Abstract
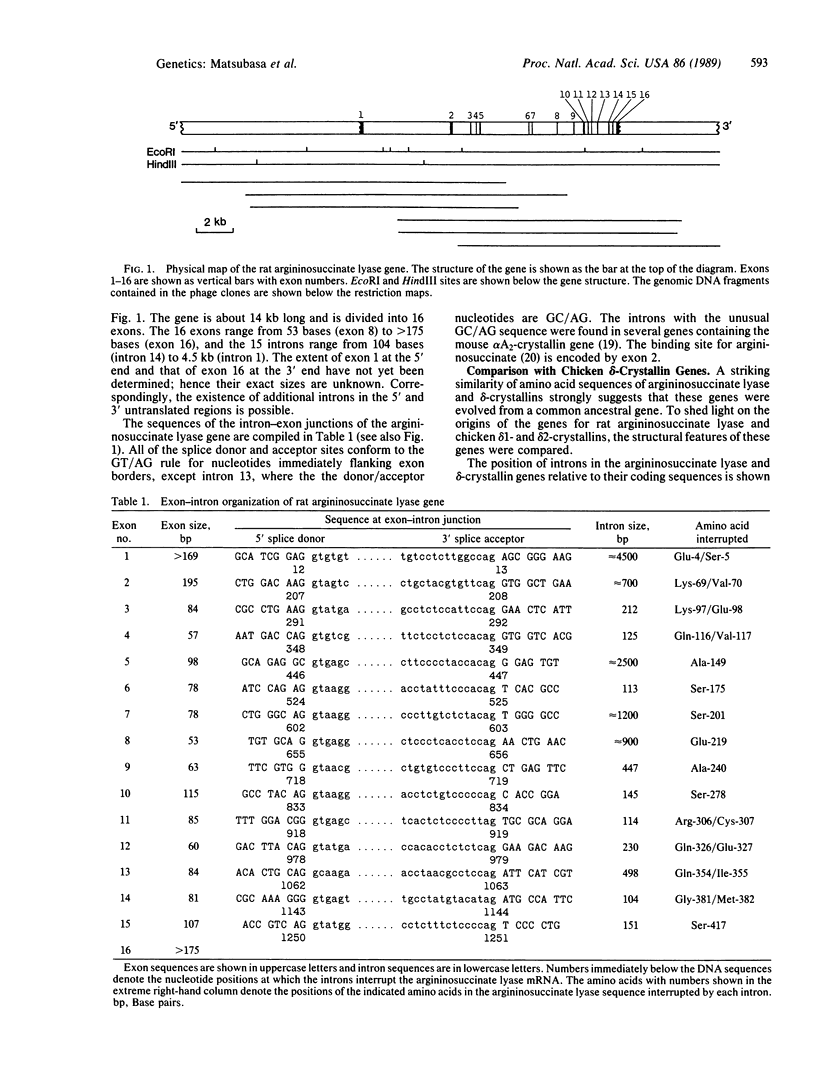
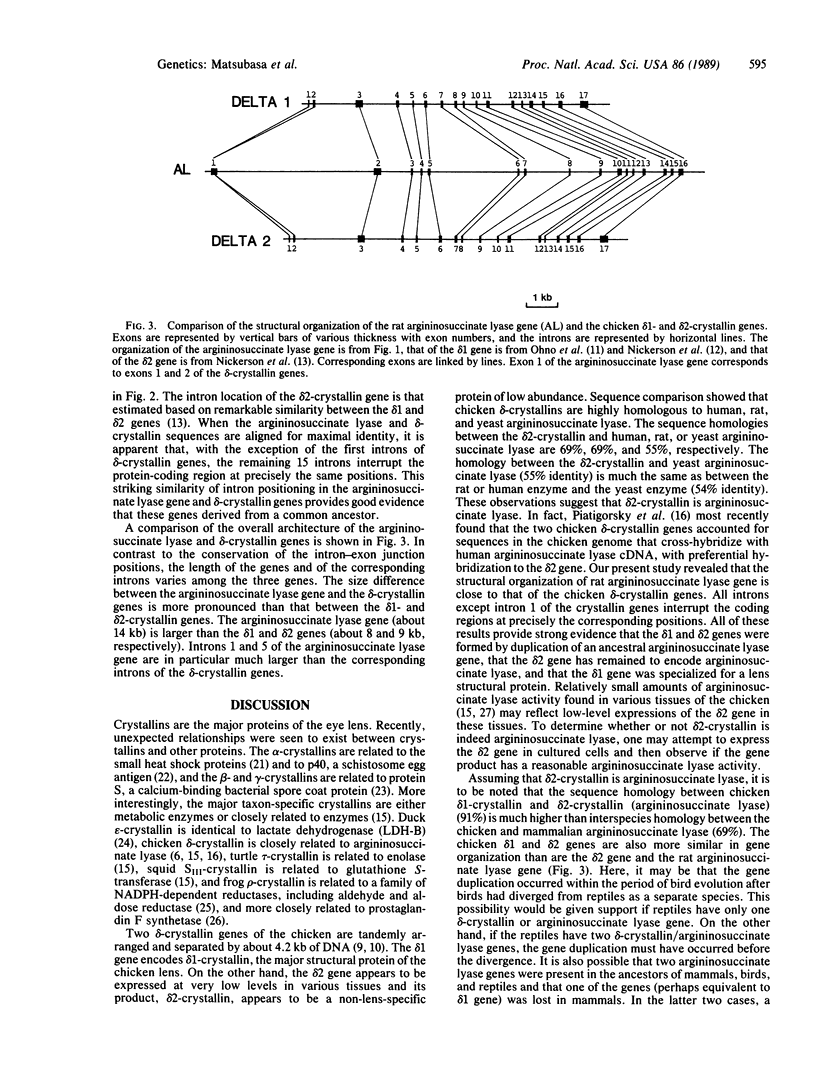
Argininosuccinate lyase (EC 4.3.2.1) is an enzyme of arginine biosynthesis and is also involved in the urea cycle in the liver of ureotelic animals. A comparison of cDNA-derived amino acid sequences revealed that argininosuccinate lyase is highly homologous with chicken delta-crystallin, a major structural protein of the eye lens. The gene for the rat argininosuccinate lyase was cloned and its structure was determined. This gene is a single-copy gene about 14 kilobases long and is split into 16 exons. A comparison with chicken delta-crystallin genes revealed that all introns interrupt the protein-coding regions at homologous positions. This close similarity in structural organization provides strong evidence for the view of Piatigorsky et al. [Piatigorsky, J., O'Brien, W. E., Norman, B. L., Kalmuck, K., Wistow, G., Borras, T., Nickerson, J. M. & Wawrousek, E. F. (1988) Proc. Natl. Acad. Sci. USA 85, 3479-3483] that chicken delta 1- and delta 2-crystallin genes evolved by recruitment and duplication of the preexisting argininosuccinate lyase gene and that delta 2-crystallin is probably the direct homologue argininosuccinate lyase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya Y., Kawamoto S., Oda T., Kuzumi T., Saheki T., Kimura S., Mori M. Molecular cloning of cDNA for argininosuccinate lyase of rat liver. Biochem Int. 1986 Sep;13(3):433–438. [PubMed] [Google Scholar]
- Amaya Y., Matsubasa T., Takiguchi M., Kobayashi K., Saheki T., Kawamoto S., Mori M. Amino acid sequence of rat argininosuccinate lyase deduced from cDNA. J Biochem. 1988 Jan;103(1):177–181. doi: 10.1093/oxfordjournals.jbchem.a122227. [DOI] [PubMed] [Google Scholar]
- Borrás T., Nickerson J. M., Chepelinsky A. B., Piatigorsky J. Structural and functional evidence for differential promoter activity of the two linked delta-crystallin genes in the chicken. EMBO J. 1985 Feb;4(2):445–452. doi: 10.1002/j.1460-2075.1985.tb03649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper D., Nishimura C., Shinohara T., Dietzchold B., Wistow G., Craft C., Kador P., Kinoshita J. H. Aldose reductase and p-crystallin belong to the same protein superfamily as aldehyde reductase. FEBS Lett. 1987 Aug 10;220(1):209–213. doi: 10.1016/0014-5793(87)80905-5. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hawkins J. W., Nickerson J. M., Sullivan M. A., Piatigorsky J. The chicken delta-crystallin gene family. Two genes of similar structure in close chromosomal approximation. J Biol Chem. 1984 Aug 10;259(15):9821–9825. [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Ratner S. Biosynthesis of urea. XIV. The quaternary structure of argininosuccinase. J Biol Chem. 1972 Nov 10;247(21):7010–7022. [PubMed] [Google Scholar]
- Lusty C. J., Ratner S. Reaction of argininosuccinase with bromomesaconic acid: role of an essential lysine in the active site. Proc Natl Acad Sci U S A. 1987 May;84(10):3176–3180. doi: 10.1073/pnas.84.10.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuo S., Tatsuno M., Kobayashi K., Saheki T., Miyata T., Iwanaga S., Amaya Y., Mori M. Isolation of cDNA clones of human argininosuccinate lyase and corrected amino acid sequence. FEBS Lett. 1988 Jul 18;234(2):395–399. doi: 10.1016/0014-5793(88)80124-8. [DOI] [PubMed] [Google Scholar]
- Nene V., Dunne D. W., Johnson K. S., Taylor D. W., Cordingley J. S. Sequence and expression of a major egg antigen from Schistosoma mansoni. Homologies to heat shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986 Nov;21(2):179–188. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- Nickerson J. M., Wawrousek E. F., Borras T., Hawkins J. W., Norman B. L., Filpula D. R., Nagle J. W., Ally A. H., Piatigorsky J. Sequence of the chicken delta 2 crystallin gene and its intergenic spacer. Extreme homology with the delta 1 crystallin gene. J Biol Chem. 1986 Jan 15;261(2):552–557. [PubMed] [Google Scholar]
- Nickerson J. M., Wawrousek E. F., Hawkins J. W., Wakil A. S., Wistow G. J., Thomas G., Norman B. L., Piatigorsky J. The complete sequence of the chicken delta 1 crystallin gene and its 5' flanking region. J Biol Chem. 1985 Aug 5;260(16):9100–9105. [PubMed] [Google Scholar]
