Abstract
Selenium (Se) is an essential trace element required for the biosynthesis of selenoproteins. Selenocysteine insertion sequence (SECIS) binding protein 2 (SBP2) represents a key trans-acting factor for the co-translational insertion of selenocysteine into selenoproteins. In 2005, we reported the first mutations in the SBP2 gene in two families in which the probands presented with transient growth retardation associated with abnormal thyroid function tests. Intracellular metabolism of thyroid hormone (TH) and availability of the active hormone, triiodothyronine, is regulated by three selenoprotein iodothyronine deiodinases (Ds). While acquired changes in D activities are common, inherited defects in humans were not known. Affected children were either homozygous or compound heterozygous for SBP2 mutations. Other selenoproteins, glutathione peroxidase, and selenoprotein P were also reduced in affected subjects. Since our initial report, another family manifesting a similar phenotype was found to harbor a novel SBP2 mutation. In vivo studies of these subjects have explored the effects of Se and TH supplementation. In vitro experiments have provided new insights into the effect of SBP2 mutations. In this review we discuss the clinical presentation of SBP2 mutations, their effect on protein function, consequence for selenoproteins, and the clinical course of subjects with SBP2 defects. Antioxid. Redox Signal. 12, 905–920.
Introduction
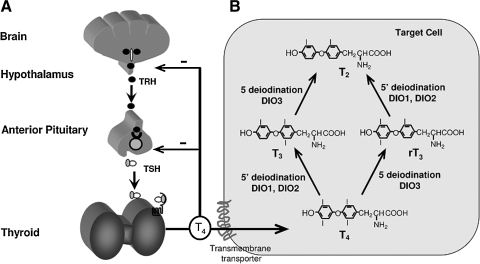
Thyroid hormones (TH) are iodinated compounds which, by controlling the expression of specific genes, affect the differentiation, growth, and metabolism of all vertebrates. TH homeostasis is maintained by a feedback system involving the hypothalamus, pituitary, and thyroid glands (Fig. 1A). Thyrotropin releasing hormone (TRH), a tripeptide secreted by the hypothalamus, stimulates the synthesis and secretion of thyroid stimulating hormone (TSH or thyrotropin) by the thyrotrophs, located in the anterior pituitary gland. TSH is a circulating glycoprotein made of two subunits that binds a G-protein coupled receptor expressed in thyrocytes to stimulate TH synthesis and secretion. TSH is also regulated by TH through a negative feedback system. Thus, a high serum TSH concentration is indicative of TH deficiency, while TH excess suppresses TSH. The effects of TH are dependent on the quantity of the active hormone that reaches peripheral tissues, their intracellular availability, and the presence of unaltered TH receptors and cofactors.
FIG. 1.
Central regulation of TH synthesis and TH metabolism. (A) Feedback system maintaining TH homeostasis. TRH secreted by the hypothalamus, stimulates the synthesis and secretion of TSH by the thyrotrophs, located in the anterior pituitary gland. TSH stimulates TH synthesis and secretion by the thyroid gland. TSH is also regulated by TH through a negative feedback system. (B) Activation and metabolism of TH. After active cellular uptake of TH through transmembrane transporters, the precursor 3,3′,5,5′-tetraiodothyronine (thyroxine, T4) is converted into the active 3,3′,5-triiodothyronine (T3) hormone or inactive 3,3′,5′-triiodothyronine metabolite (reverse T3, rT3). D1 and D2 are the principal enzymes that catalyze 5′-deiodination, converting T4 to T3 and rT3 to 3,′3-diiodothyronine (T2), while D3 catalyzes 5-deiodination, converting T4 to rT3 and T3 to T2.
Entry of TH into cells is an active process that involves several classes of TH membrane transporters with different kinetics and substrate preferences (33). After entering a cell, the hormone precursor 3,3′,5,5′-tetraiodothyronine (thyroxine, T4) is metabolized by removal of the outer ring iodine (5′-deiodination) to form the active hormone, 3,3′,5-triiodothyronine (liothyronine, T3). Alternatively, T4 and T3 are inactivated by inner ring (5-deiodination) to form 3,3′,5′- triiodothyronine (reverse T3, rT3) and 3,3′-diiodothyronine (T2), respectively (6). The deiodinases that activate TH are D1 and D2, while the enzyme that inactivates TH is principally D3 (Fig. 1B). The presence of these enzymes in changing concentrations in various cell types contributes an additional mechanism in regulating the amount of active hormone supplied to the cell (6).
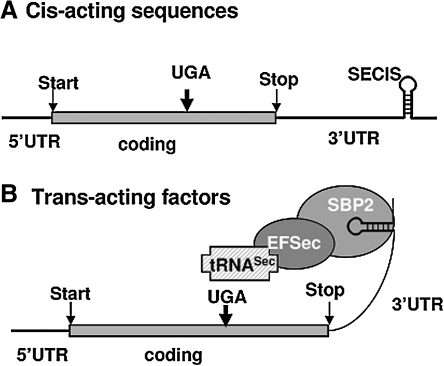
While genetic defects of thyroid gland development, TH synthesis, secretion and action have been identified, until few years ago, inherited defects in TH metabolism were not known (71). Deiodinases are selenoenzymes containing the rare amino acid selenocysteine (Sec) in their active center. Several factors are required for Sec incorporation: cis-acting sequences present in the mRNA of a selenoprotein [UGA codon and Sec insertion sequence (SECIS)] and transacting factors [Sec-specific elongation factor (eEFSec), Sec-specific tRNASec, and SECIS-binding protein (SECISBP2 or SBP2)] (26) (Fig. 2). However, the list of factors involved in this mechanism is constantly growing, the most recent members being the ribosomal protein L30 (15), the 43 KDa RNA binding protein (Secp43), and the soluble liver antigen protein (SLA) (3, 66, 97). Sec, the 21st amino acid, is structurally identical to cysteine (Cys), except for the selenium replacing sulfur. Sec has a distinct functional advantage at physiological pH. When Sec is replaced with Cys, the catalytic activity of a selenoenzyme is drastically reduced (38).
FIG. 2.
Some of the important components involved in Sec incorporation. (A) Cis-acting sequences present in the mRNA of selenoproteins: an in frame UGA codon, and Sec incorporation sequence (SECIS) element, a stem loop structure located in the 3′UTR (untranslated region). (B) Binding of the trans-acting factor SECIS-binding protein (SBP2) recruits the Sec-specific elongation factor (EFSec) and Sec-specific tRNA (tRNASec), resulting in the recoding of the UGA codon and Sec incorporation.
Sec is incorporated through recoding of a UGA codon present in the mRNAs of selenoproteins. This is achieved by the presence of SECIS, a characteristic stem-loop RNA structure in the 3′ untranslated region. Using the SECIS element as bait, the rat SECIS binding protein, SBP2, was purified and cloned in 2000 (21).
The human selenoproteome comprises at least 25 individual selenoproteins (52, 66). Although the precise function of most selenoproteins is unknown, some characterized mammalian selenoproteins were found to serve as antioxidants or oxido-reductases [glutathione peroxidases (GPx) and thioredoxin reductases], in thyroid hormone metabolism (deiodinases), selenium transport and storage (SePP), and potential protein folding (Sep15, SelN, SelM, SelS). Several approaches have been used to study the role of selenoproteins in rodents (61, 80). These include dietary restriction in Se, the knockout of individual selenoproteins (82), or targeting tRNASec (7, 11, 12, 53). That some selenoproteins must have a crucial function is supported by the observation that removal of the tRNASec gene is lethal to the embryo (7). The efficiency of Se retention during exposure to Se-depleted diets is different among tissues (82). A distinct hierarchy exists in the synthesis of selenoproteins as the expression of individual selenoproteins is differentially affected by the cellular content in Se (12). This may be due to changes in the distribution of the two isoforms of tRNASec (12), mRNA degradation by nonsense-mediated decay (94) and preferential SECIS recognition by SBP2 (9, 85). Components of the Sec incorporation machinery other than tRNASec have not been targeted in animal models and no Sec insertion defects have been described in humans.
In 2005, we described two families with mutations in the SECISBP2, (MIM 607693; also called SBP2) gene. The probands presented with growth retardation associated with abnormal thyroid function tests (28), consisting of high serum total and free T4 concentrations, a modest reduction in serum T3, high rT3 and normal or slightly elevated TSH. Because SBP2 is a major determinant in the incorporation of selenocysteine during selenoprotein synthesis (20, 67), the absence of lethality and mild phenotype were attributed, respectively, to the preservation of partial SBP2 activity and the hierarchy in the synthesis of selenoproteins (12). The latter allows the differential preservation of some selenoproteins in the presence of unfavorable conditions for their synthesis.
Since the initial description of the two families with SBP2 mutations (28), another family with a similar phenotype was reported by our laboratory (25). This family had a new SBP2 gene mutation producing an early stop codon. Another family was identified in England (59) and its full report is in progress. In addition, in vitro studies of a rat Sbp2 harboring a mutation corresponding to that identified in family A, and immunoprecipitation studies using the mutant SBP2 R540Q have been performed by Bubenik et al. (9) and Squires et al. (85), respectively. The effectiveness of Se supplementation was tested in several affected individuals (25, 79) and TH supplementation was tried in two affected children (25, 28). All these studies and observations are the subject of this review.
Clinical Presentation
The probands of three families were brought to clinical attention because of short stature. All three were boys ranging in age from 6 to 14.5 years (25, 28). The proband of a fourth family was a 32-year-old man, and infertility led to thyroid investigation (59). All affected subjects had thyroid function test abnormalities, characterized by high serum T4, low T3, high rT3, and normal or slightly elevated TSH concentrations. This unusual combination of thyroid test results, in otherwise healthy appearing individuals, were the reason for referral for further investigation. None of the subjects had an enlarged thyroid gland confirmed by ultrasound examinations.
