Abstract
Lentiviral vectors are efficient gene delivery vehicles suitable for delivering long-term transgene expression in various cell types. Engineering lentiviral vectors to have the capacity to transduce specific cell types is of great interest to advance the translation of lentiviral vectors towards the clinic. Here we provide an overview of innovative approaches to target lentiviral vectors to cells of the immune system. In this overview we distinguish between two types of lentiviral vector targeting strategies: 1) targeting of the vectors to specific cells by lentiviral vector surface modifications, and 2) targeting at the level of transgene transcription by insertion of tissue-specific promoters to drive transgene expression. It is clear that each strategy is of enormous value but ultimately combining these approaches may help reduce the effects of off-target expression and improve the efficiency and saftey of lentiviral vectors for gene therapy.
Keywords: lentiviral vector, immune cells, targeted delivery, T-cell, B-cells
INTRODUCTION
The delivery of genes of interest to cellular targets has been studied intensively over the past twenty years in hopes that autoimmune diseases such as Parkinson's disease and severe combined immunodeficiency (SCID), as well as cancers, may be cured. It has been over a decade since the first gene therapy procedure was performed on a patient.(1) The patient was born with SCID, which required her to live in relative isolation with frequent bouts of illnesses and routine administration of antibiotics. For the procedure, the patient's white blood cells were removed and cultured in the lab. Then, the missing genes were inserted into the cells and the cells were infused back into the patient's bloodstream. After this, the patient's immune system was strengthened by 40%, although the procedure was not permanent and so the corrected white blood cells had to be repeatedly infused every few months. There has been some debate whether these results were obtained solely due to the gene therapy procedure versus the other treatments which were administered; however, this study was at least able to demonstrate that gene therapy could be administered to a patient without adverse consequences.(2)
GENE DELIVERY VECTORS
There are two types of vehicles by which genes may be delivered into cells. First, synthetic delivery vehicles can be used. These usually consist of either lipids or polymers which surround the DNA, termed lipoplexes(3) and polyplexes, respectively. There are three types of lipoplexes: anionic, neutral, and cationic. Anionic and neutral lipids were initially preferred since they were safer, more compatible with body fluids, and had the potential for tissue-specific gene transfer; however, production of these lipoplexes was difficult and expression in transduced cells was relatively low(4). Cationic liposomes, on the other hand, naturally form complexes with DNA, which is negatively charged. The positive charge also facilitates the penetration of the complexes into the negatively charged cellular membrane. Most polyplexes are created with cationic polymers. Unlike lipoplexes, though, some polyplexes are unable to deposit the DNA into the cytoplasm.(5) Thus, these polyplexes need to be co-transfected with endosome-lytic agents, such as inactivated adenovirus, to be effective. Synthetic, non-viral vectors have both advantages and disadvantages. They are capable of being produced on a large scale and have low host immunogenicity; however, although recent advances in vector technology have yielded higher transfection efficiencies, they remain too inefficient for most clinical applications.
The second type of gene delivery vehicle is the viral vector. These exploit the natural ability of viruses to efficiently deliver genetic materials to cells. With the tools available to modern biology, therapeutic transgenes can be easily swapped with the original viral genes, resulting in specialized gene delivery vehicles. Several in vivo studies, animal disease models, and even clinical trials have been successfully conducted using viral delivery vectors, mostly using adenoviral, adeno-associated, retroviral, or lentiviral vectors.(6) However, several factors limit the efficacy of viral vectors for gene delivery. First, systemic barriers, such as pre-existing immunity,(7-9) and cellular barriers, such as binding to the cell surface,(10, 11) hinder the efficient delivery of genes into the cells. Also, inefficient production and purification of the viral vectors,(12, 13) as well as poor transduction efficiency to the therapeutically relevant cells(11, 14) are other barriers that must be overcome for viral gene delivery vectors.
RETROVIRAL VECTORS
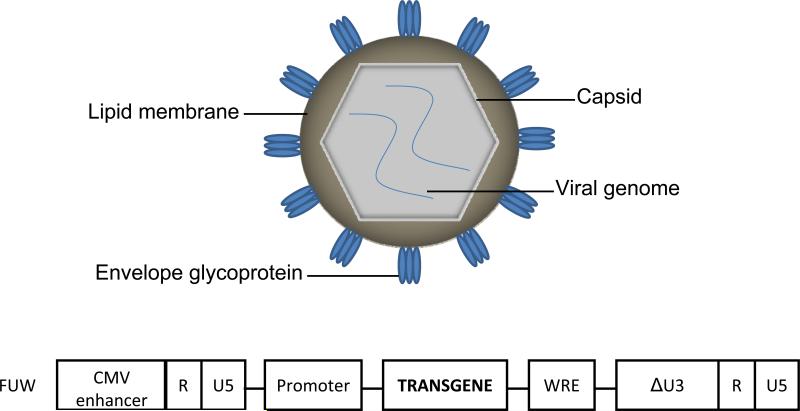
Retroviruses are enveloped viruses with diploid, single-stranded, 7-12 kb positive sense RNA genomes.(15) This genome contains gag, which encodes the structural proteins (matrix protein, capsid protein, and nucleocapsid protein), pro and pol, which encodes enzymatic proteins (protease, reverse transcriptase, and integrase), and env, which encodes the surface and transmembrane units of the envelope protein (Figure 1).(16) The genetic material is contained in the nucleocapsid, which is enveloped by a bi-lipidic membrane taken from the virus-producing cell. The glycoproteins are inserted into this membrane and are responsible for binding the virions to receptors on the cell surface. The interaction between the envelope glycoprotein and cellular receptors is what determines viral tropism. Binding leads to conformational changes in the viral envelope glycoprotein, exposing the hydrophobic fusion peptide. When inserted into cellular membrane, this peptide will mediate fusion between the viral and cellular membranes. After virus-cell membrane fusion occurs, the core nucleoprotein complex is released into the cytoplasm and reverse transcription of the viral genome into DNA occurs. This newly synthesized double-stranded DNA is then transported into the nucleus and integrated into to the host chromosome.
Figure 1.
Schematic of the retrovirus structure and a representative lentiviral vector backbone plasmid, FUW.
Since retroviruses are able to accommodate extensive changes to their genomes and integrate efficiently into the genomes of their host cells, they are excellent candidates for gene transfer vectors. The first retroviral vectors were produced with the help of replication-competent or helper viruses. In 1983, retroviral packaging cells were created that supplied the retroviral proteins but did not produce replication-competent viruses. Later, all the viral coding regions were deleted and only the vital viral elements for high-efficiency transfer were retained. Since viral entry, reverse transcription, and genome integration are not dependent on the synthesis of viral proteins, the viral vectors can contain only the genes of interest.
