71,85–89. In processing visual signals there is a general tendency for the receptive field size to be smaller in regions of the visual cortex that have a role earlier in visual processing pathways; in addition, the tuning specificity of neurons for features such as orientation and motion direction is higher at earlier than at later stages94,95. It was therefore concluded that VPL with high feature and location specificity is associated with changes in the earliest visual area, such as V1 (REF. 89). Indeed, several studies, including training on a contrast-discrimination task96 and a TDT77,78,97, have found that VPL is associated with increases in the BOLD signal in the region of V1 in humans that corresponds to the location of the trained stimulus. Furthermore, training in an orientation-discrimination task in monkeys led to enhancement of the tuning specificity of cells in V1 that are involved in discrimination of orientation14. Several physiological studies also support the hypothesis that V1 is changed in association with VPL89,98,99.
Some studies have indicated that cortical changes associated with VPL also occur in the middle visual stages (between the lowest visual stage and the decision-making stage). For example, changes in the tuning properties of cells in V4 in monkeys were found after training on an orientation discrimination task100, whereas no such tuning changes were observed in V1 (REFS 100,101). Some psychophysical studies also suggest that VPL is associated with changes in the middle visual stages. For example, some types of VPL depend on perceptual constancy (the stable representation of certain properties of an object despite variable visual input)102. Such representation is thought to occur in the middle visual stage102.
In addition, there are cases in which VPL transfers to untrained features103 and locations104. Training on a task in which subjects must discriminate between motion directions that are close to each other leads to VPL specific to the trained directions90; however, the learning that results from training on a task in which the direction differences are larger can transfer to untrained directions103. Furthermore, training to discriminate a particular feature (such as contrast) at one location followed by additional training with another feature (such as orientation) at a second location resulted in a complete transfer of the improvement in discrimination of the first feature to the second location104. These results suggest that under certain conditions, including those that do not require high location or feature specificity that need to be mainly processed in V1, VPL occurs in a middle visual stage in which location and feature signals are less specific than in V1.
The results of some experiments suggest that the stage of visual processing at which neural changes associated with VPL occur is not fixed but changes as VPL proceeds33,105. For example, it was shown that the location specificity of VPL depends on the difficulty of an orientation-discrimination task and that VPL of a difficult task can be accomplished only after VPL on an easier task occurs33. Based on these results, a reversed hierarchy model has been proposed in which, as learning proceeds, the stages that are involved move from higher to lower processing levels33,105. However, the dependency of the specificity of VPL on task difficulty has recently been challenged106.
Changes in connectivity or decision-making areas
The idea that changes associated with VPL occur exclusively in visual areas has recently been challenged by the results of some neurophysiological studies in monkeys4,5. In one of these studies4, VPL of motion did not change the responses of cells in the middle temporal area (MT) — a region highly responsive to motion107 that is regarded as one of the highest visual areas in the dorsal visual path-way — but did change the responses of cells in the lateral intraparietal area (LIP), a region that is known to represent the transformation of visual motion signals into responses by saccadic eye movements (that is, to represent a perceptual decision)108. In another monkey physiology study5, reversible inactivation of MT by injection of the GABA (γ-aminobutyric acid) agonist muscimol before training in a task requiring coarse discrimination between disparities in absolute depth of stimuli was shown to impair performance. However, MT inactivation before training on fine relative-depth discrimination did not impair coarse depth discrimination or alter the disparity tuning of MT neurons. As fine depth signals are not carried by MT109 and are probably represented in areas including V4 in the ventral pathway110, it was suggested that when MT is inactivated these areas in the ventral pathway are recruited and mediate coarse depth discrimination. These results suggest that the brain learns to put more weight on disparity signals from the ventral pathway than on those from MT5.
These neurophysiological findings are in accordance with a model in which perceptual learning is described as task-specific selective re-weighting111–113. According to this model, visual stimuli are represented by standard orientation- and frequency-tuned representational units. VPL results from changes in task-specific selective ‘weighting’ (that is, task-specific changes in the strength of neural connections) between low-level visual representation stages and a higher stage at which a decision concerning how visual signals are interpreted guides responses (that is, a decision stage)111–113.