- O'Brien W. E., Barr R. H. Argininosuccinate lyase: purification and characterization from human liver. Biochemistry. 1981 Mar 31;20(7):2056–2060. doi: 10.1021/bi00510a049. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E., McInnes R., Kalumuck K., Adcock M. Cloning and sequence analysis of cDNA for human argininosuccinate lyase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7211–7215. doi: 10.1073/pnas.83.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M., Sakamoto H., Yasuda K., Okada T. S., Shimura Y. Nucleotide sequence of a chicken delta-crystallin gene. Nucleic Acids Res. 1985 Mar 11;13(5):1593–1606. doi: 10.1093/nar/13.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palekar A. G., Mantagos S. Human liver arginiosuccinase purification and partial characterization. J Biol Chem. 1981 Sep 10;256(17):9192–9194. [PubMed] [Google Scholar]
- Piatigorsky J., O'Brien W. E., Norman B. L., Kalumuck K., Wistow G. J., Borras T., Nickerson J. M., Wawrousek E. F. Gene sharing by delta-crystallin and argininosuccinate lyase. Proc Natl Acad Sci U S A. 1988 May;85(10):3479–3483. doi: 10.1073/pnas.85.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMIR H., RATNER S. ENZYMES OF ARGININE METABOLISM IN CHICKS. Arch Biochem Biophys. 1963 Aug;102:249–258. doi: 10.1016/0003-9861(63)90178-4. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Fujii Y., Nakayama K., Ohkubo H., Kuramitsu S., Kagamiyama H., Nakanishi S., Hayaishi O. Structural similarity of bovine lung prostaglandin F synthase to lens epsilon-crystallin of the European common frog. Proc Natl Acad Sci U S A. 1988 Jan;85(1):11–15. doi: 10.1073/pnas.85.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Mulders J. W., de Jong W. W. The enzyme lactate dehydrogenase as a structural protein in avian and crocodilian lenses. Nature. 1987 Apr 9;326(6113):622–624. doi: 10.1038/326622a0. [DOI] [PubMed] [Google Scholar]
- Wistow G., Piatigorsky J. Recruitment of enzymes as lens structural proteins. Science. 1987 Jun 19;236(4808):1554–1556. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- Wistow G., Summers L., Blundell T. Myxococcus xanthus spore coat protein S may have a similar structure to vertebrate lens beta gamma-crystallins. 1985 Jun 27-Jul 3Nature. 315(6022):771–773. doi: 10.1038/315771a0. [DOI] [PubMed] [Google Scholar]
- Yasuda K., Kondoh H., Okada T. S., Nakajima N., Shimura Y. Organization of delta-crystallin genes in the chicken. Nucleic Acids Res. 1982 May 11;10(9):2879–2891. doi: 10.1093/nar/10.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]