Retroviral vectors hold many advantages over other gene delivery methods. First, they can transduce many different types of cells from different species. They can also integrate their genetic payload into cells with precision and produce high levels of transgene expression. Since the virus had been engineered to be replication-incompetent, there is no danger of the vector spreading to other cells, or of viral proteins being produced after transduction. Lastly, retroviral vectors have a relatively large payload capacity and offer low immunogenicity.(15) Drawbacks of retroviral vectors include the inability to transduce non-dividing cells, the lack of stability in the envelope proteins, and the risk of insertional mutagenesis due to the semirandom integration of genes.(17)
LENTIVIRAL VECTORS
A subclass of retroviruses that has emerged as another vehicle for gene delivery is the lentivirus. Lentiviral vectors (LVs), such as those derived from the human immunodeficiency virus (HIV) are capable of infecting nondividing cells through mitosis-independent transport of the viral DNA into the nucleus.(18, 19) This feature is particularly useful for gene transfer to nondividing cells, such as antigen presenting cells (APCs), monocytes, and neurons.(19-21) LVs also do not tend to integrate by transcriptional initiation sites, a problem faced by other viral vectors.(22) However, like retroviral vectors, high-titer production of LVs has been difficult due to the complex nature of the virus. To combat this problem, vectors have been produced which contain the vesicular stomatitis virus (VSV) envelope protein with the HIV core proteins. This has resulted in higher-titer vector production, as well as higher transduction efficiencies. Development of the LV has been geared towards improving safety by reducing the risk of insertional mutagenesis. First, the vector was made self-inactivating (SIN) and the enhancer in the LTRs has was deleted.(23) Second, to prevent read-through transcripts, a strong RNA polyadenylation sequence was added to the vector sequence.(24) Lastly, DNA insulators were inserted into the LTR to isolate the internal promoter from the neighboring genome.(25)
GENERAL TRANSDUCTIONAL TARGETING STRATEGY: DELIVERY OF GENES TO SPECIFIC CELLS
One strategy for altering the cellular tropism of lentiviruses is through the construction of phenotypically mixed particles, or pseudotypes, in which heterologous glycoproteins are incorporated into the viron as it buds out from the producing cell.(26) Pseudotyping LVs consists of engineering vector particles to incorporate envelope glycoproteins (GPs) derived from other enveloped viruses. Pseudotyped particles adopt the tropism of the virus from which the GP was derived.(26) Perhaps the most prominent glycoprotein used to pseudotype lentivectors is the vesicular stomatitis virus G (VSV-G) protein. VSV-G-pseudotyped lentivectors appear to use ubiquitous lipid-type receptors, such as phosphatidylserine, resulting in a broad cellular tropism.(27-29) This broad tropism, along with good vector stability,(30) is the reason why VSV-G is the most widely used GP for pseudotyping LVs. However, VSV-G-pseudotyped vector particles also have significant shortcomings. VSV-G expression is toxic to cells if expressed constitutively and thus complicates the development of stable packaging cell lines.(31) Furthermore, VSV-G-pseudotyped particles are inactivated by human serum complements, requiring PEGylation for in vivo applications.(32) Vectors based on HIV and other lentiviruses have also been pseudotyped with various envelope proteins to expand the host range to a variety of cell types. In addition, pseudotyping with alternative viral glycoproteins can be used to resolve other limitations such as neutralization by host immune responses, inefficiencies in production and purification, poor specificities, and poor transduction of therapeutically relevant cells.(33) Thus, pseudotyping techniques to generate viral vectors with novel and improved gene delivery properties offer a potential system to address these gene delivery shortfalls.
Entry of pseudotyped viruses is limited to cells and tissues that express the appropriate cellular receptor. The natural budding mechanism of the lentivirus and the plasticity of the envelope membrane to be altered allow pseudotyping with surface glycoproteins from a variety of different enveloped viruses. Previous virus envelopes used to pseudotype lentivectors have been review elsewhere,(26) but among others include: lyssavirus (Rabies virus), arenavirus (lymphocytic choriomeningitis virus (LCMV)), alphavirus (Sindbis virus), influenza virus (HA), coronavirus (SARS-CoV), Flavivirus (HCV), Filovirus (Ebola), Gammaretrovirus (RD117), Bacculovirus (GP64), and Measles virus. These pseudotyped vectors vary widely in their cellular tropism, titer, efficiency of packaging, stability, immune response, and inactivation by complement. All characteristics should be carefully considered when choosing a suitable glycoprotein tailored to best fit the experiment. For example, the superiority of Gibbon Ape Leukemia virus (GALV) and the cat endogenous retroviral glycoprotein (RD114) for transduction of progenitor and differentiated hematopoietic cells was established by screening a large library of pseudotyped vectors.(34-37) HIV-1 vectors pseudotyped with RD114 and amphotropic murine leukemia virus (MLV) glycoproteins were more efficient than VSV-G pseudotypes at transducing human cord blood CD34+ cells and progenitors.(34) When lentiviruses are utilized in the CNS, additional glycoprotein characteristics such as retrograde transport must be considered. While envelope proteins from VSV and Rabies come from the same viral family and exhibit similar tropism, they have very different retrograde transport activities when injected into the striatum of the mouse brain.(38) Whereas VSV-G transduces cells locally, equine infectious anemia virus (EIAV) pseudotyped with rabies envelope proteins undergo retrograde transport to the thalamus upon striatal injection.(39) Ultimately, the aim of incorporating alternative envelope glycoproteins is to produce a therapeutic, safe, and efficient LV for clinical applications.
Alphaviruses exhibit a wide cellular tropism that includes important gene therapy targets such as antigen-presenting cells, neurons, and muscle cells. The cellular receptors for the various alphavirus glycoproteins have not yet been identified; however, several receptors or receptor-coreceptor combinations may be involved in virus entry. This property allows the tropism of HIV-1-based LVs to be altered.(40) Recently, wild-type mosquito-produced Sindbis alphavirus(41) was shown to use C-type lectins as attachment receptors leading to productive transduction of dendritic cells. Additionally, several reports of efficient pseudotyping of LVs with Ross River virus (RRV), Semliki Forest virus (SFV), and Sindbis virus (SIN) glycoproteins have been reported.(42-44) Pseudotyping is an alternative straightforward method to utilize the mechanism by which alphaviral glycoproteins can mediate transduction by C-type lectins. As an important example, to narrow the tropism of LVs and enhance vector stability, Sindbis virus glycoproteins have been mutated to reduce binding to heparan sulfate and enhance dendritic cell tropism.(45, 46) High affinity interactions of viral glycoproteins with these C-type lectins might represent a strategy by which dendritic cells can be targeted by viruses. Enhanced delivery of antigen to immature dendritic cells may provide an opportunity for improvement of vaccines, particularly for gene-based vaccination approaches.
Development of methods capable of engineering LVs to be cell type-specific receptors could substantially change the current practice of gene therapy and greatly expand the scope of gene therapy for disease treatment.(47-49) Cell-specific transduction can address most of the side effects of off targeting gene transfer by the precise introduction of the therapeutic nucleic acid into expected cells.(49) A common strategy is to genetically modify envelope glycoproteins to incorporate targeting ligands into LVs. It was found that several glycoproteins have structures that are able to tolerate the insertion of binding motifs such as peptide ligands,(50-53) single chain antibodies,(54-56) growth factors,(57-60) etc. These engineered glycoproteins can retarget vectors to cells expressing their corresponding target moieties. Another popular approach is to introduce a “molecular bridge” to direct vectors to specific cells(49). The molecular bridge has dual specificities: one end can recognize viral glycoproteins and the other end can bind to the molecular determinant on the target cell. Such a molecule can direct the attachment of viral vectors to target cells for transduction. To date, ligand-receptor, avidin-biotin, and chemical conjugations have been exploited for the creation of such molecular bridges to retarget envelope vectors.(61-63) Recently, monoclonal antibodies have been introduced as a new kind of molecular bridge to allow vectors to preferentially transduce cells expressing cognate surface antigens both in vitro(44) and in vivo.(64) In such studies, the E2 protein of the Sindbis virus glycoprotein was modified to contain the Fc-binding domain of protein A. Thus, one end of the monoclonal antibody could bind to viral vectors and the antigen recognition end could direct vectors to antigen-expressing cells. Proteins overexpressed by producer cells can also be incorporated onto the vector surface. LVs have been produced that incorporated CD4 and CCR5 or CD4 and CXCR4 to target the HIV primary receptors and co-receptors of HIV-1-infected cells.(65, 66)
Functions of binding and fusion of some natural viruses such as paramyxovirus are attributed to two proteins: an attachment protein and a fusion protein.(67) Thus, mimicking such viruses to separate the binding and fusion functions as two distinct envelope molecules on the surface of enveloped vectors represents another attractive strategy for targeting. Lin et al. incorporated a binding-defective but fusion-competent hemagglutinin (HA) protein as a fusion protein and a chimeric glycoprotein engineered to contain specificity for the Flt-3 receptor as a binding protein, into gammaretroviral vectors.(68) It was shown that such two proteins could complement each other to mediate preferential modification of cells expressing Flt-3 in vitro(68). We have demonstrated successful targeting LVs by co-display of membrane-bound antibody as the binding protein and fusogenic molecule derived from Sindbis virus glycoprotein as the fusion protein.(69) Efficient and specific transduction was accomplished by a two-stage process: endocytosis induced by the antibody-antigen interaction and fusion triggered by the acidic pH within the endosomal compartment.