The noted discrepancies between studies that have implicated different regions as being altered by VPL demands explanation. One possibility is that the best strategy for performance improvement may vary depending on the task in question. For example, if a task requires finer orientation discrimination, the orientation tuning properties of neurons in the lower-level visual cortex would be changed33. Conversely, if a more global change such as noise reduction or switching pathways leads to enhancement in performance, changes may involve brain regions beyond the visual cortex.
Past, present and future
The early general view of VPL — that conscious effort such as attention is necessary2,3 — has been challenged by the results of several studies, as described in this article. In addition, a growing body of evidence indicates that consolidation processed implicitly during wakefulness69 and during sleep65,79,114 may be important in VPL, as originally suggested by Sagi and colleagues65. As we have outlined, it has also become clear that different types of VPL are associated with changes in the visual cortex and in areas responsible for decision making or with changes in the connectivity between the visual cortex and the decision-making areas4,5,111,113.
However, the newer results that we have discussed are not necessarily inconsistent with the older views. For example, conscious and implicit processing may not be mutually exclusive. The usual training procedure used to generate VPL may include both conscious processing, such as focused attention to a task-relevant feature, and reinforcement processing that includes implicit components (FIG. 1). Furthermore, as outlined above, whether changes associated with the completion of VPL occur mainly in the visual cortex or beyond may depend on which changes lead to performance improvement in the most efficient manner.
What should future research focus on? It remains unclear how conscious processing involving attention and the more implicit processing of reinforcement signals interact to produce VPL. One model indicates that diffusive reinforcement signals are regulated by attention49. Another possibility is that attentional and reinforcement signals are processed independently and that the degree to which each influences VPL depends on the situation under which VPL occurs. It is also unclear how consolidation of VPL is similar to or different from consolidation of other forms of learning and memory. As discussed above, certain aspects of VPL are distinct from other types of learning and memory. It will be necessary to systematically compare consolidation of VPL with that of other forms of learning and memory. To date, there has been a tendency for different researchers to use different parameters, such as stimuli or tasks, in their VPL studies. In some cases findings based on a particular set of parameters have been overgeneralized. However, it remains unclear whether different types of VPL have the same general underlying mechanism(s). For example, a condition that requires conscious effort during training may or may not result in a different type of consolidation and/or a change in a different cortical region to a condition that does not require conscious effort. We feel that the time has come to move research in a new direction in which the interactions and relations between different types of processing and the conditions leading to different types of VPL should be systematically investigated.
Supplementary Material
Acknowledgements
We thank G. Deangelis, B. Dosher, Z.-L. Lu and K. Shibata for valuable comments on an early draft of the paper and N. Ito for technical assistance. This study is supported by grants from the Sleep Research Society Foundation, Harvard Medical School, Massachusetts General Hospital, ERATO Shimojo Implicit Brain Project (Japan Science Technology), the National Centre for Research Resources (P41RR14075) the Mind Institute and the Athinoula A. Martinos Center for Biological Imaging to Y.S. and by grants from the US National Institutes of Health (R01 EY015980-04A2, R01 EY019466, R01 AG031941, R21 EY018925, R21 EY017737) and the National Science Foundation (BCS-0549,036) to T.W.
Glossary
- Implicit processing
Processing that occurs without a subject’s awareness.
- Vernier acuity
The ability to detect an offset from collinearity in a pair or triad of abutting lines or dots.
- Gabor patches
The two-dimensional image formed by multiplying a sine wave and a Gaussian function. Gabor patches are widely used in vision research because they have a well-defined spatial frequency, orientation and location.
- Blood oxygen level-dependent (BOLD) signal
The signal based on the relative concentration of contrast deoxygenated and oxygenated blood measured by functional MRI. The BOLD signal is thought to reflect some significant aspects of neural activity.
- Rapid eye movement (REM) sleep
The period of sleep characterized by a relatively low-voltage, mixed-frequency electroencephalogram in conjunction with episodic rapid eye movements and low-amplitude electromyogram. Breathing and heart rates are irregular during REM sleep, which is also when vivid dreaming is thought to occur.
