Abstract
Protein–protein interactions are critical to cellular processes yet the ability to predict and rationally design interactions is limited because of incomplete knowledge of the principles governing these interactions. The β-lactamase inhibitory protein (BLIP)/β-lactamase interaction has become a model system to investigate protein–protein interactions and has been the focus of several structural, thermodynamic and binding specificity studies. BLIP-II also inhibits β-lactamase but has no sequence homology with BLIP. The structure of BLIP-II in complex with TEM-1 β-lactamase revealed that BLIP-II has a completely different structure than BLIP but it interacts with the same protruding loop-helix region of TEM-1 as does BLIP. The significance of the individual interacting residues in molecular recognition by BLIP-II is currently unknown. Therefore, a phage display vector was developed with the purpose of expressing BLIP-II onto the surface of the M13 filamentous bacteriophage. The BLIP-II displayed phage bound to TEM-1 with picomolar affinity indicating that BLIP-II is properly folded while on the surface of the phage. The phage system, as well as enzyme inhibition assays with purified proteins, revealed that BLIP-II is a more potent inhibitor than BLIP for several class A β-lactamases with Ki values in the low picomolar range.
Keywords: β-lactamase, β-lactamase inhibitory protein, antibiotic resistance, phage display, protein–protein interactions
Introduction
Protein–protein interactions are involved in virtually every process in the cell and amino acid substitutions in the interface have the potential to change the binding specificity and affinity of the proteins involved (Shi et al., 1997). The ability to manipulate protein–protein interactions by design based on understanding the principles of molecular recognition is a potential means of modifying disease processes (Momand et al., 2000; Alkhatib, 2009). The current knowledge of the determinants of binding in protein–protein interactions, however, is insufficient to allow rational design of affinity in protein interfaces (Jelesarov and Bosshard, 1999; Kortemme and Baker, 2004).
β-Lactamase enzymes catalyze the hydrolysis of β-lactam antibiotics (e.g. penicillins and cephalosporins) and are the most common form of bacterial resistance to these drugs (Frere, 1995). Classes A, C and D are serine hydrolases, whereas class B β-lactamases are metallo-enzymes that utilize a zinc-coordinated water for catalysis (Paterson and Bonomo, 2005). Class A β-lactamases exhibit broad substrate hydrolysis profiles which include penicillins, cephalosporins and, for a few enzymes, carbapenems (Matagne et al., 1998; Walther-Rasmussen and Hoiby, 2007; Walsh, 2008). TEM-1 β-lactamase is a class A enzyme and is the most common plasmid-encoded β-lactamase in Gram-negative bacteria (Shah et al., 2004). TEM-1 β-lactamase has also been widely used as a model to understand how amino acid substitutions impact protein folding and stability (Huang et al., 1996; Huang and Palzkill, 1997; Sideraki et al., 2001; Bershtein et al., 2008; Kather et al., 2008; Lejeune et al., 2008; Marciano et al., 2008). Numerous class A β-lactamases such as SHV-1, SME-1, Bla1 and PC1 have broadly similar substrate profiles as TEM-1, whereas others have evolved the ability to hydrolyze extended-spectrum β-lactams such as the CTX-M type β-lactamases (Nugent and Hedges, 1979; Christensen et al., 1990; Shlaes and Currie-McCumber, 1992; Chaves et al., 1995; Zawadzke et al., 1995; Matagne et al., 1998; Giakkoupi et al., 1999; Kuzin et al., 1999; Tzouvelekis and Bonomo, 1999; Nordmann and Poirel, 2002; Chen et al., 2003; Materon et al., 2003; Majiduddin and Palzkill, 2005; Rossolini et al., 2008).
Naturally occurring, proteinaceous inhibitors of β-lactamases have been discovered in Streptomyces species (Iwai et al., 1973; Ono et al., 1973; Doran et al., 1990; Park and Lee, 1998). The first β-lactamase inhibitory protein (BLIP) to be discovered and characterized was isolated from Streptomyces clavuligerus (Doran et al., 1990). BLIP is a 17.5-kDa protein that inhibits class A β-lactamases with Ki values ranging from the picomolar to micromolar depending on the target enzyme (Zhang and Palzkill, 2004). The first BLIP-β-lactamase interface to be characterized in detail was the interaction between BLIP and TEM-1 (Strynadka et al., 1994, 1996; Zhang and Palzkill, 2003; Reichmann et al., 2007a,b). The co-crystal structure of the BLIP–TEM-1 interface indicated that BLIP competitively inhibits β-lactamase by inserting two loops into the active site. In addition, BLIP is composed of two tandem repeat domains that form a concave eight-stranded β-sheet which binds to a protruding loop-helix region (residues 99–114) of TEM-1 adjacent to the active site (Strynadka et al., 1996). Since the determination of the BLIP–TEM-1 structure, several affinity, specificity, thermodynamic and structural studies have been performed on the interactions between several class A β-lactamases and BLIP (Albeck and Schreiber, 1999; Petrosino et al., 1999; Rudgers and Palzkill, 1999; Zhang and Palzkill, 2003, 2004; Reichmann et al., 2005, 2007a,b; Reynolds et al., 2006, 2008; Hanes et al., 2009; Wang et al., 2009; Yuan et al., 2009a,b). For these reasons, the BLIP–β-lactamase interaction has become a model system to investigate molecular recognition in protein–protein interactions.
Streptomyces exfoliatus produces two BLIPs, named BLIP-I and BLIP-II (Kim and Lee, 1994). BLIP-I exhibits 38% sequence identity with BLIP and inhibits β-lactamase with a similar mechanism based on structural and site-directed mutagenesis experiments (Kang et al., 2000; Gretes et al., 2009). BLIP-II, however, is a 28-kDa protein that shares no discernable sequence identity to either BLIP or BLIP-I (Park and Lee, 1998). The co-crystal structure of BLIP-II in complex with TEM-1 β-lactamase revealed that BLIP-II is a unique seven-bladed β-propeller with each blade containing three β-strands and one α-helix (Lim et al., 2001; Stevens and Paoli, 2008). The majority of β-propeller proteins have four β-strands per blade (Fulop and Jones, 1999; Paoli, 2001; Guruprasad and Dhamayanthi, 2004). BLIP-II interacts with TEM-1 through a circular arrangement of predominately hydrophobic residues present in β-turns and loops connecting repeat units. Residue contacts on the periphery of the BLIP-II–TEM-1 interface block the substrate binding cavity of β-lactamase. Interestingly, the majority of the BLIP-II contact residues interact with the same loop-helix region (residues 99–114) of TEM-1 as that contacted by BLIP (Strynadka et al., 1996; Lim et al., 2001). Although a substantial amount of work has been done on BLIP and its interactions with class A β-lactamases, the energetically significant residues involved in the interaction between BLIP-II and class A β-lactamases have yet to be investigated.
The display of heterologous proteins on the surface of M13 bacteriophage has proven to be an excellent tool for investigating the amino acid sequence determinants of affinity in protein–protein interactions (Lowman et al., 1991; Lowman and Wells, 1993; Rudgers and Palzkill, 1999; Sidhu et al., 2000a,b; Weiss et al., 2000; Morrison and Weiss, 2001). The protein of interest is most often genetically fused to either gene III or gene VIII, which allows for the protein of interest to be displayed on the M13 phage surface either monovalently or multivalently, respectively (Parmley and Smith, 1988; Bass et al., 1990; Smith and Yu, 1996; Yu and Smith, 1996; Smith and Petrenko, 1997; Sidhu et al., 2000a,b; Kehoe and Kay, 2005; Paschke, 2006). It has been shown that libraries of mutants with amino acid substitutions at interface residues can be used in conjunction with a selection process to estimate the energetic contributions of individual residues to binding affinity (Pal et al., 2005; Pal et al., 2006; Sidhu and Koide, 2007). Therefore, a goal of this study was to establish and validate a phage display system for BLIP-II.
A new phage display plasmid or phagemid was constructed encoding BLIP-II fused at its N-terminus to the TEM-1 β-lactamase signal sequence to direct secretion to the periplasm and at its C-terminus to gene III for inclusion into the phage particle. The expression of the fusion protein is under the transcriptional control of the arabinose-inducible PBAD promoter (Huang et al., 2000). This allows for tight regulation of expression of the BLIP-II–gene III protein (g3p). Enzyme-linked immunosorbent assay (ELISA) experiments were performed using the phages displaying BLIP-II to measure binding to immobilized β-lactamase, which indicated the displayed BLIP-II binds to TEM-1 β-lactamase with picomolar affinity. This result suggests that BLIP-II is properly folded and functional while fused to g3p on the surface of the phage. The utilization of this system further revealed that BLIP-II interacts with several class A β-lactamases but not enzymes from classes C and D. These interactions were confirmed by an enzyme inhibition assay with purified proteins. In addition, these assays revealed that BLIP-II is a more potent inhibitor than BLIP with significantly lower Ki values for all the class A β-lactamases tested. These findings indicate that the BLIP-II phage display system will be useful for determination of the contributions of individual BLIP-II residues for binding β-lactamases and for directed evolution experiments to change the binding properties of BLIP-II.