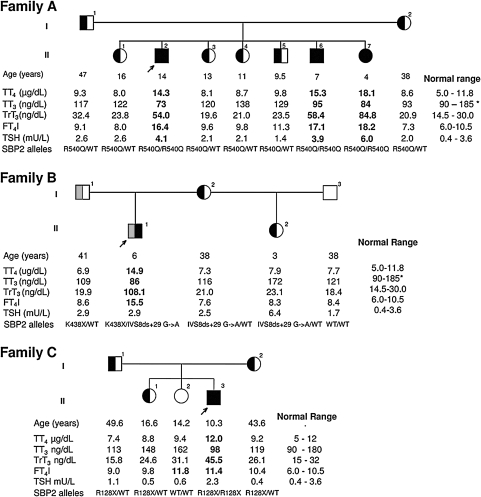
Family A is Bedouin from Saudi Arabia. Though the parents were not consanguineous, they belong to the same tribe. Three of the seven siblings of the proband had the characteristic thyroid function test abnormalities (Fig. 3A). The proband, the oldest of the three affected, was the second born. He was 14.5 years old when brought to medical attention because of short stature. Since age 11 he has been growing below the 3rd percentile and bone ages were 7.5 and 9 at chronological ages of 11.5 and 14.5, respectively, while still prepubertal (28). Insulin-like growth factor (IGF) 1 and stimulated growth hormone values were within the normal range. He was the product of full-term pregnancy and spontaneous vaginal delivery. Birth weight was 3.4 kg, length 54 cm, and head circumference 25.5 cm (all within the normal range). It is of note that, on the second day of life, because of protruding tongue suggestive of possible hypothyroidism, thyroid tests were obtained. TSH was >60 mU/L (normal <20) but total T4 was high at 21.6 μg/dl (normal range 6–14). Treatment with levothyroxine (L-T4) was not given because serum T4 concentration was not low. Growth and development appeared to be normal but at age 2 his height was 75 cm [standard deviation score (SDS) −3.0; <1 centile]. Thyroid function tests obtained at ages 2, 11.5, and 14.5 years showed TSH values that ranged from 1.8 to 4.1 mU/L, thus straddling the upper limit of normal of 3.8 mU/L; total T4 ranged from 214 to 245 nmol/L and free T4 29.2 pmol/L, both above the respective upper limits of normal of 141 nmol/L and 19 pmol/L. In contrast, a free T3 determination gave a low result of 0.65 pmol/L (normal range 1.3–2.5). The TSH response to TRH was normal, rising from a baseline level of 3.1 to a peak value of 8.9 mU/L at 30 min following the intravenous administration of TRH. Ultrasound showed that the thyroid gland was of normal size and a pertechnetate scan revealed a homogeneous uptake of 3% (normal range 0.5–4). Audiogram showed no hearing impairment.
FIG. 3.
Pedigrees and thyroid function tests of the three families. (A, B, and C) Values are aligned under each subject symbol. TT4, total T4; TT3, total T3; TrT3, total reverse T3; FT4I, free T4 index. Abnormal values are in bold characters. Note that the blood sample from the proband of family C was obtained 5 days after interruption of L-T3 treatment. *The normal range for children younger than 10 years is 100–205 μg/dL. SBP2 alleles present in each individual are indicated.
At age 13.5 years, the proband was treated for 8 months with 20 μg L-T3 daily. During this period of time his height increased from 133 to 133.8 cm and weight from 24.2 to 24.8 kg. Although T3 had no obvious effect on growth, TSH was suppressed to 0.2 mU/L. Six months later, off L-T3, TSH returned to 2.0 mU/L and his height and weight were 136 cm and 26.1 kg, respectively. The concentrations of total T4, free T4, and total rT3 were high, while that of total and free T3 were low. The same pattern of thyroid function tests was found in a 7-year-old brother and 4-year-old sister (Fig. 3A), both clinically euthyroid. However, the height of the affected brother was in the 3rd percentile. Thyroid function tests of the parents and four other siblings were normal and their stature ranged from 10 to 50 centile.
Family B is of mixed ethnic origin, the mother being Irish of European descent and the father Kenyan of African origin. Their single affected child was 6 years old when brought to medical attention by the mother concerned about his growth, because she was buying clothes for a child younger than his age. However, growth retardation was excluded by measurements. The mother reported syncopal episodes of the child after exertion. One episode witnessed in the physician's office, consisted of unresponsiveness, jerky movements of the arms and legs, followed by drowsiness for 5 min. Clinical, biochemical, and electroencephalographic investigations did not detect abnormalities. Thyroid function tests revealed a TSH at 8.1 mU/L (normal range 0.4–4) with a normal alpha subunit and a free T4 by equilibrium dialysis of 45.0 pmol/L (normal range 10–25). The possibility of resistance to thyroid hormone (RTH) was considered due to the combination of high free T4 with nonsuppressed TSH. However, RTH was excluded by sequencing the thyroid hormone receptor beta (TRβ) gene (72). Results of more detailed studies of thyroid function were similar to those of affected members of family A. The child had a maternal half sister. Her thyroid function tests results and those of the parents were within the range of normal (Fig. 3B).
Family C came to our attention after the report of families A and B (28). The proband, of African origin, was born to nonconsanguineous parents from the same town in the eastern region of Ghana. Pregnancy was normal and delivery at term. Birth weight was 3.1 kg and early development was thought to have been normal. It is not clear when his growth slowed down, as there were no records of height measurements, but short stature was noted at age 8 years, 2 months after his family moved to the United Kingdom. He was 8.9 years old when the family sought medical attention regarding his stature. He was proportionately short for age (height 113.5 cm, SDS −3.36; centile <1) and for the height of his parents (target height 170.9 cm, SDS −1.06, target centile 14). His bone age was delayed at 3.1 years (height for bone age SDS +4.7). He was slim, weighing 18.5 kg (BMI 14.4 kg.m2, weight for height 93%) whilst his head circumference was 51.0 cm (centile 2.4). No goiter or other somatic abnormalities were found and he was clinically euthyroid (25). Thyroid function tests showed high total T4, free T4 index (FT4I), and rT3 with low total T3 and normal TSH (Fig. 3C). All members of the immediate family were tested. They were of normal stature (10 to 80 centile) and tests of thyroid function were within normal range, except for the slight elevation of FT4I in one of the sisters (Subject II-2, Fig. 3C). Additional studies in the proband showed normal plasma IGF1 and IGF binding protein 3 at 11.1 nmol/L and 2.2 mg/L, respectively. Peak growth hormone response to insulin induced hypoglycemia was 11.2 mU/L and cortisol 668 nmol/L (25). Blood selenium was low but assessment for malabsorption, including upper and lower gastrointestinal endoscopy and biopsy, were negative. Immunoglobulins, CD 3:4:8, and inflammatory markers were negative. Karyotype was 46XY and normal. Electrocardiography and echocardiography showed no evidence of cardiomyopathy (25).
Initial In Vivo and In Vitro Studies
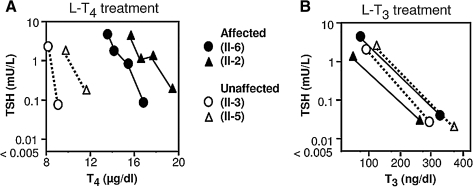
Although the clinical presentation and abnormalities of thyroid function were not typical of resistance to TH (72) or defects in serum (71) or cell membrane TH transport defects (29, 32), these were excluded by further testing (isoelectric focusing, measurement of thyroxine binding globulin and transthyretin) and sequencing of the corresponding genes. The relative ability of L-T4, compared to L-T3, to suppress TSH was used to test the hypothesis of a defect in iodothyronine metabolism. The in vivo and in vitro studies were done in members of family A, as it was the largest family available, with three affected and four unaffected siblings. When incremental doses of L-T4 were given, higher doses [up to 4.4 μg/kg body weight (BW)] were required to reduce the TSH level in the two affected children as compared to 2.2 μg/kg BW to have the same effect on the two normal siblings. These doses achieved on average 1.8-fold higher T4 levels in the affected as compared to the unaffected siblings (Fig. 4A). In contrast, similar doses of L-T3, on average 2.48 μg/kg BW compared to 2.46 μg/kg BW, used in the affected and unaffected siblings, respectively, had equal suppressive effects on TSH (Fig. 4B). These results of apparent resistance to L-T4 but not L-T3, suggest a defect in T4 to T3 conversion. However, linkage of the phenotype to loci of the three deiodinases was excluded and their coding sequences were normal (28).
FIG. 4.
Evidence for abnormal TH metabolism. In vivo studies in members of family A. Serum TSH and corresponding serum T4 and T3 levels, before and during the oral administration of incremental doses of L-T4 (A) and L-T3 (B). Note that higher concentrations of T4, but not T3, are required to reduce serum TSH in the affected subjects. Affected and normal individuals are identified in the legend and described in detail in Fig. 3A.
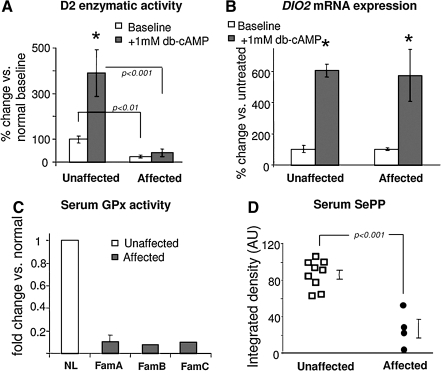
We used cultured skin fibroblasts from members of family A to test in vitro the hypothesis of abnormal T4 to T3 conversion. As D1 and D3 are not expressed in these cells, the expression and function of D2, an integral membrane ER- resident selenoenzyme (2) was measured. The synthetic analogue dibutyrylcAMP (db-cAMP) was used to stimulate D2 expression and activity, as the DIO2 gene contains a cAMP response element. Baseline D2 activity in fibroblasts from the affected subjects was reduced to the limit of detection (Fig. 5A). In fibroblasts from normal individuals, D2 mRNA and enzymatic activity increased with dbcAMP stimulation, whereas in the affected subjects, the increase in D2 mRNA was not accompanied by a corresponding increase in enzymatic activity (Fig. 5A and B). These results support the clinical findings of defective TH metabolism and provided direct evidence for abnormal D2 function. Furthermore, the reduction in D2 enzymatic activity without corresponding change in mRNA levels suggested that the defect may be post-transcriptional.
FIG. 5.
In vitro studies. (A) Deiodinase 2 enzymatic activity and (B) DIO2 mRNA expression in cultured fibroblasts from two affected children and four unaffected individuals from family A. Bars indicate ± SEM. Baseline and stimulated D2 activity is significantly lower in affected. There is significant increase of DIO2 mRNA with db-cAMP, in both normals and affected (*p < 0.001). There are no significant differences in baseline and db-cAMP-stimulated DIO2 mRNA in affected versus the unaffected. (C and D) Effect of SBP2 deficiency on selenoproteins other than deiodinases. (C) Glutathione peroxidase (GPx) enzymatic activity in serum from all five affected children and nine unaffected individuals (NL) from families A, B, and C. Results are expressed as fold change compared to unaffected subjects. Bars indicate mean ± SD. (D) SePP levels in serum from four affected and available unaffected members of families A and B were quantified by scanning the Western blot (AU, arbitrary units).