- Non-REM (NREM) sleep
The period of sleep that is not classified as REM sleep. Slow-wave sleep (SWS) is a component of deeper NREM sleep in humans. However, SWS is synonymous with NREM sleep in animals.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Yuka Sasaki’s homepage: http://www.nmr.mgh.harvard.edu/martinos/people/showPerson.php?people_id=147
Jose E. Nanez’s homepage: http://www.west.asu.edu/jnanez/
Takeo Watanabe’s homepage: http://www.bu.edu/psych/faculty/watanabe/
SUPPLEMENTARY INFORMATION
See online article: S1 (figure)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Fahle M, Poggio T. Perceptual Learning. MIT Press; 2002. [Google Scholar]
- 2.Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proc. Natl Acad. Sci. USA. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiu LP, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Percept. Psychophys. 1992;52:582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- 4. Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nature Neurosci. 2008;11:505–513. doi: 10.1038/nn2070. Performance improvement in motion-direction discrimination resulting from training was accompanied by changes in motion-driven responses of LIP but not MT neurons. The results support a model suggesting that VPL is associated with changes in connectivity between the visual and decision areas or in decision areas.
- 5. Chowdhury SA, DeAngelis GC. Fine discrimination training alters the causal contribution of macaque area MT to depth perception. Neuron. 2008;60:367–377. doi: 10.1016/j.neuron.2008.08.023. This study indicates that VPL of coarse binocular disparity, which is usually processed by MT, can occur as a result of decision units learning to put more weight on signals from ventral areas that usually process finer binocular disparity.
- 6.Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear. Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shams L, Seitz AR. Benefits of multisensory learning. Trends Cogn. Sci. 2008;12:411–417. doi: 10.1016/j.tics.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Chun MM, Marois R. The dark side of visual attention. Curr. Opin. Neurobiol. 2002;12:184–189. doi: 10.1016/s0959-4388(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 9.Turk-Browne NB, Scholl BJ, Chun MM, Johnson MK. Neural evidence of statistical learning: efficient detection of visual regularities without awareness. J. Cogn. Neurosci. 2009;21:1934–1945. doi: 10.1162/jocn.2009.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiser J, Scholl BJ, Aslin RN. Perceived object trajectories during occlusion constrain visual statistical learning. Psychon. Bull. Rev. 2007;14:173–178. doi: 10.3758/bf03194046. [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara H, Doi T, Kawato M. Systems biology perspectives on cerebellar long-term depression. Neurosignals. 2008;16:300–317. doi: 10.1159/000123040. [DOI] [PubMed] [Google Scholar]
- 12.Grossberg S. How does a brain build a cognitive code? Psychol. Rev. 1980;87:1–51. doi: 10.1007/978-94-009-7758-7_1. [DOI] [PubMed] [Google Scholar]
- 13.Ahissar M. Perceptual training: a tool for both modifying the brain and exploring it. Proc. Natl Acad. Sci. USA. 2001;98:11842–11843. doi: 10.1073/pnas.221461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. Changes were observed in tuning properties in monkey V1 neurons tuned to orientations crucial for VPL of an orientation-discrimination task.
- 15.Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nature Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Lu H, Tjan BS, Zhou Y, Liu Z. Motion perceptual learning: when only task-relevant information is learned. J. Vis. 2007;7(14):1–10. doi: 10.1167/7.10.14. [DOI] [PubMed] [Google Scholar]
- 17. Herzog MH, Fahle M. The role of feedback in learning a vernier discrimination task. Vision Res. 1997;37:2133–2141. doi: 10.1016/s0042-6989(97)00043-6. This study systematically examined the effects of trial-by-trial feedback, block feedback and incorrect feedback on VPL.