Materials and methods
Construction of the BLIP-II phagemid
The BLIP-II gene was made by total gene synthesis (Genescript) with a 6× His-tag encoded at the C-terminus. Polymerase chain reaction (PCR) was used to introduce the SalI and XbaI restriction sites, the amber stop codon and a trypsin cleavage site at the C-terminus of BLIP-II. The PCR amplified gene product encoded for residues 41–311 of BLIP-II and the C-terminal His-tag. The PCR product and the pTP184 plasmid were digested with the SalI and XbaI restriction enzymes and ligated together (Huang et al., 2000). The Sa1I restriction site allowed for the insertion to be placed in frame with the bla signal sequence for periplasmic expression and the Xba1 restriction site introduced an amber stop codon at the C-terminus. The DNA was purified by phenol–chloroform extraction and ethanol precipitation. The DNA pellet was resuspended in TE (10 mM Tris, 1 mM EDTA) and transformed into Escherichia coli XL1-Blue (Stratagene). Individual colonies were picked and plasmid DNA was purified and screened for the presence of the BLIP-II insert by restriction enzyme digestion. The sequence of the BLIP-II gene in the construct was verified by DNA sequencing (Lonestar Labs). The BLIP-II phage display plasmid is named pNK621.
Construction of the BLIP-II protein expression vector
A BLIP-II protein expression plasmid was constructed by inserting the BLIP-II gene into the previously published pET-TEM1 vector (Sosa-Peinado et al., 2000; Marciano et al., 2008). The BLIP-II gene was synthesized, as mentioned above, to include a 6× His-tag on its C-terminus. NdeI and BamHI restriction sites were introduced by PCR and the NdeI site also encodes for the ‘start’ methionine. As constructed, the BLIP-II protein product lacks a signal sequence and remains in the cytoplasm of E.coli. The PCR product and the pET-TEM1 vector were digested with NdeI and BamHI, purified and ligated as described above. The DNA was transformed into E.coli XL1-Blue cells (Stratagene) and individual colonies were screened by restriction enzyme digestion. The sequence of the BLIP-II gene was confirmed by DNA sequencing (Lonestar Labs) and the plasmid was named pET-BLIP-II.
Expression and purification of BLIP-II displayed phage
The pNK621 plasmid was electroporated into E.coli TG1, and single colonies were picked and inoculated into 5 ml 2YT broth supplemented with 12.5 µg/ml chloramphenicol. After growth overnight at 37°C, the culture was added to 50 ml of 2YT broth supplemented with 12.5 µg/ml chloramphenicol and grown at 37°C until the OD600 was between 0.4 and 0.5. A total of 2 × 1011 KM13 helper phage particles were then added, and the culture was incubated without shaking for 30 min at 37°C to allow for bacteriophage infection. The culture was centrifuged at 3000g for 10 min to pellet the cells and the supernatant was decanted. The pNK621 TG1 cells were then resuspended in 100 ml of 2YT supplemented with 12.5 µg/ml chloramphenicol, 50 µg/ml kanamycin and 1% l-arabinose. The addition of arabinose induced the expression of the BLIP-II–g3p. The culture was incubated at 30°C overnight for production of the BLIP-II-display phage particles. The E.coli TG1 host cells were pelleted by centrifugation at 3300g for 30 min, and the BLIP-II phage were precipitated from the supernatant by adding 20 ml of 20% polyethylene glycol 6000–2.5 M NaCl (PEG–NaCl). This solution was left on ice for 1 h and then centrifuged at 3300g for 30 min. The supernatant was removed and the pellet was centrifuged briefly and residual PEG–NaCl was aspirated. The phage pellet was resuspended in 4 ml of PBS and centrifuged at 11 600g for 10 min to pellet any remaining host cells. The phage titer was determined by serial dilution of the phage into a log-phase E.coli TG1 culture. A total of 0.1 ml of each of the dilutions were plated on Luria–Bertani (LB) agar plates supplemented with 12.5 µg/ml chloramphenicol, incubated overnight at 37°C and colonies were counted to determine the phage titer.
Phage ELISA experiments
A two-step phage ELISA was performed to determine the affinity and specificity of binding of BLIP-II phage with β-lactamases. TEM-1 β-lactamase was immobilized by adding 0.15 ml/well of 540 nM enzyme to a 96-well immulon 2 HB microtiter plate from Thermo Scientific. The plate was incubated without shaking at 4°C overnight. The wells were washed with 0.25 ml/well of PBS three times and blocked by gently shaking with 0.25 ml/well of MPBS (5% dry milk in PBS) for 2.5 h at room temperature. The wells were then washed again three times with 0.25 ml/well of PBS as described above. Increasing amounts of BLIP-II phage were incubated with immobilized β-lactamase with gentle shaking for 2.5 h at room temperature in a final volume of 0.15 ml/well. The wells were washed three times with 0.25 ml of PBST (PBS with 0.1% Tween-20) and the anti-M13-HRP antibody (GE healthcare) was diluted (1:3000) in MPBS and 0.15 ml was added and allowed to incubate at room temperature for 1 h. The ELISA was developed by adding 0.15 ml/well of 1-Step ABTS (Thermo Scientific) and read at OD405 after 30–45 min using a TECAN microtiter plate reader. This protocol was also used to determine BLIP-II phage specificity by immobilizing various β-lactamases and other non-β-lactamase proteins and determining the amount of bound BLIP-II phage as described above. In addition, the KM13 helper phage was also assayed as a negative control for binding. The KM13 helper phage was added to the wells at equal amounts as the BLIP-II phage to demonstrate the β-lactamase binding was a direct result of BLIP-II being displayed on the surface of the bacteriophage.
The affinity of the BLIP-II displayed phage for immobilized β-lactamase was determined using the phage ELISA method with a constant, sub-saturating level of 1.7 × 10−9 M BLIP-II phage added to each well in combination with increasing amounts of soluble TEM-1 β-lactamase. The TEM-1 dilutions and the BLIP-II displayed phage were added in a final volume of 0.15 ml/well. The plate was then washed and developed as described above. The affinity (IC50) value was defined as the concentration of soluble, competing TEM-1 β-lactamase that resulted in a half-maximal signal for BLIP-II phage bound.
BLIP-II expression and purification
The pET-BLIP-II protein expression plasmid was electroporated into E.coli BL21(DE3) cells, and a single colony was used to inoculate 10 ml of LB containing 25 µg/ml kanamycin. After overnight growth, the 10 ml culture was added to 1 l of LB supplemented with 25 µg/ml kanamycin and the culture was grown at 37°C until the OD600 was 0.4–0.5. Protein expression was induced by the addition of IPTG to a concentration of 0.2 mM and the culture was incubated at 16°C for 18–22 h. The culture was next centrifuged for 15 min at 10 000g and the cell pellet was stored at −80°C for at least 1 h. The pellet was resuspended in 20 ml of lysis buffer (25 mM phosphate buffer pH 7.4, 300 mM NaCl, 10 mM imidazole, 40 µM MgCl2, 10 µg/ml Deoxyribonuclease I (Sigma) and 1 tablet of EDTA-free Protease Inhibitor Cocktail (Roche). The cells were lysed using a French press at 1250 psi and the insoluble material was removed by centrifugation at 12 000g for 15 min. TALON Metal Affinity Resin (Clontech) was prepared by three washes of a 3-ml bed volume with 6 ml of 25 mM phosphate buffer 300 mM NaCl and 10 mM imidazole pH 7.4. The soluble protein fraction was incubated with the washed TALON resin in an end-over-end shaker for an hour at room temperature which allowed BLIP-II with the C-terminal His-tag to bind the resin. The resin was further washed with 10 and 20 mM imidazole in 25 mM phosphate buffer, 300 mM NaCl pH 7.4 to remove protein that bound less tightly than BLIP-II. The BLIP-II protein was eluted from the column using 50 and 100 mM imidazole in 25 mM phosphate buffer 300 mM NaCl pH 7.4. Approximately 7 mg of >90% pure BLIP-II was obtained for every liter of starting culture using this method as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis and a Bradford protein concentration assay.
Sequence alignment
The sequences of the class A β-lactamases used in this study were retrieved from a previously described collection of 156 class A β-lactamase sequences (Marciano et al., 2009). The CTX-M-14 sequence was retrieved from GenBank (accession number ACJ43256; Yuan et al., 2009a,b). The β-lactamase sequences were aligned using ClustalW2 multiple sequence alignment program from the European Bioinformatics Institute (www.ebi.ac.uk/clustalw2).