GENERAL TRANSLATIONAL TARGETING STRATEGY: TRANSGENE EXPRESSION IN SPECIFIC CELLS
Another strategy for targeting the genetic manipulation of specific cell types is through the construction of tissue-specific expression vectors, in which a tissue-specific promoter confers restricted transgene expression in only the target cells. When the transgene is delivered to the affected cell it would encounter the appropriate transcriptional machinery and theoretically will not be eliminated by degradation or by an immune response. Tissue-specific promoters have been widely used to restrict transgene expression using both viral vectors and non-viral vectors. Most of them aim at the production of tissue-specific expression after transduction and differentiation of hematopoietic stem cells (HSCs).
One focus of gene therapy relies on transduction of HSCs that are self-renewing and have the potential to differentiate into all blood cells, which makes them the main target for the genetic correction of hematopoietic diseases. Recently, transplantation of genetically modified HSCs has been explored for the treatment of inherited blood disorders such as SCID resulting from the lack of the common γ chain receptor (X-linked SCID),(70) adenosine deaminase-deficient SCID (ADA-SCID),(71) and chronic granulomatous disease (CGD).(72) Previous studies have revealed that MLV-derived vectors integrate in a nonrandom fashion into the host genome, favoring transcripitonally active genes, CpG islands, and transcriptional start sites.(73) The occurrence of leukemia-like disorders in patients with SCID-X1 treated by gene therapy has been associated with insertional activation of protooncogenes.(74, 75) The retroviral vectors used in the previous clinical trials possessed strong enhancer and promoter elements within the integrated viral LTR.(70-72, 76) The strong enhancer of the LTR drives expression but is known to be involved in the overexpression of the LMO2 protooncogene. Improved safety may be achieved by the third generation self inactivating LVs, in which transgene expression is driven by tissue-specific promoters. Tissue-specific promoters may prevent oncogenesis in cells of the relevant lineages by using more tightly regulated protein expression.
TARGETING IMMUNE CELLS USING CELL-SPECIFIC PROMOTORS
The goal of targeted gene delivery is precise transgene expression. The technique of driving gene expression using LVs with restricted promoters is amendable to targeting various immune cell types. Targeted expression in immune cells must produce an appropriate amount of transcription without inducing an immune response or gene silencing. Recently, systems have been developed to regulate transgene expression.(77) Clearly, transgene expression restricted to only the target cell type, controlled expression of the gene, and a limited induced immune response are desirable properties of an immune cell-specific promotor gene expression system. Tissue-specific promoters have been used to restrict transgene expression to specific cells of both non-immune and immune systems.(78) To target expression to specific cells of the hematopoietic system, most tissue-specific expression has focused on the transduction and differentiation of HSCs. Previous cell-specific lentiviral promoters have been shown to result in B cell,(79-83) T lymphoid,(84-87) and general antigen-presenting cell-specific expression.(88-94) However, the study of LV integration has pointed out the preferential insertion of the transgene in transcriptionally-active sites of the cell genome.(95) Furthermore, additional genetic elements are desirable to impede the convolution of the genome environment where the transgene will be inserted.
For B cells, previously used retro/lentiviral promoters have included an immunoglobulin (Ig) heavy chain enhancer in combination with a phosphoglycerate kinase or cytomegalovirus (CMV) promoter to increase expression,(82) and a CD19 gene promoter to drive expression of a marker gene in mice using a retroviral vector(83) and in human B cells using a LV.(79) An Ig kappa (Igκ) light chain promoter and enhancer has been described as a useful B cell-specific promoter in a LV.(80)
Given that T cells, whether CD4+ or CD8+, are prominent players in pathologic conditions such as viral infection, autoimmunity, and cancer, specific expression of therapeutic genes in T cells has important implications for gene therapy strategies. Using a LV with transgene expression restricted through the CD4 gene promoter and enhancer sequences,(85) expression was restricted to mature T cells. A LV driven by the T lymphocyte-specific proximal lck was also able to restrict expression when injected to mouse embryos.(87) In contrast to transducing HSCs then differentiating them into T cells, another strategy involves direct gene transfer to the target cells. To efficiently transduce T cells with HIV-1-based lentivectors, the central DNA flap of the wild-type virus, which acts as a cis-determinant of HIV-1 nuclear import, is important for efficient gene transfer into prestimulated CD4+, as well as CD8+ human T cells.(86) Stable high level expression of the transgene of interest is a crucial parameter for gene therapy. To enhance the gene expression of LVs in primary T cells, one strategy is to incorporate the CD2 locus control region (LCR) to regulate gene expression in T cells.(84) The use of these vector construction techniques for T cell-based gene therapy of genetic disorders appears very promising.
The technique of driving gene expression using LVs with restricted promoters is also amendable to targeting various antigen-presenting cell types. The 3.2 kb dectin-2 gene promoter fragment has been used to drive gene expression in a vaccine construct.(91) Because of its tissue distribution, the dectin-2 gene has been considered a potentially promising method to restrict gene expression to antigen-presenting cells.(88) Lopes and colleagues demonstrated that lentivectors with gene expression driven by the dectin-2 promoter exhibited restricted distribution to CD11c+ DCs after subcutaneous injection.(91) Additionally, dectin-2 lentivectors encoding the human melanoma antigen NY-ESO-1 stimulated significant CD8+ and CD4+ T cell responses in HLA-A2 transgenic mice.(91) The concept of driving gene expressing using a LV with transcriptional control of a transgene was alternatively implemented with the DC-specific DC-STAMP promoter to transduce HSCs and obtain transgene transcription predominantly in DCs and in some monocytes.(89) When injected into the brain of a mouse, a LV containing the HLA-DRα promoter was able to target a population of intraparenchymal microglia APCs.(96) Another potential application of APC-gene therapy is to prevent an immune response after the infusion of gene-modified autologous stem cells for the treatment of primary hematopoietic diseases, where the transgene had never been present in the patient. Promoter targeting can circumvent the expression of transgenes by APCs after hepatocyte gene therapy in a mouse model.(97) The immune response against the transgene was much lower in the mice injected with a hepto-specific promoter versus an ubiquiotous CMV promoter. Adoptive transfer of transgene-modified APCs or transgene-induced adaptive regulatory T cells together with LVs could induce tolerance to transgene-expressing cells.(98) However, the efficacy of promoter-specific targeting seems to depend on its precise pattern or level of expression.
The administration of LVs carrying tissue-specific promoters should be directed to the affected tissue where it would encounter the appropriate transcriptional regulatory machinery and not incur restricted expression or be silenced by the host cell immune system. This strategy has been proven to be a good method to restrict transgene expression. However, additional convolution in the genome environment where the transgene will be inserted can also restrict expression. The knowledge of LV integration tropism has been advanced enormously and revealed the preferential insertion of the transgene in transcriptionally-active sites of the cell genome. Thus, one could expect some transgene expression could take place due to the transcription of upstream genes, even if the vector contains a tissue-specific promoter.
The use of tissue-specific promoters is a good approach to restrict transgene expression. When combined with additional genetic components such as insulators to shield the promoter from neighboring regulatory elements,(99-101) inducible expression,(77) and use of non-integrating LVs,(102) the safety and controllability of tissue-specific transgene expression can be significantly enhanced leading to better designed clinical vectors.