- 18.Herzog MH, Fahle M. Modeling perceptual learning: difficulties and how they can be overcome. Biol. Cybern. 1998;78:107–117. doi: 10.1007/s004220050418. [DOI] [PubMed] [Google Scholar]
- 19.Herzog MH, Fahle M. Effects of biased feedback on learning and deciding in a vernier discrimination task. Vision Res. 1999;39:4232–4243. doi: 10.1016/s0042-6989(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 20.Shibata K, Yamagishi N, Ishii S, Kawato M. Boosting perceptual learning by fake feedback. Vision Res. 2009;49:2574–2585. doi: 10.1016/j.visres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Poggio T, Fahle M, Edelman S. Fast perceptual learning in visual hyperacuity. Science. 1992;256:1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- 22.Weiss Y, Edelman S, Fahle M. Models of perceptual learning in vernier hyperacuity. Neural Comput. 1993;5:695–718. [Google Scholar]
- 23.Zajonc R. Attitudinal effects of mere exposure. J. Pers. Soc. Psych. Mon. Suppl. 1968;9:1–27. [Google Scholar]
- 24.Skrandies W, Fahle M. Neurophysiological correlates of perceptual learning in the human brain. Brain Topogr. 1994;7:163–168. doi: 10.1007/BF01186774. [DOI] [PubMed] [Google Scholar]
- 25.Gutnisky DA, Hansen BJ, Iliescu BF, Dragoi V. Attention alters visual plasticity during exposure-based learning. Curr. Biol. 2009;19:555–560. doi: 10.1016/j.cub.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 26.Carrasco M, Rosenbaum A, Giordano A. Exogenous attention: less effort, more learning! J. Vis. 2008;8:1095a. [Google Scholar]
- 27.Nishina S, Seitz AR, Kawato M, Watanabe T. Effect of spatial distance to the task stimulus on task-irrelevant perceptual learning of static Gabors. J. Vis. 2007;7(2):1–10. doi: 10.1167/7.13.2. [DOI] [PubMed] [Google Scholar]
- 28. Watanabe T, et al. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nature Neurosci. 2002;5:1003–1009. doi: 10.1038/nn915. This study indicates that attention to a visual feature is not necessary in order to learn the feature.
- 29.Watanabe T, Nanez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- 30.Seitz AR, Watanabe T. Psychophysics: is subliminal learning really passive? Nature. 2003;422:36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- 31. Seitz A, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron. 2009;61:700–707. doi: 10.1016/j.neuron.2009.01.016. This study shows that a stimulus that was below the threshold for perception was learnt when the subject was deprived of food or water and then given water as a reward.
- 32.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis. Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 33.Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- 34.Mukai I, et al. Activations in visual and attention-related areas predict and correlate with the degree of perceptual learning. J. Neurosci. 2007;27:11401–11411. doi: 10.1523/JNEUROSCI.3002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seitz A, Lefebvre C, Watanabe T, Jolicoeur P. Requirement for high-level processing in subliminal learning. Curr. Biol. 2005;15:R753–R755. doi: 10.1016/j.cub.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Seitz A, Watanabe T. A unified model for perceptual learning. Trends Cogn. Sci. 2005;9:329–334. doi: 10.1016/j.tics.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Seitz AR, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron. 2009;61:700–707. doi: 10.1016/j.neuron.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nature Neurosci. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- 39.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- 41.Serences JT. Value-based modulations in human visual cortex. Neuron. 2008;60:1169–1181. doi: 10.1016/j.neuron.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsushima Y, Seitz AR, Watanabe T. Task-irrelevant learning occurs only when the irrelevant feature is weak. Curr. Biol. 2008;18:R516–R517. doi: 10.1016/j.cub.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol. (Amst.) 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 44.Tsushima Y, Sasaki Y, Watanabe T. Greater disruption due to failure of inhibitory control on an ambiguous distractor. Science. 2006;314:1786–1788. doi: 10.1126/science.1133197. [DOI] [PubMed] [Google Scholar]
- 45.Paffen CL, Verstraten FA, Vidnyanszky Z. Attention-based perceptual learning increases binocular rivalry suppression of irrelevant visual features. J. Vis. 2008;8(25):1–11. doi: 10.1167/8.4.25. [DOI] [PubMed] [Google Scholar]
- 46.Gal V, et al. Learning to filter out visual distractors. Eur. J. Neurosci. 2009;29:1723–1731. doi: 10.1111/j.1460-9568.2009.06724.x. [DOI] [PubMed] [Google Scholar]
- 47.Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 48.Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nature Neurosci. 2002;5:631–632. doi: 10.1038/nn876. [DOI] [PubMed] [Google Scholar]
- 49.Roelfsema PR, van Ooyen A. Attention-gated reinforcement learning of internal representations for classification. Neural Comput. 2005;17:2176–2214. doi: 10.1162/0899766054615699. [DOI] [PubMed] [Google Scholar]
- 50.Dudai Y. Memory from A to Z. Keywords, Concepts and Beyond. Oxford Univ. Press; 2002. [Google Scholar]
- 51.Meeter M, Murre JM. Consolidation of long-term memory: evidence and alternatives. Psychol. Bull. 2004;130:843–857. doi: 10.1037/0033-2909.130.6.843. [DOI] [PubMed] [Google Scholar]
- 52.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu. Rev. Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 53.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 54.Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc. Natl Acad. Sci. USA. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 57.Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- 58.Craik FI. Levels of processing: past, present. and future? Memory. 2002;10:305–318. doi: 10.1080/09658210244000135. [DOI] [PubMed] [Google Scholar]
- 59.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: a mechanism for sleep-dependent plasticity. J. Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr. Biol. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 62.Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc. Natl Acad. Sci. USA. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J. Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 65. Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. The first study to indicate that deprivation of REM sleep abolished VPL established by training before the sleep. The result suggests an important role of sleep in perceptual learning.