β-Lactamase inhibition assay
The β-lactamase purification procedures used have been described previously (Rudgers et al., 2001; Zhang and Palzkill, 2003, 2004; Adediran et al., 2005; Marciano et al., 2008) The inhibition assay utilized is similar to the assay described previously for tight binding inhibitors and for BLIP kinetic studies (Green and Work, 1953; Morrison, 1969; Empie and Laskowski, 1982; Zhang and Palzkill, 2003). Increasing amounts of BLIP-II (from 10% to 133% of the total enzyme concentration) and the various β-lactamases to be tested were incubated for 2 h at 25°C at room temperature in 50 mM sodium phosphate buffer with 0.1 mg/ml BSA. The occupancy of BLIP-II on β-lactamase was determined by measuring the initial velocity of nitrocefin hydrolysis in a TECAN microtiter plate reader at OD485. The PC1 inhibition assay was performed in a DU800 spectrophotometer at OD482. The total enzyme concentrations were 0.3 nM TEM-1, 0.7 nM SHV-1, 0.5 nM CTX-M-14, 5 nM Bla1, 1.6 nM SME-1, 8 nM PC1, 1 nM IMP-1, 1 nM P99 and 20 nM Oxa-10. The nitrocefin concentration was also varied depending on the β-lactamase enzyme used. A concentration of 40 µM, 1 µM, 15 µM, 20 µM, and 20 µM of nitrocefin was used for TEM-1, PC1, Bla1, Sme1 and CTX-M-14, respectively. Nitrocefin was used at 10 µM for the remaining enzymes, SHV1, IMP-1, P99 and Oxa-10. The nitrocefin concentrations used in the assays were below the previously published KM values for these enzyme and substrate combinations (KM values: TEM-1, 72.5 µM; PC1, 1.5 µM; Bla1, 19 µM; Sme-1, 26 µM; CTX-M-14, 25 µM; SHV-1, 21 µM; IMP-1, 27 µM; P99, 56 µM; Oxa-10, 450 µM; Faraci and Pratt, 1985; Joris et al., 1985; Ledent et al., 1993; Raquet et al., 1997; Laraki et al., 1999; Bebrone et al., 2001; Materon et al., 2003; Thomson et al., 2006; Ishii et al., 2007; Marciano et al., 2009). The Ki values were determined by fitting the initial velocities to the Morrison tight binding equation [Equation (1)] using Graphpad Prism 5 software,
 |
(1) |
where Efree is the concentration of free enzyme determined by the residual β-lactamase activity against the activity and concentration of the uninhibited β-lactamase, the [E0] is the total enzyme concentration and [I0] is the total inhibitor concentration (Green and Work, 1953; Morrison, 1969; Copeland, 2000)
| (2) |
The dissociation constant, Ki, was determined by applying the Cheng–Prusoff correction [Equation (2)] relating the  for a competitive inhibitor to the dissociation constant, Ki, and the substrate concentration, [S], and the KM values that were determined in independent experiments (Morrison, 1969; Cheng and Prusoff, 1973; Copeland, 2000).
for a competitive inhibitor to the dissociation constant, Ki, and the substrate concentration, [S], and the KM values that were determined in independent experiments (Morrison, 1969; Cheng and Prusoff, 1973; Copeland, 2000).
For all of the BLIP-II inhibitor experiments, an effort was made to establish conditions whereby the residual enzyme activity at the equivalence point with inhibitor was ∼10–15% by keeping the enzyme concentration within ∼100-fold of the Ki (Empie and Laskowski, 1982; Murphy, 2004). Note that in the case of inhibition of TEM-1, SME-1, SHV-1 and CTX-M-14 β-lactamases, it was possible to use enzyme concentrations that resulted in at least 10% residual enzyme activity at the equivalence point with BLIP. Computer simulations of inhibition curves for theoretical Ki values 10-fold above and below the measured Ki indicated that it was possible to obtain an accurate Ki with the data available. However, due to technical difficulties with the assay, the enzyme concentrations of Bla1 and PC1 were higher and therefore significantly less than 10% of residual β-lactamase activity was observed at the equivalence point. Therefore, for Bla1 and PC1, the measured Ki is an upper estimate of the actual Ki value.
Amber suppression efficiency determined by immunoblots
The pNK621 phagemid was electroporated into TG1 E.coli cells, and a single colony was used to inoculate 10 ml of LB containing 12.5 µg/ml chloramphenicol. After an overnight growth at 37°C, the culture was diluted 1:100 and grown in either the presence or the absence of 1% l-arabinose to an OD600 of 0.4. The cells were then harvested and fractionated on a SDS–PAGE gel and transferred to a nitrocellulose membrane. The recombinant His-tagged BLIP-II-g3p was detected using an anti-His-tag antibody following the protocol for the Anti-His HRP Conjugates antibody set (Qiagen).
BLIP-II display copy number determination by immunoblot
An immunoblot of phages displaying BLIP-II was used to determine the ratio of BLIP-II-g3p fusion molecules to non-displaying g3p molecules. The phage particles were fractionated on an SDS–PAGE gel and transferred to a nitrocellulose membrane. A monoclonal anti-g3p antibody (New England Biolabs) was used to probe for the presence of g3p and was visualized using a goat anti-mouse secondary antibody conjugated with HRP (Sigma). Densitometry determined the relative intensity of the bands to estimate the ratio of recombinant BLIP-II-g3p to native g3p.
BLIP-II display copy number determination by β-lactamase inhibition assay
A TEM-1 β-lactamase inhibition assay was used to determine the number of BLIP-II molecules present per phage particle. Increasing amounts of phage were incubated with a constant TEM-1 concentration of 0.3 nM. The enzyme activity was measured by adding 25 µM of a cephalosporin chromogenic substrate, nitrocefin. The initial velocities were determined at OD485 using a TECAN plate reader. The amount of BLIP-II phage that resulted in a reduction of the initial velocity of nitrocefin hydrolysis to background levels was estimated to be the concentration of BLIP-II that is equimolar to TEM-1. These reactions were performed in 0.3 ml of PBS supplemented with 0.25 mg/ml BSA.
Results
Construction of a BLIP-II expressing phagemid
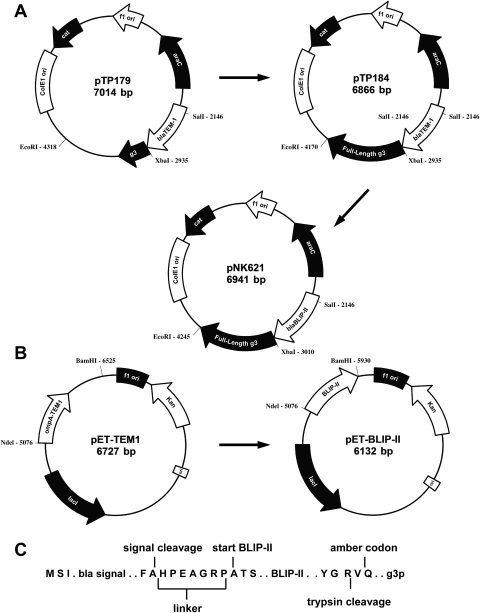
Phage display has proven to be a powerful method to study the determinants of molecular recognition in protein–protein interactions and as a method to engineer new binding properties in proteins (Pal et al., 2006; Sidhu and Koide, 2007). In order to facilitate the study of the molecular basis of the interaction between BLIP-II and class A β-lactamases, we undertook to develop a phage display system for BLIP-II. For this purpose, a phagemid vector was constructed with the BLIP-II coding sequence fused at its N-terminus to the TEM-1 β-lactamase signal sequence and at its C-terminus to the M13 bacteriophage g3p (Fig. 1). The expression of BLIP-II in this vector is tightly regulated because it is under the transcriptional control of the arabinose-inducible PBAD promoter (Fig. 1A). Tightly regulated expression is important because high level expression of free soluble BLIP-II in the periplasm is toxic to E.coli (data not shown). The phagemid therefore allows for the display of BLIP-II on the surface of the bacteriophage when both arabinose and helper phage are added to the growth medium.
Fig. 1.
Schematic representation of the plasmids constructed for this study. (A) Construction of pNK621 phage display plasmid. (B) Construction of pET-BLIP-II expression plasmid. Restriction sites utilized for the construction of the plasmids are shown. (C) The amino acid sequence of BLIP-II-g3p present in pNK621. The signal sequence and the signal peptidase cleavage site are illustrated followed by an amino acid linker segment used to introduce the SalI restriction site. The first three amino acids of the BLIP-II protein is shown as ‘start BLIP-II’. The amber codon position is marked at the C-terminus of BLIP-II as a glutamine which is followed in the plasmid by the full-length g3p with a trypsin cleavage site between the BLIP-II and g3p.