TARGETING IMMUNE CELLS USING MODIFIED ENVELOPE PROTEINS
The need for efficient and safe gene transfer to immune cells has led to a growing interest in the development of methods for targeting lentivectors to specific target cells and tissues. Development of a high titer lentivector to receptor-specific immune cells is an attractive approach to restrict gene expression and could potentially ensure therapeutic effects in the desired cells while limiting side effects caused by gene expression in non-target cells. Many attempts have been made to develop targetable transduction methods by using LVs.(47-49) Promising targeting methodologies have been developed for these vectors, but despite enticing results, limitations remain.
Lentivectors initiate infection through interactions between their envelope glycoproteins and specific cellular receptors, and this interaction is a critical determinant of viral tropism. The natural budding mechanism of the lentivectors and the plasticity of envelope membrane glycoproteins to be altered allow insertion of ligands, peptides, cytokines, and single-chain antibodies that can direct the vectors to specific cell types.(16, 103-105) One targeting strategy for gene delivery to immune cells attempts to redirect the tropism of the envelope glycoprotein of MLVs by the addition of ligands, which bind to specific molecules associated with the cell membrane.(60, 106) However, this approach has often resulted in relatively inefficient infection because the function of the chimeric envelope protein was compromised to some extent.(107) Likewise, targeting of enveloped lentiviruses using single-chain antibodies fused to the MLV envelope protein has resulted in similar limitations.(55, 93, 108-111) Another strategy involves using “bridging molecules” to target vectors. In order to target ecotropic MLVs by means of MHC class I and class II antigens, antibodies against ecotropic MLV GP are bridged by streptavidin to specific cell membrane markers on the other side.(63) The MLV-modified targeting vector's transduction remains limited by inefficiency due to the diminished fusion activity of the engineered surface protein, which reduces endosomal delivery of the viral capsid into cells. Similar methods have been used to alter the natural tropism of a lentivector surface protein to enhance transduction of MHC-1-expressing cells but have been met with similar difficulties.(112) Another approach circumvents this fusion deficiency by utilizing vectors displaying both MLV glycoproteins fused to activating ligands and VSV-G to enhance HIV-1 lentivector transduction of resting T cell lymphocytes.(58, 113, 114) However, these approaches have had limited success and future attempts must resolve fusion activity while preserving the ligand targeting, thereby producing efficient high titer lentivectors.
Systemic injection of HIV-vectors pseudotyped with various envelope proteins results in predominant transduction of the liver and spleen.(115) HSCs are often used as targets for therapy because of their self-renewal and multi-lineage differentiation capabilities. Although HSCs only represent a small fraction of cells in the bone marrow, they can fully reconstitute all blood cell elements including cells integral to the immune system such as B cells, T cells, and dendritic cells. A system to deliver genes specifically to HSCs would be a powerful tool for engineering novel therapies for the hematopoietic system. To circumvent the need for specific targeting, current strategies depend upon direct injection to a localized site with cell-specific promoters/enhancers or ex vivo isolation, purification, and transduction. While natural viral variants can offer some desirable properties for the transduction of hematopoietic cells, they possess several limitations such as poor specificities and poor transduction of therapeutically relevant cells.(26) Recently, RRV-mediated transduction of human CD34+ cord blood cells and progenitors was very inefficient.(42) Therefore, protein engineering approaches to generate viral vectors with novel and improved gene delivery properties offer attractive means to address these gene delivery problems for HSCs. Interestingly, surface proteins overexpressed by producer cells can be also efficiently incorporated into virion particles during vector production, facilitating novel targeted gene delivery opportunities.(69, 116) However, when both ecotropic MLV glycoproteins and the membrane-bound form of stem cell factor (SCF) were produced, the virus transduced cells in a inverse targeting fashion.(117) The SCF-displaying vectors failed to infect c-kit-positive hematopoietic cells, but efficiently infected c-kit-negative epithelial carcinoma cells. This was because the fusion function of MLV is dependent on the binding of the glycoprotein to the cell. Similarly, another targeting methodology incorporates avidin or streptavidin onto the viral surface along with the gp64 glycoprotein. These vectors conjugated to biotinylated ligands or antibodies can be retargeted to enhance transduction of target cell types.(118) One limitation in using this strategy is that the viral glycoproteins used for fusion retain their binding potential creating high levels of non-targeted infection. However, the fusion function remains intricately linked with the binding of the viral fusogen glycoprotein, causing problems when the binding function is separated from the fusion molecule.
One approach to limit the background infection caused by viral fusogen binding is to create a binding-defective version of the viral fusogen molecule. Cannon and coworkers created a binding-defective version of Fowl Plague Virus Rostock 34 (HAmu). When incorporated into a retrovirus displaying a functionally attenuated envelope glycoprotein targeted to murine Flt-3, HAmu could enhance viral transduction efficiency.(68) HAmu is thought to mediate fusion independent of receptor binding and, when targeted to the Flt-3 receptor, could be useful for vectors directed either to hematopoietic progenitor cells or myeloid leukemias.(47) In another strategy to target lentivectors to immune cells, Chen and coworkers created lentivectors pseudotyped with the Sindbis virus glycoprotein containing the IgG binding domain of protein A (ZZ domain). When combined with a CD4 antibody, the vectors were able to specifically transduce CD4+ lymphocyte subpopulations in human primary peripheral blood mononuclear cells (PBMCs).(44) To target dendritic cells, the natural affinity of the Sindbis virus, which naturally binds to DC-SIGN, was utilized by eliminating the binding to non-specific cellular heparan sulfate molecules, thus restricting SVGmu pseudotyped particles to DCs.(46) Further mutations by Chen and coworkers in the Sindbis glycoprotein made a binding-deficient and fusion competent molecule,(64) which, when combined with a CD34 antibody, produced vectors which specifically transduced CD34+ cells in nonpurified human mobilized PBMCs.(119) We adapted this form of Sindbis glycoprotein but decoupled the antibody binding domain and instead separated the binding and fusion functions into two separate molecules that are inserted into the viral envelope.(69) With the addition of membrane-bound anti-CD20 antibody, vector particles conferred their binding specificity to cells expressing the B cell marker CD20.(69, 120, 121) The efficiency was further enhanced by engineering several mutant forms of the Sindbis fusogen which exhibited elevated fusion functions in a pH-dependent manner.(121) This system was further expanded to deliver genes to monospecific immunoglobulin-expressing B cells,(122) CD3-positive T-cells,(123) and CD117-expressing HSCs.(124) In another approach to target CD20 human primary B lymphocytes, the measles virus binding (H protein) and fusion (F protein) functions were divided and the viral glycoprotein was retargeted using a single-chain antibody fused to the mutant binding-deficient H protein.(125) Such lentivectors re-targeted to specific immune cells are an attractive vehicle for targeted gene delivery and could potentially ensure therapeutic effects in the desired cells, while limiting side effects caused by gene expression in non-target cells.
CONCLUSION
Several promising targeting methodologies have been developed for lentivectors to modify immune cells (see Table for the summary). Although this review has focused largely on the final step of achieving targeted gene expression, other aspects are equally important. These hurdles include high costs of vector production for clinical use, immune system barriers (antibodies, complement system), and questions of systemic application and dosage.(49) However, despite these hurdles, the enticing results and the promise of cures for previously incurable diseases warrant further studies and clinical consideration.
Table 1.