Identification of SBP2 Gene Mutations
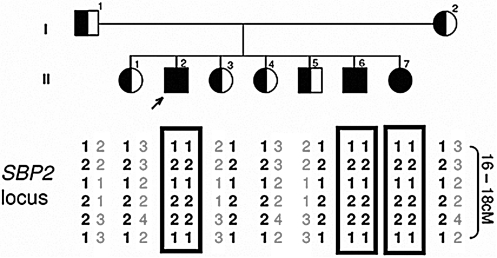
Based on the results of in vitro studies in fibroblasts and absence of co- segregation between phenotype and haplotypes of all three iodothyronine deiodinases, a post-transcriptional defect involving D2 metabolism or synthesis was considered as possibly responsible for the reduced enzymatic activity (28). Supply of active D2 enzyme is regulated by degradation in proteosomes through ubiquitination (8) and by de-ubiquitination (23). Linkage studies were carried out in family A having three affected and four unaffected siblings. There was absence of co-segregation for genes encoding proteins involved in D2 ubiquitination (UbE2G1, UbE2G2, UbE2L3) (8) and in D2 de-ubiquitination (VDU1, VDU2) (23). Defects in some components of the Sec incorporation machinery (cis sequences, tRNASec and EFSec) (26) were also excluded by co- segregation analysis or by sequencing. However, affected subjects shared homozygous haplotypes at the SBP2 locus (Fig. 6). Sequencing revealed a missense mutation in exon 12, a G to A transition in a CpG dinucleotide that changes the wild-type (WT) codon CGG to CAG. This results in the replacement of the normal arginine-540 with a glycine (R540Q) (28). Affected children were homozygous and the parents and the four unaffected siblings were heterozygous carriers (Fig. 3A). The haplotype harboring the mutation is most likely inherited identical by descent in this Bedouin family. Extensive genotyping with markers flanking the locus showed a 16–18cM segment shared by the parents, suggestive of a recent common ancestor.
FIG. 6.
Identification of a SBP2 gene mutation; Haplotyping of family A with genetic markers overlapping the SBP2 locus. Affected share homozygous haplotypes while the unaffected parents and siblings are heterozygous.
Two different SBP2 gene mutations were found in family B. The affected child was compound heterozygous. He inherited from his father an A to T transversion that changes the WT codon AAA with TAA, a nonsense mutation replacing the normal lysine-438 with a stop (K438X). From the mother he inherited an intronic mutation (IVS8ds+29 G->A) which, by altering the donor splice site, produces an alternative transcript that incorporates 26 bp into exon 8 (Fig. 3B) (28). The abnormally spliced transcripts represented 52% of the transcripts generated from the mutant maternal allele in lymphocytes and change the reading frame to produce a putative truncated protein (28). The total amount of normal transcripts in the affected child was estimated to be 24%.
For family C, an SBP2 defect was suspected based on the thyroid function tests abnormalities, growth delay, and the detection of a low serum Se concentration. Sequencing of the SBP2 gene revealed a novel mutation, a C to T transition in codon 128, located in exon 3, that results in the replacement of the normal arginine (CGA) with a stop (TGA), R128X (25). This early stop predicts an SBP2 defect in the proband and is in agreement with the observed thyroid phenotype. Both parents were heterozygous for the same mutation as was one of his sisters (subject II-1) but not the other (Subject II-2, Fig. 3C) (25).
Population screens showed that these SBP2 gene sequence differences were not polymorphic in the respective populations.
Consequences for Other Selenoproteins
As SBP2 is epistatic to selenoprotein synthesis, identification of decreased D2 activity due to recessive SBP2 defect prompted us to investigate whether other selenoproteins were also affected. As D1 and D3 are too low to measure or absent in skin fibroblasts and lymphocytes, other selenoproteins were assessed (25, 28). Compared to that of normal individuals, serum GPx activity (GPx3) was 9.9-fold lower in the three (mean value) affected subjects of family A, 7.7-fold lower in the affected of family B (28), and 9.2-fold in that of family C (25) (Fig. 5C). GPx1 mRNA expression and activity in fibroblasts of the affected subjects from family A was 7.2-fold and 3.3-fold lower, respectively, compared to normal subjects. No fibroblasts from individuals of families B and C were available. Furthermore, selenoprotein P (SePP) serum levels measured in members of families A and B were significantly lower in the affected compared with unaffected individuals (Fig. 5D).
SBP2 is expressed at low levels in all tissues tested, with a high expression and an additional smaller transcript in testis (55). The human SBP2 gene, cloned in 2002 (55), has 854 amino acids. The C-terminal domain of the protein is required for SEC IS binding, ribosome binding, and Sec incorporation (20). The role of the N-terminal region is in part unclear. Recent in vitro studies have characterized a nuclear localization signal (NLS) located in the N-terminal part and nuclear export signal (NES) in the C-terminal part. These domains enable SBP2 to shuttle between the nucleus and the cytoplasm (67) and these motifs play a role in the function of SBP2 in the nucleus, in vivo. Oxidative stress induces nuclear accumulation of SBP2 via oxidation of cysteine residues within a redox-sensitive cysteine-rich domain. Sec incorporation is reduced substantially after treatment of cells with agents that cause oxidative stress, suggesting that nuclear sequestration of SBP2 under such conditions may represent a mechanism to regulate the expression of selenoproteins (67).
There appears to be a hierarchical preservation of selenoproteins during Se deprivation, conserving the enzymatic activity of selected selenoproteins (20, 50). This hierarchy is supposedly produced by the rates of selenoprotein degradation and by the functional demands of particular selenoproteins. More recently, selective binding of SBP2 to different SECIS elements was demonstrated in vitro, thus further contributing to selenoprotein hierarchy (9,85). Therefore, SBP2 defects manifest as deficiencies in the synthesis of selective selenoproteins.
Effect of the SBP2 Gene Mutations on Protein Function
Given the expected devastating effect from complete impairment of selenoprotein synthesis (7), we hypothesized that the mild phenotype of the affected individuals of these families, limited to growth retardation, possible infertility, and partial defect in thyroid hormone metabolism, must be due to preservation of sufficient SBP2 function in the affected subjects. Indeed, the mutation IVS8ds+29 G->A of family B results in partial alternative splicing and abnormal transcripts. The total amount of normal transcripts in the affected child was estimated to be 24%, resulting in a partial, rather than a complete SBP2 defect, thus predicting a partial deficiency of selenoprotein synthesis and a mild phenotype (28). An experiment that mimics this situation was performed by Squires et al. (85) using knockdowns of SBP2 expression in cell lines to 25%–30% of normal. These studies assessed the effects on selenoprotein mRNA levels and quantified the in vivo binding of selenoprotein mRNAs by SBP2 via immunoprecipitation. SBP2 exhibited strong preferential binding to some selenoprotein mRNAs over others, and SBP2 was a major determinant in dictating the hierarchy of selenoprotein synthesis via differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. A more recent study also demonstrates loss of telomeric reserve when SBP2 expression is attenuated (84).
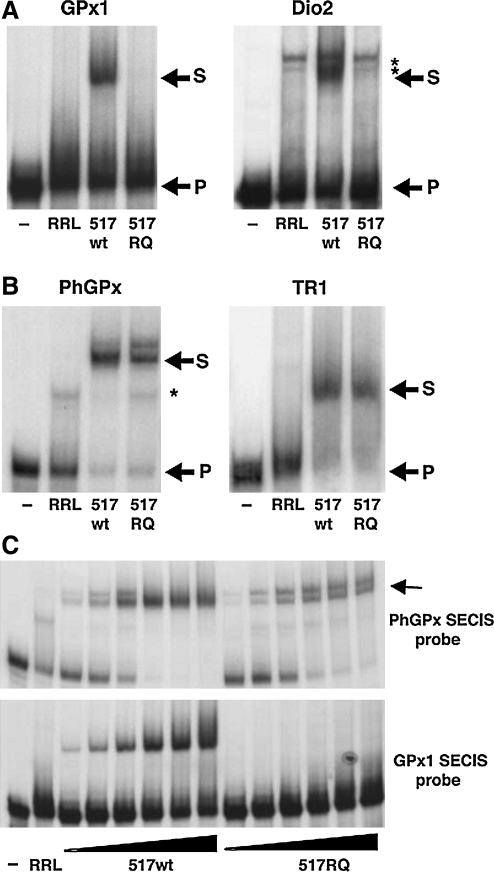
The homozygous mutation R540Q in family A is a nonsynonymous change located in a conserved amino acid across species (9) and likely creates a hypomorphic rather than a null allele. This mutation is located in the RNA- binding domain of SBP2 and suggests that the mutant protein could be defective in SECIS binding. Bubenik et al. (9) examined its ability to bind to various SECIS elements using the rat Sbp2 R531Q protein. This amino acid substitution is equivalent to the same change in humans located at position 540. In agreement with our initial findings of defects in GPx1 and D2 activity in subjects homozygous for SBP2 R540Q, RNA electrophoretic mobility shift assays (REMSA), using the rat mutant Sbp2 R531Q, showed no binding to the SECIS elements of GPx1 and Dio2 (9) (Fig. 7A), while the WT protein had normal binding. In addition, the R531Q mutant did not bind the SECIS element of Dio1 (9), which also catalyzes 5′ deiodination as D2 does (49) and this likely contributes to the overall thyroid phenotype seen in SBP2 defects.
FIG. 7.
In vitro studies using the rat Sbp2 R531Q, the equivalent mutation to the human SBP2 R540Q mutation identified in family A. Rat Sbp2 R531Q results in selective perturbations in SECIS interactions. (A) REMSA assay with in vitro translated rat Sbp2[517–777] or rat Sbp2[517–777,R531Q] using rat GPx1 and human Dio2 SECIS elements as probes. P, unbound probe; S, shift caused by the functional SBP2/SECIS interaction; *shift caused by interaction with endogenous protein in the lysate. (B) REMSA assay as above, using rat PhGPx and TR1 SECIS as probe. (C) REMSA with increasing concentrations of rat Sbp2[517–777] or Sbp2[517–777,R531Q], corresponding to a 24-fold range, with probes as indicated; wt, wild type. Arrow indicates the functional band, the lower band of the two. [Figure reproduced from Bubenik et al. (9) JBC 282: 34658, 2007, with permission from the authors and publisher].