The pTP179 phagemid was previously constructed and characterized as described (Huang et al., 2000). This phagemid encodes chloramphenicol resistance and utilizes the arabinose-inducible PBAD promoter to express TEM-1 β-lactamase fused to a truncated form of the g3p. It was shown by immunoblotting that expression levels of TEM-1 from this promoter were undetectable in the absence of arabinose but TEM-1 was efficiently expressed on the addition of arabinose. The pTP179 phagemid was modified to encode a full-length g3p to increase the accessibility of BLIP-II to bind to the target β-lactamase. This phagemid was named pTP184. The BLIP-II gene was synthesized and then amplified by PCR with primers that allowed for the insertion of an amber codon (TAG) and a trypsin cleavage site between BLIP-II and gene III as well as the addition of SalI and XbaI restriction sites for cloning the BLIP-II encoding fragment into pTP184 (Fig. 1C). The amber codon (TAG) is suppressed with insertion of glutamine at the TAG in many E.coli phage display strains such as TG1 to allow the expression of the BLIP-II–g3p. Both the tight control of the PBAD promoter and the high-efficiency suppression of the amber codon (TAG) in the BLIP-II–g3p were validated by immunoblot analysis (Fig. S1, Supplementary data are available at PEDS online). Escherichia coli TG1 cells carrying the BLIP-II–gene III phagemid (pNK621) were grown in media with and without arabinose. The BLIP-II–g3p was detected only when arabinose was present in the growth medium (Fig. S1, Supplementary data are available at PEDS online). The stringent regulation of the PBAD promoter is also necessary because the expression of full-length g3p renders E.coli cells immune to bacteriophage infection (Boeke et al., 1982). However, the helper phage infection efficiency was determined to be ∼94% by examining the percentage of clones that were both kanamycin and chloramphenicol resistant. This result indicates that the E.coli cells harboring the pNK621 phagemid can be stably infected with helper phage.
BLIP-II phages bind tightly to TEM-1 β-lactamase
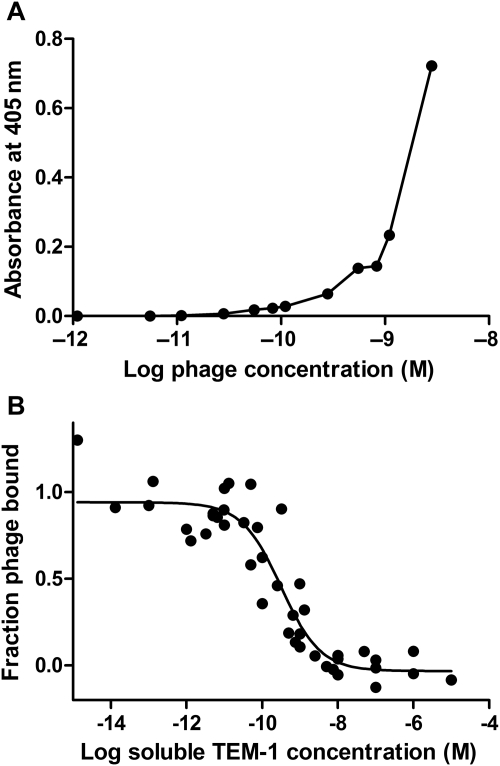
A two-step phage ELISA method was used to demonstrate that BLIP-II is displayed on the surface of the M13 bacteriophage in a functional form that has a high affinity toward the TEM-1 β-lactamase. Microtiter wells were coated with TEM-1 β-lactamase, and increasing amounts of BLIP-II phage were added to the various wells. After washing, anti-M13-antibody conjugated to horseradish peroxidase (HRP) was used to detect BLIP-II phage bound to immobilized β-lactamase. As shown in Fig. 2A, the ELISA signal increased as increasing amounts of BLIP-II phage were added to the wells suggesting that the phage was binding to the immobilized TEM-1 β-lactamase.
Fig. 2.
Bacteriophage displaying BLIP-II interacts with TEM-1 β-lactamase with binding affinity in the picomolar concentration range. (A) Phage ELISA results with a dose-response curve. The x-axis indicates the amount of BLIP-II displaying phage added to immobilized β-lactamase, and the y-axis shows the absorbance after addition of HRP reagent that reacts with the anti-M13 HRP antibody. (B) Phage ELISA with competition from soluble TEM-1 β-lactamase. A subsaturating level of BLIP-II phage (1.7 × 10−9 M) was used for binding with increasing concentrations of TEM-1 β-lactamase competitor (x-axis). The y-axis indicates the fractional amount of BLIP-II phage bound in the well. This figure reveals that the BLIP-II phage has an IC50 value of 320 pM. The IC50 value is the concentration of soluble TEM-1 competitor that results in half maximal binding of the BLIP-II phage with immobilized β-lactamase.
A competition experiment with soluble TEM-1 β-lactamase was performed to show the BLIP-II phage bind soluble enzyme and to estimate the binding affinity of the phage for β-lactamase. For this purpose, a subsaturating level of BLIP-II phage was added to wells that contained a constant amount of immobilized TEM-1 β-lactamase. Increasing amounts of soluble TEM-1 was added to the wells at the same time as the BLIP-II phage to compete with the immobilized form of TEM-1 for binding to the phage. As seen in Fig. 2B, an IC50 value of 320 pM was determined for the interaction. This value is defined as the concentration of soluble TEM-1 β-lactamase added to the wells that yielded a phage ELISA signal that is half the maximal value. These results indicate BLIP-II is displayed on the surface of the M13 bacteriophage in a form competent to interact with both the immobilized and soluble versions of TEM-1 with picomolar binding affinity.
The copy number of BLIP-II displayed on the phage was estimated by immunoblotting and as well as a TEM-1 inhibition assay using nitrocefin as the reporter substrate. Both experiments yielded a similar ratio of approximately one BLIP-II–g3p per 4–10 phages (Fig. S2, Supplementary data are available at PEDS online). Thus, the display efficiency of the BLIP-II molecule on the surface of the bacteriophage is sufficient for the use of combinatorial libraries.
Determination of the β-lactamase binding specificity of BLIP-II phage
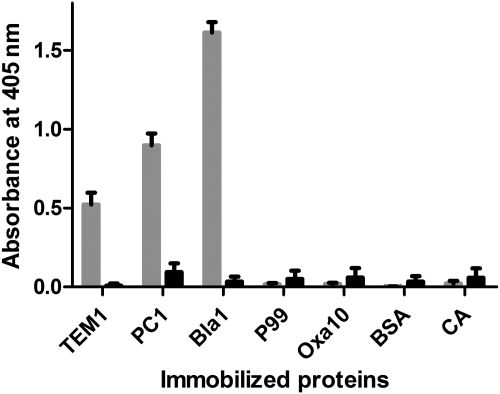
The ability of BLIP-II phage to bind other β-lactamases besides TEM-1 was tested by immobilizing various purified class A β-lactamases into microtiter wells and evaluating binding of BLIP-II phage to the enzymes by phage ELISA. In addition, bovine serum albumin (BSA) and carbonic anhydrase (CA) were immobilized and used as negative controls for BLIP-II-phage binding. Furthermore, the KM13 helper phage that does not display a heterologous protein was used as a control to confirm that the display of BLIP-II is required for binding to β-lactamase.
As seen in Fig. 3, BLIP-II phage bound to TEM-1, PC-1 (Staphylococcus aureus) (Ambler, 1975) and Bla1 (Bacillus anthracis; Chen et al., 2003; Materon et al., 2003) class A β-lactamases but did not bind to immobilized forms of either the P99 class C enzyme (Enterobacter cloacae; Joris et al., 1984) or the class D Oxa-10 β-lactamase (Pseudomonas aeruginosa; Huovinen et al., 1988). The KM13 helper phages that do not display a protein did not bind to any of the immobilized β-lactamases or to the BSA and CA negative control proteins. Therefore, it appears BLIP-II binds specifically to a wide range of class A β-lactamases and the observed binding is a direct result of BLIP-II being expressed on the surface of the bacteriophage. In addition, the results indicate that BLIP-II does not bind to β-lactamases from classes C or D.
Fig. 3.
Bacteriophage displaying BLIP-II interacts with class A β-lactamases. This diagram shows the results of phage ELISA experiments with BLIP-II phage tested for binding to immobilized β-lactamases (TEM-1, PC1, Bla1—grey bars). P99 and Oxa-10 are representative class C and D β-lactamases, respectively. The results of phage ELISA measurement of KM13 helper phage binding to the immobilized targets are shown with black bars.
Soluble BLIP-II protein binds and inhibits class A β-lactamases
The hypothesis that BLIP-II inhibits additional class A β-lactamases was examined using soluble BLIP-II protein that was expressed from E.coli and purified to homogeneity. An enzyme inhibition assay was employed whereby the colorimetric β-lactam substrate nitrocefin was used as a reporter to determine the potency of BLIP-II for inhibition of β-lactamases. Six class A β-lactamases originating from both Gram-positive and Gram-negative bacteria were assayed for BLIP-II-dependent inhibition. The assay revealed that BLIP-II inhibited all tested class A β-lactamases with a Ki value in the low picomolar range but did not inhibit representative β-lactamases from other classes (Table I).