Targeting of Lentiviral vectors to immune cells using amendable strategies by modifying the envelope targeting or by transcriptional targeting.
| Target cells | Envelope Targeting References | Transcriptional Targeting References |
|---|---|---|
| Antigen Presenting Cells | (46, 108) | (88-94) |
| T Cells | (44, 58, 106, 113, 123) | (84-87) |
| B Cells | (69, 120-122, 125) | (79-83) |
| Hematopoietic Stem Cells | (68, 107, 114, 117, 119, 124) | (126) |
| Macrophage/Other | (60, 63, 110-112) | (127, 128) |
REFERENCES
- 1.Anderson WF, Blaese RM, Culver K. The ADA human gene therapy clinical protocol: Points to Consider response with clinical protocol, July 6, 1990. Hum. Gene Ther. 1990;1(3):331–362. doi: 10.1089/hum.1990.1.3-331. [DOI] [PubMed] [Google Scholar]
- 2.Thrasher AJ, Gaspar HB, Baum C, Modlich U, Schambach A, Candotti F, Otsu M, Sorrentino B, Scobie L, Cameron E, Blyth K, Neil J, Abina SH, Cavazzana-Calvo M, Fischer A. Gene therapy: X-SCID transgene leukaemogenicity. Nature. 2006;443(7109):E5–E7. doi: 10.1038/nature05219. [DOI] [PubMed] [Google Scholar]
- 3.Dass CR. Biochemical and biophysical characteristics of lipoplexes pertinent to solid tumour gene therapy. Int. J. Pharm. 2002;241(1):1–25. doi: 10.1016/s0378-5173(02)00194-1. [DOI] [PubMed] [Google Scholar]
- 4.Gardlik R, Palffy R, Hodosy J, Lukacs J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med. Sci. Monit. 2005;11(4):110–121. [PubMed] [Google Scholar]
- 5.Forrest ML, Pack DW. On the kinetics of polyplex endocytic trafficking: implications for gene delivery vector design. Mol. Ther. 2002;6(1):57–66. doi: 10.1006/mthe.2002.0631. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007--an update. J. Gene Med. 2007;9(10):833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- 7.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6(9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 8.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, Vogels R, Bakker M, Berkhout B, Havenga M, Goudsmit J. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS. 2004;18(8):1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- 9.Muruve DA. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15(12):1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 10.Howitt J, Anderson CW, Freimuth P. Adenovirus interaction with its cellular receptor CAR. Curr. Top. Microbiol. Immunol. 2003;272:331–364. doi: 10.1007/978-3-662-05597-7_11. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Arica JR, Thomson AJ, Ansell R, Chiorini J, Davidson B, McWhir J. Infection efficiency of human and mouse embryonic stem cells using adenoviral and adeno-associated viral vectors. Cloning Stem Cells. 2003;5(1):51–62. doi: 10.1089/153623003321512166. [DOI] [PubMed] [Google Scholar]
- 12.Clemens PR, Kochanek S, Sunada Y, Chan S, Chen HH, Campbell KP, Caskey CT. In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Ther. 1996;3(11):965–972. [PubMed] [Google Scholar]
- 13.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther. 1996;7(17):2101–2212. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 14.Shenk TE. Adenoviridae: the viruses and their replication. In: Virology Fields, Knipe David M., Griffin Diane E., Martin Malcolm A., Lamb Robert A., editors. Lippincott Williams & Wilkins; New York: 2006. pp. 2111–2148. [Google Scholar]
- 15.Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press; Woodbury, NY: 1997. [PubMed] [Google Scholar]
- 16.Schaffer DV, Koerber JT, Lim KI. Molecular engineering of viral gene delivery vehicles. Annu. Rev. Biomed. Eng. 2008;10:169–194. doi: 10.1146/annurev.bioeng.10.061807.160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 18.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11(8):3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg JB, Matthews TJ, Cullen BR, Malim MH. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J. Exp. Med. 1991;174(6):1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Case SS, Price MA, Jordan CT, Yu XJ, Wang L, Bauer G, Haas DL, Xu D, Stripecke R, Naldini L, Kohn DB, Crooks GM. Stable transduction of quiescent CD34(+)CD38(-) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc. Natl. Acad. Sci. USA. 1999;96(6):2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA. 1996;93(21):11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Palma M, Montini E, Santoni de Sio FR, Benedicenti F, Gentile A, Medico E, Naldini L. Promoter trapping reveals significant differences in integration site selection between MLV and HIV vectors in primary hematopoietic cells. Blood. 2005;105(6):2307–2315. doi: 10.1182/blood-2004-03-0798. [DOI] [PubMed] [Google Scholar]
- 23.Frecha C, Szecsi J, Cosset FL, Verhoeyen E. Strategies for targeting lentiviral vectors. Curr. Gene Ther. 2008;8(6):449–460. doi: 10.2174/156652308786848003. [DOI] [PubMed] [Google Scholar]
- 24.Zaiss AK, Son S, Chang LJ. RNA 3′ readthrough of oncoretrovirus and lentivirus: implications for vector safety and efficacy. J. Virol. 2002;76(14):7209–7219. doi: 10.1128/JVI.76.14.7209-7219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emery DW, Yannaki E, Tubb J, Nishino T, Li Q, Stamatoyannopoulos G. Development of virus vectors for gene therapy of beta chain hemoglobinopathies: flanking with a chromatin insulator reduces gamma-globin gene silencing in vivo. Blood. 2002;100(6):2012–2019. doi: 10.1182/blood-2002-01-0219. [DOI] [PubMed] [Google Scholar]
- 26.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 2005;5(4):387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carneiro FA, Lapido-Loureiro PA, Cordo SM, Stauffer F, Weissmuller G, Bianconi ML, Juliano MA, Juliano L, Bisch PM, Da Poian AT. Probing the interaction between vesicular stomatitis virus and phosphatidylserine. Eur. Biophys. J. 2006;35(2):145–154. doi: 10.1007/s00249-005-0012-z. [DOI] [PubMed] [Google Scholar]
- 28.Coil DA, Miller AD. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J. Virol. 2004;78(20):10920–10926. doi: 10.1128/JVI.78.20.10920-10926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coil DA, Miller AD. Enhancement of enveloped virus entry by phosphatidylserine. J. Virol. 2005;79(17):11496–11500. doi: 10.1128/JVI.79.17.11496-11500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol. Ther. 2002;5(5 Pt 1):528–537. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]
- 31.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA. 1996;93(21):11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ, Dubensky TW., Jr. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2000;2(3):218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- 33.Schaffer DV, Koerber JT, Lim KI. Molecular engineering of viral gene delivery vehicles. Annu. Rev. Biomed. Eng. 2008;10:169–194. doi: 10.1146/annurev.bioeng.10.061807.160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanawa H, Kelly PF, Nathwani AC, Persons DA, Vandergriff JA, Hargrove P, Vanin EF, Nienhuis AW. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 2002;5(3):242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- 35.Kelly PF, Vandergriff J, Nathwani A, Nienhuis AW, Vanin EF. Highly efficient gene transfer into cord blood nonobese diabetic/severe combined immunodeficiency repopulating cells by oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. Blood. 2000;96(4):1206–1214. [PubMed] [Google Scholar]
- 36.Porter CD, Collins MK, Tailor CS, Parkar MH, Cosset FL, Weiss RA, Takeuchi Y. Comparison of efficiency of infection of human gene therapy target cells via four different retroviral receptors. Hum. Gene Ther. 1996;7(8):913–919. doi: 10.1089/hum.1996.7.8-913. [DOI] [PubMed] [Google Scholar]