Because SBP2 is required for selenoprotein synthesis, the expectation would be that a SBP2 defect would have a generalized effect on all selenoproteins. However, given the mild phenotype of the affected individuals, the hypothesis was that the expression of other selenoproteins might be in part preserved in these individuals. In particular, phospholipid hydroperoxide glutathione peroxidase (PhGPx) and thioredoxin reductase (TR1) are considered essential selenoproteins, because knock-out of these proteins are lethal to mice in the embryonic stage (45, 98). Thus, the loss of expression of these proteins in humans is expected to have more severe consequences. When the SECIS elements from these two selenoprotein mRNAs were used as probes in REMSA assays (9), the R531 Q Sbp2 mutant was able to bind both the PhGPx SECIS and TR1 SECIS. However, the R531Q Sbp2 mutant was not capable of binding the PhGPx SECIS as well as the WT Sbp2 (Fig. 7B), suggesting that the R531Q mutant has a weaker RNA binding affinity than the WT. This is further demonstrated using increasing amounts of protein in REMSA assay. When the SECIS element of PhGPx was used as probe (Fig. 7C, upper gel), increasing the concentrations of WT protein resulted in an increase of the intensity of the lower functional band and the loss of the upper band. Although the R531Q protein is able to interact with the PhGPx SECIS, the binding does not exactly recapitulate that of the WT protein because the doublet remains, even at higher concentrations. If GPx1 SEC IS is used as probe (Fig. 7C, lower gel), only the binding of the WT protein is detected. Increasing the amount of R531Q protein does not result in complex formation. Similarly, using selenocysteine insertion assays, Bubenick et al. (9) demonstrated that the R531Q mutation further decreases the affinity of SBP2 for a subset of SECIS elements, implying that in the affected children of family A harboring the equivalent mutation, this additional decrease in affinity results in the loss of a subset of selenoproteins (9).
Independently, Squires et al. (85) also tested the R540 SBP2 mutation identified in family A for its ability to immunoprecipitate selenoprotein mRNAs. The R540Q mutant exhibited significant decrease in RNA binding relative to wild- type SBP2 for most selenoproteins tested. In particular, Sel15 and GPx4 mRNAs showed the highest crossing point changes, in the range of ∼ 4, equivalent to 8-fold lower binding affinity for the mutant SBP2, while the changes for most selenoprotein mRNAs were lower, in the range of 0 to 2. Surprisingly, the mRNA level of a selenoprotein with a noncanonical SECIS element, SelO, exhibited a greater increase in crossing point (i.e., increased binding) with the R540Q mutant than in the wild-type SBP2 immunoprecipitation.
In support of these observations, a recent study by Takeuchi et al. (86) identified in human SBP2 a lysine (K) rich domain between K516 and K544, essential for SECIS and 60S ribosomal subunit binding. This domain contains the R540 amino acid which is conserved across species. Using deletion and alanine scanning mutagenesis, the amino acid sequence 526–540 was demonstrated to be essential for binding form 1 SECIS RNAs but its mutation did not affect form 2 SECIS recognition (86). Altogether these results showed that the R540Q mutation alters the binding specificity of SBP2. Because SBP2 is a limiting factor in cells (56), the relative ability of the mutant SBP2 to bind with relatively higher affinity to some SECIS elements than others would determine which members of the selenoprotein family are expressed, thus creating an imbalance in the relative amounts of selenoproteins synthesized even in selenium-adequate conditions.
The propositus of family C was homozygous for a nonsense gene mutation that produces an early stop codon (R128X). Both parents and a sister were heterozygous but showed no growth or thyroid test abnormalities (25). Despite the severity of the defect, the patient had a relatively mild phenotype, similar to that associated with partial SBP2 deficiency demonstrated in families A and B. The recent finding that SBP2 has several mRNA and protein isoforms (68) prompted us to explore the possibility that the mutation produced alternative splicing and/or the synthesis of a partial SBP2 molecule with possible functional activity that proceeded normally (25).
Exon 3, where the mutation R128X is located, is subject to alternative splicing, resulting in three different SBP2 isoforms: a) the intact isoforms containing the entire exon 3 (intact Ex3); b) an isoform lacking 121 bp of the 5′-segment of exon 3 (partial Ex3); c) an isoform lacking the entire exon 3 (absent Ex3) (68). Using quantitative PCR (qPCR) and primers sequences crossing specific exons, we tested for the relative proportion of the three isoforms of SBP2 mRNA described above in the RNA extracted from leukocytes of all family members (25). In order to quantify all three isoforms in combination, qPCR of exon 4 to 5 was also performed. Compared to the sister expressing only the WT alleles (100%), the expression of the intact Ex3, partial Ex3, absent Ex3, and exons 4 to 5 in the heterozygote parents were 74, 89, 62, and 76%, respectively, while the homozygote proband expressed 67, 79, 64, and 69% (25).
The abundance in mRNA harboring the mutant and WT sequence were quantified using cDNA derived from leukocytes of the heterozygote parents (25). The mean ratios of mutant relative to WT sequences were: 0.64/1 for intact Ex3 isoform, 0.62/1 for partial Ex3 isoform, and 0.60/1 for all isoforms combined. This suggests a modest allele-preferential expression due to nonsense-mediated decay in heterozygote subjects (13).
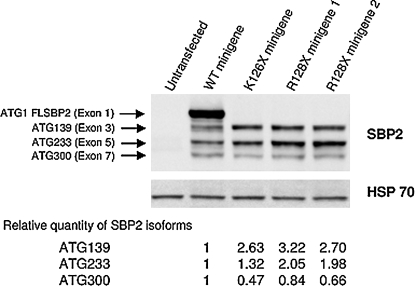
To further characterize the consequence of the SBP2 mutation R128X on protein synthesis, the transcription and translation of the mutant R128X and WT SBP2 were analyzed using minigenes as previously described (68). Two colonies of plasmids containing the minigene with the mutation (R128X) were studied along with two controls, the minigene that produces a stop at the nearby codon 126 (K126X) and the WT (68). As shown in Fig. 8, minigenes with stop codons at 126 and 128 lacked the full length protein (25). However, they synthesized the smaller fragments from downstream AUGs at codons 139, 233, and 300, also produced by the WT minigene (25). Minigenes K126X and R128X synthesized 2- to 3-fold more SBP2 molecules from AUG 139 than the WT and those from AUG 233 were 2-fold more abundant in R128X but not K126X (25). From these data it can be concluded that although mutant SBP2 mRNA in leucocytes may undergo some nonsense mediated decay, SBP2 isoforms ATG 130 and ATG 233 showed 2–3-fold increase in truncated R128X minigene, indicating that a compensatory effect might exist (25). These smaller molecules contain the functional domains, including those required for selenocysteine insertion and RNA binding and ribosome interaction (residues present in exons 12 through 16) (22).
FIG. 8.
Western blot of SBP2 synthesized by minigenes expressing the mutation identified in family C. Minigenes constructs, identified above each lane, were transiently transfected into HEK293T cells and products were electophoresed, blotted, and developed with antibodies to SBP2. Bands generated from different ATGs are identified as described previously (68). Their relative proportion was quantified by density measurement using Image J and expressed relative to the corresponding band generated by the WT full length (FL) minigene and corrected for HSP 70; HSP, heat shock protein.
Selenium Supplementation and TH Treatment
Identification of the metabolic pathway responsible for the phenotype of these patients and the demonstration of defects in the SBP2 gene provided further insight into targeted treatment possibilities. Two such options, namely administration of Se and TH, were tested.
The phenotype produced by SBP2 gene defects manifests as growth delay and abnormal thyroid function tests caused by reduced synthesis of selenocysteine-containing enzymes. As the affected individuals had decreased serum Se compared to unaffected siblings, we considered Se supplementation as a means to improve the overall Se availability and possibly also selenoprotein synthesis. Two different studies were done, one in the children from family A (two unaffected siblings serving as controls) (79), and another in the affected child of family C.
It is known from clinical studies that a high Se status confers reduced health risks and protects against some chronic diseases (70). Therefore, improving the Se status might still be of benefit to the affected individuals. Two different forms of Se supplementation were used, Se-rich yeast and selenite. Selenomethionine is the main compound in Se-rich yeast that can be incorporated nonspecifically into all circulating serum proteins (10), whereas selenite is metabolized and inserted as selenocysteine into the growing peptide chain of selenoproteins (96), therefore resulting in different Se bioavailability.
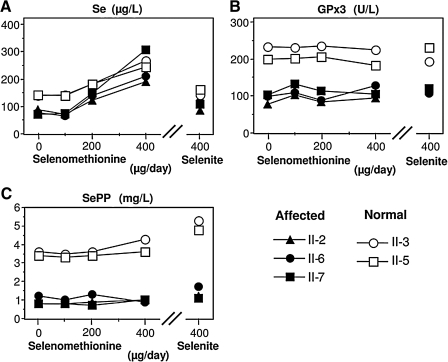
The three affected subjects of family A II-2, II-6, and II-7, ages 18, 11, and 9, respectively, and normal subjects II-3 and II-5, ages 17 and 14, respectively, were given Se supplementation (79). Initially, three incremental doses of Se-rich yeast (100, 200, and 400 μg) were given daily for one month. After a wash-out period of 3 months, 400 rig sodium selenite was given daily for another month. As shown in Fig. 9A, all subjects, irrespective of genotype, responded to treatment by increase in total serum Se concentrations after Se-rich yeast supplementation. Therefore, not only the normal siblings but also the SBP2 deficient children metabolized this Se form successfully. This positive supplementation effect was in contrast to the effect of selenite, as the affected subjects failed to show similarly strong supplementation success as the normal siblings, and displayed only slight increase in SePP concentrations (Fig. 9C) without a positive effect on circulating GPx3 activity (79) (Fig. 9B).
FIG. 9.
Effects of selenium (Se) supplementation in the form of Se-rich yeast (primarily selenomethionine) and selenite for SBP2-deficient individuals of family A and their normal siblings. (A) Serum Se concentration; (B) glutathione peroxidase (GPx) 3 enzymatic activity in serum; (C) serum selenoprotein P (SePP) concentration. Affected and normal individuals are identified in the legend and described in detail in Fig. 3A.