Table I.
Inhibition constants (Ki)
| Organism of isolation (Gram stain) | Identitya (%) | β-lactamase (Eo, nM) | BLIP-II (Ki, pM) | BLIP (Ki, pM)b |
|---|---|---|---|---|
| Escherichia coli (−) | 100 | TEM-1 (0.3) | 2.5 ± 0.9 | 500 ± 100 |
| Klebsiella pneumoniae (−) | 67 | SHV-1 (0.7) | 12 ± 3.4 | 1 130 000 ± 10 000 |
| Bacillus anthracis (+) | 38 | Bla1 (5) | <25c | 2500 ± 900 |
| Serratia marcescens (−) | 34 | Sme-1 (1.6) | 8.4 ± 3.9 | 2400 ± 200 |
| Kluyvera georgiana (−) | 34 | CTX-M-14 (0.5) | 9.1 ± 2.1 | 810 000 ± 77 000d |
| Staphylococcus aureus (+) | 31 | PC1 (8) | <16c | 350 000 ± 100 000d |
aThe sequence identity to TEM-1 β-lactamase determined by ClustalW2.
bThe Ki values presented were previously determined in Zhang and Palzkill (2003, 2004).
cThe value shown is an upper limit of the Ki. With the enzyme concentration used, it is not possible to exactly determine Ki. See Materials and Methods section for details.
dUnpublished data.
BLIP-II-mediated TEM-1 inhibition was first assayed to determine if the His-tag on the C-terminus of recombinant BLIP-II affected the interaction. The C-terminus is not in direct contact in the BLIP-II–TEM-1 interface as determined by the co-crystal structure and therefore it is not expected to interfere with the BLIP-II–β-lactamase interaction. This was confirmed with the determination of an inhibition constant (Ki) for TEM-1 β-lactamase of 2.5 pM. This result is ∼10-fold lower than the previously published inhibition constant of 27.2 pM and is significantly lower than the inhibition constant for the BLIP–TEM-1 interaction (Ki of 0.5 nM; Lim et al., 2001; Zhang and Palzkill, 2003). The difference in binding constant may be due to differences in enzyme concentrations used in the assays. The purified BLIP-II was further used in the inhibition assay to confirm the interactions shown by phage ELISAs between BLIP-II and the class A β-lactamases PC1 and Bla1 from Gram-positive bacteria. BLIP-II inhibited both PC1 and Bla1 with estimated inhibition constants of 16 and 25 pM, respectively (Table I). These values are substantially lower than the BLIP dissociation constants toward these enzymes which have Ki values of 350 nM for PC1 (unpublished data) and 2.5 nM for Bla1 (Zhang and Palzkill, 2004).
To further elucidate the binding properties of BLIP-II, inhibition assays were performed with the class A β-lactamases CTX-M-14 (Kluyvera georgiana; Olson et al., 2005; Rossolini et al., 2008), SME-1 (Serratia marcescens; Naas et al., 1994) and SHV-1 (Klebsiella pneumoniae) from Gram-negative bacteria (Simpson et al., 1980). CTX-M-14, SME-1 and SHV-1 are inhibited by BLIP with Ki values of 810 (unpublished data), 2.4 and 1130 nM, respectively (Zhang and Palzkill, 2003, 2004). It was found that BLIP-II is a much more potent inhibitor of these enzymes with Ki values of 9.1, 8.4 and 12 pM for CTX-M-14, SME-1 and SHV-1, respectively. Taken together, the results indicate BLIP-II tightly binds to a wide range of class A β-lactamases and is a more potent inhibitor than BLIP for all enzymes tested.
The purified, soluble BLIP-II protein was also used to test for inhibition of β-lactamases from classes B–D. IMP-1 is a metallo β-lactamase (class B) and was used as a negative control in this experiment because it does not share sequence or structural homology with the serine β-lactamases composing classes A, C and D (Bebrone, 2007). No significant change in the hydrolysis of nitrocefin was found for the IMP-1, P99 (class C) or Oxa-10 (class D) β-lactamases despite the addition of BLIP-II up to 1 µM concentrations. These findings support the results of the phage ELISA experiments as BLIP-II did not bind to either P99 or Oxa10 β-lactamases in either assay. Thus, the interactions observed in the phage ELISAs were the direct result of properly folded and active BLIP-II on the M13 bacteriophage surface. In addition, the results of this study indicate that soluble BLIP-II protein is a very potent inhibitor of the six class A β-lactamases tested.
Discussion
Although protein–protein interactions play a significant role in most processes of the cell, our current ability to manipulate and control these interactions remains out of reach. Phage display technology has proven to be an important method to engineer binding affinity, determine hot spots and analyze the inherent contributions of various amino acids (Rudgers and Palzkill, 1999; Pal et al., 2005, 2006; Sidhu and Koide, 2007; Birtalan et al., 2008; Gilbreth et al., 2008; Koide and Sidhu, 2009). The methodology provides a mechanism to study interactions by facilitating the selection of functional mutants from large combinatorial libraries. However, a prerequisite for the use of these libraries is to confirm that the protein of interest functions properly when expressed on the surface of the bacteriophage. In this paper, we have demonstrated that functional BLIP-II protein is expressed on the surface of phage and the system was further employed to study the binding specificity of the inhibitor for several different β-lactamase enzymes.
A phagemid construct was made with the BLIP-II gene fused to gene III of the M13 filamentous bacteriophage. Expression of the fusion product was placed under the transcriptional control of the arabinose-inducible PBAD promoter to tightly control the periplasmic expression of BLIP-II (Huang et al., 2000). The stringent regulation of the promoter is necessary because the expression of full-length g3p in E.coli cells render the cells immune to bacteriophage infection (Boeke et al., 1982). However, when the protein is not expressed, the phage is capable of infecting the E.coli. This was demonstrated when Model and co-workers used the phage shock promoter to express g3p to supplement the gene III deletion mutant helper phage, R408d3 (Rakonjac et al., 1997). They demonstrated by immunoblotting that the full-length g3p was not expressed until the cells were infected. Similarly, we demonstrate that our BLIP-II–g3p is not expressed until the addition of arabinose (Fig. S1, Supplementary data are available at PEDS online) and, therefore, does not affect the efficiency of infections of E.coli carrying the phagemid pNK621.
BLIP-II displaying phage was produced and phage ELISAs performed using immobilized β-lactamase enzymes revealed the BLIP-II phage bound to TEM-1 β-lactamase with picomolar affinity as determined by a competition assay between the immobilized and soluble TEM-1. In addition, this affinity was shown to be the direct result of the presence of BLIP-II by comparison to the lack of binding observed with the KM13 helper phage. These results indicate that BLIP-II is properly folded and active while displayed on the surface of the M13 filamentous bacteriophage.
Since the capacity of BLIP-II to inhibit other β-lactamases is largely unknown, the inhibition spectrum of the protein was evaluated. Phage ELISA experiments revealed that BLIP-II bound to two other class A β-lactamases including PC1 and Bla1 but binding was not observed for the P99 and Oxa10 β-lactamase representatives of classes C and D suggesting that BLIP-II only binds to class A β-lactamases. Thus, the binding preference of BLIP-II for class A enzymes is similar to that observed for BLIP.
Purified, soluble proteins were then used in an enzyme inhibition assay to establish an inhibition profile for BLIP-II. This assay revealed that BLIP-II did not inhibit β-lactamases other than class A enzymes. In addition, it was shown that BLIP-II inhibited every class A β-lactamase tested with a Ki in the low picomolar range (Table I). The affinity of BLIP-II for the class A β-lactamases is significantly higher than that observed for BLIP and suggests that BLIP-II is a more potent inhibitor than BLIP for most class A enzymes. When compared with other model systems of protein–protein interactions, the BLIP-II-mediated inhibition of β-lactamase is more potent than the well-studied human growth hormone–human growth hormone receptor interaction (KD of 10−9 M) but exhibits weaker binding than that observed for the barnase–barstar interaction (Ki of 10−14 M; Schreiber and Fersht, 1993; Pal et al., 2005, 2006).