- 37.Sandrin V, Boson B, Salmon P, Gay W, Negre D, Le Grand R, Trono D, Cosset FL. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100(3):823–832. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- 38.Jakobsson J, Lundberg C. Lentiviral vectors for use in the central nervous system. Mol. Ther. 2006;13(3):484–493. doi: 10.1016/j.ymthe.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Wong LF, Azzouz M, Walmsley LE, Askham Z, Wilkes FJ, Mitrophanous KA, Kingsman SM, Mazarakis ND. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol. Ther. 2004;9(1):101–111. doi: 10.1016/j.ymthe.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 40.Kahl CA, Pollok K, Haneline LS, Cornetta K. Lentiviral vectors pseudotyped with glycoproteins from Ross River and vesicular stomatitis viruses: variable transduction related to cell type and culture conditions. Mol. Ther. 2005;11(3):470–482. doi: 10.1016/j.ymthe.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 41.Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 2003;77(22):12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahl CA, Marsh J, Fyffe J, Sanders DA, Cornetta K. Human immunodeficiency virus type 1-derived lentivirus vectors pseudotyped with envelope glycoproteins derived from Ross River virus and Semliki Forest virus. J. Virol. 2004;78(3):1421–1430. doi: 10.1128/JVI.78.3.1421-1430.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD, Ratliff KL, Shen H, Barker CK, Martins I, Sharkey CM, Sanders DA, McCray PB, Jr., Davidson BL. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J. Virol. 2002;76(18):9378–9388. doi: 10.1128/JVI.76.18.9378-9388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morizono K, Bristol G, Xie YM, Kung SK, Chen IS. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 2001;75(17):8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner JP, Frolov I, Perri S, Ji Y, MacKichan ML, zur Megede J, Chen M, Belli BA, Driver DA, Sherrill S, Greer CE, Otten GR, Barnett SW, Liu MA, Dubensky TW, Polo JM. Infection of human dendritic cells by a sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 2000;74(24):11849–11857. doi: 10.1128/jvi.74.24.11849-11857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, Wang P. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 2008;26(3):326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavillette D, Russell SJ, Cosset FL. Retargeting gene delivery using surface-engineered retroviral vector particles. Curr. Opin. Biotechnol. 2001;12(5):461–466. doi: 10.1016/s0958-1669(00)00246-9. [DOI] [PubMed] [Google Scholar]
- 48.Sandrin V, Russell SJ, Cosset FL. Targeting retroviral and lentiviral vectors. Curr. Top. Microbiol. Immunol. 2003;281:137–178. doi: 10.1007/978-3-642-19012-4_4. [DOI] [PubMed] [Google Scholar]
- 49.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007;8(8):573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gollan TJ, Green MR. Redirecting retroviral tropism by insertion of short, nondisruptive peptide ligands into envelope. J. Virol. 2002;76(7):3558–3563. doi: 10.1128/JVI.76.7.3558-3563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han X, Kasahara N, Kan YW. Ligand-directed retroviral targeting of human breast cancer cells. Proc. Natl. Acad. Sci. USA. 1995;92(21):9747–9751. doi: 10.1073/pnas.92.21.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valsesia-Wittmann S, Drynda A, Deleage G, Aumailley M, Heard JM, Danos O, Verdier G, Cosset FL. Modifications in the binding domain of avian retrovirus envelope protein to redirect the host range of retroviral vectors. J. Virol. 1994;68(7):4609–4619. doi: 10.1128/jvi.68.7.4609-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guibinga GH, Hall FL, Gordon EM, Ruoslahti E, Friedmann T. Ligand-modified vesicular stomatitis virus glycoprotein displays a temperature-sensitive intracellular trafficking and virus assembly phenotype. Mol. Ther. 2004;9(1):76–84. doi: 10.1016/j.ymthe.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Somia NV, Zoppe M, Verma IM. Generation of targeted retroviral vectors by using single-chain variable fragment: an approach to in vivo gene delivery. Proc. Natl. Acad. Sci. USA. 1995;92(16):7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benedict CA, Tun RY, Rubinstein DB, Guillaume T, Cannon PM, Anderson WF. Targeting retroviral vectors to CD34-expressing cells: binding to CD34 does not catalyze virus-cell fusion. Hum. Gene Ther. 1999;10(4):545–557. doi: 10.1089/10430349950018625. [DOI] [PubMed] [Google Scholar]
- 56.Jiang A, Chu TH, Nocken F, Cichutek K, Dornburg R. Cell-type-specific gene transfer into human cells with retroviral vectors that display single-chain antibodies. J. Virol. 1998;72(12):10148–10156. doi: 10.1128/jvi.72.12.10148-10156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin F, Chowdhury S, Neil S, Phillipps N, Collins MK. Envelope-targeted retrovirus vectors transduce melanoma xenografts but not spleen or liver. Mol. Ther. 2002;5(3):269–274. doi: 10.1006/mthe.2002.0550. [DOI] [PubMed] [Google Scholar]
- 58.Maurice M, Verhoeyen E, Salmon P, Trono D, Russell SJ, Cosset FL. Efficient gene transfer into human primary blood lymphocytes by surface-engineered lentiviral vectors that display a T cell-activating polypeptide. Blood. 2002;99(7):2342–2350. doi: 10.1182/blood.v99.7.2342. [DOI] [PubMed] [Google Scholar]
- 59.Chadwick MP, Morling FJ, Cosset FL, Russell SJ. Modification of retroviral tropism by display of IGF-I. J. Mol. Biol. 1999;285(2):485–494. doi: 10.1006/jmbi.1998.2350. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen TH, Pages JC, Farge D, Briand P, Weber A. Amphotropic retroviral vectors displaying hepatocyte growth factor-envelope fusion proteins improve transduction efficiency of primary hepatocytes. Hum. Gene Ther. 1998;9(17):2469–2479. doi: 10.1089/hum.1998.9.17-2469. [DOI] [PubMed] [Google Scholar]
- 61.Snitkovsky S, Young JA. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc. Natl. Acad. Sci. USA. 1998;95(12):7063–7068. doi: 10.1073/pnas.95.12.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boerger AL, Snitkovsky S, Young JA. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc. Natl. Acad. Sci. USA. 1999;96(17):9867–9872. doi: 10.1073/pnas.96.17.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roux P, Jeanteur P, Piechaczyk M. A versatile and potentially general approach to the targeting of specific cell types by retroviruses: application to the infection of human cells by means of major histocompatibility complex class I and class II antigens by mouse ecotropic murine leukemia virus-derived viruses. Proc. Natl. Acad. Sci. USA. 1989;86(23):9079–9083. doi: 10.1073/pnas.86.23.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 2005;11(3):346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 65.Somia NV, Miyoshi H, Schmitt MJ, Verma IM. Retroviral vector targeting to human immunodeficiency virus type 1-infected cells by receptor pseudotyping. J. Virol. 2000;74(9):4420–4424. doi: 10.1128/jvi.74.9.4420-4424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Endres MJ, Jaffer S, Haggarty B, Turner JD, Doranz BJ, O'Brien PJ, Kolson DL, Hoxie JA. Targeting of HIV- and SIV-infected cells by CD4-chemokine receptor pseudotypes. Science. 1997;278(5342):1462–1464. doi: 10.1126/science.278.5342.1462. [DOI] [PubMed] [Google Scholar]
- 67.Lamb RA. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197(1):1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 68.Lin AH, Kasahara N, Wu W, Stripecke R, Empig CL, Anderson WF, Cannon PM. Receptor-specific targeting mediated by the coexpression of a targeted murine leukemia virus envelope protein and a binding-defective influenza hemagglutinin protein. Hum. Gene Ther. 2001;12(4):323–332. doi: 10.1089/10430340150503957. [DOI] [PubMed] [Google Scholar]