These studies indicate that Se-rich yeast might be the compound of choice, because it readily increased the Se concentrations in serum, and this might serve as an important Se reservoir during times of need, such as in severe illness. However, the thyroid function test abnormalities, including elevated T4 and rT3 and low T3 concentrations, persisted in the SBP2-deficient individuals with both Se preparations and at all doses (79). This study showed that the SBP2-deficient individuals do not profit from surplus Se supplementation as judged by those parameters that are readily measurable and directly related to the impairment (79). It is of note that the subjects were not residing in a Se-deplete area since the unaffected siblings displayed normal circulating Se levels.
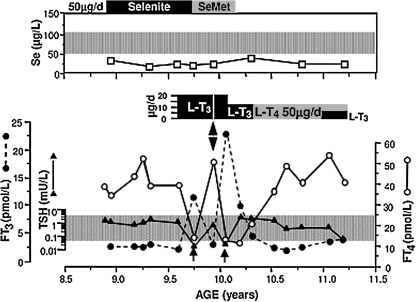
When the proband of the family C was evaluated for short stature, the only positive findings were a low serum Se and abnormal thyroid function tests consisting of high FT4, low FT3, and normal TSH. Based on these results, a defect in the conversion of T4 to T3 caused by deficiency in the Se-containing deiodinases was suspected. Thus, selenium replacement at a dose of 50 μg/day in the form of selenite (for 6 months) and later selenomethionine (for 4 months) was given. Despite this Se supplementation regimen, no changes in serum Se, thyroid function tests, or growth were observed during the initial 4 months of supplementation with selenite only, before the TH supplementation was started (Fig. 10). Of note is that this lower dose of Se was insufficient to increase serum Se levels (Fig. 10).
FIG. 10.
Clinical course of the proband of family C (subject II-3, Fig. 3C). Note the low serum Se concentration that did not respond to Se treatment in the form of selenite or selenomethionine (SeM). Normal range is for children and adults living in England and Wales as provided by the Trace Elements Laboratory Department of Clinical Biochemistry, City Hospital, Birmingham, UK. The thyroid function test abnormalities (high serum T4 and low T3 concentrations in the presence of a normal serum TSH level), characteristic of SBP2 defects, were persistent during the initial 7-month period of observation. Treatment with 20 μg L-T3, reduced the serum T4 levels but also suppressed the serum TSH concentration (arrows). Variations in serum FT3 concentrations are due to different interval of time between blood sampling and the last dose of L-T3. The double head arrow indicates cessation of L-T3 treatment for 5 days which promptly returned the baseline thyroid test abnormalities.
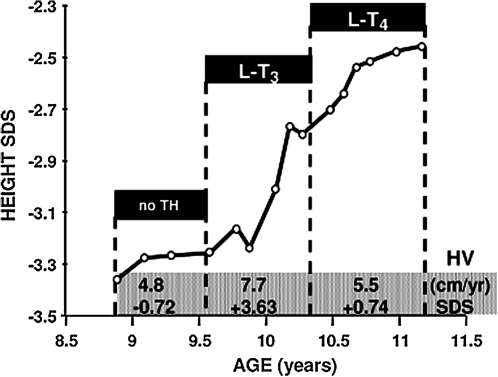
Since Se supplementation failed to correct the abnormal thyroid function tests or to improve growth (Fig. 11), the pediatrician initiated treatment with L-T3. The hormone was first given in a dose of 20 μg daily as divided doses and later, because of TSH suppression, the dose was reduced to 10 μg/day (Fig.10). During this period, in addition to an increase in height velocity (Fig. 11), there was advancement in bone age with narrowing of the gap between chronological and bone age (Table 1) (25). Although treatment with 20 μg of L-T3 reduced the serum FT4 levels and suppressed TSH below the lower limit of normal, no alterations in cardiac parameters were observed and the serum cholesterol concentration did not change. Discontinuation of L-T3 administration for only 5 days resulted in a return of thyroid tests to near pre-treatment values (Fig. 10). Treatment with 50 μg/day of L-T4 brought the serum T4 levels to baseline values and slowed down the increase in height velocity (Fig. 11), though the gap between chronological and bone age continued to narrow (Table 1). Finally, L-T3 at a lower dose was reinstated but L-T4 was continued in order to maintain the basal serum T4 level (25).
FIG. 11.
Effect of thyroid hormone treatment on linear growth of the proband of family C (subject II-3, Fig. 3C). Height is expressed as standard deviation score in the ordinate and as a function of chronological age (31). Height velocities (HV) are changes in height over a >6-month interval and expressed in cm/year (87) and as SDS. In this plot, they are averaged for the three periods defined by the treatment given.
Table 1.
Bone Age and Linear Growth in Proband of Family C
| Chronological Age (years) | Bone age1(years) | Difference2years | Height SDS3 | Treatment (months) |
|---|---|---|---|---|
| 8.9 | 3.1 | 5.8 | −3.36 | None |
| 10.2 | 5.2 | 5.0 | −2.77 | LT3 (7)4 |
| 11.1 | 6.4 | 4.7 | −2.48 | L-T4 (9)4 |
Tanner Whitehouse (TW20) (87); 2Chronological minus bone age; 3Standard deviation scores; 4Months of treatment in parenthesis.
It is of note that, in contrast to the results described above, 20 μg of L-T3 daily given to the proband of family A, for 8 months, did not increase his growth rate. This treatment was initiated at the age of 13.5 years. Evidence for compliance is the reduction of his TSH from a baseline of 1.8 to 0.19 mU/L.
Clinical Course of Subjects with SBP2 Defects
The long-term clinical evolution of partial SBP2 defects, as those described in this review, remains largely unknown. Postnatal growth retardation appears to be common. Yet the magnitude varies, as well as the age of onset and of catch up growth. Heights of the probands for each of the three families (A, B, and C), at chronological ages of 11.5, 6, and 8.9 years, were in the 1st, 97th, and <1st centile, respectively, with bone ages of 7.5, 6, and 3.1 years. Of note is that the proband of family B reached a normal stature by the age of 6 years, when the first accurate measurement became available. Longitudinal measurements available for members of family A showed that the height of three affected individuals was from <1 to 3rd centile, resulting in adult heights from 1 to 10th centile. This contrasts with that of the unaffected four siblings ranging from the 3rd to 25th centile during growth and reaching adult heights in the 10th to 50th centiles. Thus, in terms of linear and bone growth during childhood, the impairment was most severe in the subject homozygous for an early stop codon in the SBP2 gene from family C, followed by the propositus of family A with the hypomorphic missense mutation. The least and transient effect on growth was observed in the child from family B, expressing 24% of the WT SBP2.
Information about the clinical progress during adulthood is currently limited to one case, that of the 32-year-old male who presented with azoospermia and bilateral high frequency sensorineural hearing loss (59). In part, this outcome was predicted earlier (25, 28). Infertility was suspected based on the knowledge that selenoprotein P is required for the development of sperm (12, 63) and the dual role of PhGPx in sperm maturation (89). PhGPx exists as a soluble peroxidase in spermatids and as structural protein, enzymatically inactive in mature spermatozoa, representing at least 50% of the capsule material that embeds the helix of mitochondria. Selenium deficiency was found to cause mechanical instability of the mitochondrial midpiece (95). With regard to hearing impairment, D2-deficient mice were shown to have defective auditory function, retarded differentiation of the cochlear inner sulcus and sensory epithelium, and deformity of the tectorial membrane (62), an auditory phenotype similar to that caused by systemic hypothyroidism or TH receptor deletions (30). However, affected members of family A had normal audiograms, in agreement with their demonstrated partial D2 deficiency.
Similarly, it can be hypothesized that, due to impaired antioxidative protection, affected subjects may be prone to the development of malignancies (18). Recent studies by Squires et al. (84) have shown that attenuated expression of SBP2 using siRNA in MSTO and SY5Y cell lines causes increased frequency of oxidative damage-induced lesions in the telomeric DNA, without affecting telomerase, implying that selenoproteins may help protect telomeric reserve in mammalian cells. Thus, SBP2 defects could have as yet undetermined consequences and the identification of additional patients, and their long-term follow up, are important in further characterizing this recently described defect.
Unfortunately, results of early treatment experience with Se and TH are insufficient in predicting whether these agents could be favorable to the long-term outcome.
Other TH Metabolism Defects in Humans and Mice
Until recently, only the acquired forms of TH metabolism defects in humans were known to exist. Frequently encountered is the “low T3” syndrome in nonthyroidal illness that is the most common thyroid function test abnormality in patients with acute illness (88). Low serum T3 can be detected within 2 h after the onset of severe physical stress. As the severity of the illness progresses, there is a gradual development of a more complex syndrome associated with low levels of T3 and T4. Altered TH levels have been reported in starvation (36), acute and chronic medical illnesses (69, 73, 93), bone marrow transplantation (54, 92), surgery (19, 100), trauma (16), and can be seen in any severe systemic illness (58, 83). Decreased 5′-monodeiodination, reducing both the conversion of T4 to T3 and the degradation of rT3, is the principal mechanism responsible for the decrease in circulating T3 and increase in rT3 levels in severe illness (46–48, 99). With more prolonged illness, increased turnover of T3 and T4 and an alteration in TSH secretion play secondary roles. Some inflammatory cytokines such as TNFα, IL1, and IL6 have been recently implicated at both central and peripheral levels. Exogenous administration of TNFα and IL1 in humans and animals replicates the thyroid function tests changes reported in the syndrome (24, 27, 34, 39, 65, 91). Certain pharmacologic agents (dopamine, amiodarone, corticosteroids) may also alter the pattern of thyroid function tests in a similar way (14, 64, 74, 90) and this should be taken in consideration when evaluating patients with nonthyroidal illness. Multiple tests at different time points are recommended.
Another form of acquired abnormality in TH metabolism is that caused by increased D3 content in hemangiomas (44). The phenotype resolves with tumor involution or resection. Several cases in infants (1, 5, 37, 41, 44, 51), and one in an adult (43), have been reported. Hemangiomas are the most common tumors in infancy, with a prevalence of 5%–10% among one-year olds. Paraneoplastic D3 enzymatic activity was also recently demonstrated in a nonvascular tumor, in a 54-year-old man with a large malignant solitary fibrous tumor (75). The phenotype is that of consumptive hypothyroidism, with increased TSH and marked elevation of rT3 in the context of normal T3 and T4. The high level of D3 produced by the tumor inactivates T4 by conversion to rT3 at rates that exceed the synthetic capability of the thyroid gland. D3 activity of the tumor was found to be 3- to 7-fold higher than that of term placenta, the human tissue with the highest D3 activity (42).