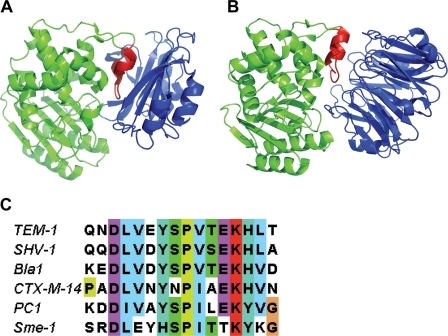
The crystal structures of the BLIP–TEM-1 and BLIP-II–TEM-1 complexes reveal that both BLIP and BLIP-II make extensive contacts with the protruding loop-helix domain (residues 99–114) of TEM-1 that is a characteristic feature of class A β-lactamases (Strynadka et al., 1996; Lim et al., 2001). BLIP uses its β-stands to encapsulate this loop-helix region and interact with the active site residues (Fig. 4A; Strynadka et al., 1996). In contrast, BLIP-II utilizes a series of β-turns and repeat connecting loops to bind to the loop-helix region of TEM-1 β-lactamase and the peripheral contacts sterically hinder the active site pocket (Fig. 4B; Lim et al., 2001). Mutational studies of BLIP-based interactions revealed several contact residues in the loop-helix domain of β-lactamase that function in both the binding affinity and specificity of these interactions (Rudgers and Palzkill, 1999; Reynolds et al., 2006, 2008). An amino acid sequence alignment of class A β-lactamases reveals a similar level of sequence identity in the 99–114 region as that found throughout the alignment of the protein sequence (Marciano et al., 2009). However, there are a few residues that are strongly conserved and could potentially be significant for interactions with BLIP-II (Fig. 4C). It will be interesting to investigate these residues to determine their contributions to binding interactions. Nonetheless, the 99–114 region of class A β-lactamases is crucial to the binding and specificity of both BLIP and, based on its co-crystal structure with TEM-1, BLIP-II (Fig. 4C).
Fig. 4.
Illustration of the structures of the (A) BLIP–TEM-1 β-lactamase (1JTG) and (B) BLIP-II–TEM-1 (1JTD) β-lactamase complexes (Strynadka et al., 1996; Lim et al., 2001). The protruding loop-helix region of β-lactamase, in red, is adjacent to the active site of TEM-1 β-lactamase, which is shown in green. BLIP and BLIP-II are shown in blue. (C) Sequence alignment of the β-lactamase loop-helix region (residues 99–114). The name of each β-lactamase used is at the left of the sequence. The residue 99–114 numbering refers to the canonical numbering scheme for class A β-lactamases.
Taken together, the BLIP and BLIP-II interactions with β-lactamases provide a rare perspective on molecular recognition in protein–protein interactions in that two proteins (BLIP and BLIP-II), which share no discernable sequence or structural homology, bind to the same loop-helix region of class A β-lactamases. The successful display of BLIP-II on the surface of M13 phage allows for the construction and screening of combinatorial libraries encompassing the amino acid positions believed to be responsible for β-lactamase binding in order to assess the determinants of BLIP-II binding affinity and specificity.
Supplementary Data
Funding
This work was supported by a training fellowship from the Keck Center Pharmacoinformatics Training Program of the Gulf Coast Consortia (National Institutes of Health, T90 DK070109-05) and by National Institutes of Health AI32956 to T.P.
Supplementary Material
Acknowledgements
We thank Wanzhi Huang, Ana Maria Cardenas, Lori Horton and Zanna Beharry for providing various proteins used in this paper.
Footnotes
Edited by Gideon Schreiber
References
- Adediran S.A., Zhang Z., Nukaga M., Palzkill T., Pratt R.F. Biochemistry. 2005;44:7543–7552. doi: 10.1021/bi050136f. doi:10.1021/bi050136f. [DOI] [PubMed] [Google Scholar]
- Albeck S., Schreiber G. Biochemistry. 1999;38:11–21. doi: 10.1021/bi981772z. doi:10.1021/bi981772z. [DOI] [PubMed] [Google Scholar]
- Alkhatib G. Curr. Opin. HIV AIDS. 2009;4:96–103. doi: 10.1097/COH.0b013e328324bbec. doi:10.1097/COH.0b013e328324bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R.P. Biochem. J. 1975;151:197–218. doi: 10.1042/bj1510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass S., Greene R., Wells J.A. Proteins. 1990;8:309–314. doi: 10.1002/prot.340080405. doi:10.1002/prot.340080405. [DOI] [PubMed] [Google Scholar]
- Bebrone C. Biochem. Pharmacol. 2007;74:1686–1701. doi: 10.1016/j.bcp.2007.05.021. doi:10.1016/j.bcp.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Bebrone C., Moali C., Mahy F., Rival S., Docquier J.D., Rossolini G.M., Fastrez J., Pratt R.F., Frere J.M., Galleni M. Antimicrob. Agents Chemother. 2001;45:1868–1871. doi: 10.1128/AAC.45.6.1868-1871.2001. doi:10.1128/AAC.45.6.1868-1871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershtein S., Goldin K., Tawfik D.S. J. Mol. Biol. 2008;379:1029–1044. doi: 10.1016/j.jmb.2008.04.024. doi:10.1016/j.jmb.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Birtalan S., Zhang Y., Fellouse F.A., Shao L., Schaefer G., Sidhu S.S. J. Mol. Biol. 2008;377:1518–1528. doi: 10.1016/j.jmb.2008.01.093. doi:10.1016/j.jmb.2008.01.093. [DOI] [PubMed] [Google Scholar]
- Boeke J.D., Model P., Zinder N.D. Mol. Gen. Genet. 1982;186:185–192. doi: 10.1007/BF00331849. doi:10.1007/BF00331849. [DOI] [PubMed] [Google Scholar]
- Chaves J., Coira A., Segura C., Reig R. J. Chemother. 1995;7(Suppl. 4):49–51. [PubMed] [Google Scholar]
- Chen Y., Succi J., Tenover F.C., Koehler T.M. J. Bacteriol. 2003;185:823–830. doi: 10.1128/JB.185.3.823-830.2003. doi:10.1128/JB.185.3.823-830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W.H. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. doi:10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Christensen H., Martin M.T., Waley S.G. Biochem. J. 1990;266:853–861. [PMC free article] [PubMed] [Google Scholar]
- Copeland R.A. Tight Binding Inhibitors Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. New York: Wiley & Sons, Inc.; 2000. pp. 310–313. [Google Scholar]
- Doran J.L., Leskiw B.K., Aippersbach S., Jensen S.E. J. Bacteriol. 1990;172:4909–4918. doi: 10.1128/jb.172.9.4909-4918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empie M.W., Laskowski M., Jr Biochemistry. 1982;21:2274–2284. doi: 10.1021/bi00539a002. doi:10.1021/bi00539a002. [DOI] [PubMed] [Google Scholar]
- Faraci W.S., Pratt R.F. Biochemistry. 1985;24:903–910. doi: 10.1021/bi00325a014. doi:10.1021/bi00325a014. [DOI] [PubMed] [Google Scholar]
- Frere J.M. Mol. Microbiol. 1995;16:385–395. doi: 10.1111/j.1365-2958.1995.tb02404.x. doi:10.1111/j.1365-2958.1995.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Fulop V., Jones D.T. Curr. Opin. Struct. Biol. 1999;9:715–721. doi: 10.1016/s0959-440x(99)00035-4. doi:10.1016/S0959-440X(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Giakkoupi P., Tzelepi E., Legakis N.J., Tzouvelekis L.S. J. Antimicrob. Chemother. 1999;43:23–29. doi: 10.1093/jac/43.1.23. doi:10.1093/jac/43.1.23. [DOI] [PubMed] [Google Scholar]
- Gilbreth R.N., Esaki K., Koide A., Sidhu S.S., Koide S. J. Mol. Biol. 2008;381:407–418. doi: 10.1016/j.jmb.2008.06.014. doi:10.1016/j.jmb.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N.M., Work E. Biochem. J. 1953;54:347–352. doi: 10.1042/bj0540347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretes M., Lim D.C., de Castro L., Jensen S.E., Kang S.G., Lee K.J., Strynadka N.C. J. Mol. Biol. 2009;389:289–305. doi: 10.1016/j.jmb.2009.03.058. doi:10.1016/j.jmb.2009.03.058. [DOI] [PubMed] [Google Scholar]