- 69.Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. USA. 2006;103(31):11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 71.Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, Morecki S, Andolfi G, Tabucchi A, Carlucci F, Marinello E, Cattaneo F, Vai S, Servida P, Miniero R, Roncarolo MG, Bordignon C. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296(5577):2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 72.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, Glimm H, Kuhlcke K, Schilz A, Kunkel H, Naundorf S, Brinkmann A, Deichmann A, Fischer M, Ball C, Pilz I, Dunbar C, Du Y, Jenkins NA, Copeland NG, Luthi U, Hassan M, Thrasher AJ, Hoelzer D, von Kalle C, Seger R, Grez M. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12(4):401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 73.Bushman F, Lewinski M, Ciuffi A, Barr S, Leipzig J, Hannenhalli S, Hoffmann C. Genome-wide analysis of retroviral DNA integration. Nat. Rev. Microbiol. 2005;3(11):848–858. doi: 10.1038/nrmicro1263. [DOI] [PubMed] [Google Scholar]
- 74.Cavazzana-Calvo M, Fischer A. Gene therapy for severe combined immunodeficiency: are we there yet? J. Clin. Invest. 2007;117(6):1456–1465. doi: 10.1172/JCI30953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 76.Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, Brouns G, Schmidt M, Von Kalle C, Barington T, Jakobsen MA, Christensen HO, Al Ghonaium A, White HN, Smith JL, Levinsky RJ, Ali RR, Kinnon C, Thrasher AJ. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364(9452):2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 77.Goverdhana S, Puntel M, Xiong W, Zirger JM, Barcia C, Curtin JF, Soffer EB, Mondkar S, King GD, Hu J, Sciascia SA, Candolfi M, Greengold DS, Lowenstein PR, Castro MG. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol. Ther. 2005;12(2):189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frecha C, Szecsi J, Cosset FL, Verhoeyen E. Strategies for targeting lentiviral vectors. Curr. Gene Ther. 2008;8(6):449–460. doi: 10.2174/156652308786848003. [DOI] [PubMed] [Google Scholar]
- 79.Moreau T, Bardin F, Imbert J, Chabannon C, Tonnelle C. Restriction of transgene expression to the B-lymphoid progeny of human lentivirally transduced CD34+ cells. Mol. Ther. 2004;10(1):45–56. doi: 10.1016/j.ymthe.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 80.Laurie KL, Blundell MP, Baxendale HE, Howe SJ, Sinclair J, Qasim W, Brunsberg U, Thrasher AJ, Holmdahl R, Gustafsson K. Cell-specific and efficient expression in mouse and human B cells by a novel hybrid immunoglobulin promoter in a lentiviral vector. Gene Ther. 2007;14(23):1623–1631. doi: 10.1038/sj.gt.3303021. [DOI] [PubMed] [Google Scholar]
- 81.Taher TE, Tulone C, Fatah R, D'Acquisto F, Gould DJ, Mageed RA. Repopulation of B-lymphocytes with restricted gene expression using haematopoietic stem cells engineered with lentiviral vectors. Gene Ther. 2008;15(13):998–1006. doi: 10.1038/gt.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lutzko C, Senadheera D, Skelton D, Petersen D, Kohn DB. Lentivirus vectors incorporating the immunoglobulin heavy chain enhancer and matrix attachment regions provide position-independent expression in B lymphocytes. J. Virol. 2003;77(13):7341–7351. doi: 10.1128/JVI.77.13.7341-7351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Werner M, Kraunus J, Baum C, Brocker T. B-cell-specific transgene expression using a self-inactivating retroviral vector with human CD19 promoter and viral post-transcriptional regulatory element. Gene Ther. 2004;11(12):992–1000. doi: 10.1038/sj.gt.3302255. [DOI] [PubMed] [Google Scholar]
- 84.Indraccolo S, Minuzzo S, Roccaforte F, Zamarchi R, Habeler W, Stievano L, Tosello V, Klein D, Gunzburg WH, Basso G, Chieco-Bianchi L, Amadori A. Effects of CD2 locus control region sequences on gene expression by retroviral and lentiviral vectors. Blood. 2001;98(13):3607–3617. doi: 10.1182/blood.v98.13.3607. [DOI] [PubMed] [Google Scholar]
- 85.Marodon G, Mouly E, Blair EJ, Frisen C, Lemoine FM, Klatzmann D. Specific transgene expression in human and mouse CD4+ cells using lentiviral vectors with regulatory sequences from the CD4 gene. Blood. 2003;101(9):3416–3423. doi: 10.1182/blood-2002-02-0578. [DOI] [PubMed] [Google Scholar]
- 86.Dardalhon V, Herpers B, Noraz N, Pflumio F, Guetard D, Leveau C, Dubart-Kupperschmitt A, Charneau P, Taylor N. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 2001;8(3):190–198. doi: 10.1038/sj.gt.3301378. [DOI] [PubMed] [Google Scholar]
- 87.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 88.Bonkobara M, Zukas PK, Shikano S, Nakamura S, Cruz PD, Jr., Ariizumi K. Epidermal Langerhans cell-targeted gene expression by a dectin-2 promoter. J. Immunol. 2001;167(12):6893–6900. doi: 10.4049/jimmunol.167.12.6893. [DOI] [PubMed] [Google Scholar]
- 89.Dresch C, Edelmann SL, Marconi P, Brocker T. Lentiviral-mediated transcriptional targeting of dendritic cells for induction of T cell tolerance in vivo. J. Immunol. 2008;181(7):4495–4506. doi: 10.4049/jimmunol.181.7.4495. [DOI] [PubMed] [Google Scholar]
- 90.Kozmik Z, Czerny T, Busslinger M. Alternatively spliced insertions in the paired domain restrict the DNA sequence specificity of Pax6 and Pax8. EMBO J. 1997;16(22):6793–6803. doi: 10.1093/emboj/16.22.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopes L, Dewannieux M, Gileadi U, Bailey R, Ikeda Y, Whittaker C, Collin MP, Cerundolo V, Tomihari M, Ariizumi K, Collins MK. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J. Virol. 2008;82(1):86–95. doi: 10.1128/JVI.01289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, Mule JJ, McDonagh KT, Fox DA. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J. Clin. Invest. 2001;107(10):1275–1284. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ageichik A, Collins MK, Dewannieux M. Lentivector targeting to dendritic cells. Mol. Ther. 2008;16(6):1008–1009. doi: 10.1038/mt.2008.95. [DOI] [PubMed] [Google Scholar]
- 94.Cui Y, Golob J, Kelleher E, Ye Z, Pardoll D, Cheng L. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood. 2002;99(2):399–408. doi: 10.1182/blood.v99.2.399. [DOI] [PubMed] [Google Scholar]
- 95.Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110(4):521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 96.Lesniak MS, Kelleher E, Pardoll D, Cui Y. Targeted gene therapy to antigen-presenting cells in the central nervous system using hematopoietic stem cells. Neurol. Res. 2005;27(8):820–826. doi: 10.1179/016164105X49454. [DOI] [PubMed] [Google Scholar]
- 97.Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG, Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103(10):3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- 98.Annoni A, Battaglia M, Follenzi A, Lombardo A, Sergi-Sergi L, Naldini L, Roncarolo MG. The immune response to lentiviral-delivered transgene is modulated in vivo by transgene-expressing antigen-presenting cells but not by CD4+CD25+ regulatory T cells. Blood. 2007;110(6):1788–1796. doi: 10.1182/blood-2006-11-059873. [DOI] [PubMed] [Google Scholar]