Several mouse models of deiodinase deficiencies have been reported. A naturally occurring D1 deficiency was identified in C3H mouse strain (78) caused by a 21-base pair insert in the promoter of Dio1 locus in the C3H strain found to co-segregate with low D1 activity in four other mouse strains (57). The D1 KO (77), D2KO (76), D3KO (40), and combined D1/D2KO (35) mice were generated by homologous recombination targeting of the respective genes. In addition, the C3H/D2KO (17) mouse was generated as well, by breeding D2KO mice in the C3H genetic background.
There is no doubt that the thyroid phenotype in subjects deficient in SBP2 is secondary to the defect in iodothyronine metabolism due to a reduced amount of iodothyronine deiodinases. Yet the serum thyroid abnormalities of high T4, low T3, and high rT3, do not perfectly match any of the mouse models of deiodinase deficiencies (4, 17, 35, 40, 76, 77). All have increased serum T4 and normal T3 concentrations except for the D3KO mouse. The latter has low T4 and T3 concentrations. Serum rT3 is high in the D1 KO (77) and double D1/D2KO (35) and TSH is increased in the D2KO (76), C3H/D2KO (17), and D1/D2KO (35). As supported by our previous findings of reduced but not absent D2 activity in subjects from family A (Fig. 5A), it is possible that the phenotype in humans with SBP2 deficiency represents the combined but partial deficiency in all three deiodinases, not fully reproduced in any of the available mouse models of total but selective deiodinase deficiencies. The finding of a predominant thyroid phenotype in SBP2 defects highlights the importance of selenoproteins for thyroid feedback regulation.
Conclusions and Speculations
The phenotype produced by SBP2 gene mutations is characterized by low T3, high T4, and high rT3 concentrations in serum, and variable but transient growth delay without other obvious abnormalities when presenting during childhood. In the families we investigated, the observed phenotype seems to be the consequence of selective reduction in selenoprotein synthesis (25, 28).
SBP2 is believed to be the major determinant of Sec incorporation as its in vitro addition increases selenoprotein synthesis by 20-fold, whereas its immunodepletion eliminates Sec incorporation (20). The absence of more prominent and generalized symptoms in the patients described above is due to the partial loss of SBP2 function and selectivity of selenoprotein deficiency.
Growth impairment is greatly variable and a genotype–phenotype correlation is not yet apparent. Similarly, the degree of impairment of thyroid function tests does not show such correlation. Serum concentrations in affected subjects of families A, B, and C were, respectively, for T4 15.9 ± 1.9 (mean ± SD for the three affected), 14.9, and 14.0 μg/dl; for T3 84 ± 11, 86, and 98 ng/dl. Similarly, no quantitative differences were observed in the reduction of the serum GPx3 activity which was 9.9 ± 6.4 (mean ± SD for the three affected), 7.7, and 9.2-fold below the normal mean in affected subjects of families A, B, and C, respectively. Unfortunately, no other selenoproteins, including deiodinases could be measured in the members of families B and C because of the limited amount of leukocytes and their low level of deiodinase expression. In addition, family C refused to provide skin samples for culture of fibroblasts.
The exact cause of early growth retardation in individuals with SBP2 gene mutations remains unknown. While the growth retardation observed in Se deficient rodents (60, 81) is associated with a reduction in GH and IGF1 (60), this was not found in two of the affected individuals in which it was measured. Treatment with Se had no effect on the affected child from family C nor had the higher Se doses given to subjects of family A (79). Based on the accelerated growth during L-T3 treatment in the proband of family C, it appears that the growth retardation might be due to reduced thyroid hormone action on peripheral tissues. Yet there was no overall correlation between the magnitude of serum T3 reduction and growth delay. Furthermore, the growth rate of the propositus of family A was not affected by L-T3 treatment and some catch-up growth was observed with the passage of time. Affected subjects reached normal adult height, though possibly lower than their genetic growth potential. It is uncertain whether delayed puberty is a characteristic for males with the syndrome or a coincidental occurrence.
Abbreviations Used
- BW
body weight
- C3H
mouse strain
- Cys
cysteine
- db-cAMP
dibutyryl-cAMP
- Ds, D1, D2, D3 or Dios
deiodinases 1, 2, 3, protein or gene, respectively
- eEFSec
Sec-specific elongation factor
- FTI
free T4 index
- GPx
glutathione peroxidases
- IGF
insulin-like growth factor
- IL1, IL6
interleukin 1 and 6
- KO
knock-out
- L-T3
levo-triiodothyronine
- L-T4
levo-thyroxine
- NES
nuclear export signal
- NL
normal
- NLS
nuclear localization signal
- PhGPx
phospholipid hydroperoxide glutathione peroxidase
- qPCR
quantitative PCR
- REMSA
RNA electrophoretic mobility shift assays
- RTH
resistance to thyroid hormone
- SBP2
selenocysteine insertion sequence (SECIS) binding protein 2
- SD
standard deviation
- SDS
standard deviation score
- Se
selenium
- Sec
selenocysteine
- SECIS
selenocysteine insertion sequence
- Secp43
43 KDa RNA binding protein
- SeM
selenomethionine
- SLA
the soluble liver antigen protein
- T2
3,3′-diiodothyronine
- T3
liothyronine or 3,3′,5-triiodothyronine
- rT3
reverse T3 or 3,3′,5′-triiodothyronine
- T4
thyroxine or 3,3′,5,5′-tetraiodothyronine
- TH
thyroid hormone
- TNFα
tumor necrosis factor alpha
- TR1
thioredoxin reductases
- TRH
thyrotropin releasing hormone
- tRNASec
Sec-specific tRNA
- TSH
thyroid stimulating hormone or thyrotropin
- UbE2G1, UbE2G2, UbE2L3
ubiquitin-conjugating enzyme E2G 1, E2G2, and E2L3 respectively
- UTR
untranslated region
- VDU1, VDU2
von Hippel-Lindau protein interacting de-ubiquitinating enzyme 1 and 2
- WT
wild-type, normal
Acknowledgments
We thank all members of the families for their willingness to participate in this study. Thanks are due to Dr. Neal H. Scherberg and his laboratory staff for performing the tests of thyroid function. We acknowledge all the clinicians who referred these families for further investigations. These include: Mohamed Abdullah, Fathia Abdul Majed, and Bassam Bin-Abbas (family A); Gerard Boran and Muhammad Adrees (family B); and Neil McLellan (family C). We are also grateful to the following investigators who participated in various phases of this work: Lutz Schomburg, Johanna Hoeflich, Josef Köhrle, Joaquin Lado-Abeal, Lars C. Moeller, Laura Papp, and Kum Kum Khanna. We also acknowledge Donna M. Driscoll for allowing us to reproduce her figure, here Fig. 7.
This work was supported by in part by Grants DK1 5070, DK20595 DK0701 1 and RR04999 from the National Institutes of Health.
References
- 1.Balazs AE. Athanassaki I. Gunn SK. Tatevian N. Huang SA. Haymond MW. Karaviti LP. Rapid resolution of consumptive hypothyroidism in a child with hepatic hemangioendothelioma following liver transplantation. Ann Clin Lab Sci. 2007;37:280–284. [PubMed] [Google Scholar]
- 2.Baqui MM. Gereben B. Harney JW. Larsen PR. Bianco AC. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology. 2000;141:4309–4312. doi: 10.1210/endo.141.11.7872. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger FP. Raman AV. Reeves MA. Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J. 2009;422:11–22. doi: 10.1042/BJ20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry MJ. Grieco D. Taylor BA. Maia AL. Kieffer JD. Beamer W. Glover E. Poland A. Larsen PR. Physiological and genetic analyses of inbred mouse strains with a type I iodothyronine 5′ deiodinase deficiency. J Clin Invest. 1993;92:1517–1528. doi: 10.1172/JCI116730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessho K. Etani Y. Ichimori H. Miyoshi Y. Namba N. Yoneda A. Ooue T. Chihara T. Morii E. Aoki T. Murakami M. Mushiake S. Ozono K. Increased type 3 iodothyronine deiodinase activity in a regrown hepatic hemangioma with consumptive hypothyroidism. Eur J Pediatr. 2009 doi: 10.1007/s00431-009-1009-x. June 23 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Bianco AC. Salvatore D. Gereben B. Berry MJ. Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 7.Bosl MR. Takaku K. Oshima M. Nishimura S. Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci USA. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botero D. Gereben B. Goncalves C. De Jesus LA. Harney JW. Bianco AC. Ubc6p and ubc7p are required for normal and substrate-induced endoplasmic reticulum-associated degradation of the human selenoprotein type 2 iodothyronine monodeiodinase. Mol Endocrinol. 2002;16:1999–2007. doi: 10.1210/me.2002-0135. [DOI] [PubMed] [Google Scholar]
- 9.Bubenik JL. Driscoll DM. Altered RNA binding activity underlies abnormal thyroid hormone metabolism linked to a mutation in selenocysteine insertion sequence-binding protein 2. J Biol Chem. 2007;282:34653–34662. doi: 10.1074/jbc.M707059200. [DOI] [PubMed] [Google Scholar]
- 10.Burk RF. Norsworthy BK. Hill KE. Motley AK. Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15:804–810. doi: 10.1158/1055-9965.EPI-05-0950. [DOI] [PubMed] [Google Scholar]
- 11.Carlson BA. Novoselov SV. Kumaraswamy E. Lee BJ. Anver MR. Gladyshev VN. Hatfield DL. Specific excision of the selenocysteine tRNA[Ser]Sec (Trsp) gene in mouse liver demonstrates an essential role of selenoproteins in liver function. J Biol Chem. 2004;279:8011–8017. doi: 10.1074/jbc.M310470200. [DOI] [PubMed] [Google Scholar]