- Guruprasad K., Dhamayanthi P. Int. J. Biol. Macromol. 2004;34:55–61. doi: 10.1016/j.ijbiomac.2004.03.003. doi:10.1016/j.ijbiomac.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hanes M.S., Jude K.M., Berger J.M., Bonomo R.A., Handel T.M. Biochemistry. 2009;48:9185–9193. doi: 10.1021/bi9007963. doi:10.1021/bi9007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Palzkill T. Proc. Natl Acad. Sci. USA. 1997;94:8801–8806. doi: 10.1073/pnas.94.16.8801. doi:10.1073/pnas.94.16.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Petrosino J., Hirsch M., Shenkin P.S., Palzkill T. J. Mol. Biol. 1996;258:688–703. doi: 10.1006/jmbi.1996.0279. doi:10.1006/jmbi.1996.0279. [DOI] [PubMed] [Google Scholar]
- Huang W., McKevitt M., Palzkill T. Gene. 2000;251:187–197. doi: 10.1016/s0378-1119(00)00210-9. doi:10.1016/S0378-1119(00)00210-9. [DOI] [PubMed] [Google Scholar]
- Huovinen P., Huovinen S., Jacoby G.A. Antimicrob. Agents Chemother. 1988;32:134–136. doi: 10.1128/aac.32.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Galleni M., Ma L., Frere J.M., Yamaguchi K. Int. J. Antimicrob. Agents. 2007;29:159–164. doi: 10.1016/j.ijantimicag.2006.09.005. doi:10.1016/j.ijantimicag.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Iwai Y., Ono H., Takeshima H., Yamaguchi N., Omura S. Antimicrob. Agents Chemother. 1973;4:222–225. doi: 10.1128/aac.4.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelesarov I., Bosshard H.R. J. Mol. Recognit. 1999;12:3–18. doi: 10.1002/(SICI)1099-1352(199901/02)12:1<3::AID-JMR441>3.0.CO;2-6. doi:10.1002/(SICI)1099-1352(199901/02)12:1<3::AID-JMR441>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Joris B., Dusart J., Frere J.M., van Beeumen J., Emanuel E.L., Petursson S., Gagnon J., Waley S.G. Biochem. J. 1984;223:271–274. doi: 10.1042/bj2230271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., De Meester F., Galleni M., Reckinger G., Coyette J., Frere J.M., Van Beeumen J. Biochem. J. 1985;228:241–248. doi: 10.1042/bj2280241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.G., Park H.U., Lee H.S., Kim H.T., Lee K.J. J. Biol. Chem. 2000;275:16851–16856. doi: 10.1074/jbc.M000227200. doi:10.1074/jbc.M000227200. [DOI] [PubMed] [Google Scholar]
- Kather I., Jakob R.P., Dobbek H., Schmid F.X. J. Mol. Biol. 2008;383:238–251. doi: 10.1016/j.jmb.2008.07.082. doi:10.1016/j.jmb.2008.07.082. [DOI] [PubMed] [Google Scholar]
- Kehoe J.W., Kay B.K. Chem. Rev. 2005;105:4056–4072. doi: 10.1021/cr000261r. doi:10.1021/cr000261r. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Lee K.J. Appl. Environ. Microbiol. 1994;60:1029–1032. doi: 10.1128/aem.60.3.1029-1032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S., Sidhu S.S. ACS Chem. Biol. 2009;4:325–334. doi: 10.1021/cb800314v. doi:10.1021/cb800314v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortemme T., Baker D. Curr. Opin. Chem. Biol. 2004;8:91–97. doi: 10.1016/j.cbpa.2003.12.008. doi:10.1016/j.cbpa.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Kuzin A.P., Nukaga M., Nukaga Y., Hujer A.M., Bonomo R.A., Knox J.R. Biochemistry. 1999;38:5720–5727. doi: 10.1021/bi990136d. doi:10.1021/bi990136d. [DOI] [PubMed] [Google Scholar]
- Laraki N., Franceschini N., Rossolini G.M., Santucci P., Meunier C., de Pauw E., Amicosante G., Frere J.M., Galleni M. Antimicrob. Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent P., Raquet X., Joris B., Van Beeumen J., Frere J.M. Biochem. J. 1993;292:555–562. doi: 10.1042/bj2920555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune A., Pain R.H., Charlier P., Frere J.M., Matagne A. Biochemistry. 2008;47:1186–1193. doi: 10.1021/bi701927y. doi:10.1021/bi701927y. [DOI] [PubMed] [Google Scholar]
- Lim D., Park H.U., De Castro L., Kang S.G., Lee H.S., Jensen S., Lee K.J., Strynadka N.C. Nat. Struct. Biol. 2001;8:848–852. doi: 10.1038/nsb1001-848. doi:10.1038/nsb1001-848. [DOI] [PubMed] [Google Scholar]
- Lowman H.B., Wells J.A. J. Mol. Biol. 1993;234:564–578. doi: 10.1006/jmbi.1993.1612. doi:10.1006/jmbi.1993.1612. [DOI] [PubMed] [Google Scholar]
- Lowman H.B., Bass S.H., Simpson N., Wells J.A. Biochemistry. 1991;30:10832–10838. doi: 10.1021/bi00109a004. doi:10.1021/bi00109a004. [DOI] [PubMed] [Google Scholar]
- Majiduddin F.K., Palzkill T. Antimicrob. Agents Chemother. 2005;49:3421–3427. doi: 10.1128/AAC.49.8.3421-3427.2005. doi:10.1128/AAC.49.8.3421-3427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano D.C., Pennington J.M., Wang X., Wang J., Chen Y., Thomas V.L., Shoichet B.K., Palzkill T. J. Mol. Biol. 2008;384:151–164. doi: 10.1016/j.jmb.2008.09.009. doi:10.1016/j.jmb.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano D.C., Brown N.G., Palzill T. Protein Sci. 2009;18:2080–2089. doi: 10.1002/pro.220. doi:10.1002/pro.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne A., Lamotte-Brasseur J., Frere J.M. Biochem. J. 1998;330:581–598. doi: 10.1042/bj3300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materon I.C., Queenan A.M., Koehler T.M., Bush K., Palzkill T. Antimicrob. Agents Chemother. 2003;47:2040–2042. doi: 10.1128/AAC.47.6.2040-2042.2003. doi:10.1128/AAC.47.6.2040-2042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momand J., Wu H.H., Dasgupta G. Gene. 2000;242:15–29. doi: 10.1016/s0378-1119(99)00487-4. doi:10.1016/S0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- Morrison J.F. Biochim. Biophys Acta. 1969;185:269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Morrison K.L., Weiss G.A. Curr. Opin. Chem. Biol. 2001;5:302–307. doi: 10.1016/s1367-5931(00)00206-4. doi:10.1016/S1367-5931(00)00206-4. [DOI] [PubMed] [Google Scholar]
- Murphy D.J. Anal. Biochem. 2004;327:61–67. doi: 10.1016/j.ab.2003.12.018. doi:10.1016/j.ab.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Naas T., Vandel L., Sougakoff W., Livermore D.M., Nordmann P. Antimicrob. Agents Chemother. 1994;38:1262–1270. doi: 10.1128/aac.38.6.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Poirel L. Clin. Microbiol. Infect. 2002;8:321–331. doi: 10.1046/j.1469-0691.2002.00401.x. doi:10.1046/j.1469-0691.2002.00401.x. [DOI] [PubMed] [Google Scholar]
- Nugent M.E., Hedges R.W. Mol. Gen. Genet. 1979;175:239–243. doi: 10.1007/BF00397222. doi:10.1007/BF00397222. [DOI] [PubMed] [Google Scholar]
- Olson A.B., Silverman M., Boyd D.A., McGeer A., Willey B.M., Pong-Porter V., Daneman N., Mulvey M.R. Antimicrob. Agents Chemother. 2005;49:2112–2115. doi: 10.1128/AAC.49.5.2112-2115.2005. doi:10.1128/AAC.49.5.2112-2115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H., Matsumae A., Iwai Y., Nakae M., Omura S. Antimicrob. Agents Chemother. 1973;4:226–230. doi: 10.1128/aac.4.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal G., Fong S.Y., Kossiakoff A.A., Sidhu S.S. Protein Sci. 2005;14:2405–2413. doi: 10.1110/ps.051519805. doi:10.1110/ps.051519805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal G., Kouadio J.L., Artis D.R., Kossiakoff A.A., Sidhu S.S. J. Biol. Chem. 2006;281:22378–22385. doi: 10.1074/jbc.M603826200. doi:10.1074/jbc.M603826200. [DOI] [PubMed] [Google Scholar]
- Paoli M. Prog. Biophys. Mol. Biol. 2001;76:103–130. doi: 10.1016/s0079-6107(01)00007-4. doi:10.1016/S0079-6107(01)00007-4. [DOI] [PubMed] [Google Scholar]
- Park H.U., Lee K.J. Microbiology. 1998;144 ( Pt 8):2161–2167. doi: 10.1099/00221287-144-8-2161. doi:10.1099/00221287-144-8-2161. [DOI] [PubMed] [Google Scholar]