- 99.Prioleau MN, Nony P, Simpson M, Felsenfeld G. An insulator element and condensed chromatin region separate the chicken beta-globin locus from an independently regulated erythroid-specific folate receptor gene. EMBO J. 1999;18(14):4035–4048. doi: 10.1093/emboj/18.14.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Schambach A, Sinclair J, Kinnon C, Gaspar HB, Antoniou M, Thrasher AJ. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110(5):1448–1457. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Recillas-Targa F, Valadez-Graham V, Farrell CM. Prospects and implications of using chromatin insulators in gene therapy and transgenesis. Bioessays. 2004;26(7):796–807. doi: 10.1002/bies.20059. [DOI] [PubMed] [Google Scholar]
- 102.Negri DR, Michelini Z, Baroncelli S, Spada M, Vendetti S, Buffa V, Bona R, Leone P, Klotman ME, Cara A. Successful immunization with a single injection of non-integrating lentiviral vector. Mol. Ther. 2007;15(9):1716–1723. doi: 10.1038/sj.mt.6300241. [DOI] [PubMed] [Google Scholar]
- 103.Gollan TJ, Green MR. Redirecting retroviral tropism by insertion of short, nondisruptive peptide ligands into envelope. J. Virol. 2002;76(7):3558–3563. doi: 10.1128/JVI.76.7.3558-3563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kueng HJ, Leb VM, Haiderer D, Raposo G, Thery C, Derdak SV, Schmetterer KG, Neunkirchner A, Sillaber C, Seed B, Pickl WF. General strategy for decoration of enveloped viruses with functionally active lipid-modified cytokines. J. Virol. 2007;81(16):8666–8676. doi: 10.1128/JVI.00682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 2005;11(3):346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 106.Maurice M, Mazur S, Bullough FJ, Salvetti A, Collins MK, Russell SJ, Cosset FL. Efficient gene delivery to quiescent interleukin-2 (IL-2)-dependent cells by murine leukemia virus-derived vectors harboring IL-2 chimeric envelope glycoproteins. Blood. 1999;94(2):401–410. [PubMed] [Google Scholar]
- 107.Fielding AK, Maurice M, Morling FJ, Cosset FL, Russell SJ. Inverse targeting of retroviral vectors: selective gene transfer in a mixed population of hematopoietic and nonhematopoietic cells. Blood. 1998;91(5):1802–1809. [PubMed] [Google Scholar]
- 108.Gennari F, Lopes L, Verhoeyen E, Marasco W, Collins MK. Single-chain antibodies that target lentiviral vectors to MHC class II on antigen-presenting cells. Hum. Gene Ther. 2009;20(6):554–562. doi: 10.1089/hum.2008.189. [DOI] [PubMed] [Google Scholar]
- 109.Karavanas G, Marin M, Bachrach E, Papavassiliou AG, Piechaczyk M. The insertion of an anti-MHC I ScFv into the N-terminus of an ecotropic MLV glycoprotein does not alter its fusiogenic potential on murine cells. Virus Res. 2002;83(1-2):57–69. doi: 10.1016/s0168-1702(01)00419-1. [DOI] [PubMed] [Google Scholar]
- 110.Marin M, Noel D, Valsesia-Wittman S, Brockly F, Etienne-Julan M, Russell S, Cosset FL, Piechaczyk M. Targeted infection of human cells via major histocompatibility complex class I molecules by Moloney murine leukemia virus-derived viruses displaying single-chain antibody fragment-envelope fusion proteins. J. Virol. 1996;70(5):2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chowdhury S, Chester KA, Bridgewater J, Collins MK, Martin F. Efficient retroviral vector targeting of carcinoembryonic antigen-positive tumors. Mol. Ther. 2004;9(1):85–92. doi: 10.1016/j.ymthe.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 112.Dreja H, Piechaczyk M. The effects of N-terminal insertion into VSV-G of an scFv peptide. Virol. J. 2006;3:69. doi: 10.1186/1743-422X-3-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verhoeyen E, Dardalhon V, Ducrey-Rundquist O, Trono D, Taylor N, Cosset FL. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood. 2003;101(6):2167–2174. doi: 10.1182/blood-2002-07-2224. [DOI] [PubMed] [Google Scholar]
- 114.Verhoeyen E, Wiznerowicz M, Olivier D, Izac B, Trono D, Dubart-Kupperschmitt A, Cosset FL. Novel lentiviral vectors displaying “early-acting cytokines” selectively promote survival and transduction of NOD/SCID repopulating human hematopoietic stem cells. Blood. 2005;106(10):3386–3395. doi: 10.1182/blood-2004-12-4736. [DOI] [PubMed] [Google Scholar]
- 115.Pan D, Gunther R, Duan W, Wendell S, Kaemmerer W, Kafri T, Verma IM, Whitley CB. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol. Ther. 2002;6(1):19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
- 116.Kueng HJ, Leb VM, Haiderer D, Raposo G, Thery C, Derdak SV, Schmetterer KG, Neunkirchner A, Sillaber C, Seed B, Pickl WF. General strategy for decoration of enveloped viruses with functionally active lipid-modified cytokines. J. Virol. 2007;81(16):8666–8676. doi: 10.1128/JVI.00682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chandrashekran A, Gordon MY, Casimir C. Targeted retroviral transduction of c-kit+ hematopoietic cells using novel ligand display technology. Blood. 2004;104(9):2697–2703. doi: 10.1182/blood-2003-10-3717. [DOI] [PubMed] [Google Scholar]
- 118.Kaikkonen MU, Lesch HP, Pikkarainen J, Raty JK, Vuorio T, Huhtala T, Taavitsainen M, Laitinen T, Tuunanen P, Grohn O, Narvanen A, Airenne KJ, Yla-Herttuala S. (Strept)avidin-displaying lentiviruses as versatile tools for targeting and dual imaging of gene delivery. Gene Ther. 2009;16(7):894–904. doi: 10.1038/gt.2009.47. [DOI] [PubMed] [Google Scholar]
- 119.Liang M, Pariente N, Morizono K, Chen IS. Targeted transduction of CD34+ hematopoietic progenitor cells in nonpurified human mobilized peripheral blood mononuclear cells. J. Gene Med. 2009;11(3):185–196. doi: 10.1002/jgm.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang H, Ziegler L, Joo KI, Cho T, Lei Y, Wang P. Gamma-retroviral vectors enveloped with an antibody and an engineered fusogenic protein achieved antigen-specific targeting. Biotechnol. Bioeng. 2008;101(2):357–368. doi: 10.1002/bit.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lei Y, Joo KI, Wang P. Engineering fusogenic molecules to achieve targeted transduction of enveloped lentiviral vectors. J. Biol. Eng. 2009;3:8. doi: 10.1186/1754-1611-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ziegler L, Yang L, Joo K, Yang H, Baltimore D, Wang P. Targeting lentiviral vectors to antigen-specific immunoglobulins. Hum. Gene Ther. 2008;19(9):861–872. doi: 10.1089/hum.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang H, Joo KI, Ziegler L, Wang P. Cell type-specific targeting with surface-engineered lentiviral vectors co-displaying OKT3 antibody and fusogenic molecule. Pharm. Res. 2009;26(6):1432–1445. doi: 10.1007/s11095-009-9853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Froelich S, Ziegler L, Stroup K, Wang P. Targeted gene delivery to CD117-expressing cells in vivo with lentiviral vectors co-displaying stem cell factor and a fusogenic molecule. Biotechnol. Bioeng. 2009;104(1):206–215. doi: 10.1002/bit.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Funke S, Maisner A, Muhlebach MD, Koehl U, Grez M, Cattaneo R, Cichutek K, Buchholz CJ. Targeted cell entry of lentiviral vectors. Mol Ther. 2008;16(8):1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Logan AC, Lutzko C, Kohn DB. Advances in lentiviral vector design for gene-modification of hematopoietic stem cells. Curr. Opin. Biotechnol. 2002;13(5):429–436. doi: 10.1016/s0958-1669(02)00346-4. [DOI] [PubMed] [Google Scholar]
- 127.Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, Acharya SA, Ellis J, London IM, Eaves CJ, Humphries RK, Beuzard Y, Nagel RL, Leboulch P. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294(5550):2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- 128.Lavenu-Bombled C, Izac B, Legrand F, Cambot M, Vigier A, Masse JM, Dubart-Kupperschmitt A. Glycoprotein Ibalpha promoter drives megakaryocytic lineage-restricted expression after hematopoietic stem cell transduction using a self-inactivating lentiviral vector. Stem Cells. 2007;25(6):1571–1577. doi: 10.1634/stemcells.2006-0321. [DOI] [PubMed] [Google Scholar]