- 12.Carlson BA. Xu XM. Gladyshev VN. Hatfield DL. Selective rescue of selenoprotein expression in mice lacking a highly specialized methyl group in selenocysteine tRNA. J Biol Chem. 2005;280:5542–5548. doi: 10.1074/jbc.M411725200. [DOI] [PubMed] [Google Scholar]
- 13.Cartegni L. Chew SL. Krainer AR. Listening to silence and understanding nonsense: Exonic mutations that affect splicing. Nat Rev Genet. 2002;3:285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- 14.Cavalieri RR. The effects of nonthyroid disease and drugs on thyroid function tests. Med Clin North Am. 1991;75:27–39. doi: 10.1016/s0025-7125(16)30470-9. [DOI] [PubMed] [Google Scholar]
- 15.Chavatte L. Brown BA. Driscoll DM. Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes. Nat Struct Mol Biol. 2005;12:408–416. doi: 10.1038/nsmb922. [DOI] [PubMed] [Google Scholar]
- 16.Cheville AL. Kirshblum SC. Thyroid hormone changes in chronic spinal cord injury. J Spinal Cord Med. 1995;18:227–232. doi: 10.1080/10790268.1995.11719400. [DOI] [PubMed] [Google Scholar]
- 17.Christoffolete MA. Arrojo e Drigo R. Gazoni F. Tente SM. Goncalves V. Amorim BS. Larsen PR. Bianco AC. Zavacki AM. Mice with impaired extrathyroidal thyroxine to 3,5,3′-triiodothyronine conversion maintain normal serum 3,5,3′-triiodothyronine concentrations. Endocrinology. 2007;148:954–960. doi: 10.1210/en.2006-1042. [DOI] [PubMed] [Google Scholar]
- 18.Chu FF. Esworthy RS. Chu PG. Longmate JA. Huycke MM. Wilczynski S. Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64:962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 19.Clark RE. Cardiopulmonary bypass and thyroid hormone metabolism. Ann Thorac Surg. 1993;56:S35–S41. doi: 10.1016/0003-4975(93)90552-s. discussion 41–42. [DOI] [PubMed] [Google Scholar]
- 20.Copeland PR. Regulation of gene expression by stop codon recoding: Selenocysteine. Gene. 2003;312:17–25. doi: 10.1016/s0378-1119(03)00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Copeland PR. Fletcher JE. Carlson BA. Hatfield DL. Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copeland PR. Stepanik VA. Driscoll DM. Insight into mammalian selenocysteine insertion: Domain structure and ribosome binding properties of Sec insertion sequence binding protein 2. Mol Cell Biol. 2001;21:1491–1498. doi: 10.1128/MCB.21.5.1491-1498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curcio-Morelli C. Zavacki AM. Christofollete M. Gereben B. de Freitas BC. Harney JW. Li Z. Wu G. Bianco AC. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Invest. 2003;112:189–196. doi: 10.1172/JCI18348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies PH. Sheppard MC. Franklyn JA. Inflammatory cytokines and type I 5′-deiodinase expression in phi1 rat liver cells. Mol Cell Endocrinol. 1997;129:191–198. doi: 10.1016/s0303-7207(97)04058-6. [DOI] [PubMed] [Google Scholar]
- 25.Di Cosmo C. McLellan N. Liao XH. Khanna KK. Weiss RE. Papp L. Refetoff S. Clinical and molecular characterization of a novel selenocysteine insertion sequence-binding protein 2 (SBP2) gene mutation (R1 28X) J Clin Endocrinol Metab. 2009;94:4003–4009. doi: 10.1210/jc.2009-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driscoll DM. Copeland PR. Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- 27.Dubuis JM. Dayer JM. Siegrist-Kaiser CA. Burger AG. Human recombinant interleukin-1 beta decreases plasma thyroid hormone and thyroid stimulating hormone levels in rats. Endocrinology. 1988;123:2175–2181. doi: 10.1210/endo-123-5-2175. [DOI] [PubMed] [Google Scholar]
- 28.Dumitrescu AM. Liao XH. Abdullah MS. Lado-Abeal J. Majed FA. Moeller LC. Boran G. Schomburg L. Weiss RE. Refetoff S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 29.Dumitrescu AM. Liao XH. Best TB. Brockmann K. Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forrest D. Erway LC. Ng L. Altschuler R. Curran T. Thyroid hormone receptor beta is essential for development of auditory function. Nat Genet. 1996;13:354–357. doi: 10.1038/ng0796-354. [DOI] [PubMed] [Google Scholar]
- 31.Freeman JV. Cole TJ. Chinn S. Jones PR. White EM. Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friesema EC. Grueters A. Biebermann H. Krude H. von Moers A. Reeser M. Barrett TG. Mancilla EE. Svensson J. Kester MH. Kuiper GG. Balkassmi S. Uitterlinden AG. Koehrle J. Rodien P. Halestrap AP. Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 33.Friesema EC. Jansen J. Milici C. Visser TJ. Thyroid hormone transporters. Vitam Horm. 2005;70:137–167. doi: 10.1016/S0083-6729(05)70005-4. [DOI] [PubMed] [Google Scholar]
- 34.Fujii T. Sato K. Ozawa M. Kasono K. Imamura H. Kanaji Y. Tsushima T. Shizume K. Effect of interleukin-1 (IL-1) on thyroid hormone metabolism in mice: Stimulation by IL-1 of iodothyronine 5′-deiodinating activity (type I) in the liver. Endocrinology. 1989;124:167–174. doi: 10.1210/endo-124-1-167. [DOI] [PubMed] [Google Scholar]
- 35.Galton VA. Schneider MJ. Clark AS. St Germain DL. Life without thyroxine to 3,5,3′-triiodothyronine conversion: Studies in mice devoid of the 5′- deiodinases. Endocrinology. 2009;150:2957–2963. doi: 10.1210/en.2008-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glass AR. Young RA. Anderson J. Decreased serum 3,5,3′-triiodothyronine (T3) and abnormal serum binding of T3 in calorie-deficient rats: Adaptation after chronic underfeeding. Endocrinology. 1986;118:2464–2469. doi: 10.1210/endo-118-6-2464. [DOI] [PubMed] [Google Scholar]
- 37.Guven A. Aygun C. Ince H. Aydin M. Pinarli FG. Baysal K. Kucukoduk S. Severe hypothyroidism caused by hepatic hemangioendothelioma in an infant of a diabetic mother. Horm Res. 2005;63:86–89. doi: 10.1159/000083879. [DOI] [PubMed] [Google Scholar]
- 38.Hatfield DL. Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermus RM. Sweep CG. van der Meer MJ. Ross HA. Smals AG. Benraad TJ. Kloppenborg PW. Continuous infusion of interleukin-1 beta induces a nonthyroidal illness syndrome in the rat. Endocrinology. 1992;131:2139–2146. doi: 10.1210/endo.131.5.1425414. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez A. Martinez ME. Liao XH. Van Sande J. Refetoff S. Galton VA. St Germain DL. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology. 2007;148:5680–5687. doi: 10.1210/en.2007-0652. [DOI] [PubMed] [Google Scholar]
- 41.Ho J. Kendrick V. Dewey D. Pacaud D. New insight into the pathophysiology of severe hypothyroidism in an infant with multiple hepatic hemangiomas. J Pediatr Endocrinol Metab. 2005;18:511–514. doi: 10.1515/jpem.2005.18.5.511. [DOI] [PubMed] [Google Scholar]
- 42.Huang SA. Dorfman DM. Genest DR. Salvatore D. Larsen PR. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 2003;88:1384–1388. doi: 10.1210/jc.2002-021291. [DOI] [PubMed] [Google Scholar]
- 43.Huang SA. Fish SA. Dorfman DM. Salvatore D. Kozakewich HP. Mandel SJ. Larsen PR. A 21-year-old woman with consumptive hypothyroidism due to a vascular tumor expressing type 3 iodothyronine deiodinase. J Clin Endocrinol Metab. 2002;87:4457–4461. doi: 10.1210/jc.2002-020627. [DOI] [PubMed] [Google Scholar]
- 44.Huang SA. Tu HM. Harney JW. Venihaki M. Butte AJ. Kozakewich HP. Fishman SJ. Larsen PR. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–189. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 45.Jakupoglu C. Przemeck GK. Schneider M. Moreno SG. Mayr N. Hatzopoulos AK. de Angelis MH. Wurst W. Bornkamm GW. Brielmeier M. Conrad M. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaptein EM. Thyroxine kinetics in nonthyroidal illnesses. J Endocrinol Invest 9 Suppl. 1986;4:37–46. [PubMed] [Google Scholar]
- 47.Kaptein EM. Kaptein JS. Chang EI. Egodage PM. Nicoloff JT. Massry SG. Thyroxine transfer and distribution in critical nonthyroidal illnesses, chronic renal failure, and chronic ethanol abuse. J Clin Endocrinol Metab. 1987;65:606–616. doi: 10.1210/jcem-65-4-606. [DOI] [PubMed] [Google Scholar]
- 48.Koenig RJ. Modeling the nonthyroidal illness syndrome. Curr Opin Endocrinol Diabetes Obes. 2008;15:466–469. doi: 10.1097/MED.0b013e32830eb838. [DOI] [PubMed] [Google Scholar]
- 49.Kohrle J. The deiodinase family: Selenoenzymes regulating thyroid hormone availability and action. Cell Mol Life Sci. 2000;57:1853–1863. doi: 10.1007/PL00000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohrle J. Selenium and the control of thyroid hormone metabolism. Thyroid. 2005;15:841–853. doi: 10.1089/thy.2005.15.841. [DOI] [PubMed] [Google Scholar]
- 51.Konrad D. Ellis G. Perlman K. Spontaneous regression of severe acquired infantile hypothyroidism associated with multiple liver hemangiomas. Pediatrics. 2003;112:1424–1426. doi: 10.1542/peds.112.6.1424. [DOI] [PubMed] [Google Scholar]
- 52.Kryukov GV. Castellano S. Novoselov SV. Lobanov AV. Zehtab O. Guigo R. Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 53.Kumaraswamy E. Carlson BA. Morgan F. Miyoshi K. Robinson GW. Su D. Wang S. Southon E. Tessarollo L. Lee BJ. Gladyshev VN. Hennighausen L. Hatfield DL. Selective removal of the selenocysteine tRNA [Ser]Sec gene (Trsp) in mouse mammary epithelium. Mol Cell Biol. 2003;23:1477–1488. doi: 10.1128/MCB.23.5.1477-1488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee WY. Kang MI. Oh KW. Oh ES. Baek KH. Lee KW. Kim SW. Kim DW. Min WS. Kim CC. Relationship between circulating cytokine levels and thyroid function following bone marrow transplantation. Bone Marrow Transplant. 2004;33:93–98. doi: 10.1038/sj.bmt.1704304. [DOI] [PubMed] [Google Scholar]
- 55.Lescure A. Allmang C. Yamada K. Carbon P. Krol A. cDNA cloning, expression pattern and RNA binding analysis of human selenocysteine insertion sequence (SECIS) binding protein 2. Gene. 2002;291:279–285. doi: 10.1016/s0378-1119(02)00629-7. [DOI] [PubMed] [Google Scholar]