- Parmley S.F., Smith G.P. Gene. 1988;73:305–318. doi: 10.1016/0378-1119(88)90495-7. doi:10.1016/0378-1119(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Paschke M. Appl. Microbiol. Biotechnol. 2006;70:2–11. doi: 10.1007/s00253-005-0270-9. doi:10.1007/s00253-005-0270-9. [DOI] [PubMed] [Google Scholar]
- Paterson D.L., Bonomo R.A. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. doi:10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino J., Rudgers G., Gilbert H., Palzkill T. J. Biol. Chem. 1999;274:2394–2400. doi: 10.1074/jbc.274.4.2394. doi:10.1074/jbc.274.4.2394. [DOI] [PubMed] [Google Scholar]
- Rakonjac J., Jovanovic G., Model P. Gene. 1997;198:99–103. doi: 10.1016/s0378-1119(97)00298-9. doi:10.1016/S0378-1119(97)00298-9. [DOI] [PubMed] [Google Scholar]
- Raquet X., Lamotte-Brasseur J., Bouillenne F., Frere J.M. Proteins. 1997;27:47–58. doi: 10.1002/(sici)1097-0134(199701)27:1<47::aid-prot6>3.0.co;2-k. doi:10.1002/(SICI)1097-0134(199701)27:1<47::AID-PROT6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Reichmann D., Rahat O., Albeck S., Meged R., Dym O., Schreiber G. Proc. Natl Acad. Sci. USA. 2005;102:57–62. doi: 10.1073/pnas.0407280102. doi:10.1073/pnas.0407280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann D., Cohen M., Abramovich R., Dym O., Lim D., Strynadka N.C., Schreiber G. J. Mol. Biol. 2007a;365:663–679. doi: 10.1016/j.jmb.2006.09.076. doi:10.1016/j.jmb.2006.09.076. [DOI] [PubMed] [Google Scholar]
- Reichmann D., Rahat O., Cohen M., Neuvirth H., Schreiber G. Curr. Opin. Struct. Biol. 2007b;17:67–76. doi: 10.1016/j.sbi.2007.01.004. doi:10.1016/j.sbi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Reynolds K.A., Thomson J.M., Corbett K.D., Bethel C.R., Berger J.M., Kirsch J.F., Bonomo R.A., Handel T.M. J. Biol. Chem. 2006;281:26745–26753. doi: 10.1074/jbc.M603878200. doi:10.1074/jbc.M603878200. [DOI] [PubMed] [Google Scholar]
- Reynolds K.A., Hanes M.S., Thomson J.M., Antczak A.J., Berger J.M., Bonomo R.A., Kirsch J.F., Handel T.M. J. Mol. Biol. 2008;382:1265–1275. doi: 10.1016/j.jmb.2008.05.051. doi:10.1016/j.jmb.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossolini G.M., D'Andrea M.M., Mugnaioli C. Clin. Microbiol. Infect. 2008;14(Suppl. 1):33–41. doi: 10.1111/j.1469-0691.2007.01867.x. doi:10.1111/j.1469-0691.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- Rudgers G.W., Palzkill T. J. Biol. Chem. 1999;274:6963–6971. doi: 10.1074/jbc.274.11.6963. doi:10.1074/jbc.274.11.6963. [DOI] [PubMed] [Google Scholar]
- Rudgers G.W., Huang W., Palzkill T. Antimicrob. Agents Chemother. 2001;45:3279–3286. doi: 10.1128/AAC.45.12.3279-3286.2001. doi:10.1128/AAC.45.12.3279-3286.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G., Fersht A.R. Biochemistry. 1993;32:5145–5150. doi: 10.1021/bi00070a025. doi:10.1021/bi00070a025. [DOI] [PubMed] [Google Scholar]
- Shah A.A., Hasan F., Ahmed S., Hameed A. Res. Microbiol. 2004;155:409–421. doi: 10.1016/j.resmic.2004.02.009. doi:10.1016/j.resmic.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Shi Y., Hata A., Lo R.S., Massague J., Pavletich N.P. Nature. 1997;388:87–93. doi: 10.1038/40431. doi:10.1038/40431. [DOI] [PubMed] [Google Scholar]
- Shlaes D.M., Currie-McCumber C. Biochem. J. 1992;284:411–415. doi: 10.1042/bj2840411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideraki V., Huang W., Palzkill T., Gilbert H.F. Proc. Natl Acad. Sci. USA. 2001;98:283–288. doi: 10.1073/pnas.011454198. doi:10.1073/pnas.011454198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu S.S., Koide S. Curr. Opin. Struct. Biol. 2007;17:481–487. doi: 10.1016/j.sbi.2007.08.007. doi:10.1016/j.sbi.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Sidhu S.S., Lowman H.B., Cunningham B.C., Wells J.A. Methods Enzymol. 2000a;328:333–363. doi: 10.1016/s0076-6879(00)28406-1. doi:10.1016/S0076-6879(00)28406-1. [DOI] [PubMed] [Google Scholar]
- Sidhu S.S., Weiss G.A., Wells J.A. J. Mol. Biol. 2000b;296:487–495. doi: 10.1006/jmbi.1999.3465. doi:10.1006/jmbi.1999.3465. [DOI] [PubMed] [Google Scholar]
- Simpson I.N., Harper P.B., O'Callaghan C.H. Antimicrob. Agents Chemother. 1980;17:929–936. doi: 10.1128/aac.17.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.P., Petrenko V.A. Chem. Rev. 1997;97:391–410. doi: 10.1021/cr960065d. doi:10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- Smith G.P., Yu J. Mol. Divers. 1996;2:2–4. doi: 10.1007/BF01718693. doi:10.1007/BF01718693. [DOI] [PubMed] [Google Scholar]
- Sosa-Peinado A., Mustafi D., Makinen M.W. Protein Expr. Purif. 2000;19:235–245. doi: 10.1006/prep.2000.1243. doi:10.1006/prep.2000.1243. [DOI] [PubMed] [Google Scholar]
- Stevens T.J., Paoli M. Proteins. 2008;70:378–387. doi: 10.1002/prot.21521. doi:10.1002/prot.21521. [DOI] [PubMed] [Google Scholar]
- Strynadka N.C., Jensen S.E., Johns K., Blanchard H., Page M., Matagne A., Frere J.M., James M.N. Nature. 1994;368:657–660. doi: 10.1038/368657a0. doi:10.1038/368657a0. [DOI] [PubMed] [Google Scholar]
- Strynadka N.C., Jensen S.E., Alzari P.M., James M.N. Nat. Struct. Biol. 1996;3:290–297. doi: 10.1038/nsb0396-290. doi:10.1038/nsb0396-290. [DOI] [PubMed] [Google Scholar]
- Tesar M., Beckmann C., Rottgen P., Haase B., Faude U., Timmis K.N. Immunotechnology. 1995;1:53–64. doi: 10.1016/1380-2933(95)00005-4. doi:10.1016/1380-2933(95)00005-4. [DOI] [PubMed] [Google Scholar]
- Thomson J.M., Distler A.M., Prati F., Bonomo R.A. J. Biol. Chem. 2006;281:26734–26744. doi: 10.1074/jbc.M603222200. doi:10.1074/jbc.M603222200. [DOI] [PubMed] [Google Scholar]
- Tzouvelekis L.S., Bonomo R.A. Curr. Pharm. Des. 1999;5:847–864. [PubMed] [Google Scholar]
- Walsh T.R. Curr. Opin. Infect. Dis. 2008;21:367–371. doi: 10.1097/QCO.0b013e328303670b. doi:10.1097/QCO.0b013e328303670b. [DOI] [PubMed] [Google Scholar]
- Walther-Rasmussen J., Hoiby N. J. Antimicrob. Chemother. 2007;60:470–482. doi: 10.1093/jac/dkm226. doi:10.1093/jac/dkm226. [DOI] [PubMed] [Google Scholar]
- Wang J., Palzkill T., Chow D.C. J. Biol. Chem. 2009;284:595–609. doi: 10.1074/jbc.M804089200. doi:10.1074/jbc.M804089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G.A., Watanabe C.K., Zhong A., Goddard A., Sidhu S.S. Proc. Natl Acad. Sci. USA. 2000;97:8950–8954. doi: 10.1073/pnas.160252097. doi:10.1073/pnas.160252097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Smith G.P. Methods Enzymol. 1996;267:3–27. doi: 10.1016/s0076-6879(96)67003-7. doi:10.1016/S0076-6879(96)67003-7. [DOI] [PubMed] [Google Scholar]
- Yuan J., Huang W., Chow D.C., Palzkill T. J. Mol. Biol. 2009a;389:401–412. doi: 10.1016/j.jmb.2009.04.028. doi:10.1016/j.jmb.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Liu J.H., Hu G.Z., Pan Y.S., Liu Z.M., Mo J., Wei Y.J. J. Med. Microbiol. 2009b;58:1449–1453. doi: 10.1099/jmm.0.012229-0. doi:10.1099/jmm.0.012229-0. [DOI] [PubMed] [Google Scholar]
- Zawadzke L.E., Smith T.J., Herzberg O. Protein Eng. 1995;8:1275–1285. doi: 10.1093/protein/8.12.1275. doi:10.1093/protein/8.12.1275. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Palzkill T. J. Biol. Chem. 2003;278:45706–45712. doi: 10.1074/jbc.M308572200. doi:10.1074/jbc.M308572200. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Palzkill T. J. Biol. Chem. 2004;279:42860–42866. doi: 10.1074/jbc.M406157200. doi:10.1074/jbc.M406157200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.