- 66.Karni A, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc. Natl Acad. Sci. USA. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 68.Plihal W, Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. Neuroreport. 1999;10:2741–2747. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- 69.Seitz AR, et al. Task-specific disruption of perceptual learning. Proc. Natl Acad. Sci. USA. 2005;102:14895–14900. doi: 10.1073/pnas.0505765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yotsumoto Y, Watanabe T, Sasaki Y. Interference and feature specificity in visual perceptual learning. Vision Res. 2009;49 doi: 10.1016/j.visres.2009.08.001. 26112-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc. Natl Acad. Sci. USA. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. A seminal study indicating a high specificity for location and the trained feature in VPL and suggesting the involvement of early visual stages in VPL.
- 72.Rechtschaffen A, Kales AA, editors. Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Bethesda, Maryland: US Department of Health, Education, and Welfare; 1968. [Google Scholar]
- 73.Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nature Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- 74.Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nature Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 75.Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol. Behav. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 76.Ribeiro S, Goyal V, Mello CV, Pavlides C. Brain gene expression during REM sleep depends on prior waking experience. Learn. Mem. 1999;6:500–508. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc. Natl Acad. Sci. USA. 2002;99:17137–17142. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker MP, Stickgold R, Jolesz FA, Yoo SS. The functional anatomy of sleep-dependent visual skill learning. Cereb. Cortex. 2005;15:1666–1675. doi: 10.1093/cercor/bhi043. [DOI] [PubMed] [Google Scholar]
- 79. Yotsumoto Y, et al. Location-specific cortical activation changes during sleep after training for perceptual learning. Curr. Biol. 2009;19:1278–1282. doi: 10.1016/j.cub.2009.06.011. Signal enhancement was observed specifically in the region of V1 corresponding to the trained location during sleep after training on a TDT, and was highly correlated with performance enhancement after sleep.
- 80.Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- 81.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Censor N, Karni A, Sagi D. A link between perceptual learning, adaptation and sleep. Vision Res. 2006;46:4071–4074. doi: 10.1016/j.visres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 83.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 84.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn. Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 85.Crist RE, Kapadia MK, Westheimer G, Gilbert CD. Perceptual learning of spatial localization: specificity for orientation, position, and context. J. Neurophysiol. 1997;78:2889–2894. doi: 10.1152/jn.1997.78.6.2889. [DOI] [PubMed] [Google Scholar]
- 86.Fahle M, Edelman S. Long-term learning in vernier acuity: effects of stimulus orientation, range and of feedback. Vision Res. 1993;33:397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
- 87.Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- 88.McKee SP, Westheimer G. Improvement in vernier acuity with practice. Percept. Psychophys. 1978;24:258–262. doi: 10.3758/bf03206097. [DOI] [PubMed] [Google Scholar]
- 89.Sagi D, Tanne D. Perceptual learning: learning to see. Curr. Opin. Neurobiol. 1994;4:195–199. doi: 10.1016/0959-4388(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 90.Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vision Res. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- 91.Koyama S, Harner A, Watanabe T. Task-dependent changes of the psychophysical motion-tuning functions in the course of perceptual learning. Perception. 2004;33:1139–1147. doi: 10.1068/p5195. [DOI] [PubMed] [Google Scholar]
- 92.Vaina LM, Belliveau JW, des Roziers EB, Zeffiro TA. Neural systems underlying learning and representation of global motion. Proc. Natl Acad. Sci. USA. 1998;95:12657–12662. doi: 10.1073/pnas.95.21.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 94.Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 1984;52:1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- 95.Maunsell JH, Newsome WT. Visual processing in monkey extrastriate cortex. Annu. Rev. Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- 96.Furmanski CS, Schluppeck D, Engel SA. Learning strengthens the response of primary visual cortex to simple patterns. Curr. Biol. 2004;14:573–578. doi: 10.1016/j.cub.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 97.Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57:827–833. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 99.Karmarkar UR, Dan Y. Experience-dependent plasticity in adult visual cortex. Neuron. 2006;52:577–585. doi: 10.1016/j.neuron.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J. Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghose GM, Yang T, Maunsell JH. Physiological correlates of perceptual learning in monkey V1 and V2. J. Neurophysiol. 2002;87:1867–1888. doi: 10.1152/jn.00690.2001. [DOI] [PubMed] [Google Scholar]