- 56.Low SC. Grundner–Culemann E. Harney JW. Berry MJ. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000;19:6882–6890. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maia AL. Berry MJ. Sabbag R. Harney JW. Larsen PR. Structural and functional differences in the dio1 gene in mice with inherited type 1 deiodinase deficiency. Mol Endocrinol. 1995;9:969–980. doi: 10.1210/mend.9.8.7476994. [DOI] [PubMed] [Google Scholar]
- 58.McIver B. Gorman CA. Euthyroid sick syndrome: An overview. Thyroid. 1997;7:125–132. doi: 10.1089/thy.1997.7.125. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell C. Robertson V. Montano S. Lu J. Clemons N. Halsall D. Rajanayagam O. Castanet M. Baguely D. Fitzgerald R. Holmgren A. Coles A. Ogunko A. Chatterjee VK. The adult phenotype of multiple selenoprotein deficiencies associated with a novel SECISBP2 gene defect. 8th International Workshop on Resistance to Thyroid Hormone and Action, Ponta Delgada, S. Miguel, Azores Portugal: p OP#1 8, 2007 (abstract)
- 60.Moreno–Reyes R. Egrise D. Neve J. Pasteels JL. Schoutens A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J Bone Miner Res. 2001;16:1556–1563. doi: 10.1359/jbmr.2001.16.8.1556. [DOI] [PubMed] [Google Scholar]
- 61.Moustafa ME. Kumaraswamy E. Zhong N. Rao M. Carlson BA. Hatfield DL. Models for assessing the role of selenoproteins in health. J Nutr. 2003;133:2494S–2496S. doi: 10.1093/jn/133.7.2494S. [DOI] [PubMed] [Google Scholar]
- 62.Ng L. Goodyear RJ. Woods CA. Schneider MJ. Diamond E. Richardson GP. Kelley MW. Germain DL. Galton VA. Forrest D. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA. 2004;101:3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olson GE. Winfrey VP. Nagdas SK. Hill KE. Burk RF. Selenoprotein P is required for mouse sperm development. Biol Reprod. 2005;73:201–211. doi: 10.1095/biolreprod.105.040360. [DOI] [PubMed] [Google Scholar]
- 64.Ormerod AD. How J. Bewsher PD. Reid IW. Effects of widespread dermatitis and topical steroid therapy on thyroid function tests. Dermatologica. 1988;176:257–259. doi: 10.1159/000248715. [DOI] [PubMed] [Google Scholar]
- 65.Ozawa M. Sato K. Han DC. Kawakami M. Tsushima T. Shizume K. Effects of tumor necrosis factor-alpha/cachectin on thyroid hormone metabolism in mice. Endocrinology. 1988;123:1461–1467. doi: 10.1210/endo-123-3-1461. [DOI] [PubMed] [Google Scholar]
- 66.Papp LV. Lu J. Holmgren A. Khanna KK. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 67.Papp LV. Lu J. Striebel F. Kennedy D. Holmgren A. Khanna KK. The redox state of SECIS binding protein 2 controls its localization and selenocysteine incorporation function. Mol Cell Biol. 2006;26:4895–4910. doi: 10.1128/MCB.02284-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papp LV. Wang J. Kennedy D. Boucher D. Zhang Y. Gladyshev VN. Singh RN. Khanna KK. Functional characterization of alternatively spliced human SECISBP2 transcript variants. Nucleic Acids Res. 2008;36:7192–7206. doi: 10.1093/nar/gkn829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plikat K. Langgartner J. Buettner R. Bollheimer LC. Woenckhaus U. Scholmerich J. Wrede CE. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007;56:239–44. doi: 10.1016/j.metabol.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 71.Refetoff S. Dumont JE. Vassart G. Thyroid disorders (Chapter 158) New York: McGraw-Hill, Inc.; 2001. pp. 4029–4075. [Google Scholar]
- 72.Refetoff S. Weiss RE. Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- 73.Rubenfeld S. Euthyroid sick syndrome. N Engl J Med. 1978;299:1414. doi: 10.1056/NEJM197812212992514. [DOI] [PubMed] [Google Scholar]
- 74.Rumolo R. Vitolo E. Tronci M. Massari D. Cavagnini F. Dubini D. Alterations in thyroid function induced by chronic administration of amiodarone. Drugs Exp Clin Res. 1987;13:29–35. [PubMed] [Google Scholar]
- 75.Ruppe MD. Huang SA. Jan de Beur SM. Consumptive hypothyroidism caused by paraneoplastic production of type 3 iodothyronine deiodinase. Thyroid. 2005;15:1369–1372. doi: 10.1089/thy.2005.15.1369. [DOI] [PubMed] [Google Scholar]
- 76.Schneider MJ. Fiering SN. Pallud SE. Parlow AF. St Germain DL. Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 77.Schneider MJ. Fiering SN. Thai B. Wu SY. St Germain E. Parlow AF. St Germain DL. Galton VA. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 78.Schoenmakers CH. Pigmans IG. Poland A. Visser TJ. Impairment of the selenoenzyme type I iodothyronine deiodinase in C3H/He mice. Endocrinology. 1993;132:357–361. doi: 10.1210/endo.132.1.8419134. [DOI] [PubMed] [Google Scholar]
- 79.Schomburg L. Dumitrescu AM. Liao XH. Bin-Abbas B. Hoeflich J. Kohrle J. Refetoff S. Selenium supplementation fails to correct the selenoprotein synthesis defect in subjects with SBP2 gene mutations. Thyroid. 2009;19:277–281. doi: 10.1089/thy.2008.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schomburg L. Schweizer U. Kohrle J. Selenium and selenoproteins in mammals: Extraordinary, essential, enigmatic. Cell Mol Life Sci. 2004;61:1988–1995. doi: 10.1007/s00018-004-4114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schweizer U. Michaelis M. Kohrle J. Schomburg L. Efficient selenium transfer from mother to offspring in selenoprotein-P-deficient mice enables dose-dependent rescue of phenotypes associated with selenium deficiency. Biochem J. 2004;378:21–26. doi: 10.1042/BJ20031795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schweizer U. Schomburg L. Savaskan NE. The neurobiology of selenium: Lessons from transgenic mice. J Nutr. 2004;134:707–710. doi: 10.1093/jn/134.4.707. [DOI] [PubMed] [Google Scholar]
- 83.Sellmeyer DE. Grunfeld C. Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev. 1996;17:518–532. doi: 10.1210/edrv-17-5-518. [DOI] [PubMed] [Google Scholar]
- 84.Squires JE. Davy P. Berry MJ. Allsopp R. Attenuated expression of SECIS binding protein 2 causes loss of telomeric reserve without affecting telomerase. Exp Gerontol. 2009;44:619–623. doi: 10.1016/j.exger.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Squires JE. Stoytchev I. Forry EP. Berry MJ. SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Mol Cell Biol. 2007;27:7848–7855. doi: 10.1128/MCB.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeuchi A. Schmitt D. Chapple C. Babaylova E. Karpova G. Guigo R. Krol A. Allmang C. A short motif in Drosophila SECIS Binding Protein 2 provides differential binding affinity to SECIS RNA hairpins. Nucleic Acids Res. 2009;37:2126–2141. doi: 10.1093/nar/gkp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanner JM. Whitehouse RH. Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966;41:613–635. doi: 10.1136/adc.41.220.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Umpierrez GE. Euthyroid sick syndrome. South Med J. 2002;95:506–513. [PubMed] [Google Scholar]
- 89.Ursini F. Heim S. Kiess M. Maiorino M. Roveri A. Wissing J. Flohe L. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–1396. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 90.Van den Berghe G. de Zegher F. Lauwers P. Dopamine and the sick euthyroid syndrome in critical illness. Clin Endocrinol (Oxf) 1994;41:731–737. doi: 10.1111/j.1365-2265.1994.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 91.van der Poll T. Romijn JA. Wiersinga WM. Sauerwein HP. Tumor necrosis factor: A putative mediator of the sick euthyroid syndrome in man. J Clin Endocrinol Metab. 1990;71:1567–1572. doi: 10.1210/jcem-71-6-1567. [DOI] [PubMed] [Google Scholar]
- 92.Vexiau P. Perez-Castiglioni P. Socie G. Devergie A. Toubert ME. Aractingi S. Gluckman E. The ‘euthyroid sick syndrome’: Incidence, risk factors and prognostic value soon after allogeneic bone marrow transplantation. Br J Haematol. 1993;85:778–782. doi: 10.1111/j.1365-2141.1993.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 93.Wartofsky L. Burman KD. Alterations in thyroid function in patients with systemic illness: The “euthyroid sick syndrome”. Endocr Rev. 1982;3:164–217. doi: 10.1210/edrv-3-2-164. [DOI] [PubMed] [Google Scholar]
- 94.Weiss Sachdev S. Sunde RA. Selenium regulation of transcript abundance and translational efficiency of glutathione peroxidase-1 and -4 in rat liver. Biochem J. 2001;357:851–858. doi: 10.1042/0264-6021:3570851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu ASH. Oldfiled JE. Shull LR. Cheeke PR. Specific effect of selenium deficiency on rat sperm. Biol Reprod. 1979;20:793–798. doi: 10.1095/biolreprod20.4.793. [DOI] [PubMed] [Google Scholar]
- 96.Xu XM. Carlson BA. Mix H. Zhang Y. Saira K. Glass RS. Berry MJ. Gladyshev VN. Hatfield DL. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2007;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu XM. Mix H. Carlson BA. Grabowski PJ. Gladyshev VN. Berry MJ. Hatfield DL. Evidence for direct roles of two additional factors, SECp43 and soluble liver antigen, in the selenoprotein synthesis machinery. J Biol Chem. 2005;280:41568–41575. doi: 10.1074/jbc.M506696200. [DOI] [PubMed] [Google Scholar]
- 98.Yant LJ. Ran Q. Rao L. Van Remmen H. Shibatani T. Belter JG. Motta L. Richardson A. Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 99.Yu J. Koenig RJ. Regulation of hepatocyte thyroxine 5′-deiodinase by T3 and nuclear receptor coactivators as a model of the sick euthyroid syndrome. J Biol Chem. 2000;275:38296–38301. doi: 10.1074/jbc.M004866200. [DOI] [PubMed] [Google Scholar]
- 100.Zaloga GP. Chernow B. Smallridge RC. Zajtchuk R. Hall-Boyer K. Hargraves R. Lake CR. Burman KD. A longitudinal evaluation of thyroid function in critically ill surgical patients. Ann Surg. 1985;201:456–464. doi: 10.1097/00000658-198504000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]