- 102.Garrigan P, Kellman PJ. Perceptual learning depends on perceptual constancy. Proc. Natl Acad. Sci. USA. 2008;105:2248–2253. doi: 10.1073/pnas.0711878105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Z. Perceptual learning in motion discrimination that generalizes across motion directions. Proc. Natl Acad. Sci. USA. 1999;96:14085–14087. doi: 10.1073/pnas.96.24.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xiao LQ, et al. Complete transfer of perceptual learning across retinal locations enabled by double training. Curr. Biol. 2008;18:1922–1926. doi: 10.1016/j.cub.2008.10.030. Training to discriminate a particular feature at one location concurrently with or followed by additional training with another feature at a second location resulted in a complete transfer of the improved discrimination of the first feature to the second location. The results suggest that at least some types of VPL are associated with changes in middle- or higher-level stages of visual processing.
- 105.Hochstein S, Ahissar M. View from the top: hierarchies and reverse hierarchies in the visual system. Neuron. 2002;36:791–804. doi: 10.1016/s0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- 106.Dosher BA, Lu ZL. The functional form of performance improvements in perceptual learning: learning rates and transfer. Psychol. Sci. 2007;18:531–539. doi: 10.1111/j.1467-9280.2007.01934.x. [DOI] [PubMed] [Google Scholar]
- 107.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shadlen MN, Newsome WT. Motion perception: seeing and deciding. Proc. Natl Acad. Sci. USA. 1996;93:628–633. doi: 10.1073/pnas.93.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uka T, DeAngelis GC. Linking neural representation to function in stereoscopic depth perception: roles of the middle temporal area in coarse versus fine disparity discrimination. J. Neurosci. 2006;26:6791–6802. doi: 10.1523/JNEUROSCI.5435-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Umeda K, Tanabe S, Fujita I. Representation of stereoscopic depth based on relative disparity in macaque area V4. J. Neurophysiol. 2007;98:241–252. doi: 10.1152/jn.01336.2006. [DOI] [PubMed] [Google Scholar]
- 111. Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc. Natl Acad. Sci. USA. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. Proposes one of the most influential models of VPL. In this model, VPL occurs in association with changes in connectivity between areas for visual representation and for decision making, rather than with changes in visual representation in early areas.
- 112.Dosher BA, Lu ZL. Mechanisms of perceptual learning. Vision Res. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- 113.Petrov AA, Dosher BA, Lu ZL. The dynamics of perceptual learning: an incremental reweighting model. Psychol. Rev. 2005;112:715–743. doi: 10.1037/0033-295X.112.4.715. [DOI] [PubMed] [Google Scholar]
- 114.Stickgold R, Walker MP. Sleep and memory: the ongoing debate. Sleep. 2005;28:1225–1227. doi: 10.1093/sleep/28.10.1225. [DOI] [PubMed] [Google Scholar]
- 115.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 116.Censor N, Sagi D. Benefits of efficient consolidation: short training enables long-term resistance to perceptual adaptation induced by intensive testing. Vision Res. 2008;48:970–977. doi: 10.1016/j.visres.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 117.Ofen N, Moran A, Sagi D. Effects of trial repetition in texture discrimination. Vision Res. 2007;47:1094–1102. doi: 10.1016/j.visres.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 118.Mednick SC, et al. The restorative effect of naps on perceptual deterioration. Nature Neurosci. 2002;5:677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- 119.Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nature Neurosci. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- 120.Mednick SC, Drummond SP, Arman AC, Boynton GM. Perceptual deterioration is reflected in the neural response: fMRI study of nappers and non-nappers. Perception. 2008;37:1086–1097. doi: 10.1068/p5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 124.Peigneux P, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.